Back to Journals » Journal of Asthma and Allergy » Volume 10
Anaphylaxis across two Canadian pediatric centers: evaluating management disparities
Authors Lee AYM, Enarson P, Clarke AE, La Vieille S, Eisman H, Chan ES, Mill C, Joseph L, Ben-Shoshan M
Received 23 September 2016
Accepted for publication 4 November 2016
Published 30 December 2016 Volume 2017:10 Pages 1—7
DOI https://doi.org/10.2147/JAA.S123053
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Luis Garcia-Marcos
Alison YM Lee,1 Paul Enarson,2 Ann E Clarke,3 Sébastien La Vieille,4 Harley Eisman,5,6 Edmond S Chan,7 Christopher Mill,7 Lawrence Joseph,8 Moshe Ben-Shoshan9
1Pediatric Residency Program, Department of Pediatrics, University of British Columbia, BC Children’s Hospital, 2Division of Emergency Medicine, Department of Pediatrics, University of British Columbia, Vancouver, BC, 3Division of Rheumatology, Department of Medicine, Cumming School of Medicine, University of Calgary, Calgary, AB, 4Food Directorate, Health Canada, Ottawa, ON, 5Emergency Department, 6Department of Pediatrics, Montreal Children’s Hospital, Montreal, QC, 7Division of Allergy and Immunology, Department of Pediatrics, BC Children’s Hospital, University of British Columbia, Vancouver, BC, 8Department of Epidemiology and Biostatistics, McGill University, 9Division of Allergy and Clinical Immunology, Department of Pediatrics, Montreal Children’s Hospital, Montreal, QC, Canada
Background: There are no data on the percentage of visits due to anaphylaxis in the emergency department (ED), triggers, and management of anaphylaxis across different provinces in Canada.
Objective: To compare the percentage of anaphylaxis cases among all ED visits, as well as the triggers and management of anaphylaxis between two Canadian pediatric EDs (PEDs).
Methods: As part of the Cross-Canada Anaphylaxis Registry (C-CARE), children presenting to the British Columbia Children’s Hospital (BCCH) and Montreal Children’s Hospital (MCH) EDs with anaphylaxis were recruited. Characteristics, triggers, and management of anaphylaxis were documented using a standardized data entry form. Differences in demographics, triggers, and management were determined by comparing the difference of proportions and 95% confidence interval.
Results: Between June 2014 and June 2016, there were 346 visits due to anaphylaxis among 93,730 PED visits at the BCCH ED and 631 anaphylaxis visits among 164,669 pediatric visits at the MCH ED. In both centers, the majority of cases were triggered by food (BCCH 91.3% [88.7, 94.0], MCH 82.4% [79.7, 85.3]), of which peanuts were the most common culprit (24.7% [20.9, 29.9] and 19.0% [15.8, 22.7], respectively). Pre-hospital administration of epinephrine (BCCH 27.7% [23.2, 32.8], MCH 33.1% [29.5, 37.0]) and antihistamines (BCCH 50.6% [45.2, 56.0], MCH 47.1% [43.1, 51.0]) was similar. In-hospital management differed in terms of increased epinephrine, antihistamine, and steroid use at the BCCH (59.2% [53.9, 64.4], 59.8% [54.4, 65.0], and 60.1% [54.7, 65.3], respectively) compared to the MCH (42.2% [38.3, 46.2], 36.2% [32.5, 40.1], and 11.9% [9.5, 14.8], respectively). Despite differences in management, percentage of cases admitted to the intensive care unit was similar between the two centers.
Conclusion: Compared to previous European and North American reports, there is a high percentage of anaphylaxis cases in two PEDs across Canada with substantial differences in hospital management practices. It is crucial to develop training programs that aim to increase epinephrine use in anaphylaxis.
Keywords: anaphylaxis, emergency department, epinephrine, triggers of anaphylaxis, management
Introduction
Anaphylaxis is an acute, severe, and potentially life-threatening systemic allergic reaction.1 In the United States, the rate of anaphylaxis presentations to pediatric emergency departments (PEDs) ranges from 0.18% to 0.45%,2–4 and globally, the incidence of anaphylaxis is increasing.3,5–7 In Canada, the overall prevalence of anaphylaxis is unknown, but we have previously reported that 0.21% of PED visits are due to anaphylaxis and that this percentage almost doubled over a 4-year period at the emergency department (ED) in Montreal.8,9 However, it is not clear if this high rate of anaphylaxis is reflective of PEDs in other Canadian provinces. For children presenting with anaphylaxis, food is the most common trigger,8–10 though specific triggers vary by geography and ethnicity.7 A Canadian cross-sectional survey identified peanuts and tree nuts as the most common allergens.11
While Canadian guidelines exist for diagnosing and managing anaphylaxis,12 there is a paucity of research on actual pre-hospital and ED management variations across Canada. Knowledge gaps pertaining to anaphylaxis management have previously been identified,13 with the most concern relating to epinephrine administration for anaphylaxis. A Canadian survey found that 25% of pediatric health care providers would not give epinephrine for severe anaphylaxis.13 Currently, there have not been any multicenter studies examining practice variations that may exist between centers despite availability of guidelines.
The goal of this study was to compare the percentage, triggers, and management of anaphylaxis between two Canadian tertiary hospitals, the British Columbia Children’s Hospital (BCCH) and the Montreal Children’s Hospital (MCH). We hypothesized that variations would exist between patient characteristics, triggers, and management between the centers.
Methods
Study population and setting
The BCCH in Vancouver is the only dedicated tertiary-level PED in the province of BC and receives >40,000 visits annually. The MCH in Montreal is one of three tertiary PEDs in the province of Quebec and receives 80,000 visits annually. Both these EDs are primarily staffed by board-certified pediatric emergency medicine subspecialists, and in both hospitals, there is an allergy division providing consultations by phone and/or in person.
As part of the Cross-Canada Anaphylaxis Registry (C-CARE), patients presenting to the BCCH and MCH with a diagnosis of anaphylaxis were prospectively recruited. The diagnosis of anaphylaxis was based on consensus expert criteria from the Second Symposium on the Definition and Management of Anaphylaxis,1 including involvement of two organ systems and/or the presence of hypotension in response to a potential allergen. Parents or legal guardians of patients recruited at the ED provided written informed consent at each site between June 2014 and June 2016. During this time period, cases that were missed prospectively were identified via chart review with the International Classification of Disease codes (ICD-10) or written discharge diagnoses relating to anaphylaxis or allergic reaction and retrospectively captured as previously described.9,14 Two independent teams (three reviewers at the BCCH and two reviewers at the MCH) analyzed all cases to verify that they met the definition of anaphylaxis.
Study protocol
During prospective recruitment after obtaining the consent, a questionnaire was completed by the treating ED physician. Data collected included demographics (age and sex), comorbid conditions (history of atopy including asthma, eczema, and prior diagnosis of food allergy), presenting clinical characteristics, potential allergic triggers, route of exposure and time interval between exposure and development of symptoms, presence of cofactors (use of nonsteroidal anti-inflammatory drugs, alcohol, exercise within 2 hours of reaction, use of medications), and pre-hospital and ED management. For cases missed during prospective recruitment, the same information was collected retrospectively through chart review.
Anaphylaxis severity was classified according to a modified grading system published by Brown.15 Signs and symptoms of a mild anaphylactic reaction included sudden generalized pruritus or urticaria, itching of the eyes and nose, oral pruritus or tingling, throat pruritus or tightness, flushing, angioedema, mild lip swelling, nausea or emesis, mild abdominal pain, nasal congestion or sneezing, mild wheezing, and tachycardia. A moderate reaction was defined by crampy abdominal pain, recurrent emesis, hoarseness, barky cough, difficulty swallowing, stridor, dyspnea, or moderate wheezing. A severe reaction included loss of bowel control, cyanosis, desaturation <92%, or respiratory arrest.
Analytic approach
Descriptive statistics with percentages and 95% binomial or multinomial (for variables with more than two categories) confidence intervals (CIs) were used to calculate the rates of anaphylaxis among all ED visits and to describe the comorbid conditions, triggers, severity, and pre-hospital and ED management. Age was presented as medians with interquartile ranges (IQRs). Differences in demographics, triggers, and pre-hospital and in-hospital management were determined by comparing the difference of proportions and 95% CI. Univariate and multivariate regression models were compared to identify demographic factors, anaphylaxis triggers, and comorbidities associated with use of epinephrine.
Statistical analysis was conducted using R version 2.12.0, Vienna, Austria. Ethics approval was obtained from the Children’s and Women’s Health Centre of British Columbia Research Ethics Board and the McGill University Health Centre Ethics Review Board for this study.
Results
Between June 2014 and June 2015, there were 158 cases of anaphylaxis among 46,321 visits at the BCCH ED versus 323 cases among 83,684 visits at the MCH ED (0.34% [95% CI, 0.29%, 0.40%] versus 0.39% [0.34%, 0.43%], respectively, with a difference of −0.05% [−0.1%, 0.02%]).
Between June 2015 and June 2016, there were 188 cases of anaphylaxis among 47,409 visits at the BCCH ED versus 308 cases among 80,985 visits at the MCH ED (0.40% [95% CI, 0.34%, 0.46%] versus 0.38% [0.34%, 0.43%], respectively, with a difference of 0.02% [−0.06%, 0.09%]).
Patient demographics and atopic comorbidities were similar between the two sites apart from higher percentage of males and history of food allergy in BCCH ED (Table 1). Comparison of prospective and retrospective cases (Table 2) did not reveal substantial differences apart from higher percentage of eczema in the MCH prospective cases.
Although food was the principal trigger at both sites (91.3% [95% CI, 88.7%, 94.0%] of all cases of anaphylaxis at the BCCH and 82.4% [79.7%, 85.3%] at the MCH), BCCH’s percentage was almost 10% higher (likely due to higher percentage of unknown and other triggers at the MCH). Peanuts were the most common culprit at both sites, accounting for almost 20% of all food-triggered reactions, followed by tree nuts. The rates of anaphylaxis from venom, drugs, and other triggers were similar (Table 3). The distribution of anaphylaxis severity was similar between sites, with the majority of cases classified as moderate anaphylaxis (Table 4).
  | Table 3 Anaphylaxis triggers Abbreviations: BCCH, British Columbia Children’s Hospital; MCH, Montreal Children’s Hospital; Diff, difference; CI, confidence interval; PN, peanut; TNs, tree nuts. |
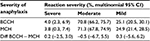  | Table 4 Severity of anaphylaxis between BCCH and MCH Abbreviations: BCCH, British Columbia Children’s Hospital; MCH, Montreal Children’s Hospital; CI, confidence interval; Diff, difference. |
Pre-hospital anaphylaxis management was comparable in terms of epinephrine, antihistamine, and steroid use between both centers (Table 5). However, there were substantial disparities in the management of anaphylaxis in the ED. Epinephrine was used more often at the BCCH. This difference was mainly related to higher percentages of epinephrine use in the BCCH ED during mild and moderate reactions (Table 5). This higher usage of medications in the BCCH ED was even more prominent for antihistamines and steroids. Steroids were used almost five times more often in the BCCH ED. Less than 3% of cases were admitted to the intensive care unit or hospital ward in both centers (Table 6).
At both the BCCH and MCH, epinephrine use inside the ED was less likely (adjusted odds ratio [aOR]: 0.06 [95% CI, 0.03, 0.12] and 0.2 [0.11, 0.25], respectively) among cases that used epinephrine prior to ED arrival and more likely in severe cases (aOR: 3.9 [1.03, 14.91] and aOR: 2.7 [1.08, 6.91], respectively). Older children at the MCH ED were more likely to receive epinephrine (aOR: 1.03 [1.00, 1.07])(Table 7).
Discussion
This is the first multicenter comparison study assessing anaphylaxis characteristics, triggers, and management differences between two Canadian tertiary PEDs. Our results indicate that the high burden of anaphylaxis among all ED visits is comparable, but substantial differences in management practices exist between these centers.
Visits due to anaphylaxis in both centers were higher than previous reports in North America and Europe.2,8,16 Furthermore, a recent study by our group reveals almost a doubling in anaphylaxis over a 4-year period in Montreal.9 The high burden of anaphylaxis in the ED, in particular from food-induced reactions, is in keeping with reports on increased prevalence of food allergy, especially peanut allergy.17 Previous literature has found food as the main culprit of anaphylaxis, accounting for 37–85% of all reactions.8,18 Our findings at the BCCH and MCH showed that food triggers were responsible for >80% of anaphylactic presentations, further supporting previous findings. The higher proportion of food triggers, of which peanuts and tree nuts were the most common culprits, suggests that Canada continues to have a higher burden of nut allergies compared to other parts of the world.17 The BCCH and MCH had similar distributions of identifiable allergic triggers, of which peanuts, tree nuts, milk, and egg were the most common, which is also in keeping with data found in the SCAAALAR survey (Surveying Canadians to Assess the prevalence of food Allergies and Attitudes towards food LAbelling and Risk).11
Previous surveys have found low rates of epinephrine administration (7–19%) across North America.19,20 The relatively higher rates of epinephrine administration across both the BCCH and MCH EDs may be related to the fact that both are tertiary academic centers with access to pediatric allergists and high volumes of academically rigorous learners. Despite this, there was greater use of epinephrine, antihistamines, and steroids in the BCCH. Although guidelines for anaphylaxis management include all three medications, epinephrine is the only medication capable of stopping the progression of anaphylaxis regardless of reaction severity.21 Given that severe reaction and pre-hospital use of epinephrine were the only factors associated with epinephrine use in the ED and given that these variables did not differ between sites, it is likely that unmeasured factors such as care-provider-related factors account for the differences. Care-provider factors likely include training and access to educational programs. Prior work by our group specifically at the MCH ED revealed that pre-hospital use of antihistamines and/or steroids often replaces early use of epinephrine8,9 and that prompt use of epinephrine prior to hospital arrival is crucial in the management of anaphylaxis and reduces the risk for uncontrolled reactions requiring multiple doses of epinephrine.9 It is possible that the findings of these earlier studies that have been presented to physicians at the MCH ED led to lower use of these medications at the MCH ED compared to the BCCH ED.
Pre-hospital epinephrine administration continues to be an area of challenge, with low rates observed in both Vancouver and Montreal. This is likely multifactorial,22 including lack of comfort from parents or child supervisors and education gaps for emergency medical service personnel, as previously found in multiple surveys.23 Pre-hospital administration rates of second-line antihistamines were higher than first-line epinephrine medication in both Vancouver and Montreal, further substantiating previous studies,19,20 which is an ongoing concern given the lack of evidence for antihistamine use in anaphylaxis treatment.24 Antihistamine and steroid use in the ED were significantly higher in Vancouver compared to Montreal, which may be reflective of local teaching and practice. While H1 antihistamines can help alleviate cutaneous symptoms of anaphylaxis, they are not lifesaving and should not replace or delay epinephrine administration.1 A 2007 Cochrane review found no randomized or quasi-randomized control trials assessing the effectiveness of H1 antihistamines in anaphylaxis and thus could not make any recommendations.24 Similarly, a recent Cochrane study showed inconclusive evidence for steroid use in anaphylaxis.25 Given that studies by our group and others7,9 suggest that early epinephrine use is key in the management of anaphylaxis and given that epinephrine use at the pre-hospital setting was similar between the two centers, it is not surprising that in-hospital differences in the use of epinephrine did not increase the risk of negative outcomes including admission to the intensive care unit or hospital ward. Univariate and multivariate logistic regression analyses did not reveal any significant associations between admission and patient’s demographics, comorbidities, type of anaphylaxis trigger, or timing of epinephrine/antihistamines/steroid administration (pre-arrival or at the ED).
Limitations to this study include its inclusion of only two tertiary pediatric centers, which may not be representative of practice variations across other tertiary pediatric centers across Canada. Another potential limitation is that we did not measure height and therefore were unable to calculate body mass index, which may influence the risk of atopic conditions.26 In addition, both institutions, staffed by subspecialty pediatric emergency medicine physicians, had high rates of epinephrine use, which may not be consistent with rates seen in community hospitals. Although effort was made to capture all patients prospectively, ~50% of cases were captured retrospectively, which may lead to misclassification bias due to different quality of information between comparison groups.27 However, this is likely non-differential and did not bias our results given that retrospective and prospective cases were similar in demographics, reaction characteristics, and management.
Conclusion
This is the first multicenter comparative study that examines anaphylaxis rate, characteristics, triggers, and management. Overall, there was a high burden of anaphylaxis in both EDs with similar demographics and comorbidities across both populations. Anaphylactic triggers and common food culprits were comparable across both centers and consistent with previous literature. The high rates of epinephrine administration were encouraging, though significant practice differences with antihistamine and steroid treatment were observed. Although physicians at BCCH ED use more antihistamines and steroids, these are not replacing the use of epinephrine, which is also used more frequently at BCCH ED. There is controversy surrounding the role of both of these second-line treatments in the literature, and this likely results in the observed practice variation between the two sites.
Acknowledgments
Funding for this project was provided by the Allergy, Genes and Environment Network of Centres of Excellence (AllerGEN NCE) and Health Canada. The authors have no financial relationships relevant to this article to disclose.
Disclosure
The authors report no conflicts of interest in this work.
References
Sampson HA, Munoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report – second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. Ann Emerg Med. 2006;47(4):373–380. | ||
Huang F, Chawla K, Jarvinen KM, Nowak-Wegrzyn A. Anaphylaxis in a New York City pediatric emergency department: triggers, treatments, and outcomes. J Allergy Clin Immunol. 2012;129(1):162–168. | ||
Lieberman P. Epidemiology of anaphylaxis. Curr Opin Allergy Clin Immunol. 2008;8(4):316–320. | ||
Russell S, Monroe K, Losek JD. Anaphylaxis management in the pediatric emergency department: opportunities for improvement. Pediatr Emerg Care. 2010;26(2):71–76. | ||
Lieberman P, Nicklas RA, Oppenheimer J, et al. The diagnosis and management of anaphylaxis practice parameter: 2010 update. J Allergy Clin Immunol. 2010;126(3):477–480. | ||
Wood RA, Camargo CA Jr, Lieberman P, et al. Anaphylaxis in America: the prevalence and characteristics of anaphylaxis in the United States. J Allergy Clin Immunol. 2014;133(2):461–467. | ||
Simons FE, Ardusso LR, Dimov V, et al. World Allergy Organization Anaphylaxis Guidelines: 2013 update of the evidence base. Int Arch Allergy Immunol. 2013;162(3):193–204. | ||
Ben-Shoshan M, La VS, Eisman H, et al. Anaphylaxis treated in a Canadian pediatric hospital: incidence, clinical characteristics, triggers, and management. J Allergy Clin Immunol. 2013;132(3):739–741. | ||
Hochstadter E, Clarke A, De SS, et al. Increasing visits for anaphylaxis and the benefits of early epinephrine administration: a 4-year study at a pediatric emergency department in Montreal, Canada. J Allergy Clin Immunol. 2016;137(6):1888–1890. | ||
de Silva IL, Mehr SS, Tey D, Tang ML. Paediatric anaphylaxis: a 5 year retrospective review. Allergy. 2008;63(8):1071–1076. | ||
Soller L, Ben-Shoshan M, Harrington DW, et al. Overall prevalence of self-reported food allergy in Canada. J Allergy Clin Immunol. 2012;130(4):986–988. | ||
Cheng A. Emergency treatment of anaphylaxis in infants and children. Paediatr Child Health. 2011;16(1):35–40. | ||
Desjardins M, Clarke A, Alizadehfar R, et al. Comparison between allergists and non-allergists on issues related to food-induced anaphylaxis. J Allergy Clin Immunol Pract. 2013;1(3):289–294. | ||
Asai Y, Yanishevsky Y, Clarke A, et al. Rate, triggers, severity and management of anaphylaxis in adults treated in a Canadian emergency department. Int Arch Allergy Immunol. 2014;164(3):246–252. | ||
Brown SG. Clinical features and severity grading of anaphylaxis. J Allergy Clin Immunol. 2004;114(2):371–376. | ||
Vetander M, Helander D, Flodstrom C, et al. Anaphylaxis and reactions to foods in children – a population-based case study of emergency department visits. Clin Exp Allergy. 2012;42(4):568–577. | ||
Ben-Shoshan M, Turnbull E, Clarke A. Food allergy: temporal trends and determinants. Curr Allergy Asthma Rep. 2012;12(4):346–372. | ||
Braganza SC, Acworth JP, Mckinnon DR, Peake JE, Brown AF. Paediatric emergency department anaphylaxis: different patterns from adults. Arch Dis Child. 2006;91(2):159–163. | ||
Clark S, Bock SA, Gaeta TJ, et al. Multicenter study of emergency department visits for food allergies. J Allergy Clin Immunol. 2004;113(2):347–352. | ||
Gaeta TJ, Clark S, Pelletier AJ, Camargo CA. National study of US emergency department visits for acute allergic reactions, 1993 to 2004. Ann Allergy Asthma Immunol. 2007;98(4):360–365. | ||
Gold MS, Sainsbury R. First aid anaphylaxis management in children who were prescribed an epinephrine autoinjector device (EpiPen). J Allergy Clin Immunol. 2000;106(1 pt 1):171–176. | ||
Waserman S, Chad Z, Francoeur MJ, et al. Management of anaphylaxis in primary care: Canadian expert consensus recommendations. Allergy. 2010;65(9):1082–1092. | ||
Jacobsen RC, Toy S, Bonham AJ, Salomone JA 3rd, Ruthstrom J, Gratton M. Anaphylaxis knowledge among paramedics: results of a national survey. Prehosp Emerg Care. 2012;16(4):527–534. | ||
Sheikh A, Ten Broek V, Brown SG, Simons FE. H1-antihistamines for the treatment of anaphylaxis: Cochrane systematic review. Allergy. 2007;62(8):830–837. | ||
Choo KJ, Simons FE, Sheikh A. Glucocorticoids for the treatment of anaphylaxis. Cochrane Database Syst Rev. 2012;4:CD007596. | ||
Silverberg JI, Simpson EL. Association between obesity and eczema prevalence, severity and poorer health in US adolescents. Dermatitis. 2014;25(4):172–181. | ||
Delgado-Rodriguez M, Llorca J. Bias. J Epidemiol Community Health. 2004;58(8):635–641. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





