Back to Journals » Journal of Asthma and Allergy » Volume 17
Analysis of the Construction of a Predictive Model for Eosinophilic Chronic Rhinosinusitis
Received 28 November 2023
Accepted for publication 20 February 2024
Published 28 February 2024 Volume 2024:17 Pages 133—141
DOI https://doi.org/10.2147/JAA.S450514
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Luis Garcia-Marcos
Shuo Wu,1,2 Jiahong Lao,1 Feitong Jian1
1E.N.T. Department, the 3rd Affiliated Hospital, Sun Yat-Sen University, Guangzhou, People’s Republic of China; 2School of Biomedical Engineering, Sun Yat-Sen University, Shenzhen, People’s Republic of China
Correspondence: Shuo Wu, E.N.T. Department, the 3rd Affiliated Hospital, Sun Yat-Sen University, Guangzhou, People’s Republic of China, Tel +86-20-85252239, Email [email protected]
Purpose: This study aimed to determine indices to diagnose and predict eosinophilic chronic rhinosinusitis (ECRS) during the initial clinic visit.
Patients and Methods: We retrospectively analyzed 116 patients with chronic rhinosinusitis who underwent endoscopic sinus surgery and were classified according to the postoperative pathological diagnosis. General data and various clinical indicators were analyzed, and indicators with statistically significant differences between groups were further incorporated into a multivariate logistic regression to establish a comprehensive prediction model. The receiver operating characteristic (ROC) curve was used to compare the two significant valuable single factors from previous studies, the difference in CT scores between the ethmoid sinus and the sum difference of the maxillary sinus (EM difference) and the absolute value of peripheral blood eosinophil (bEOS), with a comprehensive prediction model.
Results: There were significant differences in history of allergic asthma (p < 0.001), visual analog scale (VAS) score (p=0.005), sino-nasal outcome test-22(SNOT-22) scale score (p=0.004), Lund-Mackay scale score (p=0.017), EM difference (p=0.002), percentage of bEOS (%)(p=0.001), and absolute value of bEOS (× 109/L) (p=0.000) between the two groups (p< 0.05). The history of allergic disease, VAS and bEOS were screened out and included in the comprehensive prediction model. The area under the curve (AUC) of the comprehensive prediction model (0.804)> the AUC of the absolute value of the bEOS (0.764)>the AUC of the EM difference (0.655). The AUC of the EM difference and the comprehensive prediction model were statistically different (P=0.025). There was no statistical difference between the absolute value of bEOS and the AUC of the comprehensive prediction model.
Conclusion: The comprehensive prediction model covering the three aspects of allergic asthma history, VAS score, and bEOS count had the highest AUC compared to the other predictors and had good predictive power for the diagnosis of ECRS.
Keywords: eosinophilic chronic rhinosinusitis, non-eosinophilic chronic rhinosinusitis, prediction model, the difference of the CT scores between the ethmoid sinus and maxillary sinus, European position paper on rhinosinusitis and nasal polyps 2020
Introduction
Rhinosinusitis is a highly heterogeneous disease with complex pathogenesis and a wide range of treatment options. The treatment options and prognosis for different types of rhinosinusitis vary greatly.1 The European Position Paper on Rhinosinusitis and Nasal Polyps 20202 (EPOS-2020), based on the 2012 version,3 incorporated a large number of research findings and reclassified chronic rhinosinusitis according to its pathogenesis, extent of the lesions, intrinsic phenotype, etc. The classification was not based on the presence or absence of associated nasal polyps, but on the degree of tissue eosinophil percentage (tEOS) infiltration. They are divided into type II (eosinophilic chronic rhinosinusitis, ECRS) and non-type II (non-eosinophilic chronic rhinosinusitis, nECRS).4 Patients with ECRS are more likely to have nasal polyps than those with nECRS and show a higher rate of asthma comorbidity and postoperative recurrence.5,6 Following the European Forum for Research and Education in Allergy and Airway Diseases (EUFOREA) treatment algorithm for rhinosinusitis, there are significant differences between the treatment modalities of ECRS and nECRS.7 Thus, typing diagnosis is an urgent issue for all rhinologists. tEOS ≥10/HPF was recently proposed as a criterion for the diagnosis of ECRS by EPOS-2020.3 However, this diagnosis requires a local biopsy and an experienced pathologist to evaluate the number of eosinophils in 10 random high-magnification fields before a typing diagnosis can be made. This makes it difficult to carry out this work in primary care hospitals, and it is difficult for primary care physicians to make a precise typing diagnosis in the early stages of a patient’s visit so that targeted treatment can be administered. Therefore, it is particularly important to establish a predictive model for typing diagnosis based on clinical characteristics as early as possible. In this study, we retrospectively analyzed patients with chronic rhinosinusitis who underwent endoscopic sinus surgery at the Third Affiliated Hospital of Sun Yat-sen University from June 2020 to March 2022 and were classified by pathological diagnosis after surgery to construct a predictive model of ECRS by comparing and analyzing their general data and various clinical indicators to provide some help in classifying and diagnosing chronic rhinosinusitis through non-invasive examination.
Materials and Methods
Study Design
The primary clinical data of the study participants were collected, and the patients were divided into ECRS and nECRS groups according to whether the number of eosinophils in 10 random high-power fields (HPF) was greater than 10 on postoperative pathological examination. The VAS was used to assess preoperative nasal symptoms and peripheral blood inflammatory cell counts, and serum total immunoglobulin (IgE) tests were performed. The sinus CT and nasal endoscopy findings were scored using the Lund-Kennedy and Lund-Mackay scoring systems. General data and various clinical indicators were analyzed, and indicators with statistically significant differences between groups were further incorporated into multivariate logistic regression to establish a comprehensive prediction model. The receiver operating characteristic (ROC) curve was used to compare the two significant valuable single factors from previous studies, the difference in the EM difference and the absolute value of peripheral blood eosinophil (bEOS), with a comprehensive prediction model.
Study Participants
A retrospective analysis was performed on 116 patients hospitalized with primary chronic rhinosinusitis who underwent endoscopic sinus surgery at the Third Affiliated Hospital of Sun Yat-sen University from June 2020 to March 2022. The inclusion criteria were as follows: (i) meeting the diagnostic criteria of EPOS-2020; (ii) no oral hormonal drugs, intranasal steroid sprays, antibiotics, or biologics within the last four weeks for treating CRS or other diseases. The exclusion criteria were as follows: (i) fungal rhinosinusitis; (ii) age <18 or >70 years; (iii) choanal polyp, primary ciliary immobility syndrome, and cystic fibrosis; and (iv) history of malignant tumor of the nasal cavity and paranasal sinuses. This study was approved by the Ethics Committee of the Clinical Research Center of the Third Affiliated Hospital of Sun Yat-sen University (batch no. [2020] 02001 01), and the written informed consent for publication was obtained from all participants.
Definition of Variables
Intraoperative polyps or ethmoid sinus mucosa were obtained from patients for hematoxylin-eosin (HE) staining, and ten fields were randomly recorded by a pathologist under a 400× microscope. tEOS ≥10/HPF was the diagnostic threshold, and the two groups were divided into ECRS and nECRS. Participants were identified as having a history of allergic asthma if they confirmed being diagnosed by a physician with the question, “Have you been diagnosed with allergic asthma by a physician?” Other medical conditions were also assessed based on self-reported physician diagnoses. A history of nasal surgery was determined by asking patients, “Have you undergone nasal surgery?” Smoking history was established by asking, ‘Do you currently smoke?’ And how often?” If the response was “Yes, frequently”, the patient was considered to have a smoking history.
Data Sources/Measurement
General clinical data from the patients were collected and the VAS was used to assess nasal symptoms of nasal obstruction, olfactory loss, runny nose, and head/face pain. All patients underwent peripheral blood inflammatory cell counts and serum total IgE tests, nasal endoscopy was performed, and the Lund-Kennedy scale was used to score nasal polyps preoperatively (scoring criteria: polyp: 0, no polyps;1, polyps only in the middle nasal meatus; 2, polyps beyond the middle nasal meatus; edema:0, none;1, mild;2, severe; rhinorrhea:0, none;1, clear and thin rhinorrhea;2, thick and purulent rhinorrhea; 0–12 points per side, total score 0–24 points), spiral CT four-dimensional sinus reconstruction was performed and the sinuses were scored according to the Lund-Mackay scoring system. The Lund-Mackay scoring system was used to score the maxillary sinus, anterior ethmoid sinus, posterior ethmoid sinus, sphenoid sinus, frontal sinus, and ostiomeatal complex (OMC) (scoring criteria: sinuses: 0, normal; 1, partially shadowed (mucosal hypertrophy); 2, completely shadowed (or highly shadowed); OMC lesions were scored as 0–2:0, no obstruction; 2, obstruction; 0–12 points per side, total score 0–24 points). Two additional elements were added simultaneously: (i) the difference in EM difference, the difference between the total score of the ethmoid sinus (0–8 points) and the total score of the maxillary sinus (0–4 points); E represents the sum of the ethmoid sinus scores, and M represents the sum of the maxillary sinus scores. (ii) Olfactory cleft area score (0–2 points for unilateral lesions: 0, no obstruction; 2, obstruction). All scores were evaluated by the same associate physician.
Statistical Analysis
SPSS17.0 statistical software was used for data analysis. Data for normally distributed measures are expressed as mean ± standard deviation, and an independent sample t–test was applied for comparison between groups. Enumeration data were expressed as frequencies and percentages (cases [%]), and the test was used to compare the two groups. For nonnormally distributed variables, the median and upper and lower quartiles were used for description, and the rank sum test (Mann–Whitney U) was used to compare the groups. Indicators with statistically significant differences between group comparisons were also included in the multivariate logistic regression, and the forward LR method was used for the screening of variables. The ROC curve was used to assess the predictive value of the EM difference, the absolute value of bEOS, and the integrated model. The area under the curve (AUC) was used to determine the predictive value. When AUC was > 0.9, the predictive value was excellent; 0.8 < AUC < 0.9, the predictive value was good; 0.7 < AUC < 0.8, the predictive value was moderate; 0.6 < AUC < 0.7, the predictive value was weak; 0.5 < AUC < 0.6, the predictive value was very weak.8 The optimal cutoff value corresponding to the maximum Youden index (sensitivity + specificity − 1) was selected as the best diagnostic threshold. The Delong test and the bootstrap method were used to compare the differences in AUC of EM difference, bEOS absolute value, and comprehensive prediction model. The DeLong test and bootstrap were completed using R3.6.2, and the test level was set at 0.05 with a two-sided test.
Results
General Characteristics of the Study Population
A total of 268 patients with primary chronic rhinosinusitis were admitted for endoscopic sinus surgery at the Third Affiliated Hospital of Sun Yat-sen University between June 2020 and March 2022. Of these, 116 patients met the inclusion criteria for the study (81 male and 35 female. They were divided into two groups according to postoperative pathological results: the ECRS group comprised 40 males and 15 females with an average age of 40.02±12.55 years, and the nECRS group included 41 males and 20 females with an average age of 40.03±14.7 years. A statistical analysis of the general information of the 116 patients was conducted to identify potential risk factors for nECRS. The study revealed no significant differences between the ECRS and nECRS groups in terms of gender, smoking history, family history, history of nasal surgery, age, BMI, length of medical history, serum total IgE, Lund-Kennedy scale, score of the area of the olfactory cleft, percentage of bNEU, and percentage of bLY, with all P values greater than 0.05. However, significant differences were observed in the history of allergic asthma, VAS score, SNOT-22 scale, Lund-Mackay score, EM difference, percentage of bEOS, absolute value of bEOS (×109/L), bENR, and bELR, with all p-values below 0.05, suggesting that these factors are potential predictors of ECRS. The differences in general characteristics between the two groups are detailed in Table 1.
 |
Table 1 Univariate Analysis of ECRS Predictors |
Multidimensional Analysis of ECRS Risk Factors
To establish a comprehensive prediction model for the diagnosis of ECRS, statistically significant indicators of univariate analysis were included in the multivariate logistic regression, and the forward LR method was used to select the variables of the above indicators. Finally, three variables, VAS score(OR=1.054, 95% confidence interval [CI]=1.014–1.097), absolute value of bEOS(OR=44.263, 95% confidence interval [CI]=3.878–505.157), and allergic asthma history(OR=4.016, 95% confidence interval [CI]=1.627–9.915), were selected and included in the comprehensive prediction model (Table 2). After adjusting for potential confounders such as gender, smoking history, family history, history of nasal surgery, age, BMI, length of medical history, serum total IgE, Lund-Kennedy scale, the score of olfactory cleft area, bNEU percentage, and percentage of bLY, the calibration curve indicated that the predicted values of the comprehensive prediction model closely aligned with the diagonal line (indicating a perfect match between the predicted and actual values), demonstrating the accuracy of the prediction.
 |
Table 2 Multivariate Logistics Regression of ECRS Predictors |
The results of the bootstrap 1000 corrections were consistent with the uncorrected results, and the adjusted AUC value was 0.789, indicating that the stability of the model was good (Figure 1).
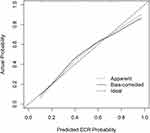 |
Figure 1 Calibration curve of the comprehensive prediction model. |
Assessment of the Prediction Performance of the Comprehensive Prediction Model
To evaluate the effectiveness of the comprehensive prediction model for nECRS, we used the receiver operating characteristic (ROC) curve to compare the comprehensive model with two significant single factors identified in previous studies: the EM difference and the absolute value of bEOS. The comprehensive prediction model had a better predictive value (AUC: 0.888). The predictive value of the single-factor bEOS was moderate (AUC:0.721). The predictive value of the EM difference was weak (AUC: 0.660), as shown in Figure 2. When the maximum Youden index of the comprehensive prediction model was 0.520, the corresponding best cut-off value was 0.410, with a sensitivity of 80.0% and a specificity of 72.0%. The sensitivity and specificity of the three cutoff values are compared in Table 3. To better evaluate the predictive performance of the ECRS diagnosis, the Delong method was used to compare the difference in EM and absolute value of the bEOS with the comprehensive prediction model. The results showed that the AUC of the EM difference and the comprehensive prediction model were statistically different (P=0.025). The mean difference between the results of bootstrap1000 calculations based on the percentile method and the comprehensive prediction model is 0.157 (0.119, 0.197). There was no statistical difference between the absolute value of bEOS and the AUC of the comprehensive prediction model. The bootstrap mean difference between the results 1000 times based on the percentile method and the AUC of the integrated prediction model is 0.087 (0.063,0.118). Compared to the single-factor EM difference and the absolute value through the ROC curve, it was found that the comprehensive prediction model > bEOS absolute value > EM difference in terms of predictive value. When the cutoff value was 0.41, the sensitivity of the comprehensive prediction model in predicting ECRS was 80.0% higher than that of the absolute value of bEOS (70.4%) and the difference in EM (65.5%), indicating that the comprehensive prediction model has an advantage in predicting ECRS.
 |
Table 3 Stability Analysis of ECRS Predictors |
 |
Figure 2 Comparison of AUC curves of relevant indicators and comprehensive prediction models. |
Discussion
CRS is a multifactorial disease. The EPOS-2020 typing of primary chronic rhinosinusitis no longer distinguishes between CRSsNP and CRSwNP according to their phenotypes, but rather in ECRS and nECRS according to the intrinsic phenotype. A large number of previous studies have focused on the prediction of eosinophilic and non-eosinophilic models for CRSwNP.9–13 Patients with chronic eosinophilic rhinosinusitis with nasal polyps (ECRSwNP) showed significant specificity in terms of symptoms, sinus CT sinus findings, incidence of olfactory dysfunction, allergic asthma comorbidity, and postoperative recurrence rate,14,15 the difference in the Lund-Mackay score (EM difference or E/M value) between the ethmoid sinus and maxillary sinus and the absolute value of peripheral blood eosinophils (bEOS) had an excellent predictive value for ECRSwNP.16,17 Currently, after the reclassification of primary chronic rhinosinusitis in EPOS-2020, there is a lack of predictive model analysis for ECRS, and the original predictive model for ECRSwNP may not be applicable to predict newly typed ECRS in EPOS-2020. To establish a comprehensive predictive model for the diagnosis of ECRS, this study compared the general and clinical characteristics of the patients in the ECRS and nECSRS groups who met the inclusion criteria. Significant differences were found between the two groups in terms of history of allergic asthma, VAS score, SNOT-22 scale, Lund-Mackay scale, EM difference, bEOS percentage (%), bEOS absolute value, bENR, and bELR. Multivariate logistic regression was performed and the three variables of allergic asthma history, VAS score, and bEOS absolute value were included in the comprehensive prediction model.
In recent years, it has been suggested that the human body produces a type II inflammatory response, resulting in allergic asthma and ECRSwNP, due to a combination of genetic and environmental factors. Therefore, ECRSwNP is associated with a history of allergic diseases.18–20 In this study, a history of allergic asthma was included in the comprehensive prediction model using multivariate logistic regression analysis, and a history of allergic asthma was also found to be associated with ECRS. However, previous studies are inconclusive in predicting peripheral blood IgE levels for ECRSwNP, and some scholars believe that it has predictive diagnostic value.16 Others have suggested that there is no significant correlation between EOS counts and total serum IgE levels in nasal polyp tissues of patients with CRSwNP. Sakuma et al found no significant differences in serum total IgE levels between patients with ECRSwNP and nECRSwNP.12 In the present study, there were no statistically significant differences in total serum IgE levels between the ECRS and nECRS groups, suggesting that serum total IgE is not significant in the diagnosis of ECRS. The present study showed that although a history of allergic asthma was associated with ECRS, serum total IgE levels were not associated with ECRS. Considering the large number of previous studies on ECRSwNP, chronic rhinosinusitis is a localized mucosal lesion, and changes in serum total IgE do not accurately reflect the local inflammatory condition of the nasal mucosa.
Based on the previous stage, a large number of studies have found that the ratio or sum difference of the CT scores of the nasal sinus between the ethmoid sinus and the maxillary sinus has some value in predicting ECRSwNP.21–24 Meng et al found that E/M values have an excellent predictive value for diagnosing ECRSwNP, with an AUC as high as 0.938.23
In the present study, EM was used to represent the difference in imaging findings between the ethmoid and maxillary sinuses in the presentation of nasal sinus CT. The EM difference had a weaker predictive value for ECRS in this study, with an AUC of 0.660. In contrast, patients with nECRSwNP had more pronounced lesions than those with maxillary sinus involvement. The new EPOS-2020 staging system no longer distinguishes between the presence or absence of concomitant nasal polyps, and many cases of chronic rhinosinusitis of the dominant eosinophil type do not show concomitant nasal polyps in the early stages of the disease and do not yet show typical imaging manifestations of heavier shadows in the ethmoid sinus area. The total difference between the ethmoid and maxillary sinuses has not yet been revealed, leading to unsatisfactory results for the EM difference as a single factor predictor.
Previous studies have shown that the bEOS count can be used as a diagnostic marker for ECRSwNP,25,26 and Hu et al reported an AUC of 0.871 for the bEOS count in diagnosing ECRSwNP.27 In the present study, peripheral blood EOS count as a single factor had a moderate predictive value of 0.721 AUC for the diagnosis of ECRS, and this multivariate logistic indicator regression analysis was included in the comprehensive prediction model. The bEOS count can be used as a predictor of ECRS, regardless of the degree of disease and the presence of nasal polyps. The AUC usually indicates the diagnostic performance of an indicator; the larger the AUC, the higher the diagnostic accuracy. An indicator with good diagnostic accuracy should generally have an AUC greater than 0.8.8 In this study, the AUC value of 0.804 for the comprehensive prediction model was higher than that of the bEOS count of a single factor. Although there was no statistical difference between the two, considering that bEOS tissue infiltration does not simply migrate from peripheral blood to local lesions, the bEOS count is not highly specific for the diagnosis of ECRS, and multiple chemokines and cytokines are involved in the local EOS infiltration process. The bEOS count can be affected by a variety of factors, including allergies, autoimmune diseases, parasitic infections, and drug use.28 Previous studies have shown that when multiple predictors are included in a model, they are more useful for diagnosis than a single predictor.21 Therefore, the use of a single-factor peripheral blood EOS count as a diagnostic predictor of ECRS may not be complete.
This study has some limitations. First, the relatively small sample size and the fact that this was a single-center retrospective study may have introduced bias. This study cannot explain the direction of causality due to its cross-sectional nature. To clarify this issue, a prospective long-term multicenter cohort study should be conducted in the future. Second, since all participants in this study were hospitalized for sinusitis surgery, the absence of outpatients with mild symptoms treated with medication might introduce bias. Future research should consider including patients with milder conditions.
Conclusion
In conclusion, the comprehensive prediction model covering the three aspects of allergic asthma history, VAS score, and bEOS count had the highest AUC compared to other predictors and had good predictive power for ECRS diagnosis. Previous asthma history representing individual characteristics, patients’ subjective perception, and the objective bEOS index could be used as a predictive model for predicting chronic rhinosinusitis typing criteria. The three indices in this comprehensive model are economical and convenient and are suitable for the classification and diagnosis of patients with initial outpatient visits, further staging treatment, and prognostic assessment.
Statement of Ethics
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the Clinical Research Center of the Third Affiliated Hospital of Sun Yat-sen University (batch number: [2020]02,001 01).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wang W, Gao Y, Zhu Z, et al. Changes in the clinical and histological characteristics of Chinese chronic rhinosinusitis with nasal polyps over 11 years. Int Forum Allergy Rhinol. 2019;9(2):149–157. doi:10.1002/alr.22234
2. Fokkens WJ, Lund VJ, Hopkins C, et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology. 2020;58(Suppl S29):1-464. doi: 10.4193/Rhin20.600
3. Fokkens W, Lund VJ, Mullol J, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology Jl. 2012;50(1):1–12. doi:10.4193/Rhino12.000
4. Yang Q, Sun YQ, Wu QW, et al. Interpretation of 2020 European position paper on rhinosinusitis and nasal polys. ChinJ Otorhinol Head Neck Sur. 2020;55(3):304–308. doi:10.3760/cma.j.issn.1673-0860.2020.03.024
5. Zhang Y, Gevaert E, Lou H, et al. rhinosinusitis in Asia. J Allergy Clin Immunol. 2017;140(5):1230–1239. doi:10.1016/j.jaci.2017.09.009
6. Jiang W, Cao -P-P, Li Z-Y, et al. A retrospective study of changes of histopathology of nasal polyps in adult Chinese in central China. Rhinology. 2019;57(4):261–267. doi:10.4193/Rhin18.070
7. Pw H, Alobid I, Wt A, et al. EUFOREA/EPOS2020 statement on the clinical considerations for CRSwNP care. Allergy. 2023;2023:1.
8. Zhu M, Gao X, Zhu Z, et al. The roles of nasal nitric oxide in diagnosis and endotypes of chronic rhinosinusitis with nasal polyps. J Otolaryngol Head Neck Surg. 2020;49(1):68. doi:10.1186/s40463-020-00465-y
9. Hauser L, Chandra RK, Li P, et al. Role of tissue eosinophils in chronic rhinosinusitis-associated olfactory loss. Int Forum Allergy Rhinol. 2017;7(10):957–962. doi:10.1002/alr.21994
10. Wang E. Eosinophilic chronic rhinosinusitis in east Asians. World J Clin Cases. 2014;2(12):873–882. doi:10.12998/wjcc.v2.i12.873
11. Tokunaga T, Sakashita M, Haruna T, et al. Novel scoring system and algorithm for classifying chronic rhinosinusitis: the JESREC study. Allergy. 2015;70(8):995–1003. doi:10.1111/all.12644
12. Sakuma Y, Ishitoya J, Komatsu M, et al. New clinical diagnostic criteria for eosinophilic chronic rhinosinusitis. Auris Nasus Larynx. 2011;38(5):583–588. doi:10.1016/j.anl.2011.01.007
13. Pan L, Liu Z. Classification of chronic rhinosinusitis with nasal polyps based on eosinophilic inflammation. ChinJ Otorhinol Head Neck Sur. 2019;54(3):222–226. doi:10.3760/cma.j.issn.1673-0860.2019.03.013
14. Shah S, Ishinaga H, Takeuchi K. Pathogenesis of eosinophilic chronic rhinosinusitis. J Inflamm. 2016;13(1):11. doi:10.1186/s12950-016-0121-8
15. Kim D, Kim S, Basurrah M, Hwang S. Clinical and laboratory features for various criteria of eosinophilic chronic rhinosinusitis: a systematic review and meta-analysis. Clin Exp Otorhinolaryngol. 2022;15(3):230–246. doi:10.21053/ceo.2022.00052
16. Lou H, Meng Y, Piao Y, et al. Predictive significance of tissue eosinophilia for nasal polyp recurrence in the Chinese population. Am J Rhinol Allergy. 2015;29(5):350–356. doi:10.2500/ajra.2015.29.4231
17. Pan X, Zhang Y, Wang C, et al. Evaluation of nasal symptoms to distinguish eosinophilic from noneosinophilic nasal polyps based on peripheral blood. Allergy Asthma Proc. 2021;42(3):214–221. doi:10.2500/aap.2021.42.210004
18. Romagnani S. The role of lymphocytes in allergic disease. J Allergy Clin Immunol. 2000;105(3):399–408. doi:10.1067/mai.2000.104575
19. Yamada T, Miyabe Y, Ueki S, et al. Eotaxin-3 as a plasma biomarker for mucosal eosinophil infiltration in chronic rhinosinusitis. Front Immunol. 2019;10:74. doi:10.3389/fimmu.2019.00074
20. Castillo J. Asthma, rhinitis, and nasal polyp multimorbidities. ArchBronconeumol. 2019;55:146–155. doi:10.1016/j.arbres.2018.09.001
21. Meng Y, Lou H, Wang C, Zhang L. The value of sinonasal CT scan in diagnosing of eosinophilic chronic rhinosinusitis with nasal polyps. ChinJ Otorhinol Head Neck Sur. 2017;52:93–98. doi:10.3760/cma.j.issn.1673-0860.2017.02.004
22. Kim D, Jin HR, Eun KM, et al. Non-eosinophilic nasal polyps shows increased epithelial proliferation and localized disease pattern in the early stage. PLoS One. 2015;10(10):e0139945. doi:10.1371/journal.pone.0139945
23. Meng Y, Lou H, Wang C, et al. Predictive significance of computed tomography in eosinophilic chronic rhinosinusitis with nasal polyps. Int Forum Allergy Rhinol. 2016;6(8):812–819. doi:10.1002/alr.21749
24. Mattos J. Mechanisms and treatment of olfactory dysfunction in chronic rhinosinusitis. Ann Allergy Asthma Immunol. 2020;124(4):307–308. doi:10.1016/j.anai.2020.01.014
25. Brescia G, Barion U, Zanotti C, et al. Blood eosinophil-to-basophil ratio in patients with sinonasal polyps: does it have a clinical role? Ann Allergy Asthma Immunol. 2017;119(3):223–226. doi:10.1016/j.anai.2017.06.008
26. Brescia G, Sfriso P, Marioni G. Role of blood inflammatory cells in chronic rhinosinusitis with nasal polyps. Acta Otolaryngol. 2019;139(1):48–51. doi:10.1080/00016489.2018
27. Hu Y, Cao -P-P, Liang G-T, et al. Diagnostic significance of blood eosinophil count in eosinophilic chronic rhinosinusitis with nasal polyps in Chinese adults. Laryngoscope. 2012;122(3):498–503. doi:10.1002/lary.22507
28. Liao B, Liu J-X, Li Z-Y, et al. Multidimensional endotypes of chronic rhinosinusitis and their association with treatment outcomes. Allergy. 2018;73(7):1459–1469. doi:10.1111/all.13411
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
