Back to Journals » ClinicoEconomics and Outcomes Research » Volume 16
Analysis of Medicare Patients Treated with Pimavanserin versus Other Atypical Antipsychotics: A Cost-Offset Model Evaluating Skilled Nursing Facility Stays and Long-Term Care Admissions in Parkinson’s Disease Psychosis
Authors Rajagopalan K , Rashid N, Yakkala V , Doshi D
Received 12 December 2023
Accepted for publication 12 February 2024
Published 11 March 2024 Volume 2024:16 Pages 149—159
DOI https://doi.org/10.2147/CEOR.S452162
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Samer Hamidi
Krithika Rajagopalan,1 Nazia Rashid,2 Vinod Yakkala,1 Dilesh Doshi2
1Anlitiks Inc., Windermere, FL, USA; 2Medical Affairs, Acadia Pharmaceuticals Inc., San Diego, CA, USA
Correspondence: Krithika Rajagopalan, Anlitiks Inc., Email [email protected]
Background: Patients with Parkinson’s disease psychosis (PDP) treated with pimavanserin (PIM) versus other atypical antipsychotics (AAPs) including quetiapine (QUE) may have health-care cost savings due to fewer skilled nursing facility-stays (SNF-stays) and long-term care admissions (LTCA).
Methods: A decision analytic model was developed using the 2019 Medicare Patient Driven Payment Model (PDPM) to estimate SNF-stays and LTCA associated per-patient- per-year (PPPY) facility and rehabilitation costs among patients that initiated PIM vs QUE or vs other-AAPs (i.e, quetiapine, risperidone, olanzapine, aripiprazole). Model inputs were derived for: (i) annual SNF-stay and LTCA rates from an analysis of Medicare beneficiaries with PDP, and (ii) annual mean rehabilitation and resident care-stay costs from PDPM case-mix adjusted value-based payment rates for 5 rehabilitation components (ie, physical-therapy, occupational-therapy, nursing, speech-language pathology, non-therapy ancillary), and an additional variable-per-diem for room/board services. PPPY costs were estimated from (i) SNF-stay and (ii) LTCA rates multiplied by annual mean costs of stay in 2022 USD. Probabilistic sensitivity analysis (PSA) was performed using 1000 Monte Carlo simulations.
Results: Overall SNF-stay rates of 20.2%, 31.4%, and 31.7%, and LTCA rates of 23.2%, 33.8%, 34.6% were observed for PIM, QUE, and other-AAPs, respectively. Based on annual mean costs, PPPY SNF-stay rehabilitation and resident related costs for PIM ($41,808) vs QUE ($65,172) or vs other-AAPs ($65,664), resulted in $23,364 and $23,856 PPPY cost savings, respectively. Similarly, PPPY LTCA rehabilitation and resident related costs for PIM ($47,957) vs QUE ($70,091) or vs other-AAPs ($71,566) resulted in $22,134 and $23,609 PPPY cost-savings for PIM, respectively. PSA suggested PIM would provide cost-savings vs QUE or other-AAPs in > 99% of iterations.
Conclusion: In this analysis, PIM demonstrated nearly 36% and 32% lower PPPY SNF-stays and LTCA costs, respectively, vs QUE or other-AAPs. Research examining additional cost-offsets (i.e., fewer falls/fractures) associated with SNF-stay or LTCA may be needed.
Keywords: nursing homes, psychosis, quetiapine, rehabilitation costs, cost savings
Background
Parkinson’s disease (PD), the second most common neurodegenerative disease among the elderly, is estimated to impact 1.2 million Americans in the United States (US) by 2030.1 Approximately 90,000 patients are newly diagnosed each year in the US.1 While clinicians commonly focus on the motor symptoms of PD (i.e., tremor at rest, bradykinesia, rigidity, and postural instability), nonmotor symptoms include neuropsychiatric features related to hallucinations and delusions, a characteristic hallmark of Parkinson’s disease psychosis (PDP) which can be more troublesome than motor symptoms in terms of quality of life and financial burden.1–5 The prevalence of PDP is estimated to range from 16% to 75% among patients with PD.6 Prior research suggest that PDP exists on a spectrum that ranges from minor phenomena and progressing to hallucinations with retained insight and, finally, to hallucinations with loss of insight.7 As PDP and other neuropsychiatric symptoms become severe, they are known to contribute to nursing home (NH) placement in skilled nursing facilities (SNF), long-term care admissions (LTCA) and institutionalization.8 Additionally, the presence of PDP is highly associated with NH placement, with PDP patients being more than two times as likely to be placed in NH than those with only PD.9 A research study of community and NH patients with PD demonstrated that nearly 25% of patients reside in nursing homes.10 Interestingly, other studies suggest that 5.2% to 6.8% of LTC residents have PD.11–13 The psychological and physical burden of NH placement, associated rehabilitation and resident care costs can impose a significant burden on patients, their families, caregivers, as well as the public payors.14–16
To date, pimavanserin (PIM), a 5-HT2A inverse agonist, is the only FDA approved therapy for the treatment of hallucinations and delusions associated with PDP.17 However, other atypical antipsychotics (AAPs) are often used as off-label to treat PDP.18 Recent published research of patients treated with PIM vs other off-label AAPs have shown fewer hospital admissions, lower mortality risk and overall better outcomes.14,19–23 Additionally, a recent real-world study has examined the risk of SNF-stays and LTCA among patients on PIM vs other AAPs including quetiapine (QUE), showing that the risk of NH placement may be lower with PIM.24
Though there is research demonstrating decreased burden due to fewer LTCA and NH stays with use of PIM vs other-AAPs or QUE, the extent to which this translates to medical cost-savings in terms of rehabilitation and room/board costs from an overall public payor or patient perspective is still unknown. Thus, the objective of this analysis is to determine total resident and rehabilitation related cost-savings within the healthcare system due to fewer SNF stays or LTCA among PDP patients treated with PIM vs (i) QUE and (ii) PIM vs other-AAPs (ie, quetiapine, risperidone, olanzapine, aripiprazole).
Methods
Model Structure, Approach and Assumptions
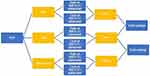
A cost-offset model was developed to demonstrate differences in annual SNF-stays and LTCA rehabilitation and resident care costs among patients newly initiating on PIM vs QUE or PIM vs other AAPs for the treatment of PDP. In a Microsoft excel based decision-tree model, cost differences between PDP patients on PIM, QUE, or other-AAPs (Figure 1) were calculated by multiplying the rates of SNF-stay and rates LTCA with the cost of rehabilitation services and resident care among patients using the Medicare Patient Driven Payment Model (PDPM).25
PDPM is a new value-based reimbursement system for NH and LTC facilities that was implemented by the Center for Medicare and Medicaid Services (CMS) in 2019.25 The payment system was intended to replace the older volume-based Resource Utilization Group-Version 4 (RUG-IV) system to improve the accuracy and quality of need-based care while ensuring alignment of reimbursement incentives. Accordingly, payments for resident care and rehabilitation services under the PDPM is determined through the combination of six payment components. Of the six components, five of them are case-mix adjusted payment groups based on their primary diagnosis and clinical status in contrast to RUG-IV that categorized payment for residents into a single group.25 These five components include physical therapy (PT), occupational therapy (OT), speech-language pathology (SLP), non-therapy ancillary (NTA) services, and nursing component. Additionally, the sixth component is non-case-mix adjusted to cover utilization of SNF resources that do not vary according to patient characteristics.25
While each SNF-stay/LTC resident is assigned to only one group for the five case-mix adjusted components during a single stay, variations in resource consumption during a resident’s stay are accommodated with the variable per diem payment adjustment to three of the case-mix components, PT, OT, and NTA.25 The total per diem rate for a particular resident is then calculated by adding the adjusted PT, OT, and NTA per diem rates with the unadjusted SLP and nursing component rates plus the non-case-mix component.25
Thus each derived payment group in the PDPM model are associated with a case-mix index derived from unadjusted and per-diem urban/rural rates (Table 1).25 The payment for each of the case mix adjusted components is calculated by multiplying the component case mix index (CMI) for the resident’s classification group by the component base rate, then by the specific day in the variable per diem adjustment schedule, when applicable. These payments are then added together along with the non-case-mix component payment rate to create a resident’s total SNF PPS per diem rate under the PDPM, which is then wage-adjusted in the same manner as rates under RUG-IV.25,26
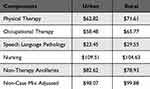 |
Table 1 PDPM Components and Unadjusted Federal Rates per Diem for Fiscal Year 2022 |
To ensure a standardized and simple approach for the model comparators, a few simplifying assumptions were made during the development of the model. First, all patients entered the model on the admission date of SNF- stay or LTCA. Second, all patients admitted to SNF or LTC facilities were assumed to stay for at least one year after admission. Third, the resident care rates beyond the first 100 days were assumed to remain the same as the Medicare rate for the first 100 days. Fourth, mortality, and all-cause discontinuation of PIM or other-AAPs for any reason were not taken into consideration.
Since the model did not include a direct observational assessment of patients, ethics committee review and approval were not required. However, the clinical inputs used in the model were derived from a study that was conducted in compliance with Health Insurance Portability and Accountability Act (HIPAA) under a CMS data use agreement that was established pursuant to a New England Institutional Review Board’ review and approval.
Model Inputs
Clinical Inputs: Risk of SNF-Stay and LTCA
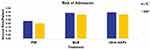
Annual risk of admission to LTC and SNF among PDP Medicare beneficiaries initiating continuous monotherapy for at least 12 months using PIM, QUE, or other-AAPs was derived from a recently concluded retrospective research study of Parts A, B, and D claims of a 100% Medicare sample from January 2014 to December 2019.23,24 In this analysis of propensity score matched overall all-cause LTCA and SNF-stay rates were 23.2% and 20.2% for PIM vs 33.8% and 31.4% for QUE, and 34.6% and 31.7% for other AAPs respectively (p<0.05, for each) (Figure 2). Hazard ratio (95% CI) for risk of SNF-stay and overall LTCA was 0.78 (0.61, 0.98) and 0.80 (0.66, 0.97), respectively, for PIM vs QUE patients (p<0.05).23,24
Cost Inputs: Patient Driven Payment Model Costs
Cost inputs for rehabilitation and resident care costs were calculated as a sum of the mean standard non-case-mix value per diem (VDP) costs and the five case-mixed adjusted mean component costs based on estimated patient conditions during the inpatient stay. All case-mixed mean component costs were derived from a PDPM cost calculator based on federal fiscal year 2022 rates implemented through a case-mix index factor and wage index factor (1.0) for each group.25,26 The methods used to derive the 5 PDPM case-mix component groups and per-diem cost inputs are described below.25–27
Physical Therapy and Occupational Therapy
Cost component estimates for PT and OT groups within the PDPM are generally calculated based on four types of case-mix groups (ie, joint replacement surgery, other orthopedic groups, medical management, and non-orthopedic neurologic group). Each of the four types of case-mix groups in turn are categorized into four sub-groups based on level of care required by the patients. Each care-burden level is determined by their functional status scores ranging from 0–24 (ie, score 1–6, 7–12, 13–18, 19–24). Thus, a total of 16 case-mix groups have been designated by CMS for calculating the costs based on the type of level of care needed by the resident patients. Given that patients with PDP have a neurologic condition, the model assumed all patients’ case mix for PT and OT will be calculated based on the patients’ assignment into one of the four non-orthopedic neurologic groups based on the level of care required by them. Based on the cost calculations, total per diem rate (Tables 2 and 3) for PT and OT ranged from $149.03 to $212.94 with a mean cost of $144.95.26,27
 |
Table 2 Case-Mix Groups Selected and Mean per Diem Rates for SNF/LTC Care |
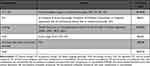 |
Table 3 Case-Mix Component and Total per Diem Rate of Rehabilitation on Annualized Basis |
Speech Language Pathology
Cost estimates for the SLP under the PDPM is derived from case-mix scoring based on the presence of certain primary conditions (eg, acute neurologic condition, comorbidities related to presence or absence of cognitive impairment). Furthermore, this case mix is further grouped into sub-categories with or without medically altered diet or swallowing disorder such as dysphagia. Given that PDP involves both a neurologic condition and cognitive impairment issues, the model assumed the base case potential case-mix groups to be level non-extensive orthopedic procedures with major complications or comorbidities (SH) severity signifying that all patients in the model would be those requiring SL support for at least 2 of the following primary conditions (eg, presence of Acute Neurologic Condition, SLP-Related Comorbidity, or Cognitive Impairment) as well as a Mechanically Altered Diet or Swallowing Disorder. It should be noted that the model assumed that a mechanically altered diet or swallowing disorder that may be typical of advanced PD patients would also be present. Since only the SH group was selected for SLP in the model, the per diem rate for SLP was $84.51 (Tables 2 and 3).26,27
Non-Therapy Ancillary
Similarly, for the calculation of costs for non-therapy, it was assumed that patients would have up to two other comorbid conditions requiring ancillary therapy (ie, NTA score range of 0–2). For the purposes of the model, patient per diem ancillary care rates were adjusted based on the day of the stay, with days 1–3 being three times the rate of any following day. Per diem rate for NTA ranged between $56.83 and $75.77; mean per diem for NTA was estimated to be $56.83 for the purposes of the model (Tables 2 and 3).26,27
Nursing
Under PDPM, the nursing case mix component utilizes the use of extensive services, certain clinical conditions, the presence of depression, restorative nursing services provided, and the patient’s functional score to assign a patient to a nursing case-mix group. For the purposes of the model, nursing care costs were classified into 4 nursing case-mix sub-groups. Two of the four case mix sub-groups had their case-mix index identified as PD with low level of special care in the presence or of coexisting depression resulting in a functional score of 0–5. The remaining 2 case-mix subgroups were identified as PD with cognitive behavioral issues and presence or absence of coexisting depression resulting in a functional score of 6–14. Per diem rate for all 4 case-mix subgroups ranged from $149.62 to $217.63, and mean cost t for the case-mix per diem was $182.06 (Tables 2 and 3).26,27
Non-Case Mix VDP Costs
The non-case mix non-adjustable costs were related to room and board related resident care costs. These resident care costs are a standard rate for all patients and only varies by geography (ie, urban or rural). Mean of rural and urban costs were derived and included the mean cost was estimated to be $98.98 per diem (Table 2).
Overall Cost Inputs
The overall cost input was the mean total per diem SNF-stay or LTCA costs of $567.33, which was derived as a sum of all the 5 adjustable case-mix and 1 non-adjustable case-mix on an annualized basis, the annual cost of SNF-stay and LTCA without considering the admission rates was $207,075.
Model Outputs
Annual SNF-stay and LTCA resident care and rehabilitation service costs, reported in 2022 USD, were calculated by multiplying by rates of SNF-stay and LTCA to determine the mean total and mean per patient per year (PPPY) cost-savings from PDP patients treated with PIM versus QUE or PIM vs other AAPs. A probabilistic sensitivity analysis (PSA) was performed using 1000 Monte Carlo simulations to determine model robustness.
Model Results
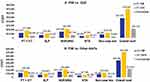
The mean PPPY LTCA rehabilitation and resident care costs for PIM, QUE and other-AAPs were $47,957, $70,091, and $71,566, respectively (Figure 3A and B). Similarly, the total mean PPPY SNF-stay related rehabilitation and resident care costs for PIM, QUE and AAPs were $41,808, $65,172, and $65,664, respectively (Figure 4A and B). Thus, PIM was associated with PPPY cost-savings of $22,134 and $23,364 compared to QUE and $23,609 and $23,855 compared to other-AAPs due to fewer LTCA or SNF-stays, respectively. Probabilistic sensitivity analysis (PSA) suggests PIM would provide cost-savings compared to QUE or other-AAPs in >99% of iterations (Figure 5).
Discussion
This cost offset decision analytic model demonstrated that PIM, in comparison to QUE, lowers PPPY rehabilitation and resident care costs by 35.8% and 31.5%, due to fewer SNF-stays and LTCA, respectively. Similarly, PIM demonstrated lower PPPY rehabilitation and resident care costs versus other-AAPs, with 36.3% and 32.9% lower PPPY costs due to fewer SNF-stays and LTCA. This reduction in NH admissions translates into more than $22,000 per patient cost savings in a year.
Results of this cost model align with previously published research demonstrating the association between PIM use and lower health care resource utilization, fewer SNF-stays, and lower mortality compared to patients on other-AAPs or on QUE for the treatment of PDP.19–24,28 In the published research of Alipour-Haris et al, PDP patients treated with PIM compared to QUE were found to have a lower risk of hospitalization and lower mortality rates at 365 days follow-up. Consistent with the above mentioned research, studies by Rajagopalan and Kumar et al also found that inpatient hospitalization, SNF-stay and LTCA admissions were lower among patients on PIM versus other-AAPs or QUE.19,23,24 Mosholder et al found PIM use was associated with lower mortality than AAP use during the first 180 days of treatment, in community-dwelling patients.22 These results were supported by another study of Medicare patients that reported decreased mortality at 180 day follow-up among patients with PIM compared to comparator AAPs.28 Reasons for the fewer SNF-stays and LTCA observed with PIM versus comparator AAPs including QUE are unknown, it is plausible that PIM’s clinical effect in reducing hallucinations and delusions associated with PDP may result in patients staying longer in the community without the need for SNF-stays and LTCA. While claims data (eg, Medicare 100% claims) do not carry information about clinical symptoms that allow us to examine the relationship between level of clinical symptoms and SNF-stay/LTCA rates, PIM is the only AAP to have demonstrated significant improvements in reducing hallucinations and delusions associated with PDP in confirmatory trials to date. Future studies examining the relationship between treatment effect (ie, reduction in hallucination and delusions associated with PDP) and rates of SNF-stay and LTCA may be helpful in explaining these findings.
The economic burden of Parkinson’s disease in the US is high, with annual direct medical costs estimated at $25.4 billion; nearly 30% of direct care costs or nearly $7.1B annually, representing NH care costs.29 While NH placement is an added economic burden in an already costly condition such as PDP, potential cost differences in NH rehabilitation and resident care costs among patients treated with various antipsychotics has not been known. This research is the first analysis to quantify potential rehabilitation and resident care related cost savings due to fewer NH stays (ie, SNF-stay or LTCA) and associated residential and rehabilitation service costs among patients treated with PIM vs other-AAPs including QUE. In the current study, we have observed that annualized cost of rehabilitation and resident care of NH admission for a typical PDP patient is over $200K, with most of the costs contributed by nursing care and PT/OT components of the PDPM system. On the other hand, costs of NH care for PDP were substantially lower (between $82,128 and $92,376) for an average NH patient in the previously published cost model by Yang et al.29 Therefore, it is possible that the previously estimated annual nursing home costs of $7.1B is likely an underestimation—actual burden may be more than twice this figure, with a substantial portion of this due to PDP.
While this model only examined the lower costs of rehabilitation and resident care costs, it is anticipated that total health care cost savings due to fewer SNF-stays and LTCA may be substantially higher if other direct medical costs (eg, falls and fractures, ER, outpatient, office visits, etc.) among PIM vs other AAPs or QUE were included. Additionally, it should be noted that this model does not include the potentially lower indirect costs of caregiver burden due to greater community participation among patients with fewer SNF-stays and LTCA. Finally, the differences in intangible costs associated with potentially better quality of life for patients and caregivers are also not included in the model. Overall, these model results suggest that nearly 99.99% of the simulations demonstrate cost savings with PIM vs QUE; and the cost savings would be substantially higher if other direct and indirect medical costs were included.
Limitations
As with any models that include specific assumptions, this model too is susceptible to limitations, both due to model assumptions and narrowly focusing on cost of nursing care only and not on total costs of care. We have assumed a one-year duration of stay for all patients admitted to LTC/SNF without accounting for discharges or deaths in the model. Therefore, it is likely that the overall rehabilitation and residential care service costs are overestimated. On the other hand, prior research examining mortality in NH patients with PD suggest that patients with hallucinations and delusions are never discharged and most likely die without getting discharged.9 Therefore, to the extent that the resident care cost estimates are over-estimated, all comparator groups are likely to be overestimated at similar levels. While mortality was not included in the model for simplicity purposes, it should be noted that much of the emerging research indicates that patients treated with PIM has numerically lower 1-year mortality rates compared to other-AAPs. Since this model only focused on rehabilitation and residential care costs, other costs resulting from inpatient readmissions during NH-stay, cost of falls, fractures, and stroke associated care, and cost of medications were not examined. Therefore, it is likely that the overall cost savings are underestimated in this regard. While the PDPM case-mix component group selection in the model was based on evaluation of CMS criteria, it is plausible that real-world case-mix for PDP patients may be higher or lower. Notwithstanding the limitations, this model demonstrates that treatment with PIM in the community has the potential to result in residential and rehabilitation related cost savings for Medicare (ie, first 100 days), Medicaid and/or patients (ie, after 100 days-stay) due to fewer NH admissions vs being treated with other-AAPs or QUE.
Conclusion
In this analysis, the medical cost-offsets of SNF-stay and LTCA related rehabilitation and resident care costs among PDP patients treated with PIM versus QUE or other AAPs are higher; with treatment using PIM translating into over $20,000 savings PPPY. These results complement prior comparative studies which found reduced need for hospitalizations and emergency department visits among PDP patients treated with PIM versus other AAPs.19–21 Though psychotic features in Parkinson’s disease are an added economic burden to an already costly condition,4,5 treatment with appropriately approved AAPs (eg, PIM) in community settings may result in fewer NH stays and reducing costs of NH placement among these patients. Given the goal of reducing expensive and debilitating NH stays among patients suffering from chronic conditions such as PDP, these results may provide the first insights about the implications related to appropriate treatment choices in improving health care quality among patients with PDP.
Abbreviations
AAP, atypical antipsychotic; CMS, Centers for Medicare and Medicaid Services; HCRU, healthcare resource utilization; PD, Parkinson’s disease; PDP, Parkinson’s disease psychosis; LTC, long-term care; SNF, skilled nursing facility.
Author Contributions
All authors made a significant contribution to the work reported, whether in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas, took part in drafting, revising, or critically reviewing the article, gave final approval of the version to be published, have agreed on the journal to which the article has been submitted, and have agreed to be accountable for all aspects of the work.
Funding
This study was financially sponsored by Acadia Pharmaceuticals.
Disclosure
Krithika Rajagopalan and Vinod Yakkala are the current employees of Anlitiks Inc., a company that received funding from Acadia Pharmaceuticals to conduct this study. Nazia Rashid and Dilesh Doshi are employees of Acadia Pharmaceuticals. The authors report no other conflicts of interest in this work.
References
1. Parkinson’s Foundation. Statistics on Parkinson’s; 2023. Available from: https://www.parkinson.org/understanding-parkinsons/statistics/prevalence-incidence.
2. Jankovic J. Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79(4):368–376. doi:10.1136/jnnp.2007.131045
3. Martinez-Martin P, Rodriguez-Blazquez C, Kurtis MM, Chaudhuri KR; NMSS Validation Group. The impact of non-motor symptoms on health-related quality of life of patients with Parkinson’s disease. Mov Disord. 2011;26(3):399–406. doi:10.1002/mds.23462
4. Hermanowicz N, Edwards K. Parkinson’s disease psychosis: symptoms, management, and economic burden. Am J Manag Care. 2015;21(10 suppl):s199–s206.
5. Fredericks D, Norton JC, Atchison C, Schoenhaus R, Pill MW. Parkinson’s disease and Parkinson’s disease psychosis: a perspective on the challenges, treatments, and economic burden. Am J Manag Care. 2017;23(5 suppl):s83–s92.
6. Chang A, Fox SH. Psychosis in Parkinson’s disease: epidemiology, pathophysiology, and management. Drugs. 2016;76(11):1093–1118. doi:10.1007/s40265-016-0600-5
7. Schneider RB, Iourinets J, Richard IH. Parkinson’s disease psychosis: presentation, diagnosis and management. Neurodegener Dis Manag. 2017;7(6):365–376. doi:10.2217/nmt-2017-0028
8. Brent RJ. Life expectancy in nursing homes. Appl Econ. 2022;54(16):1877–1888. doi:10.1080/00036846.2021.1983138
9. Goetz CG, Stebbins GT. Mortality and hallucinations in nursing home patients with advanced Parkinson’s disease. Neurology. 1995;45(4):669–671. doi:10.1212/WNL.45.4.669
10. Safarpour D, Thibault DP, DeSanto CL, et al. Nursing home and end-of-life care in Parkinson disease. Neurology. 2015;85(5):413–419. Epub 2015 Jul 2. PMID: 26138947; PMCID: PMC4534080. doi:10.1212/WNL.0000000000001715
11. Zarowitz BJ, O’Shea T. Reassessment of the prevalence, clinical characteristics, and pharmacologic treatment of nursing facility residents with Parkinson’s disease. Consult Pharm. 2013;28(9):556–568.
12. Mitchell SL, Kiely DK, Kiel DP, Lipsitz LA. The epidemiology, clinical characteristics, and natural history of older nursing home residents with a diagnosis of Parkinson’s disease. J Am Geriatr Soc. 1996;44(4):394–399. doi:10.1111/j.1532-5415.1996.tb06408.x
13. Lapane KL, Fernandez HH, Friedman JH. Prevalence, clinical characteristics, and pharmacologic treatment of Parkinson’s disease in residents in long-term care facilities. SAGE Study Group. Pharmacotherapy. 1999;19(11):1321–1327. doi:10.1592/phco.19.16.1321.30877
14. Wetmore JB, Li S, Yan H, et al. Increases in institutionalization, healthcare resource utilization, and mortality risk associated with Parkinson disease psychosis: retrospective cohort study. Parkinsonism Relat Disord. 2019;68:95–101. doi:10.1016/j.parkreldis.2019.10.018
15. Aarsland D, Larsen JP, Karlsen K, Lim NG, Tandberg E. Mental symptoms in Parkinson’s disease are important contributors to caregiver distress. Int J Geriatr Psychiatry. 1999;14(10):866–874. doi:10.1002/(SICI)1099-1166(199910)14:10<866::AID-GPS38>3.0.CO;2-Z
16. Martinez-Martin P, Rodriguez-Blazquez C, Forjaz MJ, et al. Neuropsychiatric symptoms and caregiver’s burden in Parkinson’s disease. Parkinsonism Relat Disord. 2015;21(6):629–634. doi:10.1016/j.parkreldis.2015.03.024
17. Kitten AK, Hallowell SA, Saklad SR, Evoy KE. Pimavanserin: a novel drug approved to treat Parkinson’s disease psychosis. Innov Clin Neurosci. 2018;15(1–2):16–22.
18. Yohanna D, Cifu AS. Antipsychotics to treat agitation or psychosis in patients with dementia. JAMA. 2017;318(11):1057–1058. doi:10.1001/jama.2017.11112
19. Alipour-Haris G, Armstrong MJ, Okun M, Brown JD. Comparison of pimavanserin versus quetiapine for hospitalization and mortality risk among medicare beneficiaries with Parkinson’s disease psychosis. Mov Disord Clin Pract. 2023;10(3):406–414. doi:10.1002/mdc3.13652
20. Hwang YJ, Alexander GC, An H, Moore TJ, Mehta HB. Risk of hospitalization and death associated with pimavanserin use in older adults with Parkinson disease. Neurology. 2021;97(13):e1266–e1275. doi:10.1212/WNL.0000000000012601
21. Ganesh A, Galetta S, Galetta S, Ganesh A, Lewis A, Siegler JE. Editors’ note: risk of hospitalization and death associated with pimavanserin use in older adults with Parkinson disease. Neurology. 2022;98(1):48. doi:10.1212/WNL.0000000000013041
22. Mosholder AD, Ma Y, Akhtar S, et al. Mortality among Parkinson’s disease patients treated with pimavanserin or atypical antipsychotics: an observational study in medicare beneficiaries. Am J Psychiatry. 2022;179(8):553–561. doi:10.1176/appi.ajp.21090876
23. Rajagopalan K, Rashid N, Kumar S, Doshi D. Health care resource utilization patterns among patients with Parkinson’s disease psychosis: analysis of Medicare beneficiaries treated with pimavanserin or other-atypical antipsychotics. J Med Econ. 2023;26(1):34–42. doi:10.1080/13696998.2022.2152600
24. Kumar S, Rashid N, Doshi D, et al. Risk of long-term care admissions among medicare patients treated with pimavanserin or other atypical antipsychotics for Parkinson’s disease psychosis [abstract]. Mov Disord. 2022;37(suppl 1):2022.
25. CMS. PDPM Calculation Worksheet for SNFs. Center for Medicare and Medicaid Services; 2019.
26. Association AH. Skilled nursing facility PPS final rule for FY 2022; 2021. Available from: https://www.aha.org/system/files/media/file/2021/08/skilled-nursing-facility-pps-final-rule-for-fy-2022-advisory-8-26-21.pdf.
27. (CLA) C. Skilled nursing facility PDPM PPS rate calculator; 2022.
28. Layton JB, Forns J, McQuay LJ, et al. Mortality in patients with Parkinson’s disease-related psychosis treated with pimavanserin compared with other atypical antipsychotics: a cohort study. Drug Saf. 2023;46(2):195–208. doi:10.1007/s40264-022-01260-6
29. Yang W, Hamilton JL, Kopil C, et al. Current and projected future economic burden of Parkinson’s disease in the U.S. NPJ Parkinsons Dis. 2020;6(1):15. doi:10.1038/s41531-020-0117-1
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.