Back to Journals » Journal of Asthma and Allergy » Volume 14
Analysis of Peanut Allergen Components Sensitization and Cross Reaction with Pollen Allergen in Chinese Southerners with Allergic Rhinitis and/or Asthma
Authors Luo W, Yang S, Huang H, Wu L, Cheng ZJ , Zheng P , Zheng J , Sun B
Received 24 August 2021
Accepted for publication 13 October 2021
Published 28 October 2021 Volume 2021:14 Pages 1285—1293
DOI https://doi.org/10.2147/JAA.S335265
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Luis Garcia-Marcos
Wenting Luo,* Shuwen Yang,* Huimin Huang, Liting Wu, Zhangkai J Cheng, Peiyan Zheng, Jinping Zheng, Baoqing Sun
Department of Allergy and Clinical Immunology, State Key Laboratory of Respiratory Disease, National Clinical Research Center for Respiratory Disease, Guangzhou Institute of Respiratory Health, First Affiliated Hospital of Guangzhou Medical University, Guangzhou Medical University, Guangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Baoqing Sun; Jinping Zheng Email [email protected]; [email protected]
Objective: Peanut is one of the most frequently reported allergens causing severe allergies in western countries. In China, however, there have been few reports of severe allergies caused by peanuts. We investigated the peanut allergen components sensitization and cross-reaction with pollen allergen in Chinese Southerners with allergic rhinitis and/or asthma.
Methods: Total IgE (tIgE) and specific IgE (sIgE) antibodies against Ara h 1, Ara h 8, Juglans pollen, Platanus pollen, birch pollen, Bet v 1, Bet v 4, and cross-reactive carbohydrate determinant (CCD) of 58 allergic patients, of whom 33 were peanut-sIgE positive and 25 were negative, were detected by the ImmunoCAP system. The relationships between peanut allergen and pollen allergens were analyzed.
Results: A 9.1% (3/33) of the patients with peanut sensitization were sensitized to Ara h 8, while 21.2% (7/33) were sensitized to Ara h 1. The peanut-sensitized group had significantly higher positive rates for sIgE antibodies against CCD (69.7% vs 4.0%), Juglans pollen (87.9% vs 12.0%), Platanus pollen (90.9% vs 16.0%), and birch pollen (60.6% vs 4.0%) than the peanut tolerance group (all P < 0.05). Spearman correlation showed that peanut-sIgE were significantly correlated with sIgE to CCD (rs=0.859), Juglans pollen (rs=0.772), Platanus pollen (rs=0.838) and birch pollen (rs=0.816).
Conclusion: The majority of patients sensitized to peanut allergen in Southern China tested positive for multiple pollen allergens. Peanut sensitization was highly correlated with Platanus, Juglans, and birch pollen sensitization, which suggested there may be cross-reactions between peanut and pollen allergens. Clinicians should pay attention to distinguish diagnosis in clinical peanut allergy diagnosis and treatment.
Keywords: peanut sensitization, pollen allergen, specific IgE, cross-reactivity, CCD
Introduction
The prevalence of allergic diseases has been increasing in recent years, imposing a significant economic burden on society. The prevalence rates of two common allergic diseases, food allergies and respiratory allergic diseases have increased drastically in both Western and Eastern countries. Many plant allergens, such as pan-allergens, cross-react with homologous proteins found in plant-derived foods and pollen allergens,1 causing pollen food allergy syndrome, a common allergic disease mediated by immunoglobulin E (IgE).2 This raises the question of whether cross-reactivity between allergens plays a role in the prevalence of allergic symptoms in allergic populations.
Peanut, one of the eight major food allergens, causes peanut allergy (PA) which is increasing dramatically in Asian countries.3,4 The immunologic cross-reaction between peanuts and tree nuts has been extensively researched.5,6 Although pollen allergen-induced IgE cross-reaction is the most common cause of sensitization to fruits, vegetables, tree nuts, and legumes,7 these cross-reactions were traditionally assumed to have no association with severe allergies to tree nuts such as peanuts previously. Recent studies, however, have found co-sensitization and cross-react between peanut allergen and pollen allergens.9–11 Asarnoj et al8 reported that some patients with grass pollen allergy who also had special IgE (sIgE) antibodies to peanuts did not develop any clinical symptoms. Ara h 8, a pathogenesis-related protein (PR)-10 family allergen, is a Bet v 1-homologous allergen from peanut and is thought to be the major allergen causing cross-reactions between peanut and birch.12 It has been shown that Ara h 2 and Ara h 6 are marker allergens for peanut allergy.13 But in our preliminary experiment, patients with peanut-sIgE positive had a low positive rate of Ara h 2, and none of them was positive for Ara h 3 or Ara h 6. Furthermore, cross-reactive carbohydrate determinants (CCDs) may induce IgE, leading to cross-reactivity between peanut and pollen allergens, which can cause sIgE positive to peanut despite the absence of positive peanut SPT responses. This condition may result in misdiagnosis during in vitro allergy diagnosis. Therefore, it’s necessary to distinguish a true peanut allergy from peanut sensitization alone.
Previous research found that in Guangdong, a positive peanut-sIgE test result was frequently accompanied by a positive result sIgE antibody test for pollen allergens, but no obvious clinical symptoms were associated with peanut food consumption. However, little is known about peanut allergen co-sensitization and cross-reactivity with pollen allergens.This study aimed to investigate the pollen allergen positivity rate in peanut-sIgE positive patients in Southern China, as well as the relationship between peanut and pollen allergen sensitization. Correlation analysis between peanut and birch components was also used to determine whether peanut allergens have co-sensitization or cross-reaction with pollen allergens. Our findings are expected to be utilized to discriminate the positive peanut sIgE result without clinical symptoms and peanut allergy, to avoid unnecessary misdiagnosis.
Materials and Methods
Patients
Over 58 patients with allergic diseases whose data were obtained from the Biobank for Respiratory Diseases in the National Clinical Research Center for Respiratory Disease (BRD-NCRCRD, Guangzhou, Southern China) were retrospectively enrolled in this study, with 33 patients with peanut-sIgE positive and 25 with peanut-sIgE negative. Patients with a) allergies to at least one inhaled allergen and b) physician-diagnosis of allergic rhinitis, allergic asthma, or allergic rhinitis with asthma were included in the study. There was no statistically significant difference in the proportion of asthma (27.3% vs 32.0%), allergic rhinitis (30.3% vs 44.0%), and allergic rhinitis with asthma (44.2% vs 24.0%) in the peanut-sIgE positive group and negative group, (Table 1). Through clinical records and questionnaire survey, none of these patients exhibit obvious systemic symptoms, gastrointestinal e.g vomiting, nausea or diarrhea, or oral allergic syndrome (OAS) after peanut consumption. The diagnosis of allergic rhinitis were determined based on the Allergic Rhinitis and Its Impact On Asthma (2015) and Global Initiative for Asthma (2015) guidelines. Patients with parasitic infection, immunodeficiency, or specific immunotherapy were excluded. This study was approved by the ethics committee of the First Affiliated Hospital of Guangzhou Medical University (approval number: GYFYY-2017-18). Data is in compliance with relevant data protection and privacy legislation. The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request. Written informed consent was collected from all the patients. For subjects younger than 18 years of age, written informed consents were obtained from their parents or legal guardian.
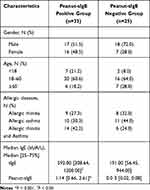 |
Table 1 Clinical Characteristics of Patients in the Peanut-sIgE Positive and Peanut-sIgE Negative Groups |
Serum Allergen-sIgE Measurement
In our preliminary experiment (unpublished), patients with peanut-sIgE positive had a low positive rate of Ara h 2 (less than 2%), and none of them was positive for Ara h 3 or Ara h 6. In this study, Ara h 1 and Ara h 8 with higher positive rate were selected for detection and analysis. Serum samples were tested for the presence of total IgE (tIgE) and sIgE antibodies against peanut (f13), Ara h 1, Ara h 8, Juglans pollen (t10), Platanus pollen (t11), birch pollen (t3), Bet v 4, and Bet v 1, and cross-reactive carbohydrate determinant (CCD) using the ImmunoCap system (Thermo Fisher Scientific Inc., California, USA). Correlation analysis was performed to determine the relationship between peanut allergen and pollen allergens.
Positive reactivity was defined as an sIgE level ≥ 0.35 kUA/L (Class 1 or above).14,15 According to the concentration ofsIgE antibody, reactivity was categorized quantitatively into six classes: Class 1 (≥0.35 to <0.70 kUA/L), Class 2 (≥0.70 to <3.50 kUA/L), Class 3 (≥3.50 to <17.50 Â kUA/L), Class 4 (≥17.50 to <50.00 kUA/L), Class 5 (≥50.00 to <100.00 kUA/L), and Class 6 (≥100.00 kUA/L).
Statistical Analysis
Data were analyzed using the SPSS 13.0 software (IBM Corp., Armonk, NY, USA). Parametric quantitative data were expressed as mean ± standard deviation. Non-parametric quantitative data were expressed as medians (interquartile ranges). Categorical data were reported as percentages showing the proportion of positive results. Proportions were compared between groups using the chi-square test (χ2). Comparisons between the two parametric groups of data were performed using unpaired t-tests. Non-parametric rank-sum tests were used to compare non-parametric data. A hierarchical cluster test was used to classify all variables by analyzing the similarity or dissimilarity of the data. Correlation analyses between non-parametric data were performed using Spearman’s tests, with the correlation coefficients presented as “rs.” The statistical significance level was set at P < 0.05.
Results
sIgE and tIgE Antibody Levels of Patients Based on Disease and Demographic Details
The levels of tIgE and peanut-sIgE antibodies in patients with peanut-sIgE positive were significantly higher than those with peanut-sIgE negative (tIgE: 592.00 kUA/L vs 191.00 kUA/L, P = 0.012; peanut-sIgE: 1.14 kUA/L vs 0.03 kUA/L, P < 0.001) (Table 1). The peanut sensitization was most common in adults (18–60-year-old group: 60.6%), followed by children (<18-year-old: 21.2%) and elderly patients (≥ 60-year-old group: 18.2%).
Differences in the Positivity Rates of Sensitization to Pollen Allergens Between the Peanut-sIgE Positive Group and Peanut-sIgE Negative Group
9.1% (3/33) of patients with peanut-sIgE positive were sensitized to Ara h 8 and 21.2% (7/33) were sensitized to Ara h 1. The positivity rate of CCD-sIgE in the peanut-sIgE positive group (69.7%, 23/33) was significantly higher than that in the peanut-sIgE negative group (4.0%, 1/25) (P < 0.001) (Figure 1). The peanut-sIgE positive group showed significantly higher positive rates of Juglans pollen (87.9%, 29/33 vs 12.0%, 3/25), Platanus pollen (90.9%, 30/33 vs 16.0%, 4/25), and birch pollen (60.6%, 20/33 vs 4.0%, 1/25) than the peanut-sIgE negative group. However, for Bet v 4 and Bet v 1, no significant difference was observed between the two groups.
 |
Figure 1 Percentage of IgE-positive responses to allergen components in the peanut-sIgE positive group and peanut-sIgE negative group. *P < 0.001, †P < 0.05. |
Relationship Between the sIgE Levels of Peanut Allergen- and Other Pollen Allergens
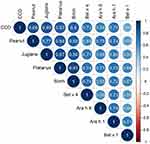
Spearman’s rank correlation analysis was used to investigate the sIgE concentration of peanut allergen and pollen allergens. Peanut-sIgElevels were strongly correlated with sIgE of Platanus pollen (rs=0.838), birch pollen (rs=0.816), Juglans pollen (Juglans: rs=0.772) and Bet v 4 (Bet v 4: rs=0.646), while it was moderately correlated with Bet v 1 (rs=0.556, all P < 0.001), as seen in Figure 2. A significant association was also found between CCD and peanut allergen (rs=0.859, P < 0.001).
The co-sensitization rate of peanut, Platanus pollen, Juglans pollen, and birch pollen was further analyzed using a Venn diagram. Of the 38 patients with peanut or pollen allergen sensitization, 50% (19/38) were sIgE positive to peanut, Platanus pollen, Juglans pollen, and birch pollen; 23.7% (9/38) of the patients were sensitized to peanut, Platanus pollen, and Juglans pollen (Figure 3). Only 2 patients were sensitized to peanut-sIgE alone, 2 to Platanus-sIgE alone, and 1 to Juglans-sIgE alone.
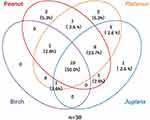 |
Figure 3 Venn diagram of component co-sensitization among the peanut, Platanus, birch, and Juglans allergens. |
Positivity Rates of Pollen Allergens in Patients Sensitized to Peanut Alone or to Both Peanut and Birch
Based on the status of peanut- and birch-associated allergic reactions, all 58 patients were further divided into four subgroups, as shown in Table 2. None of the Peanut-sIgE positive and Birch-sIgE negative patients were sensitized to Ara h 8. The sIgE response to Ara h 8 and Ara h 1 commonly occurred in group of co-sensitization of peanut and birch. sIgE-positive to both peanut and birch Group had a higher positivity rate of allergy to Platanus pollen (100%), which was significantly higher than that in IgE-positive to peanut and sIgE-negative to birch group (76.9%).
 |
Table 2 Percentages of Patients Sensitized to Peanut Alone or to Both Peanut and Birch Who Tested Positive for Allergens and CCD |
Hierarchical Cluster Analysis of the Connection Among Nine Allergen Components
Using hierarchical cluster analysis, nine allergen components were classified into three different sensitization clusters. The first cluster included three allergens (Bet v 1, Ara h 8, and birch pollen). The second cluster included five allergens (Juglans pollen, Platanus pollen, CCD, and Bet v 4). Additionally, Ara h 1 was independent of the third cluster (Figure 4).
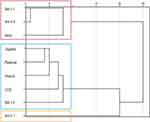 |
Figure 4 Hierarchical cluster of peanut allergens, pollen allergens, and CCD. |
Discussion
This study aimed to investigate the allergenicity of peanut and pollen allergens, as well as the correlation between peanut allergen and pollen allergen components in patients with allergic diseases. Peanut sensitization is frequently associated with allergies to beans, tree nuts, seeds, fruits, and pollen.16 Ara h 8, a homolog of Bet v 1 from birch pollen, can cause PA or OAS. Through clinical records and questionnaire survey, none of these patients exhibit obvious systemic symptoms, gastrointestinal e.g vomiting, nausea or diarrhea, or OAS after peanut consumption. This means that sIgE positive peanut results in patients in Southern China are not accompanied by clinical symptoms, and they may be false positive due to cross-reactions of CCD or other pollen allergens. In patients with PA symptoms, determining the presence of peanut sIgE antibodies is not sufficient for the diagnosis of PA caused by cross-react of tree pollen.17
All the patients included in this study were from Southern China, a subtropical region that is suitable for tree growth and has a high prevalence of tree pollen allergy. The proportion of those sensitized to CCD, Juglans pollen, Platanus pollen, and birch pollen was significantly higher in the 33 peanut-sIgE positive patients than that negative patients. In our study, half of the peanut-sensitized patients were also allergic to Juglans pollen, Platanus pollen, and birch pollen. These findings suggest that there is a link between peanut allergen and pollen allergens.Furthermore, the correlation analysis revealed that peanut, Juglans pollen, and Platanus pollen sIgE antibody levels were highly correlated. Nine of the 38 peanut-sensitized patients (23.7%) were also sensitized to Juglans and Platanus pollen. This could be due to a cross-reaction between non-specific lipid transfer protein (nsLTP) and the Platanus pollen allergen. Platanus pollen component Pla a 3 and peanut component Ara h 9 are both members of the nsLTPs of the prolamin superfamily. Because of their structural similarity, single nsLTPs exhibit IgE cross-reactivity.18–20 In the nsLTP allergic group, Scala et al found a significant correlation between the immune response to Platanus pollen component Pla a 3 and nsLTPs in tree nuts and peanuts. Meanwhile, in peanut-sensitized patients, allergy to Platanus pollen was also related to sensitivity to Ara h 9.21 In a study of nsLTP sensitization, according to the homology of sequences, Platanus pollen component Pla a 1, peanut allergen component Ara h 9, and Juglans pollen component Jug r 3 were grouped into one subset and were considered to be related to allergies to plant-derived foods.22 The result of hierarchical cluster analysis in our study shows that the peanut, Platanus, and Juglans pollen allergies were all grouped in the same subset. Unfortunately, no sIgE antibody levels of peanut allergen Ara h 9 and Platanus pollen allergen components were found in this study. As a result, the correlation between specific components could not be determined, but our results could be used as a starting point for future research on the cross-react between peanut and Platanus.
Both the Peanut allergen component Ara h 8 and birch pollen component Bet v 1 belong to the PR-10 protein family, and their high cross-reactivity has long been documented. In this study, the levels of sIgE antibodies for peanut and birch pollen allergens were highly correlated (rs=0.816), which was consistent with previous findings.12 Patients were divided into four groups based on their level of peanut and birch pollen sensitization. All patients sensitized to Ara h 8 were assigned to the peanut- and birch-sensitized groups, whereas none of the patients in the peanut-positive or birch-negative groups were sensitized to Ara h 8. The findings confirmed that Ara h 8 may play an important role in the birch-peanut cross-reaction. However, the allergen correlation analysis revealed a moderate correlation between Ara h 8 and Bet v 1 in terms of sIgE levels (rs=0.558), which could be attributed to the lower number of Ara h 8-sensitized patients in this study.
The positivity rate for Platanus pollen sensitization was significantly higher in the peanut- and birch-sensitized groups (100%) than in the peanut- and birch-sIgE negative groups (76.9%). Although the structures of Platanus pollen allergen, peanut allergen, and birch pollen allergen are similar, no study has reported the occurrence of cross-reaction or cross-reactivity among them, which requires further study. Platanus pollen and birch pollen also showed a high correlation (rs=0.907), as previously reported. Fernández-González et al demonstrated a strong correlation between the levels of sIgE against Platanus pollen allergen and birch pollen in the serum of Platanus pollen-sensitized patients.23 This could be explained by the similarities in amino acid sequences, molecular weights, and taxonomy between Pla a 1 and Bet v 1.
The first cluster included birch pollen, Bet v 1, and Ara h 8. Bet v 1 is one of the main allergens in birch. Approximately 95% of birch allergic patients were sensitized to Bet v 1.24 In a survey of children with PA in South Korea, no difference was observed in the proportion of children with sIgE antibodies to Ara h 8 or the median level of peanut sIgE antibodies between the peanut-sensitized group and peanut-tolerant group.25 In children sensitized to peanut and birch pollen, the correlation between the positive IgE response to peanut and PA is weak, especially when the birch pollen sIgE antibody level is higher than the peanut sIgE antibody level.26
The second cluster included peanut, Platanus pollen, Juglans pollen, CCD, and Bet v 4. Bet v 4 is a birch pan-allergen that belongs to the polcalcins protein family27 and can cause multiple sensitization and cross-reactivity. As a result, cross-reactivity with Bet v 4 may strongly promote the occurrence of Platanus pollen, peanut, and Juglans pollen allergies in patients sensitized to the aforementioned allergens. In the peanut-sensitized positive patients, 69.7% (23/33) were CCD positive. CCD, a cross-reactive carbohydrate determinant, can cause a wide range of allergen cross-reactions. As a potential source of interference, CCD may cause non-specific false-positive results in in vitro tests. Van der Veen et al studied a group of CCD-positive grass pollen-sensitized patients who were positive for peanut sIgE antibodies but not allergic to peanuts and showed no or low bioactivity against CCD sIgE antibodies.28 The report also explains why 50% (19/38) of peanut-sensitized patients were positive for sensitization to both Platanus and Juglans pollen, which could be due to the presence of common CCD epitopes. Ara h 1 was independently divided into five clusters. Ara h 1 is a major peanut allergen,29 and Ara h 1 sensitization can lead to more serious clinical manifestations in the presence of Ara h 2 sensitization.30 In our previous published paper, about 85% peanut-sIgE turned negative after use the CCD inhibitors in Southern China patients, suggesting that most of these peanut positives were false positives caused by CCD.31 This also explains why the patients in our study were not accompanied by clinical symptoms. And it also may be one of the reasons why the positive rates of Ara h 1, 2, 3 and 6, the major components of peanut allergy, are low in peanut allergen-positive patients in Southern China. In China, peanut sIgE can be detected in the serum of many patients, but they often do not have clinical symptoms as a result of peanut consumption. We suggest that it’s necessary to evaluate the case history and detect the sIgE of pollen allergen and CCD when a positive peanut-sIgE is found in patients in Southern China. Because a positive sIgE to peanut extract could be a false positive caused by cross-reactions of CCD or other pollen allergens. This study had several limitations. Cross-inhibition experiments could provide more information to distinguish cross-reactivity between peanut allergen and pollen allergens.
Conclusion
The majority of patients sensitized to peanut allergen in Southern China tested positive for multiple pollen allergens. Peanut sensitization was highly correlated with Platanus, Juglans, and birch pollen sensitization. There may be cross-reactions between peanut and pollen allergens. Clinicians should pay attention to distinguish diagnosis in clinical peanut allergy diagnosis and treatment. This could provide valuable research data for the prevention, diagnosis, and treatment of allergic diseases in Southern China, as well as contributing to better allergy diagnosis and treatment.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (81802076 and 81871736), SKLRD (MS-201906, Z-202209), and the Guangzhou Science and Technology Foundation (202102010327). Also thanks to the Biobank for Respiratory Diseases in the National Clinical Research Center for Respiratory Disease (BRD-NCRCRD, Guangzhou, Southern China).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
None of the authors report having any potential conflicts of interest related to this manuscript.
References
1. Eriksson NE, Formgren H, Svenonius E. Food hypersensitivity in patients with pollen allergy. Allergy. 1982;37:437–443. doi:10.1111/j.1398-9995.1982.tb02323.x
2. Mastrorilli C, Cardinale F, Giannetti A, et al. Pollen-food allergy syndrome: a not so rare disease in childhood. Medicina. 2019;55:641.
3. Sicherer SH, Sampson HA. Peanut allergy: emerging concepts and approaches for an apparent epidemic. J Allergy Clin Immunol. 2007;120:
4. Shek LP, Cabrera-Morales EA, Soh SE, et al. A population-based questionnaire survey on the prevalence of peanut, tree nut, and shellfish allergy in 2 Asian populations. J Allergy Clin Immunol. 2010;126:324–31, 331. doi:10.1016/j.jaci.2010.06.003
5. Cousin M, Verdun S, Seynave M, et al. Phenotypical characterization of peanut allergic children with differences in cross-allergy to tree nuts and other legumes. Pediatr Allergy Immunol. 2017;28:245–250. doi:10.1111/pai.12698
6. Stiefel G, Anagnostou K, Boyle RJ, et al. BSACI guideline for the diagnosis and management of peanut and tree nut allergy. Clin Exp Allergy. 2017;47:719–739. doi:10.1111/cea.12957
7. Dooper MM, Plassen C, Holden L, et al. Immunoglobulin E cross-reactivity between lupine conglutins and peanut allergens in serum of lupine-allergic individuals. J Investig Allergol Clin Immunol. 2009;19:283–291.
8. Asarnoj A, Moverare R, Ostblom E, et al. IgE to peanut allergen components: relation to peanut symptoms and pollen sensitization in 8-year-olds. Allergy. 2010;65:1189–1195.
9. Mortz CG, Andersen KE, Bindslev-Jensen C. The prevalence of peanut sensitization and the association to pollen sensitization in a cohort of unselected adolescents–The Odense Adolescence Cohort Study on Atopic Diseases and Dermatitis (TOACS). Pediatr Allergy Immunol. 2005;16:501–506. doi:10.1111/j.1399-3038.2005.00302.x
10. Kim M, Ahn Y, Yoo Y, et al. Clinical manifestations and risk factors of anaphylaxis in pollen-food allergy syndrome. Yonsei Med J. 2019;60:960–968. doi:10.3349/ymj.2019.60.10.960
11. Enrique E, Alonso R, Bartolome B, et al. IgE reactivity to profilin in Platanus acerifolia pollen-sensitized subjects with plant-derived food allergy. J Investig Allergol Clin Immunol. 2004;14:335–342.
12. Mittag D, Akkerdaas J, Ballmer-Weber BK, et al. Ara h 8, a Bet v 1-homologous allergen from peanut, is a major allergen in patients with combined birch pollen and peanut allergy. J Allergy Clin Immunol. 2004;114:1410–1417. doi:10.1016/j.jaci.2004.09.014
13. Kukkonen AK, Pelkonen AS, Mäkinen-Kiljunen S, et al. Ara h 2 and Ara 6 are the best predictors of severe peanut allergy: a double-blind placebo-controlled study. Allergy. 2015;70:1239–1245. doi:10.1111/all.12671
14. Luo W, Wang D, Zhang T, et al. Prevalence patterns of allergen sensitization by region, gender, age, and season among patients with allergic symptoms in Mainland China: a Four-year Multicenter Study. Allergy. 2021;76:589–593. doi:10.1111/all.14597
15. Luo W, Zeng G, Sun B, et al. Component-resolved diagnostic: study of dermatophagoides pteronyssinus major allergen molecules in a Southern Chinese Cohort. J Allergy Clin Immunol. 2015;135S:B245.
16. Sicherer SH, Munoz-Furlong A, Godbold JH, et al. US prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up. J Allergy Clin Immunol. 2010;125:1322–1326. doi:10.1016/j.jaci.2010.03.029
17. Park KH, Son YW, Lee SC, et al. Clinical significance of component allergens in fagales pollen-sensitized peanut allergy in Korea. Allergy Asthma Immunol Res. 2016;8:505–511. doi:10.4168/aair.2016.8.6.505
18. Asero R, Pravettoni V. Anaphylaxis to plant-foods and pollen allergens in patients with lipid transfer protein syndrome. Curr Opin Allergy Clin Immunol. 2013;13:379–385. doi:10.1097/ACI.0b013e32835f5b07
19. Deng S, Yin J. Mugwort pollen-related food allergy: lipid transfer protein sensitization and correlation with the severity of allergic reactions in a Chinese Population. Allergy Asthma Immunol Res. 2019;11:116–128. doi:10.4168/aair.2019.11.1.116
20. Skypala IJ, Cecchi L, Shamji MH, et al. Lipid transfer protein allergy in the United Kingdom: characterization and comparison with a matched Italian cohort. Allergy. 2019;74:1340–1351. doi:10.1111/all.13747
21. Scala E, Cecchi L, Abeni D, et al. Pla a 2 and Pla a 3 reactivities identify plane tree-allergic patients with respiratory symptoms or food allergy. Allergy. 2017;72:671–674. doi:10.1111/all.13121
22. Scala E, Till SJ, Asero R, et al. Lipid transfer protein sensitization: reactivity profiles and clinical risk assessment in an Italian cohort. Allergy. 2015;70:933–943. doi:10.1111/all.12635
23. Fernandez-Gonzalez D, Gonzalez-Parrado Z, Vega-Maray AM, et al. Platanus pollen allergen, Pla a 1: quantification in the atmosphere and influence on a sensitizing population. Clin Exp Allergy. 2010;40:1701–1708. doi:10.1111/j.1365-2222.2010.03595.x
24. Dehus O, Zimmer J, Doring S, et al. Development and in-house validation of an allergen-specific ELISA for quantification of Bet v 4 in diagnostic and therapeutic birch allergen products. Anal Bioanal Chem. 2015;407:1673–1683. doi:10.1007/s00216-014-8418-z
25. Kim HY, Han Y, Kim K, et al. Diagnostic value of specific IgE to peanut and Ara h 2 in Korean children with peanut allergy. Allergy Asthma Immunol Res. 2016;8:156–160. doi:10.4168/aair.2016.8.2.156
26. Asarnoj A, Ostblom E, Ahlstedt S, et al. Reported symptoms to peanut between 4 and 8 years among children sensitized to peanut and birch pollen - results from the BAMSE birth cohort. Allergy. 2010;65:213–219. doi:10.1111/j.1398-9995.2009.02138.x
27. Engel E, Richter K, Obermeyer G, et al. Immunological and biological properties of Bet v 4, a novel birch pollen allergen with two EF-hand calcium-binding domains. J Biol Chem. 1997;272:28630–28637. doi:10.1074/jbc.272.45.28630
28. van der Veen MJ, van Ree R, Aalberse RC, et al. Poor biologic activity of cross-reactive IgE directed to carbohydrate determinants of glycoproteins. J Allergy Clin Immunol. 1997;100:327–334. doi:10.1016/S0091-6749(97)70245-8
29. Burks AW, Williams LW, Helm RM, et al. Identification of a major peanut allergen, Ara h I, in patients with atopic dermatitis and positive peanut challenges. J Allergy Clin Immunol. 1991;88:172–179. doi:10.1016/0091-6749(91)90325-I
30. Astier C, Morisset M, Roitel O, et al. Predictive value of skin prick tests using recombinant allergens for diagnosis of peanut allergy. J Allergy Clin Immunol. 2006;118:250–256. doi:10.1016/j.jaci.2006.04.053
31. Luo W, Huang H, Zheng P, et al. CCD inhibition test can improve the accuracy of the detection of pollen and seed food allergen- specific IgE in Southern China. J Asthma Allergy. 2021;14:439–447. doi:10.2147/JAA.S302920
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.