Back to Journals » Patient Preference and Adherence » Volume 17
Analysis of the Current Status and Factors Influencing Compliance with Colonoscopic Monitoring After Endoscopic Surgery for Advanced Colorectal Adenoma
Authors Wang F, Han Q, Sun RJ, Tu HM, Yang YL, Ren YL
Received 25 August 2023
Accepted for publication 7 November 2023
Published 7 December 2023 Volume 2023:17 Pages 3195—3204
DOI https://doi.org/10.2147/PPA.S437092
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Fei Wang,1 Qian Han,2 Ren-Juan Sun,3 Hui-Ming Tu,4 Yu-Ling Yang,5 Yi-Lin Ren4
1Wuxi School of Medicine, Jiangnan University, Wuxi, 214122, People’s Republic of China; 2Center of Endoscopy, Affiliated Hospital of Jiangnan University, Wuxi, 214062, People’s Republic of China; 3Department of Nutrition, Affiliated Hospital of Jiangnan University, Wuxi, 214062, People’s Republic of China; 4Department of Gastroenterology, Affiliated Hospital of Jiangnan University, Wuxi, 214062, People’s Republic of China; 5Department of Oncology, Affiliated Hospital of Jiangnan University, Wuxi, 214062, People’s Republic of China
Correspondence: Hui-Ming Tu, Department of Gastroenterology, Affiliated Hospital of Jiangnan University, No. 200 Huihe Road, Binhu District, Wuxi City, Jiangsu Province, 214062, People’s Republic of China, Tel +86-13861753621, Email [email protected]
Background: Advanced colorectal adenomas are at a risk of malignant transformation following endoscopic resection, and colonoscopic monitoring interval after polypectomy have been widely used. This study aims to investigate the prevailing state of compliance with postoperative colonoscopic surveillance among patients with advanced colorectal adenomas and its’ influencing factors at Affiliated Hospital of Jiangnan University between November 2020 and April 2021.
Methods: A retrospective analysis was conducted on patients who underwent endoscopic treatment for ACA at Affiliated Hospital of Jiangnan University from November 2020 to April 2021. Compliance with postoperative colonoscopic surveillance was assessed based on established guidelines. Factors such as sociodemographic features, medical histories, and health beliefs were analyzed to determine their influence on compliance. Univariate analysis, survival analysis, and multi-factor Cox regression analysis were used for statistical evaluation.
Results: A total of 511 patients were included in the study. The compliance rate was found to be 43.2%. The univariate analysis indicated that factors such as gender, education level, work status, type of health insurance, place of residence, marital status, type of consultation, presence of gastrointestinal symptoms, number of polyps, and the maximum diameter of polyps significantly affected compliance. Multi-factor Cox regression analysis revealed that female gender, absence of gastrointestinal symptoms, outpatient endoscopic treatment, and solitary polyps were independent factors influencing compliance. Reasons for poor compliance included underestimating the severity of the disease, fear of colonoscopy, and procedural complexities.
Conclusion: Patients with advanced colorectal adenomas had poor compliance with postoperative colonoscopy monitoring. Tailored health education programs should be designed, targeting women, outpatients undergoing endoscopic procedures, and patients with solitary polyps to enhance their compliance with colonoscopy monitoring.
Keywords: advanced colorectal adenomas, compliance with colonoscopic monitoring introduction, factors, colorectal cancer
Corrigendum for this paper has been published.
Introduction
Colorectal cancer (CRC) ranks second globally in cancer incidence, trailing only breast and lung cancer. In China, it constitutes approximately 12.2% of new cancer cases, holding the second position in incidence and the fifth in mortality.1 The early identification and removal of adenomatous polyps during colonoscopy significantly diminish morbidity and mortality related to CRC.2 Advanced colorectal adenoma (ACA), a pivotal precursor to CRC, is defined as a conventional adenoma ≥10 mm in size or exhibiting advanced histology (high-grade dysplasia or villous/tubulovillous histology).3 Colonoscopy, an endoscopic procedure traversing from the anal cavity to the ileocecal region, affords a comprehensive view of the bowel and remains the premier approach for colorectal cancer screening, lesion surveillance, and minimally invasive endoscopic bowel surgery.4
Multiple long-term follow-up studies provide compelling evidence that patients with ACA face a heightened risk of recurrent ACA or CRC compared to those with non-adenomas or non-advanced adenomas.5 Regular postoperative surveillance substantially curtails the incidence of colorectal cancer.6 Colonoscopy emerges as the most pertinent and cost-effective modality for monitoring ACA patients. Regrettably, however, certain studies indicate suboptimal utilization of postoperative colonoscopy surveillance among ACA patients, with a mere 30.7% to 73.6% adhering to regular colonoscopy appointments.7,8 Several factors affecting compliance with colonoscopy monitoring have been identified in previous literature, such as patient demographics, health beliefs, physician recommendations, insurance coverage, and accessibility of health services.9–11 However, these studies were conducted in different settings and populations, and may not reflect the current situation and challenges in China. Therefore, this study aims to investigate the prevailing status and influencing factors pertaining to postoperative colonoscopic surveillance in ACA patients in China, with the ultimate goal of enhancing compliance with colonoscopic management among patients with ACA.
Methods
Subjects
This retrospective analysis focused on patients who underwent endoscopic treatment for advanced colorectal adenomas at Affiliated Hospital of Jiangnan University between November 2020 and April 2021. Inclusion criteria: (1) Diagnosis of ACA according to the Chinese Expert Consensus on Strategies for the Management of Precancerous Lesions and Pre-cancerous States of Colorectal Cancer;8 (2) Complete resection of colorectal adenomas by colonoscopic polypectomy; (3) Demonstrated clear consciousness, coherent speech, and informed consent. Exclusion criteria: (1) Presence of Lynch syndrome, genetic colonic polyposis, or inflammatory bowel disease; (2) History of CRC, synchronous CRC, or partial colectomy; (3) Absence of pathology reports; (4) Existence of significant medical conditions (eg, severe cardiovascular disease, severe liver disease, uremia, severe Parkinson’s disease); (5) Death resulting from disease-related or unrelated factors following colonoscopic polypectomy. The study followed the Declaration of Helsinki and was approved by the Ethics Committee of the Affiliated Hospital of Jiangnan University (Ethics approval number: LS2022060).
The inclusion and exclusion criteria were chosen to ensure that the study population was homogeneous and representative of patients with ACA who underwent colonoscopic polypectomy. Patients with Lynch syndrome, genetic colonic polyposis, or inflammatory bowel disease were excluded because they have different genetic and clinical characteristics that may affect their compliance with colonoscopic surveillance. Patients with a history of CRC, synchronous CRC, or partial colectomy were excluded because they may have different indications and intervals for colonoscopic surveillance than patients with ACA. Patients without pathology reports were excluded because the histological features of the adenomas are essential for determining the risk level and surveillance recommendations. Patients with significant medical conditions or death were excluded because they may have other factors that influence their compliance or survival.
Data Collection
Data collection Based on an extensive literature review and preliminary investigations, a meticulously designed questionnaire was formulated to explore a spectrum of patient-specific sociodemographic features, medical histories, compliance levels, and associated influencing factors. The formulation of the questionnaire was a result of comprehensive discussions among the researchers. The questionnaire consisted of four sections: personal information, health status, health behavior, and health beliefs. The personal information section included questions about gender, age, education, work status, medical insurance, residence, and marital status. The health status section included questions about type of consultation, adenoma attributes encompassing size and histological nature, presence of symptoms or complications, comorbidities, and medication use. The health behavior section included questions about smoking, alcohol consumption, physical activity, dietary habits, and adherence to colonoscopic surveillance. The health beliefs section included questions about perceived susceptibility, severity, benefits, barriers, cues to action, and self-efficacy regarding colonoscopic surveillance. These questions were based on the Health Belief Model (HBM), which is a widely used theoretical framework for understanding health-related behaviors.12 The questionnaire items were measured using different scales depending on the nature of the questions. For example, some items used dichotomous yes/no responses, some used Likert-type scales ranging from 1 to 5 or 1 to 7, and some used open-ended responses.
The data acquisition process involved the utilization of both an electronic medical case system and questionnaires. These instruments collectively encompassed a wide array of parameters, including general demographic information (gender, age, education, work status, medical insurance, residence, and marital status), detailed disease diagnosis (such as type of consultation, adenoma attributes encompassing size and histological nature), as well as telephonic follow-up interactions to discern patients’ progress, hospital visit frequencies, temporal patterns, and outcomes. In pursuit of comprehensive data accuracy, this study incorporated measures to mitigate information bias. To this end, follow-up calls were meticulously executed by trained interviewers using a standardized protocol to guide patients through the questionnaire. In scenarios where clarity was compromised or independent completion was hindered due to hearing impairment or cognitive impairment, the research investigators liaised with patients’ family members or caregivers for additional clarifications. Notably, in an effort to preserve the authenticity of the study, the researchers consciously abstained from using suggestive language during patient interactions. The process of completing the questionnaire, on average, required approximately 10 minutes. Instances of unanswered, disconnected, or unresponsive calls exceeding three consecutive attempts were classified as missed visits. The internal consistency reliability of the questionnaire was assessed using McDonald’s omega coefficient. The omega coefficient of this questionnaire was 0.95.
Compliance Criteria of Colonoscopic Surveillance
The compliance of postoperative colonoscopic surveillance among patients with ACA was evaluated through a synthesis of guidelines and consensus from China, the United States, and Europe.3,13–16 Following endoscopic resection of ACA, adenomas encompassing a villous component and ≥10 mm in diameter were advised to undergo review within one to two years post-surgery. Similarly, adenomas displaying high-grade intraepithelial neoplasia and ≥20 mm in diameter warranted review one year after surgery. In this study, compliance with colonoscopic surveillance was gauged by defined criteria as following: patients who underwent the recommended endoscopic review intervals (within 1 month) were classified as having good compliance. Conversely, those who failed to follow the stipulated review intervals (exceeding 1 month) or those who completely bypassed colonoscopic monitoring were defined as poor compliance.
Statistical Analyses
Statistical analysis was conducted using SPSS 26.0 software. Descriptive statistics were employed for measurement data, expressed as “x±s” for normally distributed variables. Count data were presented as frequencies and percentages, and subsequently analyzed using the χ2 test or Fisher’s exact probability method. Following univariate analysis, variables displaying statistically significant associations were retained. These selected factors were then subjected to multifactorial logistic regression analysis to identify independent risk factors influencing compliance with colonoscopy monitoring. p-values less than 0.05 indicating meaningful differences.
Statistical analysis was conducted using SPSS 26.0 software. Descriptive statistics were employed for measurement data, expressed as “x±s” for continuous data. The normality assumption for the continuous data was checked using the Kolmogorov–Smirnov test and found to be acceptable. Count data were presented as frequencies and percentages, and subsequently analyzed using the χ2 test or Fisher’s exact test. Survival analysis was performed to examine the time to noncompliance with colonoscopy monitoring among patients with ACA, using the Kaplan-Meier method and the Log rank test to compare survival curves across different groups. Cox proportional hazards regression was used to identify independent risk factors influencing compliance with colonoscopy monitoring, adjusting for potential confounders. The proportional hazards assumption was checked using Schoenfeld residuals. Variables with a p-value less than 0.2 in the univariate analysis were included in the multivariate model and selected using the backward elimination method based on the Akaike information criterion. p-values less than 0.05 indicating meaningful differences.
Results
The Patients’ Compliance
A total of 579 patients with ACA underwent endoscopic resection in our hospital from November 2020 to April 2021, including 6 patients who were excluded because of incomplete personal information, 38 patients who were excluded according to the exclusion criteria, and 24 patients who did not complete the questionnaire content. The response rate of the questionnaire was 89.5% (511/571) and the completion rate was 95.3% (511/535). Finally, 511 patients were included in the study, including 333 males and 178 females; ages ranged from 24 to 85 years, with a mean of (58.55±10.65) years. The details are shown in Figure 1. Out of 511 patients, 221 (43.2%) patients showed good compliance and 290 (56.8%) patients had poor compliance with colonoscopic surveillance.
 |
Figure 1 Study flow chart. |
Univariate Analysis of Compliance with Colonoscopic Surveillance Among ACA Patients
The univariate analysis revealed that no statistically significant differences in the comparison of age and the pathological nature of polyps between the two groups (p > 0.05). Conversely, factors such as gender, education level, work status, type of health insurance, place of residence, marital status, type of consultation, presence of gastrointestinal symptoms, the number of polyps, and the maximum diameter of polyps exhibited significant effects on the compliance with postoperative colonoscopic monitoring among patients with ACA (p < 0.05), as detailed in Table 1.
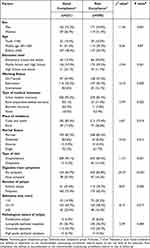 |
Table 1 Univariate Analysis of Compliance with Colonoscopic Surveillance Among ACA Patients |
Survival Analysis of Compliance with Colonoscopic Surveillance Among ACA Patients
The survival analysis showed that the median time to noncompliance with colonoscopic surveillance among ACA patients was 14 months (95% CI: 12–16 months). The survival curves for different groups based on gender, gastrointestinal symptoms, type of visit, and number of polyps are shown in Figure 2. The Log rank test indicated that there were significant differences in the survival curves across these groups (p < 0.05).
 |
Figure 2 Simulated survival curves for patient compliance. |
Multi-Factor Cox Regression Analysis of Compliance with Colonoscopic Surveillance Among ACA Patients
Ten statistically significant factors in the univariate analysis were used as independent variables, including five unordered multi-categorical variables concerning the first category to set dummy variables, namely, elementary school and below, employed, urban medical insurance, married, and polyps <10 mm in diameter. Multi-factor Cox regression analysis showed that female gender (HR = 1.63, 95% CI: 1.06–2.50), no gastrointestinal symptoms (HR = 0.36, 95% CI: 0.23–0.57), outpatient endoscopic treatment (HR = 2.28, 95% CI: 1.10–4.70), and solitary polyps (HR = 0.64, 95% CI: 0.42–0.98) were independent factors influencing the compliance with colonoscopic surveillance among ACA patients after surgery (p < 0.05). For details, see Table 2.
 |
Table 2 Results of Cox Regression Analysis of Compliance with Colonoscopic Surveillance Among ACA Patients (n = 511) |
Reasons Affecting Patient Review
The summary results of the 511 questionnaires on factors affecting patients “colonoscopic surveillance and patients” self-reports are shown in Table 3. In terms of good compliance, the top three items mentioned by patients were “fear of disease recurrence”, “doctor’s recommendation”, and “regular physical examination”. In terms of poor compliance, the top three options were “feeling that the disease is not serious”, “fear of colonoscopy and fear of taking bowel cleansing drugs”, and “complicated consultation process”. For details, see Table 3.
 |
Table 3 Responses of Participants on the Reasons Affecting Colonoscopy Monitoring by Independent Factors (n = 511) |
We also explored the association between the reasons affecting patient review and the independent factors influencing compliance with colonoscopic surveillance identified by the Cox regression analysis. We found that female patients were more likely to report fear of colonoscopy and fear of taking bowel cleansing drugs as a reason for poor compliance than male patients (χ2 = 6.32, p = 0.012). Patients who had no gastrointestinal symptoms were more likely to report feeling not seriously ill as a reason for poor compliance than those who had gastrointestinal symptoms (χ2 = 9.87, p = 0.002). Patients who received outpatient endoscopic treatment were more likely to report forgetting the review time as a reason for poor compliance than those who received inpatient endoscopic treatment (χ2 = 4.56, p = 0.033). Patients who had solitary polyps were more likely to report not being informed of the review time as a reason for poor compliance than those who had multiple polyps (χ2 = 5.21, p = 0.022).
Discussion
In this study, the compliance rate of colonoscopic surveillance in patients with ACA was only 43.2%, which is lower than the average compliance rate of 54.6% in the surveillance guidelines for high-risk adenoma patients in North America and 73.6% in the European guidelines.8 This discrepancy could potentially be attributed to variations in disease awareness levels and disparities in social health care systems across different countries. Notably, the absence of systematic links between the health care system and patients for comprehensive colonoscopy monitoring might contribute to these variations. Furthermore, these differences could also stem from the referenced colonoscopy monitoring standard in this study. Notably, the recommended postoperative colonoscopy monitoring interval for ACA patients stood at 1–2 years, in contrast to the more extended range of 1–3 years prevalent in Europe and the United States. This variation in temporal parameters for reviewing patients with ACA lacks clarity and consistency, underscoring the necessity for further clinical investigations to provide definitive insights.15
In this study, female patients were independent risk factors for patient compliance with the colonoscopic surveillance. The rate of good compliance was 48.6% (162/333) in male patients and 33.1% (59/178) in female patients, which is also consistent with the findings of Tae et al.16 Despite the widespread availability of painless colonoscopy techniques, it remains undeniable that women, from a physiological standpoint, possess longer total colon lengths compared to men. Consequently, women often encounter more challenging colonoscopy procedures, characterized by extended insertion times, heightened utilization of abdominal pressure, and increased sedation requisites. These factors collectively contribute to a higher likelihood of abdominal pain and increased procedural discomfort for women. Additionally, these difficulties might be compounded by prior hysterectomy procedures.17 Moreover, the inherent lower pain threshold among women could potentially function as an impediment to their completion of colonoscopy procedures.
Gastrointestinal symptoms were one of factors promoting participation in follow-up colonoscopic surveillance in patients with ACA. This inclination can be attributed to the discomfort experienced by patients due to symptoms such as abdominal pain, diarrhea, constipation, and hematochezia. Such symptoms not only adversely impact their overall well-being but also engender concerns about the potential development of colorectal cancer. Specifically, the presence of blood in stool tends to evoke anxiety and apprehension, prompting patients to proactively seek timely follow-up appointments. Consequently, this dynamic contributes to enhanced adherence to postoperative colonoscopy monitoring. However, in practice, even CRC may be asymptomatic or less symptomatic until it progresses to an advanced stage.18 Hence, the presence or absence of gastrointestinal symptoms is not a basis for determining whether colorectal polyps have recurred. The best evidence is found in cancer and colorectal adenoma populations in the global colorectal cancer screening program for asymptomatic people.19 For patients with ACA, healthcare providers should provide them with the necessary scientific education to help them establish the correct awareness of postoperative review, make them clear the significance of regular postoperative colonoscopic surveillance, which is to monitor their changes in condition and timely deal with the original recurrence, regeneration elsewhere, and missed intestinal polyps to reduce the occurrence rate of cancer.20
In addition, the detection of colorectal adenomas is predominantly accomplished through physical examinations or outpatient assessments. The size and number of lesions are essential references for selecting different treatment modalities under endoscopy.21 For smaller-diameter and fewer adenomas, the outpatient setting is often chosen for their removal, typically achieved through procedures such as biopsy forceps or cold snare polypectomy,21,22 This approach curtails the necessity for patients to undergo repeated bowel cleansing regimens and mitigates the depletion of ward resources. Consequently, patients experience lesser discomfort and tend to exhibit diminished compliance with colonoscopic monitoring. In contrast, patients who choose to be hospitalized for resection of colorectal adenomas often have a large number of adenomas or adenomas ≥10 mm in diameter and above, requiring endoscopic mucosal resection or endoscopic submucosal dissection.21,22 These procedures entail greater complexity and carry inherent risks of complications such as postoperative abdominal pain, bleeding and perforation.23 Notably, patients discharged following adenoma removal while hospitalized are frequently provided with written discharge summaries, serving as reminders for their scheduled follow-up appointments. In contrast, outpatient individuals predominantly receive verbal reminders from their physicians, which in turn seems to contribute to a relatively decreased adherence to colonoscopy monitoring.
Polyposis constitutes an additional affirmative aspect that ACA patient engagement in colonoscopic surveillance.24 A study by Lee et al25 showed that patients with multiple high-risk ACA patients superimposed on polyposis have a higher likelihood of future advanced neoplasia than single high-risk patients. At the same time, the rate of polyp leakage is influenced by the number of polyps detected during baseline colonoscopy.26 Despite colonoscopy’s comprehensive visualization of the intestinal lumen, the numerous folds characterizing the entire colon, coupled with stringent bowel preparation requisites, frequently culminate in the non-observation of certain polyps. Furthermore, the risk of incomplete observation is exacerbated if the endoscopist swiftly withdraws the scope or lacks experience. To prevent heterochronic lesions, endoscopists may prefer a more cautious follow-up strategy. They will advise patients to shorten the monitoring interval of colonoscopy monitoring guidelines, emphasizing the importance of colonoscopy monitoring. Patients are also more vigilant and will pay more attention to their bowel condition than solitary polyps.
The reasons affecting patients’ colonoscopy monitoring in 511 questionnaires showed that two subjective factors, “fear of disease recurrence” and “feeling that the disease is not serious”, were the top reasons affecting patients’ compliance, respectively. This alignment with Murphy et al27 establishes a parallel between patients’ perceptions of “colonoscopy benefits” and “cancer concerns” with augmented adherence rates to colonoscopy surveillance. These insights underscore the necessity for heightened patient education concerning the prevention and management of precancerous lesions, constituting a pivotal facet in enhancing compliance with colonoscopy surveillance. According to a survey of gastrointestinal endoscopists in Canada,28 patient compliance with colonoscopic surveillance is closely related to physician knowledge of intestinal polyp surveillance guidelines. Consequently, the elevation of physician training assumes paramount importance in promoting their role as guiding medical practitioners. “Fear of colonoscopy and fear of taking bowel cleansers” can promote the examination under anesthesia and continuously improve the operation level of endoscopists and the nursing staffs care to reduce patients’ fear of follow - up colonoscopy monitoring due to postoperative complications such as abdominal pain, bleeding, and perforation. Regarding bowel preparation, the conventional polyethylene glycol electrolyte powder (PEG) regimen is associated with substantial intake and unpalatable taste. A 2020 meta-analysis29 demonstrated the potential of adjuncts like citrus reticulate peel, orange juice, menthol candy drops, dimethicone oil and sugar-free chewing gum to enhance palatability. Furthermore, these adjuncts foster a willingness to repeat bowel preparation, enhancing its quality through reduced risk of vomiting and bloating, particularly through split-dose regimens, controlled dosing rates, abdominal compressions, and increased physical activity during dosing. When feasible, alternative or combined drug regimens, such as sulfates, sodium phosphate salts, mannitol, herbal preparations, and motility drugs, are chosen.
Our study has several potential limitations. First, this study is a single-center study, which may lead to some bias, and the relevant influencing factors have yet to be validated in a multicenter study. Second, the follow-up period in this study was shorter, and the colonoscopic surveillance interval was more stringent for ACA. Notably, the consideration of excessive colonoscopy monitoring was not encompassed within our study. While shortening the monitoring interval might prove advantageous at an individual level for disease prevention, the tendency towards over-screening may inadvertently unveil heterochronic lesions. Nonetheless, it further exacerbates challenges like squandering medical resources and heightening the economic burden.
Conclusion
In conclusion, the poor compliance with postoperative colonoscopic surveillance among ACA patients were associated with gender, outpatient-based endoscopic treatment, the absence of gastrointestinal symptoms, and solitary polyps. Notably, patients’ attitudes toward the disease were found to be the primary factor facilitating and hindering patients’ colonoscopy monitoring. The healthcare personnel can carry out regular follow-up and scientific guidance to ensure the effective implementation of the guidelines for post-polypectomy colonoscopy monitoring.
Data Sharing Statement
All data generated or analysed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Ethics Approval and Consent to Participate
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Affiliated Hospital of Jiangnan University (Ethics approval number: LS2022060). Written informed consent was obtained from all participants.
Funding
The work was funded by Wuxi Taihu Lake Talent Plan, Supports for Leading Talents in Medical and Health Profession.
Disclosure
All of the authors had no any personal, financial, commercial, or academic conflicts of interest separately.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clinicians. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394(10207):1467–1480. doi:10.1016/S0140-6736(19)32319-0
3. Gupta S, Lieberman D, Anderson JC, et al. Recommendations for follow-up after colonoscopy and polypectomy: a consensus update by the US multi-society task force on colorectal cancer. Gastroenterology. 2020;158(4):1131–1153.e5. doi:10.1053/j.gastro.2019.10.026
4. Healthgrades. Benefits and risks of colonoscopy | side effects after colonoscopy. Healthgrades1 Published February 1, 2021; 2023.
5. Zhu Y, Huang Y, Hu Y, et al. Long‐term risk of colorectal cancer after removal of adenomas during screening colonoscopies in a large community‐based population in China. Int J Cancer. 2022;150(4):594–602. doi:10.1002/ijc.33835
6. Wieszczy P, Kaminski MF, Franczyk R, et al. Colorectal cancer incidence and mortality after removal of adenomas during screening colonoscopies. Gastroenterology. 2020;158(4):875–883. e5. doi:10.1053/j.gastro.2019.09.011
7. Djinbachian R, Dubé AJ, Durand M, et al. Adherence to post-polypectomy surveillance guidelines: a systematic review and meta-analysis. Endoscopy. 2019;51(07):673–683. doi:10.1055/a-0865-2082
8. Dong J, Wang LF, Ardolino E, Feuerstein JD. Real-world compliance with the 2020 US multi-society task force on colorectal cancer polypectomy surveillance guidelines: an observational study. Gastrointest Endosc. 2023;97(2):350–356. e3. doi:10.1016/j.gie.2022.08.020
9. Cheng SY, Li MC, Chia SL, et al. Factors affecting compliance with confirmatory colonoscopy after a positive fecal immunochemical test in a national colorectal screening program. Cancer. 2018;124(5):907–915. doi:10.1002/cncr.31145
10. Jones RM, Devers KJ, Kuzel AJ, et al. Patient-reported barriers to colorectal cancer screening: a mixed-methods analysis. Am J Prev Med. 2010;38(5):508–516. doi:10.1016/j.amepre.2010.01.021
11. Myers RE, Sifri R, Hyslop T, et al. A randomized controlled trial of the impact of targeted and tailored interventions on colorectal cancer screening. Cancer. 2007;110(9):2083–2091. doi:10.1002/cncr.23022
12. Jones CJ, Smith H, Llewellyn C. Evaluating the effectiveness of health belief model interventions in improving adherence: a systematic review. Health Psychol Rev. 2014;8(3):253–269. doi:10.1080/17437199.2013.802623
13. National Clinical Research Center for Digestive Diseases (Shanghai), Gastrointestinal Endoscopy Branch of the Chinese Medical Association, Tumor Endoscopy Committee of the Chinese Anti-Cancer Association; et al. Expert consensus on management strategies for colorectal cancer precancerous lesions and precancerous states in China. Chin J Dig Endosc. 2022;39(1):1–18.
14. Hassan C, Antonelli G, Dumonceau JM, et al. Post-polypectomy colonoscopy surveillance: European Society of Gastrointestinal Endoscopy (ESGE) guideline–update 2020. Endoscopy. 2020;52(08):687–700. doi:10.1055/a-1185-3109
15. Wang R, Zhao S, Pan P, et al. Investigation on the development of colonoscopy and the cognition of colonoscopy physicians in China. Chin J Dig Endosc. 2021;38(2):115–119.
16. Tae CH, Moon CM, Kim SE, et al. Risk factors of nonadherence to colonoscopy surveillance after polypectomy and its impact on clinical outcomes: a KASID multicenter study. J Gastroenterol. 2017;52:809–817. doi:10.1007/s00535-016-1280-3
17. Takahashi Y, Tanaka H, Kinjo M, Sakumoto K. Prospective evaluation of factors predicting difficulty and pain during sedation-free colonoscopy. Dis Colon Rectum. 2005;48(6):1295–1300. doi:10.1007/s10350-004-0940-1
18. Pecere S, Ciuffini C, Chiappetta MF, et al. Increasing the accuracy of colorectal cancer screening. Expert Rev Anticancer Ther. 2023;23(6):583–591. doi:10.1080/14737140.2023.2207828
19. Zhao S, Wang S, Pan P, et al. FIT-based risk-stratification model effectively screens colorectal neoplasia and early-onset colorectal cancer in Chinese population: a nationwide multicenter prospective study. J Hematol Oncol. 2022;15(1):162. doi:10.1186/s13045-022-01378-1
20. Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366(8):687–696. doi:10.1056/NEJMoa1100370
21. Ferlitsch M, Moss A, Hassan C, et al. Colorectal polypectomy and endoscopic mucosal resection (EMR): European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline. Endoscopy. 2017;49(3):270–297. doi:10.1055/s-0043-102569
22. Tanaka S, Saitoh Y, Matsuda T, et al. Evidence-based clinical practice guidelines for management of colorectal polyps. J Gastroenterol. 2021;56(4)::323–335. doi:10.1007/s00535-021-01776-1
23. He Y, Wei L, Tong Z. Comparison of endoscopic mucosal resection and mucosal dissection in patients with early colorectal cancer and precancerous lesions. China J Endosc. 2016;22(10):66–69.
24. Colquhoun P, Chen HC, Kim JI, et al. High compliance rates observed for follow up colonoscopy post polypectomy are achievable outside of clinical trials: efficacy of polypectomy is not reduced by low compliance for follow up. Colorectal Dis. 2004;6(3):158–161. doi:10.1111/j.1463-1318.2004.00585.x
25. Lee SM, Kim JH, Sung IK, Hong SN. The risk of metachronous advanced colorectal neoplasia rises in parallel with an increasing number of high-risk findings at baseline. Gut Liver. 2015;9(6):741. doi:10.5009/gnl14210
26. Leufkens AM, van Oijen MG, Vleggaar FP, Siersema PD. Factors influencing the miss rate of polyps in a back-to-back colonoscopy study. Endoscopy. 2012;44(05):470–475. doi:10.1055/s-0031-1291666
27. Murphy CC, Lewis CL, Golin CE, Sandler RS. Underuse of surveillance colonoscopy in patients at increased risk of colorectal cancer. Am J Gastroenterol. 2015;110(5):633–641. doi:10.1038/ajg.2014.344
28. van Kooten H, de Jonge V, Schreuders E, et al. Awareness of post polypectomy surveillance guidelines: a nationwide survey of colonoscopists in Canada. Can J Gastroenterol. 2012;26(2):79–84. doi:10.1155/2012/919615
29. Kamran U, Abbasi A, Tahir I, Hodson J, Siau K. Can adjuncts to bowel preparation for colonoscopy improve patient experience and result in superior bowel cleanliness? A systematic review and meta-analysis. United European Gastroenterol J. 2020;8(10):1217–1227. doi:10.1177/2050640620953224
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
