Back to Journals » Therapeutics and Clinical Risk Management » Volume 19
Analysis of Patients’ Characteristics and Treatment Profile of People Who Use Drugs (PWUDs) with and without a Co-Diagnosis of Viral Hepatitis C: A Real-World Retrospective Italian Analysis
Authors Nava FA, Mangia A , Riglietta M, Somaini L, Foschi FG, Claar E, Maida I , Ucciferri C, Frigerio F , Hernandez C, Dovizio M, Perrone V, Degli Esposti L , Puoti M
Received 14 March 2023
Accepted for publication 23 July 2023
Published 4 August 2023 Volume 2023:19 Pages 645—656
DOI https://doi.org/10.2147/TCRM.S409134
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Felice Alfonso Nava,1 Alessandra Mangia,2 Marco Riglietta,3 Lorenzo Somaini,4 Francesco Giuseppe Foschi,5 Ernesto Claar,6 Ivana Maida,7 Claudio Ucciferri,8 Francesca Frigerio,9 Candido Hernandez,10 Melania Dovizio,11 Valentina Perrone,11 Luca Degli Esposti,11 Massimo Puoti12
1U.O. Sanità Penitenziaria e Area Dipendenze, Azienda ULSS 6 Euganea, Padova, Italy; 2UOS Epatologia, Istituto di Ricovero e Cura “Casa Sollievo della Sofferenza”, S. Giovanni Rotondo, Italy; 3UOC Dipendenze, ASST Papa Giovanni XXIII, Bergamo, Italy; 4Servizio per le Dipendenze, SERT di Cossato, Biella, Italy; 5UOC Medicina Interna, Ospedale di Faenza, Faenza, Italy; 6UOC Medicina Interna, Ospedale Evangelico “Villa Betania”, Napoli, Italy; 7UOC Malattie Infettive e Parassitarie, Azienda Ospedaliero Universitaria di Sassari, Sassari, Italy; 8Clinica di Malattie Infettive Ospedale “SS Annunziata”, Chieti, Italy; 9Gilead Sciences, Milano, Italy; 10Gilead Sciences, Global Medical Affairs, Stockley Park, London, UB11 1BD, UK; 11CliCon S.R.L. Società Benefit, Health Economics and Outcomes Research, Bologna, Italy; 12SC Malattie Infettive, ASST Grande Ospedale Metropolitano Niguarda, Milano, Italy
Correspondence: Luca Degli Esposti, CliCon S.r.l. Società Benefit, Health, Economics & Outcomes Research, Via Murri, 9, Bologna, 40137, Italy, Tel +39 544 38393, Fax +39 544 212699, Email [email protected]
Purpose: Hepatitis C virus (HCV) spreads from contact with blood of an infected person. HCV infections are common among people who use drugs (PWUDs), when sharing needles, syringes, or other equipment for injected drugs. The advent of pangenotypic direct-antiviral agents (DAA) in 2017 transformed the treatment landscape for HCV, but PWUDs remain a complex and hard-to-treat population with high risk of HCV reinfection. The aim of this real-world analysis was to characterize the demographic and clinical features of PWUDs in Italy, also focusing on comorbidity profile, treatment with DAAs, resource consumptions for the National Health System (NHS).
Patients and Methods: During 01/2011– 06/2020, administrative databases of Italian healthcare entities, covering 3,900,000 individuals, were browsed to identify PWUDs with or without HCV infection. Among HCV+ patients, a further stratification was made into treated and untreated with DAAs. The date of PWUD or HCV first diagnosis or DAA first prescription was considered as index-date. Patients were then followed-up for one year. Alcohol-dependency was also investigated.
Results: Total 3690 PWUDs were included, of whom 1141 (30.9%) PWUD-HCV+ and 2549 (69.1%) PWUD-HCV-. HCV-positive were significantly older (43.6 vs 38.5 years, p < 0.001), had a worse comorbidity profile (Charlson-index: 0.8 vs 0.4, p < 0.001), and high rates of psychiatric, respiratory, dermatological, musculoskeletal diseases and genitourinary (sexually transmitted) infections. Moreover, they received more drug prescriptions (other than DAAs, like anti-acids, antiepileptics, psycholeptics) and had undergone more frequent hospitalization, predominantly for hepatobiliary, respiratory system and mental disorders. DDA-untreated had significantly higher Charlson-index than DAA-treated (0.9 vs 0.6, p = 0.003). Alcoholism was found in 436 (11.8%) cases.
Conclusion: This Italian real-world analysis suggests that PWUDs with HCV infection, especially those untreated with DAAs, show an elevated drug consumption due to their complex clinical profile. These findings could help to ameliorate the healthcare interventions on PWUDs with HCV infection.
Keywords: drug abuse, hepatitis C virus, alcohol dependency, real-world evidence
Introduction
The advent of direct-antiviral agents (DAA), pangenotypic and efficacious treatments for hepatitis C virus (HCV) allowed to envisage a worldwide HCV elimination as determined during the 69th World Health Assembly and set as WHO’s goal of resolving viral hepatitis as a primary public healthcare emergency by 2030.1 Although HCV is still a “silent pandemic”, the ambitious goal of its elimination might be achievable also through the implementation of country-based prevention practices that should point out the close link between HCV and social marginalization.2
Recent experiments have proven that HCV is highly stable in water, thus routinely used injection equipment can represent a common route for viral transmission among people who use drugs (PWUDs).3 In Italy, in 2018 has been reported that 70% of HCV infections were found among PWUDs, a historically complex and hard-to-treat population due to low adherence, poor tolerability, and high HCV reinfection rates.4
Diagnosis and therapeutic interventions on HCV infection in PWUDs are therefore of central importance among public healthcare emergencies to achieve the WHO’s goal of defeating hepatitis epidemic by 2030. Hence, the proper management of HCV infection in injecting or not only injecting drug abusers denotes a priority for several reasons: (i) they represent a risky reservoir for viral transmission4 (ii) PWUDs have high morbidity and mortality due to limited access to treatments;5 (iii) therapy aimed at keeping viral load under control serves as a preventive measure to control virus circulation and transmission, new infections and re-infections.6
Before 2014, the interferon-based HCV therapy, characterized by 24–48 weeks treatment, a complex adverse event profile and relatively low cure rates, made HCV cure arduous, mainly for fragile patients such as PWUDs.7,8 The introduction of oral interferon-free direct-acting agents (DAAs) provided significant benefits to achieve sustained virologic response (SVR) rates of more than 95% with shorter treatment length and a less demanding patient management.6,9,10 Nevertheless, despite the advantages of a high virologic response, DAAs might also expose patients to the risk of drug–drug interactions (DDIs), feasibly related to the metabolic pathway requiring P450 cytochromes and/or transport through P-glycoprotein (P-gp).11,12 It is estimated that 30–60% of patients taking DAAs are at risk of clinically significant DDIs: this might be explicated by the fact that these patients are commonly burdened by several comorbidities requiring polypharmacy treatment regimens.13
To date, limited real-world studies are available on the clinical and therapeutic management of PWUDs, and this gap of information needs to be filled in view of the everchanging epidemiological scenario of HCV, especially after the introduction of pangenotypic DAAs in 2017. Over the last two decades, Italy has shown dramatically high numbers in HCV infections, and mortality rates from cirrhosis and hepatocellular carcinoma, among the highest in Western Europe.14 So far, updated epidemiological estimates either for the general population or for PWUDs in Italy are currently inconsistent. Some modelling approaches based on Italian real-world data have been proposed to forecast HCV burden in upcoming years and the chances to achieve to reach the WHO elimination goals established within 2030.15
Thus, the present analysis was undertaken to describe the current epidemiology of PWUDs in Italy, focusing on demographic features, clinical status, comorbidity profile, pharmacological treatments, and the deriving healthcare resource consumptions for the National Health System (NHS). A comparative evaluation was carried out firstly between on PWUDs with and without HCV infection, then the HCV positive population was analysed after stratifying patients into treated and untreated with DAAs. The additional investigation on HCV-infected PWUDs has been carried out to understand the possible epidemiological differences with the non-infected subjects and to describe treatment behaviours in the two cohorts in terms of both DAAs and comedications.
Materials and Methods
Data Source
This retrospective observational analysis utilized data retrieved from administrative databases, as previously described.16 Briefly, the data were extrapolated from the administrative flows of a pool of Local Health Units (LHUs) geographically distributed in the Italian territory, covering approximately 3.9 million health-assisted residents. These databases include the healthcare services provided by the Italian NHS, which is funded on the principle of universal coverage of healthcare expenses for all national and legal foreign residents. Therefore, these databases are repositories of information to be used for reimbursement purposes. Specifically, the following databases were used: i) demographic database, which contains patients’ demographic data, such as gender, age, date of death; ii) pharmaceuticals database, which collects information on drugs reimbursed by the NHS, namely the Anatomical Therapeutic Chemical (ATC) code, number of packages, number of units for each package, unit package cost, and date of prescription; iii) hospitalization database, which encloses all hospitalizations data, like discharge diagnosis codes based on ICD-9-CM (International Classification of Diseases, Ninth Revision, Clinical Modification) codes, Diagnosis-Related Group (DRG) and DRG-related charge (provided by the NHS); iv) outpatient specialist services database, which includes information on specialist visits and diagnostic tests (date and type of delivery, description activity and laboratory assay or specialist visit fees); v) payment exemption database, which provides data of exemption codes that allow to waive the contribution charge for services/treatments in case of specific disease diagnoses.
In order to ensure data privacy, an anonymous and univocal numeric code was assigned to each patient, in full compliance with the European General Data Protection Regulation (GDPR) (2016/679). The patient code allowed the electronic linkage between databases. All the results of the present analysis were provided in aggregated form, thus never attributable to a single institution, department, doctor, individual, or individual prescribing behaviours. Informed consent was waived because obtaining it was not possible due to organizational reasons (pronouncement of the Data Privacy Guarantor Authority, General Authorization for personal data treatment for scientific research purposes – n.9/2014). The participating LHUs are the owners of their data and CliCon S.r.l. Società Benefit collected those that were necessary to perform the analyses. Each LHU was responsible for data extraction from its administrative databases and provided them to CliCon in an anonymized form.
Ethics Statement
The study was conducted in compliance with the principles of the Declaration of Helsinki and approved by the following Institutional Review Boards (or Ethics Committees) of the Healthcare Departments involved in the study: i) Comitato Etico Interprovinciale Area 1 (A.O.U. Foggia, ASL Foggia, ASL BAT) (Prot. N 62/seqCE/20, 2/12/2020; Prot. N 63/seqCE/20, 3/12/2020); ii) Comitato Etico per le Sperimentazioni Cliniche (CESC) della Provincia di Vicenza (Prot. N 1627, 28/10/2020; Prot. N 0036999, 28/04/2021); iii) Comitato Etico per le province di Chieti e Pescara (Prot N. 05, 4/03/2021); iv) Comitato Etico Lazio 2 (Prot N 0216084/2020, 16/12/2020); v) Comitato Indipendente di Etica Medica (Prot N 48144, 28/5/2021); vi) Comitato Etico regionale Umbria (Prot N 19414/20/ON, 16/09/2020); vi) Comitato Etico Interaziendale (Prot N AslVC.FarmT.21.01, 18/03/2021).
Study Design, Study Population and Patients’ Cohort Definition
During all period of data availability from January 2011 to June 2020, PWUDs were identified by exemption code 014.304 or hospitalization discharge diagnosis with ICD-9-CM code 304 and included in the analysis. Among them, HCV infection was detected through the hospitalization discharge diagnosis for HCV (ICD-9-CM codes 070.4, 070.5, 070.7) or exemption code (016) or by the prescription of DAAs (ATC code J05AP) (the detailed list of DAAs is provided in Supplementary Table 1), during all the available period. Based on the presence or absence of HCV diagnosis, PWUDs were divided into PWUD-HCV+ and PWUD-HCV- cohorts, respectively. HCV infection was searched throughout the period of data availability, therefore during the observation period no HCV negative subject switched his/her HCV status. After 2017, when pangenotypic DDAs were introduced in the clinical practice, PWUD-HCV+ with at least one prescription of DAAs (ATC code J05AP), were assigned into the PWUD-HCV+ treated cohort, while those without any DAA prescription were identified as untreated (regardless of the presence of therapies other than DAAs). The date of PWUD status detection or HCV first diagnosis or DAA first prescription was considered as index-date. Patients were characterized for their baseline variables during all the available period before the index-date and were followed-up for all the available period after the index-date, at least 12 months. Health-assisted individuals without continuous data availability during the study period or with missing data (ie those who moved to another LHU) were excluded.
In a subanalysis, PWUD-HCV+ were stratified according to the date of first HCV diagnosis: before and after 2017 (year of introduction of pangenotypic DAAs).
Analysis of Demographic and Clinical Characteristics of the Study Population
For all study subjects, gender, age at index-date and 10-year age range categories (18–29, 30–39, 40–49, 50–59, above 60 years) were recorded. Clinical characteristics at baseline were assessed through the Charlson Comorbidity Index (CCI), a scoring system derived by summing the assigned weights to each concomitant disease.17 CCI was determined during the 12-month period before the index-date, by evaluating drug treatments and hospitalizations.
Demographic and clinical features collected at baseline and during the characterization period were comparatively analysed in PWUD-HCV+ vs PWUD-HCV- cohorts, and among the PWUD-HCV+, in DAA-treated vs untreated subjects. Moreover, a subanalysis was also carried out to investigate the baseline characteristics and previous medical history of PWUDs with a co-diagnosis of alcohol dependency (identified by the exemption code 014.303, or by hospitalization ICD-9-CM code 303 for alcohol-dependence syndrome).
Pharmacoutilization Analysis: Treatment Patterns
The prescription of all possible treatments (excluding DAA-anti HCV, ATC code J05AP) was searched and classified according to the first or second level of ATC code during the available follow-up. The most frequent treatments and the percentage of patients prescribed with a specific drug class were recorded.
Healthcare Resource Consumption
During follow-up, the healthcare resource consumptions were assessed for alive patients in terms of drug treatments (number of prescriptions per patient), all-cause hospitalization admissions (number per patient) and all-cause outpatient specialized services (number of delivered specialistic visits, laboratory assays, diagnostic procedures per patient).
Statistical Analysis
Continuous variables were expressed as mean ± standard deviation (SD), and median with range (min-max); categorical variables were given as numbers with percentages. Patients were grouped according to: (i) presence/absence of HCV infection (HCV+ vs HCV-), (ii) (only for HCV+) presence/absence of DAA treatment (DAA-treated vs DAA-untreated). A further comparison was then made based on the time of inclusion, before 2017 vs after 2017. For each of above-mentioned stratification criteria, comparisons were performed using chi-square test for categorical variables and Student’s t-test or non-parametric Mann–Whitney U-test for continuous variables in case of normal and non-normal distributions, respectively.
A p value below 0.05 was considered as statistically significant and Stata SE version 17.0 (StataCorp, College Station, TX, USA) was used for all the analyses.
Results
Baseline Patient Characteristics
Overall, 3690 PWUDs were included, 1141 (30.9%) with a co-diagnosis of HCV (PWUD-HCV+) and 2549 (69.1%) without a co-diagnosis of HCV (PWUD-HCV-), considering all available period of the database. Among HCV+ patients, 593 (52%) were treated with DAAs, while 548 (48%) had no DAA prescription (Figure 1A). This proportion significantly changed when analysing the period after 2017 (upon pangenotypic, panfibrotic DAA advent) showing 65.8% of HCV+ PWUD treated with DAAs (Figure 1B).
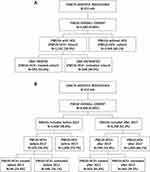 |
Figure 1 Flow-chart reporting study population identification and derived cohorts: (A) study on the overall inclusion period; (B) study on the inclusion period divided into before and after 2017. |
The baseline characteristics of the whole PWUDs, PWUD-HCV+ and PWUD-HCV- cohorts are described in Table 1. Overall, PWUDs consisted of a young population, with an average age of 40.1 years, those with HCV diagnosis were significantly older (p < 0.001), 81% of patients were in the age ranges below 50 years and the majority were males (82.1%). The mean CCI was 0.5, and unsurprisingly higher in the HCV+ subjects, among whom patients with CCI 1 or ≥2 were significantly more represented than in the HCV- cohort.
 |
Table 1 Baseline Characteristics of PWUD Population, Overall and Stratified in PWUD-HCV+ and PWUD-HCV |
Table 2 reports the baseline characteristics of PWUD-HCV+ patients, treated or not with DAAs. While no significant differences emerged for age between the two subgroups (DAA-treated vs untreated: 44.0 ± 9.0 vs 43.2 ± 9.0 years), the patients who received treatment with DAAs had a significantly lower CCI compared to the untreated (0.6 ± 1.3 vs 0.9 ± 1.9, p < 0.003).
 |
Table 2 Baseline Characteristics of PWUD-HCV+ Patients, treated and Untreated with DAA-Anti HCV Medications |
The clinical profile was further investigated analysing the main causes of hospitalization during the characterization period in PWUDs stratified according to the presence/absence of HCV and, among HCV+, to the presence/absence of DAA treatment (Table 3). The group PWUD-HCV+ underwent more frequently previous hospitalization due to complications related to nervous, respiratory, circulatory, digestive, hepatobiliary systems, pancreas, musculoskeletal system, connective tissue, skin, subcutaneous tissue and breast, infectious and parasitic diseases, mental diseases, mental disorders related to alcohol/drug, and HIV infection (these differences did not meet statistical significance in the comparisons between PWUD HCV+ and PWUD HCV- for digestive system complications and hospitalizations related to the alcohol or drug use). Considering PWUD-HCV+ in relation to DAA treatment, the hospitalization rates were higher in the untreated subjects for complications of the musculoskeletal system and connective tissue, mental disorders, alcohol/drug use or induced mental disorders (p < 0.05).
 |
Table 3 Main Causes of Previous Hospitalization in PWUDs Stratified According to the Presence/Absence of HCV and, Among HCV+, to the Presence/Absence of DAA Treatment |
A sub-analysis comparing patients included before and after year 2017 (1432 and 2258 respectively of the overall PWUDs) revealed a significant difference (p = 0.003) in CCI distribution: before 2017, 82.8% of PWUDs had CCI = 0, 11.9% had CCI = 1, and 5.2% had CCI ≥ 2, while after 2017 86.4% of PWUDs had CCI = 0, 8.5% had CCI = 1, and 5.0% had CCI ≥ 2 (Supplementary Table 2).
Among PWUDs, 436 (11.8%) individuals with a co-diagnosis of alcohol dependency were identified. In this subpopulation, age at inclusion averaged 41.3 ± 10.2 years, 82.3% were males and the CCI was 0.7 ± 1.5 (Supplementary Table 3).
Treatment Patterns
As shown in Table 4A, during the first year of follow-up, the mean number of drugs other than DAAs prescribed in the overall PWUD population was 3.7 ± 4.4 per patient: specifically, 4.0 ± 4.6 for PWUD-HCV+ and 3.6 ± 4.3 for PWUD-HCV- patients (p = 0.011). The comparison according to DAA-treatment in the PWUD-HCV+ group, reported in Table 4B, revealed a lower number of concomitant drug prescriptions in DAA-treated than untreated patients (3.7 ± 4.2 vs 4.3 ± 5.0, p = 0.028), consistent with the higher comorbidity index in the untreated subgroup.
During the same period of observation, by, the treatments most frequently prescribed among PWUDs (excluding DAAs) were those belonging to N class of ATC1 (drugs for nervous system, 47.1%), J class of ATC1 (systemic anti-infective, 42.6%), A class of ATC1 (drugs for alimentary tract and metabolism, 27.7%), B class of ATC1 (drugs for blood and blood forming organs, 13.5%), and C class of ATC1 (cardiovascular system, 15.3%). All the mentioned drugs were more frequently prescribed in HCV+ patients with exception for N class medications, which were prescribed to 42.1% of PWUD-HCV+ and to 49.4% of PWUD-HCV- (p < 0.001) (Figure 2).
The analysis at the second level of ATC codes (that indicates the therapeutic subgroup) revealed that a larger proportion of PWUD-HCV+ patients received drugs for acid-related disorders (ATC A02, 26.5% vs 20.6%, p < 0.001), diuretics (ATC C03, 7.9% vs 3.2%, p < 0.001), and antiepileptics (N03), psycholeptics (N05), and psychoanaleptics (N06), compared to PWUD-HCV- (Figure 3).
DAA-treated and untreated PWUD-HCV+ patients had mostly comparable prescription rates of the evaluated drugs; nevertheless, a more extensive use of agents acting on the renin–angiotensin system (C09, 9.6% vs 6.4%, p = 0.046) and a lower use of antiepileptics (N03, 16.2% vs 23.2%, p = 0.003) was observed in the DAA-treated cohort (Supplementary Figure 1).
Healthcare Resource Consumptions
The analysis of healthcare resource consumption is shown in Table 5. PWUD-HCV+ were characterized by higher number of drug prescriptions (4.6 ± 4.6 vs 3.6 ± 4.3, p < 0.001), specialist services (6.8 ± 12.2 vs 2.9 ± 4.5, p < 0.001), and hospitalizations (0.5 ± 1.4 vs 0.3 ± 0.9, p < 0.001) in comparison to patients without HCV. Among HCV+, DAA-treated patients revealed higher consumptions of drug prescriptions and specialist services delivery, but lower number of hospitalizations during the first year of follow-up (0.3 ± 0.9 vs 0.8 ± 1.8).
 |
Table 5 Analysis of Healthcare Resources Consumption During First Year of Follow-Up Period |
Discussion
This real-world analysis among the Italian population investigated the demographic and clinical characteristics of PWUDs with and without a co-diagnosis of HCV. Particularly, a special focus was given to the impact of comorbidity profiles in infected subjects, the role of treatment patterns and the subsequent rebounds on the consumptions of healthcare resources for the Italian NHS.
Among the study sample, 3690 PWUDs have been identified. They resulted to be a young population of around 40 years, consistently with a very recent Italian survey conducted from July 2019 to March 2020, that reported a prominent use of opioids in subjects aged between 18 and 40 years.18 By considering all available periods across the database, nearly 30% of the included PWUDs had a co-diagnosis of HCV. A systematic review by Degenhardt et al reported a worldwide prevalence of injecting drug users in 2015 of 15.6 million people in the age range of 15–64 years, and 52.3% of them resulted HCV-antibody positive.19 This discrepancy might be due to the variable percentage of PWUDs tested for HCV among Italian Regions that might underestimate the actual prevalence.20
In the present analysis, 52% of PWUD-HCV+ were treated with anti-HCV DAA medications. Despite the growing spectrum of therapeutic options against HCV infection since 2015, a high number of people with chronic C hepatitis have not yet been treated, although access to care is warranted to all subjects with HCV infection, in view of the principle of equal healthcare coverage to every citizen warranted by the Italian NHS. A recent Italian survey reported that the number of treated PWUDs currently represents nearly half of the total,21 and underlined the heavy stigma still surrounding this population together with possible constraints due to the complexity of such patients and the risk of DDIs. Though, our analysis pointed out a significant increase of PWUD-HCV+ patients treated with DAAs after 2017 (from 23.9% before 2017 to 67.8% after 2017) suggesting an improvement in HCV management and a broader accessibility to medications due to the advent of new-generation antiviral agents.22,23
Based on AIFA (Agenzia Italiana del Farmaco) data, above 68,000 HCV patients received DAAs as to February 2017.24 According to the former AIFA rules, DAA treatment had to be restricted to patients with more advanced liver disease, namely those in the waiting list for liver transplant, with HCV recurrence after liver/solid organ transplant, and with severe extra-hepatic complications related to HCV infection. Reimbursability of DAAs was therefore granted only based on urgency of the hepatic clinical status, excluding patients with less advanced fibrosis (METAVIR F0–F2).25 More recently, AIFA broadened the eligibility of HCV patients to DAA therapy up to 12 criteria including less severe clinical conditions.24
In our study, PWUDs were showed a complex comorbidity profile and an elevated number of concomitant treatments, mainly regarding anti-infective, neurological and cardiovascular medications and those related to musculoskeletal system, alimentary tract and metabolism. The trend was more evident among PWUD-HCV+, who showed a worse clinical picture supposedly due to underlying HCV infection, especially those untreated with DAAs. This is also reflected by a large utilization of co-medications in the same population, together to a remarkably higher rate of hospitalizations, due to co-infections and a variety of disorders in different districts and systems. Our local data are consistent with previous international reports stating that, in addition to blood-borne infections (ie HIV), PWUD-HCV+ are affected by several comorbidities including psychiatric (33.9%), respiratory (31.5%), dermatological (28.2%), musculoskeletal (26.6%), genitourinary (including other sexually transmitted infections, 25.8%), endocrine/metabolic (21.0%), cardiovascular (19.4%), neurological (17.7%), gastrointestinal (16.9%), hepatobiliary (15.3%), and renal systems (8.9%) conditions. Only 10% had no comorbidities, while 95% had multiple comorbidities.25 The comorbidity and comedication profile of PWUD-HCV+ suggests a more challenging therapeutic choice for HCV treatment based on the described risk of DDIs between DAAs and underlying therapies.11,12,26 This occurrence is potentially very impactive in patients with comorbidities treated with polypharmacy or in specific populations like PWUDs, often affected by coinfections and/or assuming illicit or substitutive substances and for whom the personalization of treatment remains important for minimizing the risk of clinically relevant DDIs while optimizing efficacy. Indeed, the higher number of comedications in the PWUD-HCV+ not treated with DAAs (compared to PWUD-HCV+ treated with DAA) might suggest a conservative prescriptive behaviour based on the avoidance of treating too complex patients at risk of adverse events and/or treatment failure due to DDIs. However, the worse comorbidity profile among DAA-treated HCV+ subjects might also feasibly reflect an early decision by the clinicians to first treat those with more advanced hepatitis C disease. The lower prescription of antiepileptics in PWUD-HCV+ treated with DAA might corroborate this hypothesis, despite recent evidence supports the efficacy of DAAs in achieving SVR despite concomitant ASM (antiseizure medication) use.27 PWUD-HCV+ group untreated with DAAs displayed a significantly more severe comorbidity profile, feasibly explicated by a more severe addiction-related pathology, but also by a higher medical visits number (on average, 8.5 vs 4.8 during first year of follow-up period), excluding that the lower DAA prescription rate might be due to a poorer medical assistance on this subgroup.
The analysis of healthcare resource consumptions enlightened that the management of PWUDs with a co-diagnosis of HCV is associated with higher number of drug prescriptions, specialist service deliveries, and hospitalizations with respect to patients without HCV diagnosis. Likewise, among the HCV+ patients, DAA-treated patients had higher healthcare resource consumption in terms of drug and specialist service prescriptions, in front of a lower hospitalization rate. This finding is in line with the current literature, reporting that PWUDs, especially those with HCV diagnosis, are frequently hospitalized.28,29
These results should be interpreted taking some limitations into account, above all lying in its observational design and the unavoidable flaws of the use of data retrieved from administrative databases. In fact, this approach might lead to a potential underestimation of clinical information on comorbidities and/or concomitant treatments that cannot be extrapolated from the database (also because underreported by patients) together with other potential confounding factors that might have affected the results. Besides, the comorbidities considered here were collected through the available data prior inclusion (using a proxy of diagnosis), thus there might have been an incomplete capture of some variables (ie disease severity). Moreover, primary care data cannot be extrapolated from the administrative database. Lastly, to select HCV positive population, we used hospitalization discharge ICD-9-CM codes, exemption code or prescription of DAA, and not molecular assays like anti-HCV antibody testing and HCV RNA that represent the golden standard for HCV diagnosis.30
Conclusion
This real-world analysis conducted on a representative sample of the Italian population provided a description of the demographic and clinical profile of PWUDs with and without a co-diagnosis of HCV, and the healthcare resource consumption for their management. These findings can give novel insights on PWUD population, informing physicians, addiction centers and healthcare providers about the elevated risk of DDIs in these patients and about the complexity of their disease management. PWUDs are known to be burdened by several comorbidities that imply the use of polypharmacy regimens. This was particularly true in PWUD-HCV+ patients who showed a more severe comorbidity profile, especially if untreated with anti-HCV-DDA medications, and a challenging therapeutic management with an elevated number of prescribed co-medications. Moreover, an elevated consumption of healthcare resources was observed, especially among PWUD-HCV+ patients, in front of a reduced rate of hospitalizations rate in those treated with DAAs. Hence, given the primary importance of future HCV elimination, further efforts are required in the upcoming years to reduce the HCV virus reservoir, improve PWUD-HCV+ quality of life and limit healthcare resource consumption linked to HCV clinical burden. These data derived from the real clinical practice could be helpful to ameliorate the medical management of PWUDs with HCV infection.
Acknowledgments
The authors are grateful to all the study group components of the participating Italian LHUs: Domenica Daniela Ancona (Dipartimento Farmaceutico, ASL BAT, Andria), Margherita Andretta (UOC Assistenza Farmaceutica Territoriale, Azienda ULSS 8 Berica, Vicenza), Antonietta Barbieri (SC Farmaceutica Territoriale, ASL VC, Vercelli), Fausto Bartolini (Dipartimento Farmaceutico, USL Umbria 2, Terni), Andrea Ciaccia (Servizio Farmaceutico Territoriale, ASL Foggia), Alberto Costantini (UOC Farmacia Ospedaliera, P.O. “Santo Spirito”, ASL Pescara), Stefania Dell’Orco (UOC Farmaceutica Territoriale, ASL RM 6, Albano Laziale, RM), Rossella Moscogiuri (Dipartimento Farmaceutico, Ospedale “SS. Annunziata”, ASL Taranto), Elena Mosele (UOC Assistenza Farmaceutica Territoriale, Azienda ULSS 7 Pedemontana, Bassano del Grappa, VI), Cataldo Procacci (Dipartimento Farmaceutico, ASL BAT, Andria), Fiorenzo Santoleri (UOC Farmacia Ospedaliera, P.O. “Santo Spirito”, ASL Pescara). The precious cooperation of Diego Sangiorgi, biostatistician at CliCon S.r.l. is also acknowledged.
Funding
Gilead purchased the study report that is the basis for this manuscript. This manuscript was developed with Gilead and CliCon S.r.l. Società Benefit. The views expressed here are those of the authors and not necessarily those of the supporters. The agreement signed by CliCon S.r.l. Società Benefit and Gilead does not create any entityship, joint venture, or any similar relationship between parties. CliCon S.r.l. Società Benefit is an independent company. Neither CliCon S.r.l. Società Benefit nor any of their representatives are employees of Gilead for any purpose.
Disclosure
A.M. declares teaching and speaking and research support: Angelini, Gilead Sciences, Polifarma. F.F and C.H are employees of Gilead Sciences. M.P. reports grants, personal fees, non-financial support from Gilead, AbbVie; personal fees from Merck, during the conduct of the study; personal fees from Angelini, Astra Zeneca, GSK, Menarini, Janssen, Roche, and Novartis, outside the submitted work. All other authors report no conflicts of interest in this work.
References
1. World Health Organization. Global health sector strategy on viral hepatitis 2016–21. Available from: https://apps.who.int/iris/handle/10665/246177.
2. Papatheodoridis GV, Hatzakis A, Cholongitas E, et al. Hepatitis C: the beginning of the end-key elements for successful European and national strategies to eliminate HCV in Europe. J Viral Hepat. 2018;25(Suppl 1):6–17. doi:10.1111/jvh.12875
3. Doerrbecker J, Behrendt P, Mateu-Gelabert P, et al. Transmission of hepatitis C virus among people who inject drugs: viral stability and association with drug preparation equipment. J Infect Dis. 2013;207(2):281–287. doi:10.1093/infdis/jis677
4. Mangia A, Rina MF, Canosa A, et al. Increased Hepatitis C virus screening, diagnosis and linkage to care rates among people who use drugs through a patient-centered program from Italy. United Eur Gastroenterol J. 2021;9(10):1109–1118. doi:10.1002/ueg2.12156
5. Beaulieu T, Hayashi K, Milloy MJ, et al. HIV serostatus and having access to a physician for regular hepatitis C virus care among people who inject drugs. J Acquir Immune Defic Syndr. 2018;78(1):93–98. doi:10.1097/QAI.0000000000001651
6. Martin NK, Vickerman P, Dore GJ, Hickman M. The hepatitis C virus epidemics in key populations (including people who inject drugs, prisoners and MSM): the use of direct-acting antivirals as treatment for prevention. Curr Opin HIV AIDS. 2015;10(5):374–380. doi:10.1097/COH.0000000000000179
7. Fried MW, Hoofnagle JH. Therapy of hepatitis C. Semin Liver Dis. 1995;15(1):82–91. doi:10.1055/s-2007-1007265
8. Ghany MG, Strader DB, Thomas DL, Seeff LB; American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49(4):1335–1374. doi:10.1002/hep.22759
9. Alimohammadi A, Holeksa J, Thiam A, Truong D, Conway B. Real-world efficacy of direct-acting antiviral therapy for HCV infection affecting people who inject drugs delivered in a multidisciplinary setting. Open Forum Infect Dis. 2018;5(6):ofy120. doi:10.1093/ofid/ofy120
10. Grebely J, Hajarizadeh B, Dore GJ. Direct-acting antiviral agents for HCV infection affecting people who inject drugs. Nat Rev Gastroenterol Hepatol. 2017;14(11):641–651. doi:10.1038/nrgastro.2017.106
11. Kiser JJ, Burton JR, Anderson PL, Everson GT. Review and management of drug interactions with boceprevir and telaprevir. Hepatology. 2012;55(5):1620–1628. doi:10.1002/hep.25653
12. Talavera Pons S, Boyer A, Lamblin G, et al. Managing drug-drug interactions with new direct-acting antiviral agents in chronic hepatitis C. Br J Clin Pharmacol. 2017;83(2):269–293. doi:10.1111/bcp.13095
13. Curry MP, Flamm SL, Milligan S, et al. Prevalence of drug-drug interactions with pangenotypic direct-acting antivirals for hepatitis C and real-world care management in the United States: a retrospective observational study. J Manag Care Spec Pharm. 2021;27(9):1239–1248. doi:10.18553/jmcp.2021.20550
14. Guadagnino V, Stroffolini T, Rapicetta M, et al. Prevalence, risk factors, and genotype distribution of hepatitis C virus infection in the general population: a community-based survey in southern Italy. Hepatology. 1997;26(4):1006–1011. doi:10.1002/hep.510260431
15. Kondili LA, Robbins S, Blach S, et al. Forecasting Hepatitis C liver disease burden on real-life data. Does the hidden iceberg matter to reach the elimination goals? Liver Int. 2018;38(12):2190–2198. doi:10.1111/liv.13901
16. Mangia A, Scaglione F, Toniutto P, et al. Drug-drug interactions in Italian patients with chronic hepatitis C treated with pangenotypic direct acting agents: insights from a real-world study. Int J Environ Res Public Health. 2021;18(13):7144. doi:10.3390/ijerph18137144
17. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi:10.1016/0021-9681(87)90171-8
18. Stigliano G, Miuli A, Lalli A, et al. An Italian survey of opioids misuse: epidemiological and psychopathological aspects. Emerg Trends Drugs Addict Health. 2021;1:100029. doi:10.1016/j.etdah.2021.100029
19. Degenhardt L, Peacock A, Colledge S, et al. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. Lancet Glob Health. 2017;5(12):e1192–e1207. doi:10.1016/S2214-109X(17)30375-3
20. Relazione annuale al Parlamento sul fenomeno delle tossicodipendenze in Italia-2021 (dati 2020). Available from: https://antidroga.interno.gov.it/d-p-A-relazione-annuale-al-parlamento-sul-fenomeno-delle-tossicodipendenze-in-italia-anno-2021-dati-2020/.
21. Teti E. Hepatitis C management and treatment among people who inject drugs in Italy: an exploratory pilot survey. Mission. 2020. doi:10.3280/mis54-2020oa9744
22. Alkhouri N, Lawitz E, Poordad F. Novel treatments for chronic hepatitis C: closing the remaining gaps. Curr Opin Pharmacol. 2017;37:107–111. doi:10.1016/j.coph.2017.10.001
23. Hepatitis B and C testing in the EU/EEA: progress in reaching the elimination targets. Available from: https://www.ecdc.europa.eu/sites/default/files/documents/Hepatitis-B-and%20C-Testing-in-The-EU-EEA-and-UK.pdf.
24. Registri AIFA per il monitoraggio dei farmaci anti-HCV. Available from: https://www.aifa.gov.it/aggiornamento-epatite-c.
25. Viganò M, Perno CF, Craxì A; AdHoc (Advancing Hepatitis C for the Optimization of Cure) Working Party. Treatment of Hepatitis C virus infection in Italy: a consensus report from an expert panel. Dig Liver Dis. 2017;49(7):731–741. doi:10.1016/j.dld.2017.03.027
26. Fagiuoli S, Toniutto P, Coppola N, et al. Italian real-world analysis of the impact of polypharmacy and aging on the risk of multiple Drug-Drug Interactions (DDIs) in HCV patients treated with Pangenotypic Direct-Acting Antivirals (pDAA). Ther Clin Risk Manag. 2023;19:57–65. doi:10.2147/TCRM.S394467
27. Marcos-Fosch C, Cabezas J, Crespo J, Buti M. Anti-epileptc drugs and hepatitis C therapy: real-world experience. J Hepatol. 2021;75(4):984–985. doi:10.1016/j.jhep.2021.05.040
28. Lim J, Pavalagantharajah S, Verschoor CP, et al. Infectious diseases, comorbidities and outcomes in hospitalized people who inject drugs (PWID) infections in persons who inject drugs. PLoS One. 2022;17(4):e0266663. doi:10.1371/journal.pone.0266663
29. Wurcel AG, Burke DJ, Wang JJ, et al. The burden of untreated HCV infection in hospitalized inmates: a hospital utilization and cost analysis. J Urban Health. 2018;95(4):467–473. doi:10.1007/s11524-018-0277-z
30. Chevaliez S, Pawlotsky JM. Hepatitis C virus serologic and virologic tests and clinical diagnosis of HCV-related liver disease. Int J Med Sci. 2006;3(2):35–40. doi:10.7150/ijms.3.35
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



