Back to Journals » Research and Reports in Urology » Volume 13
Analysis of Ureteral Tumour Stents for Malignant Ureteral Obstruction: Towards Reshaping an Optimal Stent
Authors Vogt B , Blanchet LH
Received 20 August 2021
Accepted for publication 18 October 2021
Published 27 October 2021 Volume 2021:13 Pages 773—782
DOI https://doi.org/10.2147/RRU.S334277
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Panagiotis J Vlachostergios
Benoît Vogt,1 Laure-Hélène Blanchet2
1Department of Urology, Polyclinique de Blois, La Chaussée Saint-Victor, 41260, France; 2Department of Public Health, Hôpital Saint-Antoine (AP-HP), Paris, 75012, France
Correspondence: Benoît Vogt
Department of Urology, Polyclinique de Blois, 1 Rue Robert Debré, La Chaussée Saint-Victor, 41260, France
Tel +33 254906511
Fax +33 254906566
Email [email protected]
Introduction: Ureteral obstruction hinders the management of malignant diseases. Adequate stent placement does not necessarily guarantee renal decompression. The stent stiffness may play a major role to maintain patency. We carried out the present study in order to evaluate drainage efficiency by using stents with distinctive degrees of stiffness and to identify the physical factors that could prevent obstruction of the stent in patients with malignant ureteral obstruction (MUO).
Materials and Methods: We performed an analysis of 150 patients with MUO drainage at a single institution from June 2009 to June 2019. A progressive choice of stents was shaped to overcome each failure by focusing on the criterion of increasingly stiff stents.
Results: During the study period, 556 ureteral stent procedures (USP) were analysed separately. The stent failure with obstruction occurred in 23.0% (128/556) of USP at a mean of 4.4± 3.6 months and depended on the type of stent. Stent failure occurred in 34.2% (70/205) of Vortek® stents, in 42.9% (15/35) of Urosoft stents, in 15.4% (39/254) of Superglide or ureteral catheters and in 6.5% (4/62) of tandem stents. No significant differences were found between Vortek® and Urosoft stents regarding stent failures, but there were significant differences between Superglide or Tandem stents and Vortek® or Urosoft stents (p< 10− 7). The study demonstrated that ureteral stent obstruction significantly decreased with a larger lumen or a stiffer stent (p< 10− 7).
Conclusion: In the present study, Superglide and tandem stents were the best stents against stent failure, and the lumen and the stiffness of the stent have been shown to be critical factors in controlling patency. The results suggest that the lumen seems more important than the stiffness, and the stiffness would be the only means of keeping the lumen intact. Future stents for MUO should integrate the importance of the lumen of the stent.
Keywords: malignant ureteral obstruction, tandem ureteral stent, ureteral stent, stent failure, diameter, prognostic factors
Introduction
Malignant ureteral obstruction (MUO) caused by malignant diseases may require indwelling stent. Ureteral obstruction may induce renal failure. Chronic renal insufficiency is a barrier to several therapies including chemotherapy. Indwelling double-pigtail stent is the common method to release renal obstruction, but adequate stent insertion across an obstructed ureter does not necessarily guarantee renal decompression.1–3 Most studies report an approximately 28% failure rate, which hampers the management of malignant diseases and the need for repeated stent changes may cause a significant reduction in overall quality of life.1,4 Thus, the urologist must be aware of the relatively high rate of stent failure in patients with MUO.
Tandem stents or Resonance stents have been developed as alternatives to single ureteral stents, while maintaining internal drainage.1,5,6 In a review about drainage of MUO, Elsamra et al concluded that heterogeneity of MUO, low number of patients, retrospective studies and the lack of a standard method to assess the actual degree of compression limit robust comparative analysis.1 Contemporary reinforced stents may probably contribute to successful stent function as suggested by Chung et al,7 but the name of polymer stent used in these studies was rarely specified and, individual stent characteristics were not evaluated.7–10
Moreover, the use of tandem stents allowed releasing the renal obstruction in the case of failure of a single stent, testifying that the very design of the stent is clearly involved in the success of drainage.6 It is therefore essential to physically assess the characteristics of the stent before comparing it to other stents in patients.
A previous study comparing in vitro, the physical characteristics and stiffness of several commercialized stents (Vortek®, Urosoft and Superglide stents), identified the physical factors that could lead to the obstruction of the stent. In conclusion, the stiffness of the stent appeared to be an important factor to maintain patency with respect to radial compression forces. However, the stent lumen and its preservation may be essential parameters to increase the volumetric flow rate.11
Another study, using the same stents in vivo, concluded that it was possible to avoid losing survival by choosing the stent stiffness best adapted to the patient.12 The results of our clinical study using the same stents may be a better indicator of the actual role of physical factors of the stent (stiffness and lumen) in preventing stent obstruction.
We carried out the present study in order to evaluate drainage efficiency by using stents with distinctive degrees of stiffness, and to identify the physical factors that could prevent their obstruction in patients with MUO.
Materials and Methods
From June 2009 to June 2019 in a single institution, a total of 150 consecutive patients requiring indwelling stent for MUO were fitted with commercially available reinforced ureteral tumour stents (Vortek®, Urosoft, Superglide) which had previously been physically and clinically evaluated.11,12 In order to study the obstructive character of the stents, the patients were divided into three groups. Group 1 with patients died soon after only one USP (n = 37). Group 2 with patients had more than one USP and no obstruction (n = 41). Patients in Group 3 had more than one USP and at least one stent obstruction (n = 62). The study population and the overall survival have been described in a previous study.12 Due to the repetition of high rate of stent failures with obstruction in patients with MUO, the progressive and prospective choices of stents were shaped by a single surgeon (BV) to overcome each failure by focusing on the criterion of increasingly stiff stents.
The efficiency of drainage was confirmed postoperatively by regression of pain or renal failure with decreased serum creatinine, and improvement in the degree of hydronephrosis.
Stent failure was defined as the presence of renal colic or renal failure with increased serum creatinine or worsening hydronephrosis during routine oncologic surveillance with/without pyelonephritis.
The patient received a document advising her/him to perform a renal ultrasound during the fifth and sixth month for routine oncologic surveillance and to change the stent every 6 months. Indeed, given the greater risk of stent obstruction after 6 months and the serious consequences (fever, pain, and renal failure), the choice was to change the stent every 6 months. Obstruction of the stiffest stent in less than 6 months motivated the switch to 8F tandem stents. The occurrence of the stent obstruction or the programmed stent change allowed the calculation of the mean time of stenting.
A successful stent insertion attempt contributed to one ureteral stent procedure (USP). Patients with bilateral stents contributed only one observation to the dataset. Bilateral stents in the same patient with unilateral or bilateral stent failure were counted as a single stent failure.
There was no selection or exclusion, and all patients were included, even those with poor performance status.
Double-pigtail stents without holes such as Tumor Stent Vortek® (7F, Coloplast, Denmark), Urosoft Tumor Stent (7F and 8F, Bard Angiomed, Germany), Superglide Tumour DD Ureter Stent (8F, Teleflex Medical, Ireland), and ureteral drainage catheter (8F, Coloplast, Denmark) were used in our study. The progressive choice of stents was shaped to overcome each failure by focusing on the criterion of increasingly stiff stents. As the previous study confirmed, the radial compressive stress of the Superglide stents and ureteral drainage catheter (5.4 N.mm−2) was higher than with the Urosoft stents (around 2.8 N.mm−2) and the Vortek® stents (1.4 N.mm−2).11 Due to the progressive choice of stents, the Vortek® stents was mostly used at the beginning of our study. Due to the repetition of Vortek® stent failures, the Urosoft stents were used in 2014 and 2015. Due to the repetition of stent failures with the Urosoft stents, the patients were fitted with the Superglide stents and the other stents were rarely used from 2015 to 2019. Consecutively, patients with stent failure were fitted with a stiffer stent in the following order: Vortek®, Urosoft, and thereafter Superglide stents.
Obstruction may occur even with stiff stents. The use of tandem stents allowed releasing the renal obstruction in the case of failure of a single stent.6,12 In case of tandem insertion, the tandem stents were pushed simultaneously into the ureter and the wire guides were a stiff and an extra stiff guide (Lunderquist Extra Stiff Wire Guide, 0.89 mm, Cook Medical). As described by Zimskind et al, a ureteral drainage catheter was shortened, so that after placement a few centimeters protruded from the ureteral orifice into the bladder.13
The data are presented as mean ± SD. Data were analysed using Student t-test, Fisher’s exact test, Pearson’s Chi-squared test and logistic regression analysis. Statistical analyses were performed using software R. Values of p < 0.05 were considered significant.
Results
The distribution of stents within groups was comparable except that patients in Group 2 were more often fitted with a Superglide stent than those in Group 3 (Table 1).
 |
Table 1 Characteristics of the 556 Ureteral Stent Procedures of the Three Groups |
During the study period, 556 USPs were analysed separately. The stents were reinforced ureteral tumour stents and was constituted of 207 Vortek® (37.2%), 39 Urosoft (7.0%, predominantly 8F (n=27) and 7F (n=12)), 287 Superglide (51.7%) and 23 ureteral catheters (4.1%). Sixty-two tandem stents consisted of a ureteral catheter that had been cut off and internalized simultaneously alongside a Vortek® stent (2 cases), an Urosoft stent (4 cases) or a Superglide stent (56 cases). Overall, the mean time of stenting was 6.0 ± 3.4 months.
The stent failure with obstruction occurred in 23.0% (128/556) of USP at a mean of 4.4 ± 3.6 months and depended on the type of stent. Stent failure occurred in 34.2% (70/205) of Vortek® stents after 4.3 ± 3.7 months, in 42.9% (15/35) of Urosoft stents after 5.1 ± 3.8 months, in 15.4% (39/254) of Superglide or ureteral catheters after 4.0 ± 3.5 months and in 6.5% (4/62) of tandem stents after 5.0 ± 4.6 months.
No significant differences were found between Vortek® and Urosoft stents regarding stent failures but, there were significant differences between Superglide stents and Vortek® or Urosoft stents (p < 10−7) and, tandem stents and Vortek® or Urosoft stents (p < 10−8). The characteristics of the USPs are described in Table 1. Following the stent-choice strategy by selecting stiffer stents, the incidence of stent failure over time decreased.
All procedures performed and stent failure among the 150 patients is summarized in Figures 1 and 2. Figure 1 provides a quick overview of stent failure over time, while the other shows the detail of each type of stent.
 |
Figure 2 Representation of the ureteral stent procedures performed with details of the stent used (colored rectangle) in the 150 patients of Group 1, 2 and 3. Vortek® stent (brown), Urosoft stent (green), Superglide stent (blue), ureteral catheter (gray), tandem (yellow), death (black), derivation (olive). The red dot in the center of the rectangle indicates a stent failure. Patients 1, 3, 5, 7, 8, 10, 12, 13, 15–19, 21, 23, 26–28, 30, 32, 34–38, 41, 44, 47, 52–55 and 57–62 with stent failure were fitted with a stiffer stent such as a ureteral catheter, an Urosoft or a Superglide stent. Patients 9, 15, 19, 24, 31, 37, 43–45, 50, 52–54, 56–58, 61 and 62 were fitted with tandem stent for obstruction of a stiff stent. Patient 24 and 57 were previously separately published for recurrent stent failure. Stent replacement with tandem stents allowed normalization of renal function.2,3 Tandem stent failure occurred in two patients (patients 31 and 45). The patients were fitted with replacement of tandem. Patients 23, 25, 49 and 56 chose nephrectomy or urinary diversion after stent change. The 10 patients with nephrectomy (n=1) or an ileal conduit urinary diversion (n=9) are shown at the bottom of the figure. |
Tandem
Prior to the decision to switch to tandem stents, the time elapsed until stent failure was 2.5 ± 1.9 months. After stenting, the tandem was changed every 6 months on an outpatient basis and, four attempts failed (6.5%) at 2.2, 2.3, 3.7 and 11.9 months in two patients. Among the four tandem stent failures, three occurred in the same patient with insufficient fluid intake and whose urine culture showed a chronic infection with Candida albicans. The patients were managed with replacement of tandem.
Stiffness and Lumen
The results of a previous study allowed physical factors such as stiffness and inner diameter (stent lumen) to be combined with stent failure. Permeability of stents was correlated with stiffness (p < 10−9) and stent lumen (p < 10−7) (Table 2 and Figure 3). A univariate analysis showed that the significant indicators of stent failure were stent lumen (odds ratio [OR] 0.29, 95% confidence interval [CI95] 0.17–0.47, p < 0.001) and stent stiffness (OR 0.74, CI95 0.66–0.82, p < 0.001). Although not significant, multivariate analysis showed that the decrease in stent failure seems more related to the increase in lumen (Table 3).
 |
Table 2 Stent Characteristics and Relationship Between Stent Obstruction, Stiffness and Stent Lumen |
 |
Table 3 Univariate and Multivariate Logistic Regression Analysis for Stent Failure |
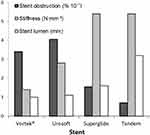 |
Figure 3 Relationship between stent obstruction, stiffness (p < 10−9 versus stent obstruction) and stent lumen (p < 10−7 versus stent obstruction). Stiffness and stent lumens were published in a previous study.11 Stent failure dropped when lumen increased from 1.0 to 3.2 mm. In addition, for identical stiffness, stent failure decreased even more as lumen increased. It seemed that stent lumen was a critical factor in controlling patency. |
Discussion
Stent Failure
Liu et al indicated that there was a paucity of data regarding the use of reinforced polymeric stent in patients with MUO.14 However, some studies provide information about the stent. Docimo at al reported 45.8% of stent failure at Day 30 with silicone stents,4 while 16% of failure at 3.2 months was observed by Jeong et al with an Endo-Sof,15 23% with a Percuflex at 5.9 months by Rosevear et al16 and 34% at a median time of 21 days with Percuflex HydroPlus Coating by Kamiyama et al.17 Clinical efficacy of the reinforced polymeric stent is generally presumed to outperform the regular polymeric stent and Liu et al reported a mean patency at 5.8 months with reinforced Urosoft 7F.14 Most studies reported stent failure with a mean of 28.7% at a mean time of 3.0 months.4,8,16,18,19
The results at the start of our study were in agreement with these data. However, in our study, the mean of reinforced stent failure over time was lower and stent failures decreased at the end of our study with rates of between 6.5% and 15.4% by a particular selection of reinforced stents. According to the manufacturer, the recommended indwelling time can be up to 1 year for the reinforced stents used in our study. During the period, the choice was to change the stent every 6 months. The possible inconsistent time for replacement exceeding 6 months corresponded to a lack of scheduled consultation or more rarely a decision dependent on an individual urologist’s judgment.
Tandem Stenting
Obstruction of the stiffest stent in less than 6 months motivated the switch to 8F tandem stents. Metallic Resonance stent or tandem stent have been developed as an alternative to single ureteral stent. The technique of tandem stent was introduced by Liu et al in 1998. For the authors, the combination of two ureteral stents increases the stiffness and reduces the likelihood of kinking from extrinsic forces.20 Several studies showed the benefits of tandem stents but the stents used were variable and the stents were not reinforced. The permeability was more than 80% at 3 months and 50–100% at 6 months.14,21–23
Stent failure rate at 6 months with Resonance in MUO was reported in 19–47.5% and even up to 82% in the case of circumferential compression by lymphatic metastasis.5,24 Wang et al observed that patients with previous radiotherapy had a higher stent failure rate in comparison with non-radiation.24 However, Resonance stent was often highlighted as capable of remaining safely in situ for one year, thus decreasing the frequency of stent changes and the cost of the stent. Elsamra et al concluded that quality evidence, on the whole, is lacking with most data retrospective and too few patients involved.1
Prior to the decision to switch to tandem stents, the time elapsed until stent failure was 2.5 months. After tandem stenting, changes occurred only every 6 months. The need to repeat stent changes may cause a significant reduction in overall quality of life.9 However, after recurrent stent obstruction and acute pyelonephritis, stent replacement with tandem stent every 6 months was viewed by patient 57 as a relief in his health-related quality of life.2 Patient 58 has Castleman's disease controlled with treatment and has a good prognosis, but stent-related symptoms and recurrent pyelonephritis prompted him to claim nephrectomy. Stent replacement with JFil® tandem stent every 6 months enabled him to work.25 Moreover, in patient 24 of our study, the stents failed to maintain kidney function after six stent failure procedures. The patient was fitted with tandem ureteral stent on both sides and renal function and health-related quality of life were improved.3 However, cachexia did not allow the patient to recover and she died 6 months later without stent failure. Several authors insisted that urologists should be actively involved in the management of patients with MUO to monitor for stent failure.8,9
As for the stent patency, tandem ureteral stenting performed significantly better than single ureteral stenting. No significant differences were found between Superglide stents and tandem stents regarding stent failures when procedures are taken into account, but there were significant differences between Superglide stents and tandem stents regarding stent failures when it was patients who were taken into account (p < 10−5).12 The difference can be explained by the occurrence of 3 of the 4 tandem stent failures in the same patient.
Reinforced Stent
Our study showed that all models of reinforced stents of the study were potentially effective, but some patients could require stents of different constitutions. Thus, the urologist must be aware of the relatively high rate of stent obstruction in patients with MUO. In order to reduce drainage failures, a useful tool would be to provide practising urologists information that may help them select a more appropriate stent if the stent currently in place is obstructed.11 Hübner et al recommended hard stents rather than large-diameter stents in patients with MUO.26 However, in the study of Docimo et al, there appeared to be an advantage to a larger lumen in the 7F silicone stent, perhaps by decreasing the hazard for occlusion by debris. The authors suggested that either a larger silicone stent or a different catheter type, perhaps of solid design might be more effective in patients with MUO.4
Towards Reshaping an Optimal Stent
Despite the clinical studies carried out, it is still difficult to conclude whether the most important characteristic is the stiffness or the lumen of the stent. During our study, the choice of the stents was driven by the increase in stiffness, but this criterion was not necessarily the factor actually involved in stent failure and MUO. For example, silicone material is known to be soft and a previous study confirmed that the inner diameter of a silicone stent is easily occluded for low radial compression. Logically, one would expect greater success from a stiffer stent like the Superglide stent, which was most effective against radial compression.11 Thus, the stiffness characteristic was chosen as criterion. Interestingly, ureteral stent obstruction significantly decreased with a large lumen of the stent. Indeed, the results in Table 2 and Figure 3 show that stent failure rate dropped sharply when lumen and stiffness increase with the Superglide stents, but the rate dropped again with tandem stents with a lumen twice as large while the stiffness remained unchanged. These results suggest that the lumen seems more important than the stiffness and, the stiffness would be the only means of keeping the lumen intact. In the study, the risk of failure stent decreases by 71% when the lumen increases by 1 mm. The preservation of native lumen as a perfect circle is therefore essential.11
In fluid mechanics, the wall shear stress in a tube is proportional to the viscosity, to the maximum linear velocity and is inversely proportional to the diameter (stent lumen). Thus, the reduction in diameter or lumen leading to a rise in velocity causes an increase in wall shear stress. The increase in wall shear stress can have consequences on bacterial incrustation and the appearance of vortices in the stent lumen. Saur et al used a rotating annular reactor to measure the impact of wall shear stress and evaluate the adhesion of bacteria clusters. The number of attached bacteria globally increased with the wall shear stress.27 De Grazia et al used microfluidic devices and observed that the formation of cavities with vortices in areas located in the proximity of a ureteral obstruction caused bacterial attachment.28 Morever, Shilo et al noted that several in vitro studies used unrealistic external forces to fully compress the stent lumen, and therefore argued that lumen could be more important than stiffness.29 By using a mechanical ureteral model, they observed that the lumen of the 8F ureteral stent offered better patency to colloid solutions than smaller lumen stents.30 The authors concluded that other factors such as urine composition and viscosity may be a major contributor to stent failure. The debris accumulation or, for example, the chronic infection with Candida albicans, are possible risk factors for stent encrustation suggesting that larger lumen stents are less likely to become occluded with debris.4,30
Using computational fluid dynamic simulations, it would be useful to know whether the obstruction of the stents used in this study correlate with the tubular constriction zones described in the in vitro study and whether it testifies that the regularity and smoothness of the lumen are possible crucial factors.11
For a better stent patency, the very design of the stent could potentially help via a wide lumen and a high degree of stiffness. However, the pressure loss along the stent increases with the length of the tube.11 Thus, the stent in itself represented an obstruction of the flow rate in the ureter and a short length of the stent may decrease the risk of an encrustation of the stent. A potential solution could come from the design of the stent itself, by shortening it to the exact length of the ureter as in the method previously described with a silicone end-piece at the bottom of the stent. Moreover, this stent was known to decrease stent-related symptoms.31
Limitations
Our Study Has Several Limitations
First, the heterogeneity of patients who were subjected to different treatments according to the severity of the disease and the heterogeneity of MUO with cases with more extensive or severe ureteral obstruction may be subject to selection bias. It would have been worth knowing if the constriction degree of ureteral stenosis was not greater in Group 3 than in Group 2 with urine flowing exclusively through the stent lumen and no possibility of passage through the extraluminal space between the stent and the ureter wall.
Second, our study is neither prospective nor randomized, but the large number of stent procedures and our 10-year-long follow-up have made it possible to obtain powerful statistical results.
Third, patients in Group 2 were more often fitted with a Superglide stent than those in Group 3, but the difference did not affect the comparison between the physical characteristics of the stents.
Finally, not all types of reinforced stents were analysed in our study, which took neither metal nor metal mesh stents into account. However, this series of 556 stent procedures is the first to focus exclusively on stent failure depending on the physical characteristics of the stents in MUO.
Conclusion
In the present study, Superglide and tandem stents were the best stents against stent failure, and the lumen and the stiffness of the stent have been shown to be critical factors in controlling patency. The results suggest that the lumen seems more important than the stiffness and that the stiffness would be the only means of keeping the lumen intact. Future stents for MUO should integrate the importance of the lumen of the stent. It would be interesting to assess the impact of the stent stiffness or tandem stent with wide lumen and silicone end-piece on overall survival and stent-related symptoms in patients with MUO in a prospective randomized controlled trial.
Abbreviations
MUO, malignant ureteral obstruction; USP, ureteral stent procedure.
Ethics Approval and Informed Consent
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (Sud-Est V French Ethical Committee: CPP 17-VOGT-01, National Medicine Safety Agency: 2017-A00205-48, and Ile-de-France II French Ethical Committee: IRB registration: 00001072) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Written informed consent was obtained from the patients included in the study.
Acknowledgments
I thank Professor Janine Dove-Rumé, English Department at the University of Tours, for re-reading the text.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors certify that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript are the following: Benoît Vogt received royalties from Rocamed for the treatment of ureteral stones but there are no financial competing interests in the manuscript. The authors report no other conflicts of interest in this work.
References
1. Elsamra SE, Leavitt DA, Motato HA, et al. Stenting for malignant ureteral obstruction: tandem, metal or metal-mesh stents. Int J Urol. 2015;22(7):629–636. doi:10.1111/iju.12795
2. Vogt B, Desfemmes FN, Desgrippes A. Improving comfort of patients with ureteral obstruction and malignant disease should be of concern. J Palliat Med. 2016;19(11):1132–1133. doi:10.1089/jpm.2016.02763
3. Vogt B. Ureteral stent obstruction and stent’s discomfort are not irreparable damages. Urol Case Rep. 2018;29(20):100–101. doi:10.1016/j.eucr.2018.07.025
4. Docimo SG, Dewolf WC. High failure rate of indwelling ureteral stents in patients with extrinsic obstruction: experience at 2 institutions. J Urol. 1989;142(2 Pt 1):277–279. doi:10.1016/s0022-5347(17)38729-3
5. Chow PM, Hsu JS, Wang SM, Yu HJ, Pu YS, Liu KL. Metallic ureteral stents in malignant ureteral obstruction: short-term results and radiological features predicting stent failure in patients with non-urological malignancies. World J Urol. 2014;32(3):729–736. doi:10.1007/s00345-013-1143-y
6. Elsamra SE, Motato H, Moreira DM, et al. Tandem ureteral stents for the decompression of malignant and benign obstructive uropathy. J Endourol. 2013;27(10):1297–1302. doi:10.1089/end.2013.0281
7. Chung SY, Stein RJ, Landsittel D, et al. 15-year experience with the management of extrinsic ureteral obstruction with indwelling ureteral stents. J Urol. 2004;172(2):592–595. doi:10.1097/01.ju.0000130510.28768.f5
8. Yoon JH, Park S, Park S, Moon KH, Cheon SH, Kwon T. Renal function is associated with prognosis in stent-change therapy for malignant ureteral obstruction. Investig Clin Urol. 2018;59(6):376–382. doi:10.4111/icu.2018.59.6.376
9. Goldfarb RA, Fan Y, Jarosek S, Elliott SP. The burden of chronic ureteral stenting in cervical cancer survivors. Int Braz J Urol. 2017;43(1):104–411. doi:10.1590/S1677-5538.IBJU.2016.0667
10. Hyams ES, Shah O. Malignant extrinsic ureteral obstruction: a survey of urologists and medical oncologists regarding treatment patterns and preferences. Urology. 2008;72(1):51–56. doi:10.1016/j.urology.2008.01.046
11. Vogt B. Stiffness analysis of reinforced ureteral stents against radial compression: In Vitro Study. Res Rep Urol. 2020;12:583–591. doi:10.2147/RRU.S285031
12. Vogt B, Blanchet LH. 10-year experience with reinforced ureteral stents for malignant ureteral obstruction. Res Rep Urol. 2021;13:581–589. doi:10.2147/RRU.S326274
13. Zimskind PD, Fetter TR, Wilkerson JL. Clinical use of long-term indwelling silicone rubber ureteral splints inserted cystoscopically. J Urol. 1967;97(5):840–844. doi:10.1016/s0022-5347(17)63130-6
14. Liu KL, Lee BC, Ye JD, et al. Comparison of single and tandem ureteral stenting for malignant ureteral obstruction: a prospective study of 104 patients. Eur Radiol. 2019;29(2):628–635. doi:10.1007/s00330-018-5560-6
15. Jeong IG, Han KS, Joung JY, Seo HK, Chung J. The outcome with ureteric stents for managing non-urological malignant ureteric obstruction. BJU Int. 2007;100(6):1288–1291. doi:10.1111/j.1464-410X.2007.07172.x.
16. Rosevear HM, Kim SP, Wenzler DL, Faerber GJ, Roberts WW, Wolf JS
17. Kamiyama Y, Matsuura S, Kato M, et al. Stent failure in the management of malignant extrinsic ureteral obstruction: risk factors. Int J Urol. 2011;18(5):379–382. doi:10.1111/j.1442-2042.2011.02731.x
18. Ganatra AM, Loughlin KR. The management of malignant ureteral obstruction treated with ureteral stents. J Urol. 2005;174(6):2125–2128. doi:10.1097/01.ju.0000181807.56114.b7
19. Rosenberg BH, Bianco FJ
20. Liu JS, Hrebinko RL. The use of 2 ipsilateral ureteral stents for relief of ureteral obstruction from extrinsic compression. J Urol. 1998;159(1):179–181. doi:10.1016/s0022-5347(01)64050-3
21. Ozyer U, Dirim A. Tandem ureteral stents in the management of double-J stent dysfunction in gynecological malignancies. Diagn Interv Imaging. 2017;98(9):601–608. doi:10.1016/j.diii.2017.07.005
22. Varnavas M, Bolgeri M, Mukhtar S, Anson K. The role of tandem double-J ureteral stents in the management of malignant ureteral obstruction. J Endourol. 2016;30(4):465–468. doi:10.1089/end.2015.0670
23. Rotariu P, Yohannes P, Alexianu M, et al. Management of malignant extrinsic compression of the ureter by simultaneous placement of two ipsilateral ureteral stents. J Endourol. 2001;15(10):979–983. doi:10.1089/089277901317203047
24. Wang HJ, Lee TY, Luo HL, et al. Application of resonance metallic stents for ureteral obstruction. BJU Int. 2011;108:428–432. doi:10.1111/j.1464-410X.2010.09842.x
25. Vogt B, Desgrippes A, Desfemmes FN. Changing the double-pigtail stent by a new suture stent to improve patient’s quality of life: a prospective study. World J Urol. 2015;33:1061–1068. doi:10.1007/s00345-014-1394-2
26. Hübner WA, Plas EG, Stoller ML. The double-J ureteral stent: in vivo and in vitro flow studies. J Urol. 1992;148(2 Pt 1):278–280. doi:10.1016/s0022-5347(17)36572-2
27. Saur T, Morin E, Habouzit F, Bernet N, Escudié R. Impact of wall shear stress on initial bacterial adhesion in rotating annular reactor. PLoS One. 2017;12(2):e0172113. doi:10.1371/journal.pone.0172113
28. De Grazia A, LuTheryn G, Meghdadi A, et al. A microfluidic-based investigation of bacterial attachment in ureteral stents. Micromachines. 2020;11(4):408. doi:10.3390/mi11040408
29. Shilo Y, Modai J, Leibovici D, Dror I, Berkowitz B. The impact of ureteral deformation and external ureteral pressure on stent failure in extrinsic ureteral obstruction: an In Vitro Experimental Study. J Endourol. 2020;34(1):68–73. doi:10.1089/end.2019.0465
30. Shilo Y, Modai J, Leibovici D, Dror I, Berkowitz B. Comparative study of renal drainage with different ureteral stents subject to extrinsic ureteral obstruction using an in vitro ureter-stent model. BMC Urol. 2021;21(1):100. doi:10.1186/s12894-021-00865-w
31. Vogt B, New Customized A. Ureteral stent with nonrefluxing silicone end-piece to alleviate stent-related symptoms in malignant diseases. Urology. 2020;137:45–49. doi:10.1016/j.urology.2019.12.022
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

