Back to Journals » Patient Preference and Adherence » Volume 17
Analysis of Nurse and Patient Preferences for Pre-Filled Pen Devices for Self-Injection of Highly Purified Human Menopausal Gonadotropin (HP-hMG, MENOPUR®)
Authors De Mesmaeker G, Calles B, Smith JA
Received 6 August 2022
Accepted for publication 2 May 2023
Published 17 May 2023 Volume 2023:17 Pages 1281—1292
DOI https://doi.org/10.2147/PPA.S385247
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Video abstract presented by Judith A Smith.
Views: 769
Guy De Mesmaeker,1,* Brigitte Calles,2,* Judith A Smith3,*
1Centre for Reproductive Medicine, Universitair Ziekenhuis Brussel, Brussels, Belgium; 2Ferring Pharmaceuticals, Gentilly, France; 3CARE Fertility Group, Nottingham, UK
*These authors contributed equally to this work
Correspondence: Judith A Smith, CARE Fertility Nottingham, John Webster House, 6 Lawrence Drive, Nottingham Business Park, Nottingham, NG8 6PZ, UK, Tel + 44 0 115 852 8100, Email [email protected]
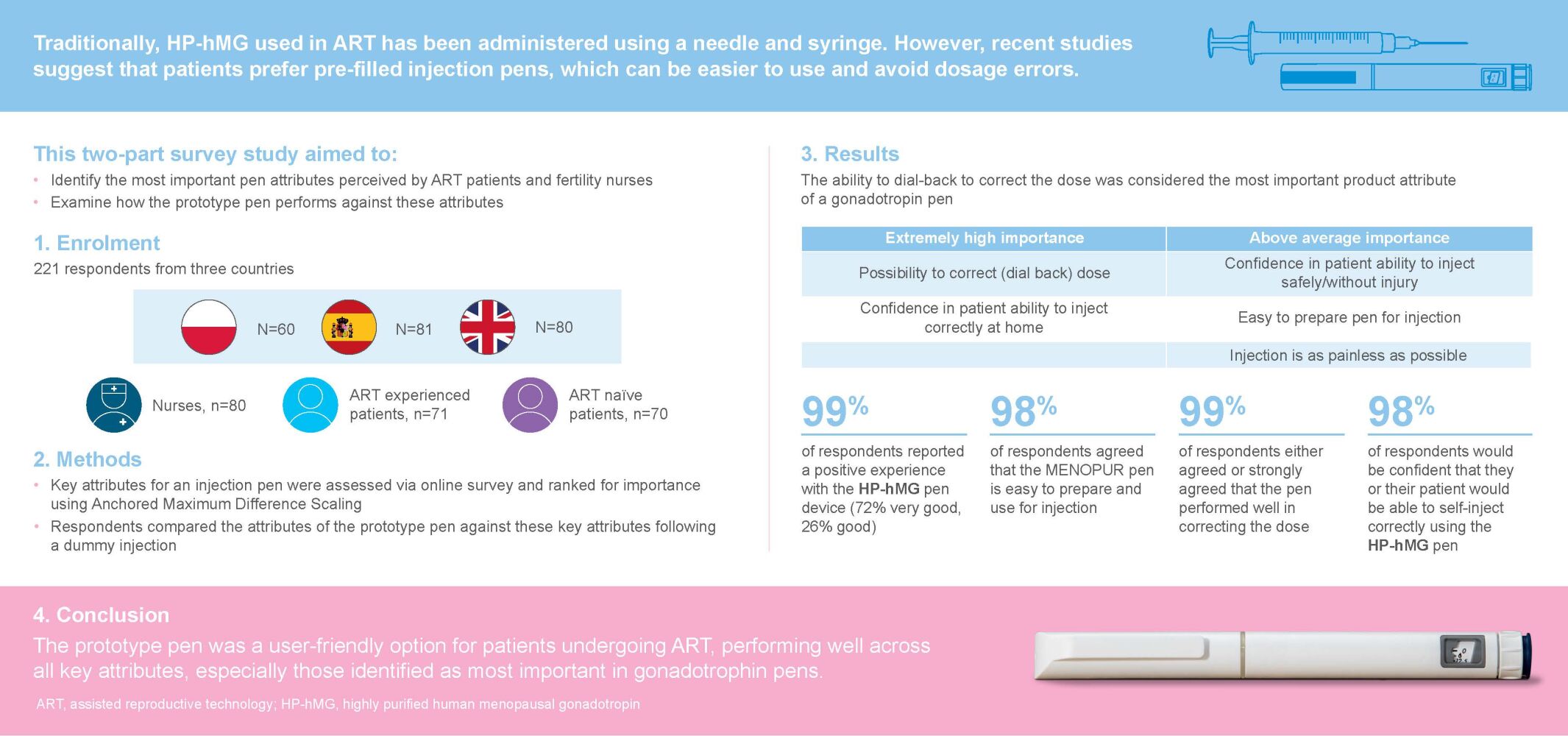
Purpose: This study aimed to identify the most important attributes for a gonadotropin pen as perceived by assisted reproductive technology (ART) patients and fertility nurses, and to examine how well a prototype HP-hMG (MENOPUR®) pen reflects these preferences.
Patients and Methods: This market research study incorporated a two-part survey with respondents (N=221) from Poland, Spain and the UK. Respondents included patients (n=141) who consulted a fertility specialist in the previous 2 years, and fertility nurses (n=80) who assisted in at least 75 ART cycles/year. Patients were divided into two subgroups depending on their experience with ART (experienced and naïve). Key attributes for an injection pen, as perceived by patients and nurses, were assessed via an online survey and ranked by their relative importance using Anchored Maximum Difference Scaling. After performing a dummy injection, respondents compared the attributes of an unbranded prototype pen against the key attributes identified.
Results: Across all survey respondents, the ability to correct the dialed dose was considered to be the most important product attribute of a gonadotropin pen. Confidence in the patient’s ability to inject correctly at home was also identified as a key attribute, considered by both nurses and naïve patients as extremely high. When considering the prototype pen device, almost all study respondents reported a positive experience (99%) with 72% rating it as “very good”. The prototype pen was perceived to possess the key attributes considered important for a gonadotropin pen by patients and nurses, including correcting the dose, the ability to self-inject safely and correctly, ease of preparation and use, and an injection which appeared to be as painless as possible.
Conclusion: The prototype pen was found to perform well across all key attributes, especially those considered most important in gonadotropin pens, suggesting that it is a user-friendly option for patients undergoing ART.
Keywords: fertility, ovarian stimulation, patient preference, assisted reproductive technology, gonadotropin-injection pen
Graphical Abstract:
Plain Language Summary
Highly purified human menopausal gonadotropin (HP-hMG) is a combination of hormones used in fertility treatments to induce follicular development. Traditionally, HP-hMG is injected by women or their partners using a syringe and needle; however, recent studies show that patients often prefer to use injection ‘pens’ that are already filled with their medication, as they are easier to use and avoid dosage errors. This study examined the features that women undergoing assisted reproductive technology (ART) and fertility nurses consider to be the most important for a gonadotropin-injection pen, and compared an unbranded prototype HP-hMG (MENOPUR®) pen against these key features. Using a questionnaire to assess the importance of the key features for an injection pen, the ability to correct the dialed dose was found to be the most important feature identified by both women undergoing ART and fertility nurses. Other important features cited were the ability to self-inject safely and correctly, that the injection pen could be prepared and used easily, and an injection that looks like it will be as painless as possible. The prototype pen performed well across all key features identified, with 99% of those questioned reporting a positive experience. This study demonstrates that the prototype pen device was perceived to possess the key features considered important by both fertility nurses and patients undergoing ART.
Introduction
Highly purified human menopausal gonadotropin (HP-hMG) comprises follicle-stimulating hormone (FSH) and luteinizing hormone (LH) activity provided mostly by human chorionic gonadotropin (hCG).1 A combination of both FSH and LH activity is fundamental to natural folliculogenesis and works to induce the growth, development and maturation of ovarian follicles in women with infertility.2,3 The efficacy of HP-hMG has been described in a number of multicenter, randomized-controlled trials.4–8 When compared to recombinant FSH (rFSH), HP-hMG has been shown to provide a comparable efficacy and safety profile, with fewer adverse events reported, including pregnancy loss and lower risk of ovarian hyperstimulation syndrome (OHSS).3,9 HP-hMG has been approved for use in assisted reproductive technology (ART) treatment in women undergoing controlled ovarian hyperstimulation.10
HP-hMG is administered by subcutaneous injection to patients undergoing ART, usually injected by the patient themselves or their partner, and, if appropriate, at home.10,11 Traditionally, patients undergoing ART have been trained by their fertility nurse to self-inject, often using a syringe and needle, and either single- or multi-dose vials or ampoules.11–13 However, errors in reconstitution or administration can have a negative effect on oocyte retrieval and subsequently influence ART cycle success.12,14 This may cause patients to feel anxious when preparing their medication for injection.12,13 As a consequence, previous studies have shown that patients undergoing ART and fertility nurses often express a preference for the use of pre-filled pens compared to the traditional use of syringe and needle to self-inject.11–13,15–18 Patients have reported that they were more confident of accurate dosing when using a pre-filled pen, it reduced preparation time and required less training in its correct usage.11,12
In addition, pre-filled pens minimize drug loss and reduce the waste caused by the use of multiple syringes and vials.19,20 Pre-filled pens can be available in several forms, including ready-to-use, single-dose disposable pens and reusable single- or multi-dose devices that can be loaded with cartridges containing the correct dose of medication.11 European studies that have investigated the perception of pre-filled pens found that ease of use is the most important attribute for patients undergoing ART.13,21 However, interestingly, differences were found between fertility nurses and patients with regard to key attributes. Patients place greater value on factors that influence their daily life, such as injection site pain and device reusability, while nurses value pens that are easier for patients to learn to use correctly, and which carry minimal risks of incorrect use.21 The aim of the current study was to determine and conclude how a prototype multi-dose, prefilled HP-hMG (MENOPUR®) pen is perceived against the desired pen features identified by patients and fertility nurses, according to their relative importance.
Materials and Methods
Sample Composition and Recruitment
Patients were eligible to enter the study if they were females aged 18–46 years and had consulted a fertility specialist in the previous 2 years to discuss undergoing controlled ovarian stimulation for ART. Patients were divided into two subgroups depending on their experience with ART. Patients were designated as “experienced” if they had undergone at least one previous ART cycle in the previous 2 years, including self-injection with gonadotropins. Naïve patients were defined as those who were scheduled to undergo ART, but who had yet to commence treatment and had no prior history of ART. Eligibility criteria for fertility nurses included assisting in a minimum of 75 ART cycles per year, being personally involved in the management of patients undergoing ART and personally teaching patients to prepare and self-inject gonadotropins. The sample size was determined with the aim of achieving a reasonable precision in terms of the width of the 95% confidence interval, a sample size of 80 nurses and 70 patients would lead to a precision of ±11–12%. A sample size of 30 nurses was sufficient to allow for country-level comparisons with a precision of ±18%.
Survey respondents were recruited by Research Partnership, a specialist healthcare market research firm, using either email or telephone contact obtained via the nurse/patient database of local agencies. Social-media campaigns were also implemented to drive recruitment and posters describing the study were displayed in fertility clinics with QR codes linked to study information. Additional recruitment methods included nurse referrals to contact other nurses or patients via letter or email. Following initial engagement, potential respondents were screened using an online survey to ensure eligibility and then online interview appointments were arranged. Online consent forms were completed prior to dispatch of the prototype pen device with all respondents providing informed consent. Institutional Review Board or Independent Ethics Committee approval was not required because this was a nonclinical, simulated-use study, with all injections performed into injection pads. The participants were not aware of the company sponsoring the study, or of the drug with which the prototype pen device was intended for use.
Study Design
This was a two-part survey study carried out in Poland, Spain and the UK between May and June 2021. It included fertility nurses, patients undergoing ART and patients scheduled to undergo ART. All participants were initially asked to rank their preferred key attributes for a pre-filled pen injection device. Respondents then handled a prototype MENOPUR pen and compared its attributes against their identified preferred key attributes.
Interview Process and Flow
The interview flow is shown in Figure 1A. Full details of the nurse and patient interview questions and process are given in Supplementary Tables 1 and 2. Survey respondents participated in 30-minute online interviews. In the initial part of the interview, some of the respondents’ screening answers were confirmed and an online survey to explore the importance of key attributes for pre-filled injection pens completed. Respondents were then shown a video demonstration of the prototype pen. Under instruction from the remote moderator, respondents then handled a prototype pen and performed a dummy injection using a demo injection cushion before assessing the attributes of the prototype pen.
 |
Figure 1 (A) Interview flow for study respondents (B) MENOPUR® pre-filled injection pen. |
Assessment of Pre-Filled Pen Attributes
In the current study, a list of attributes of a pre-filled gonadotropin self-injection pen device was prepared which contained a total of 14 options (Table 1). The options were selected to reflect standard attributes investigated in prior research and similar publications examining pre-filled injection pens13,20,21 and were validated by feedback obtained from fertility specialist nurses regarding features of pen devices that were considered to be of greatest importance. Attributes were intended to cover key areas including training, injection preparation, injection experience, how the injection fits into the patient’s daily routine and the overall appearance/look of the injection pen.
 |
Table 1 Product Attributes Examined in the Maximum Difference Scaling |
Respondents were shown a group of four different attributes and asked to select which of these was the most important attribute and which was least important when considering the attributes of a pre-filled gonadotropin self-injection pen device. This exercise was repeated using 11 different combinations of the 14 attributes.
Anchored Maximum Difference Scaling was used to identify the hierarchy of attributes from the list and the importance of attributes relative to a standard or anchor point and relative to one another.
Question 3, which examined the must-have attributes of a gonadotropin pen, was used as a dual response for scaling the attributes relative to a threshold anchor of importance or desirability, which allowed the relative importance of each attribute to be assessed against all other attributes and a negative or positive impact to be assigned. Utility scores derived from the Anchored Maximum Difference scaling >200 indicate that an attribute has extremely high importance. Scores ≥100–200 have above average importance; scores <100 have below average importance and scores <50 are of low importance.
Assessment of Prototype MENOPUR Pen Attributes
The MENOPUR pre-filled injection pen is a non-sterile disposable device containing a 3-mL cartridge with solution for injection of 600 IU HP-hMG/0.96 mL containing 600 IU of FSH bioactivity and 600 IU of LH bioactivity. The pen has a 31G needle (8 mm long with 6-bevel geometry) and is capable of delivering doses from 6.25 to 450 IU HP-hMG in increments of 6.25 IU.22 A prototype unbranded pen containing a placebo cartridge (Figure 1B) was provided to study respondents together with a demo cushion to enable a practice injection to be performed under supervision from the remote interview moderator. Respondents were required to attach the needle to the prototype pen and prime the device before performing the practice injection. The attributes of the prototype pen were then compared by the study respondents against the identified key attributes in an online survey.
Statistical Analyses
To determine any statistically significant differences between the means of more than two independent (unrelated) groups, the one-way analysis of variance (ANOVA) was used to understand whether different groups of respondents have different preferences for the items included in the MaxDiff experiment. A p-value less than 0.05 indicated a statistically significant difference between groups. To examine which groups differed from each other, Tukey post hoc test Multiple Comparisons were used. If p<0.05, there is a statistically significant difference between the groups.
Results
Study Respondents
A total of 221 respondents from Poland (n=60), Spain (n=81) and the UK (n=80) completed the study (Table 2). Overall, 80 fertility nurses were recruited, with 20 from Poland, 30 from Spain and 30 from the UK. On average, the fertility nurses had spent 11 years in practice, with nurses in the UK having the longest service (14 years) and those in Poland the shortest (8 years). While the majority of nurses in Spain and Poland worked in the private sector, under half of UK nurses (43%) worked in private practice.
 |
Table 2 Details of Survey Respondents |
Across all three countries, 84% of patients were aged between 31 and 45 years. Of 141 respondent patients, 71 were treatment-experienced (50%). Approximately two-thirds (68%) of experienced patients had undergone two cycles of ART in the past 2 years, with 66% having previous experience of using a pre-filled pen.
Preferred Device Attributes
A multi-dose pre-filled pen was shown to be the preferred type of gonadotropin injection for nurses across all markets, with 53% of nurses ranking it as their first choice (n=80). Single-use pre-filled pens and multi-dose reusable pens with replaceable cartridges were ranked as their first choice by 19% and 16% of nurses, respectively. Single- and multi-dose vials and syringes were the least preferred options, ranked as fifth choice by 30% and 49% of nurses, respectively (Figure 2).
 |
Figure 2 Fertility nurse preference for delivery device for gonadotropin injection (all markets, N=80). |
The key attributes for pre-filled pens identified as extremely important were consistent across markets and respondent types. Across all survey respondents, including both nurses and patients, the ability to dial-back to correct the dose was considered the most important product attribute of a gonadotropin pen (Figure 3). In addition, confidence in the patient’s ability to inject correctly at home was considered to be of extremely high importance to both nurses and naïve patients, and of above average importance to experienced patients. However, some differences were observed between respondents from different countries. In Spain, the ability to dial-back to correct the dose was the only attribute considered extremely important, with patients’ confidence in self-injection considered to be of above average importance. In addition, respondents in Poland considered injection with the pen being as painless as possible and having a short, thin and sharp needle as more important attributes than respondents in the UK and Spain (Figure 4A).
 |
Figure 3 Importance of pre-filled pen attributes across all survey respondents ranked using the Maximum Difference Scaling (N=221). |
 |
Figure 4 Relative importance of pre-filled pen attributes by (A) country and (B) respondent type. |
Differences in attribute importance were also identified between respondent types. Both experienced and naïve patients identified the attribute of the pen being as painless as possible as significantly more important (above average importance) compared to nurses, who scored this attribute as below average importance (p<0.05). A difference was also observed between the patients’ scores, with naïve patients considering pen injection being as painless as possible as more important compared to experienced patients (utility scores of 172 and 121, respectively; p<0.05) (Figure 4B). Another difference was seen in regard to the attributes of ease of use and ease to learn, with nurses rating these attributes as being above average importance, while both experienced and naïve patients considering them to be less important (p<0.05) (Figure 4B).
Experience with the Prototype MENOPUR Pen
Almost all respondents (99%) reported a positive experience with the prototype pen. All nurses and naïve patients reported a positive experience, with the majority reporting their experience to be “very good” (88% and 70% respectively). Just over half (58%) of experienced patients reported their experience of the prototype pen to be “very good”, with 4% reporting their experience to be poor. Differences between respondent groups in the proportion of patients reporting their experience of the prototype pen to be “very good” were not statistically significant. Respondent experience of the prototype pen across countries was positive and consistent, with similar proportions of respondents reporting the experience as “very good” (Figure 5A).
 |
Figure 5 (A) Respondent experience of the prototype pen and (B) assessment of how closely the prototype pen performs across the key attributes identified. |
When compared with the key attributes identified by the Maximum Difference Scoring, the prototype pen performed well across all key attributes. Almost all (99%) respondents either agreed or strongly agreed that the pen performed well in correcting the dose and 98% of respondents agreed that the prototype pen is easy to prepare and use for injection. In addition, the majority (97%) of respondents considered that they would be confident that they or their patient would be able to self-inject correctly using the prototype pen (Figure 5B). Overall, 98% of the patients stated that they would recommend the prototype pen to any friends or family members requiring fertility treatment.
Discussion
This study involving fertility nurses and patients undergoing ART revealed that the most important attribute for a gonadotropin pen was perceived to be the possibility to dial back to correct the dose. Other important attributes identified in the current study included confidence in the ability to inject correctly and safely at home, ease of preparation, and an injection which is as painless as possible. Our findings identified some differences in perception of attributes between the countries studied. Compared with respondents from Poland and the UK, respondents in Spain placed greater importance on the ability to correct the dose. This could potentially be a consequence of the widespread use in Spain of a gonadotropin pen that does not currently have a simple dial-back option23 and is less widely used in the UK and Poland. In addition, respondents from Poland placed greater importance on the injection being as painless as possible than respondents from either Spain or the UK. Differences in perception were also observed between nurses and patients, with fertility nurses placing greater importance on ease of use of the pen and its use being easy to learn, compared to patients. Furthermore, fertility nurses considered an injection which is as painless as possible to be less important than did patients, with naïve patients conferring greater importance on this attribute than experienced patients potentially due to their inexperience with the injection process. Almost all study respondents reported a positive experience with the prototype pen, with approximately three-quarters of respondents rating it as “very good”. Interestingly, a smaller proportion of experienced patients compared with treatment naïve patients reported their experience to be “very good”; it is possible that a higher expectation for ease of use of the prototype pen might underlie this observed difference owing to the patient familiarity with injection pen devices. The prototype pen was found to perform well across all key attributes identified as most important in gonadotropin pens.
The findings reported here reflect those of other studies, including a European study where the ability to dial back the dose was identified to be of great importance, and ranked the third most important attribute of a pre-filled injection pen by patients and second by nurses.21 A second study conducted among patients undergoing ART also reported that avoiding dosage mistakes was of great importance.13 Ease of use was identified in previous studies as the most important attribute by patients undergoing ART, however, in the current study this attribute was perceived as of below average importance.13,21 Similar differences between patient and nurse perceptions were found in a previous study, which examined FSH injection pen perception in the European market.21 This implies a possible trend in perception differences between nurses and patients, where each group tends to place a greater value on attributes that affect their daily lives.
The use of pre-filled pens has been demonstrated to be preferred over vials, syringes and needles, not only in patients undergoing ART, but also in patients with musculoskeletal conditions or diabetes who are required to self-inject.11–13,24–26 Ease of use and avoiding dosage errors have both been identified as key attributes of pre-filled pens rather than syringe and needle for self-injection.11,13 A survey conducted in Italy to examine usability and satisfaction with pre-filled pen devices among patients undergoing ART demonstrated that, compared with the use of syringes and needles, pre-filled pens are perceived to provide major advantages in terms of ease of use and prevention of dosage errors.13 Similar findings have been found with the use of pre-filled pens for methotrexate administration in treatment of juvenile idiopathic arthritis, and pre-filled insulin pens in patients with diabetes.24–26
Reusable and multi-dose pens have also been perceived by patients to reduce waste and contribute to the protection of the environment.20 However, there is evidence that patients may be more likely to make handling errors with multi-dose pens compared with single-dose pens,27 for example, dialing a dose too high in error. Therefore, there is a preference for devices where it is possible to “dial-back” the dose to ensure correct dosing with each use.21 The widespread acceptance of pre-filled injection devices is, therefore, driven by a range of benefits including convenience, simplicity, suitability for home use, reduction in wasted products and associated environmental benefits, and greater dose precision.12,13,19,20
An analysis of patient and nurse preferences for self-administered FSH injection devices conducted in France, Italy, Spain, Germany, the UK, the Netherlands, the Czech Republic and Belgium also used Maximum Difference Scaling to examine the relative importance of the selected FSH injection device attributes.21 Similar to the findings of our study, this analysis demonstrated both agreements and differences in nurse and patient priorities for the ideal injection device, with shared key attributes including the ability to dial back to correct the dose, and a device that is easy to use and teach/learn.21 In contrast to our findings, where patients were highly motivated by the potential result of their ART treatment, this study reported that patients value factors that minimize the impact of self-injection on their daily life.21
Limitations of this study include the small number of countries in which the study was conducted, and the relatively small pool of nurses and patients interviewed, although the sample size chosen was considered sufficiently robust, and the homogeneous nature of the patient population given that both experienced and naïve patients were recruited. Strengths of the study include the use of the Anchored Maximum Difference Scaling methodology that allowed an assessment of the relative importance of different attributes of a pre-filled self-injection pen.
Conclusion
The prototype pen fulfilled the most important criterion of a pre-filled gonadotropin pen that was identified by study respondents, namely the ability to correct the dose. The ability to inject correctly and safely, ease of preparation and use, and an injection device which gives the patient confidence that the injection looks like it will be as painless as possible were also attributes considered to be of above average importance by study respondents, and were all perceived to be met by the prototype pen device. In conclusion, the prototype pen device evaluation demonstrated that the device was perceived to possess the key attributes considered important by both fertility nurses and patients undergoing ART, suggesting that it is a user-friendly option in this treatment setting.
Acknowledgments
The authors would like to thank Paula Coyle, Ellie Merrigan, Zoe Clark, Evelyn Watson, and Luke Stoneman at Research Partnership for their assistance with conducting the nurse and patient interviews and preparation of the study report. Editorial support for the preparation of this manuscript (in the form of writing assistance, collating author comments, assembling tables/figures, grammatical editing and referencing) was provided by Sarah Birch, PhD and Yarden Cohen-Jones, at Makara Health Communications Ltd, UK and was funded by Ferring Pharmaceuticals.
Disclosures
BC is an employee of Ferring Pharmaceuticals. GDM and JS have no disclosures.
Funding
This study was funded by Ferring Pharmaceuticals.
References
1. Lunenfeld B, Bilger W, Longobardi S, Alam V, D’Hooghe T, Sunkara SK. The development of gonadotropins for clinical use in the treatment of infertility. Front Endocrinol. 2019;10:429. doi:10.3389/fendo.2019.00429
2. Alviggi C, Conforti A, De Rosa P, Riccardi R, Abbamondi A, De Placido G. Drugs Used for Controlled Ovarian Stimulation. In: Skinner Michael K, editor. Encyclopedia of Reproduction.
3. Deeks ED. Highly purified human menopausal gonadotropin (Menopur®): a profile of its use in infertility. Clin Drug Investig. 2018;38(11):1077–1084. doi:10.1007/s40261-018-0703-8
4. The European and Israeli Study Group on Highly Purified Menotropin versus Recombinant Follicle-Stimulating Hormone. Efficacy and safety of highly purified menotropin versus recombinant follicle-stimulating hormone in in vitro fertilization/intracytoplasmic sperm injection cycles: a randomized, comparative trial. Fertil Steril. 2002;78(3):520–528. doi:10.1016/s0015-0282(02)03250-8
5. Alviggi C, Cognigni GE, Morgante G, et al. A prospective, randomised, investigator-blind, controlled, clinical study on the clinical efficacy and tolerability of two highly purified hMG preparations administered subcutaneously in women undergoing IVF. Gynecol Endocrinol. 2013;29(7):695–699. doi:10.3109/09513590.2013.788641
6. Andersen AN, Devroey P, Arce JC. Clinical outcome following stimulation with highly purified hMG or recombinant FSH in patients undergoing IVF: a randomized assessor-blind controlled trial. Hum Reprod. 2006;21(12):3217–3227. doi:10.1093/humrep/del284
7. Devroey P, Pellicer A, Nyboe Andersen A, Arce JC; Menopur in Gn RHACwSETTG. A randomized assessor-blind trial comparing highly purified hMG and recombinant FSH in a GnRH antagonist cycle with compulsory single-blastocyst transfer. Fertil Steril. 2012;97(3):561–571. doi:10.1016/j.fertnstert.2011.12.016
8. Koo HS, Kwon H, Choi DS, Han S, Seo JY, Yang KM. Clinical utility of newly developed highly purified human menopausal gonadotrophins: a randomized controlled trial. Reprod Biomed Online. 2017;34(5):499–505. doi:10.1016/j.rbmo.2017.02.009
9. Witz CA, Daftary GS, Doody KJ, et al. Randomized, assessor-blinded trial comparing highly purified human menotropin and recombinant follicle-stimulating hormone in high responders undergoing intracytoplasmic sperm injection. Fertil Steril. 2020;114(2):321–330. doi:10.1016/j.fertnstert.2020.03.029
10. Ferring Pharmaceuticals. Menopur 75IU - Summary of Product Characteristics (SmPC); 2019. Available from: https://www.medicines.org.uk/emc/product/1294/smpc#gref.
11. Welcker JT, Nawroth F, Bilger W. Patient evaluation of the use of follitropin alfa in a prefilled ready-to-use injection pen in assisted reproductive technology: an observational study. Reprod Biol Endocrinol. 2010;8:111. doi:10.1186/1477-7827-8-111
12. Buhler K. Managing infertility with the follitropin alfa prefilled pen injector - patient considerations. Ther Clin Risk Manag. 2015;11:995–1001. doi:10.2147/TCRM.S64222
13. Dallagiovanna C, Mensi L, Di Gesaro L, La Vecchia I, Reschini M. Satisfaction and usability of the recombinant chorionic gonadotropin prefilled pen: a survey in Italy. Drugs Context. 2022;11:1–4. doi:10.7573/dic.2021-10-3
14. Bustillo M. Unsuccessful oocyte retrieval: technical artefact or genuine ‘empty follicle syndrome’? Reprod Biomed Online. 2004;8(1):59–67. doi:10.1016/s1472-6483(10)60498-1
15. Longobardi S, Seidler A, Martins J, Beckers F, MacGillivray W, D’Hooghe T. An evaluation of the use and handling errors of currently available recombinant human follicle-stimulating hormone pen injectors by women with infertility and fertility nurses. Expert Opin Drug Deliv. 2019;16(9):1003–1014. doi:10.1080/17425247.2019.1651290
16. Schertz J, Worton H. Patient evaluation of the redesigned follitropin alfa pen injector. Expert Opin Drug Deliv. 2017;14(4):473–481. doi:10.1080/17425247.2017.1289174
17. Schertz J, Worton H. Nurse evaluation of the redesigned fertility pen injector: a questionnaire-based observational survey. Expert Opin Drug Deliv. 2018;15(5):435–442. doi:10.1080/17425247.2018.1450386
18. Utsunomiya T, Tanaka A, Tatsumi K, Ezcurra D. A questionnaire-based survey to assess patient satisfaction, ease-of-learning, ease-of-use, injection site pain and overall patient satisfaction of the follitropin-alpha (Gonal-f) filled-by-mass (FbM) prefilled pen compared with other systems of gonadotrophin administration. Reprod Biol Endocrinol. 2012;10:93. doi:10.1186/1477-7827-10-93
19. Makwana S, Basu B, Makasana Y, Dharamsi A. Prefilled syringes: an innovation in parenteral packaging. Int J Pharm Investig. 2011;1(4):200–206. doi:10.4103/2230-973X.93004
20. Sauer M, Abbotts C. A new pen device for injection of recombinant human growth hormone: a convenience, functionality and usability evaluation study. Patient Prefer Adherence. 2018;12:27–34. doi:10.2147/PPA.S149412
21. Zitoun P, Parikh J, Nijs M, Zhang W, Levy-Toledano R, Tang B. Analysis of patient and nurse preferences for self-administered FSH injection devices in select European markets. Int J Womens Health. 2019;11:11–21. doi:10.2147/IJWH.S175775
22. Jonker DM, Koch M, Larsson P, et al. First pre-filled pen device with highly purified human menopausal gonadotropin (HP-hMG, Menopur) in liquid is shown to be bioequivalent to powder for reconstitution. Int J Clin Pharmacol Ther. 2021;59(12):794–803. doi:10.5414/CP204040
23. Barrenetxea G, Garcia-Velasco JA, Aragon B, et al. Comparative economic study of the use of corifollitropin alfa and daily rFSH for controlled ovarian stimulation in older patients: cost-minimization analysis based on the PURSUE study. Reprod Biomed Soc Online. 2018;5:46–59. doi:10.1016/j.rbms.2018.01.001
24. Ahmann A, Szeinbach SL, Gill J, Traylor L, Garg SK. Comparing patient preferences and healthcare provider recommendations with the pen versus vial-and-syringe insulin delivery in patients with type 2 diabetes. Diabetes Technol Ther. 2014;16(2):76–83. doi:10.1089/dia.2013.0172
25. Lasalvia P, Barahona-Correa JE, Romero-Alvernia DM, et al. Pen devices for insulin self-administration compared with needle and vial: systematic review of the literature and meta-analysis. J Diabetes Sci Technol. 2016;10(4):959–966. doi:10.1177/1932296816633721
26. Roszkiewicz J, Swacha Z, Smolewska E. Prefilled pen versus prefilled syringe: a pilot study evaluating two different methods of methotrexate subcutaneous injection in patients with JIA. Pediatr Rheumatol Online J. 2020;18(1):64. doi:10.1186/s12969-020-00455-4
27. Saunders H, Bjargestad Lamp L, Donat H, Messner M, Reder M, Kendrew H. Risk of dosing errors in ART treatment: user experience of single- vs multi-use follitropin alfa pens. Expert Opin Drug Deliv. 2021;18(5):643–654. doi:10.1080/17425247.2021.1863944
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
