Back to Journals » Risk Management and Healthcare Policy » Volume 16
Analysis of Adverse Drug Reaction Reports from a Public Hospital in Shanxi Province in 2022
Authors Zhang XJ, Zhou JG, Pan M, Yuan W, Gao B
Received 8 May 2023
Accepted for publication 18 July 2023
Published 4 August 2023 Volume 2023:16 Pages 1391—1401
DOI https://doi.org/10.2147/RMHP.S418386
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jongwha Chang
Xiao-Jie Zhang,1 Jian-Guo Zhou,1 Miao Pan,1 Wei Yuan,2 Bo Gao1
1Department of Pharmacy, General Hospital of Yangquan Coal Industry Group, Yangquan, People’s Republic of China; 2Department of Oncology and Interventional Radiology, Yangquan Hospital of Shanxi Medical University, Yangquan, People’s Republic of China
Correspondence: Wei Yuan, Department of Oncology and Interventional Radiology, Yangquan Hospital of Shanxi Medical University, No. 218 of North Street, Mining District, Yangquan, 045000, People’s Republic of China, Tel/Fax +86 131 6212 9135, Email [email protected] Bo Gao, Department of Pharmacy, General Hospital of Yangquan Coal Industry Group, Yangquan, People’s Republic of China, Tel/Fax +86 139 3539 8859, Email [email protected]
Objective: Through analyzing the characteristics and influencing factors of adverse drug reactions/adverse events (ADR/ADE) in a hospital to promote rational drug use in the clinic.
Methods: A total of 1221 ADR/ADE reports collected from a hospital in 2022 were retrieved through the National Adverse Drug Reaction Monitoring Center. The effective reports were screened according to the Guiding Principles for Collection and Reporting of Individual Adverse Drug Reactions, and classified the standardized drugs. The systems/organs and main clinical symptoms affected by ADR/ADE were classified according to the WHO Glossary of Adverse Drug Reaction Terms. The severity, age and gender, occupational distribution, drug category, route of administration, drug dosage form, system/organ involved, and main clinical symptoms of ADR/ADE reports were analyzed.
Results: Among 1221 ADR/ADE reports, 890 cases (75.27%) reported by doctors; 144 cases (11.79%) were serious; Precisely 49.22% of ADR/ADE occurred in patients aged 51 to 70 years old; The highest incidence of adverse reactions was 636 cases (52.09%) by intravenous infusion, 406 cases (33.25%) by oral administration. The top categories of reported cases were anti-infective drugs (29.40%) and anti-tumor drugs (27.52%); Systems/organs involved in ADR/ADE were mainly the skin and its accessories (24.96%) and blood system (21.35%). 166 cases were cured, 893 cases were symptomatic, 160 cases were unknown, and 2 cases had sequelae.
Conclusion: The occurrence of ADR/ADE is related to many influencing factors such as age, drug categories, and route of administration. Therefore, it is recommended that hospitals strengthen the monitoring of ADR/ADE, especially the elderly, anti-infective drugs and intravenous administration.
Keywords: adverse drug reactions, adverse events, report analysis, the rational use of drugs
Introduction
Adverse drug reactions (ADR) have been defined as an unintended, harmful response to a drug which occurs at doses normally used in humans for prophylaxis, diagnosis, or therapy of disease, or for the modification of physiological function.1 Following an ADR, the overall treatment effectiveness for a patient’s disease can be negatively impacted, potentially raising treatment costs, and increasing patient risk during the course of medication. Adverse drug events (ADE), however, encompass a broader range of harmful outcomes from the use of a drug. ADEs include ADRs as well as events due to treatment failures and medication errors. Notably, ADEs can be further divided into preventable ADEs, those that arise from medication errors, and non-preventable ADEs, for which the term ADR is sometimes exclusively used. This use of terminology, however, can often lead to inconsistency in how ADEs and ADRs are understood and discussed.
Reporting of ADR/ADEs is crucial in maintaining and enhancing the safety profile of drugs, as it is not intended to monitor the efficacy of drugs, but to observe and document any potential risks and adverse reactions. It represents an essential tool in guiding the safety of clinical medication, aiding the science of assessing and monitoring the risk/benefit profiles of medications throughout their lifecycle.2 The risk associated with drug use can be significantly reduced by analyzing relevant reporting data, exploring factors influencing ADR/ADE occurrences, and creating and implementing a corresponding clinical medication plan based on these factors.
In an era of continual drug research development, a multitude of medication categories often exist for the same disease. Hence, the analysis of ADR/ADE reports can guide future treatment strategies for the disease. In this research, we aim to provide an in-depth analysis of the ADR/ADE reports and rational drug use in a hospital in Shanxi Province. In this study, we analyze 1221 ADR/ADE reports from a Shanxi hospital in 2022 to understand their characteristics and influence factors, promoting more rational drug use in clinical settings.
Information and Methods
Study Design
This study is a secondary analysis of the National Adverse Drug Reaction Monitoring Platform from January 2022 to December 2022. A total of 1221 ADR/ADE reports were collected from a 500-bed public hospital in Yangquan, an urban area. This hospital utilizes Electronic Health Records (EHR) and the reports include both inpatients and outpatients. The effective reports were screened, and the standardized drugs were classified according to the Guiding Principles for Collection and Reporting of Individual Adverse Drug Reactions. The systems/organs and main clinical symptoms affected by ADR/ADE were classified according to the WHO Glossary of Terms for Adverse Drug Reactions.3
The hospital is required to report ADR/ADEs to the National Adverse Drug Reaction Monitoring Platform. Reports are typically submitted through the electronic health record system. The platform data is regularly used by the hospital for quality improvement and patient safety interventions.
Statistical Projects and Methods
This study use descriptive and retrospective analysis methods. Excel software was used for data collation. SPSS 22.0 software was used to descriptive statistics on ADR/ADE results, age and sex, reporter’s occupation, drug class, route of administration, affected system/organ and main clinical symptoms. The data retrieval was performed by the researchers with a background in pharmacology, and each report was reviewed by more than one person.
Observation Indicators and Evaluation Criteria
Severity
Severity was classified according to the National ADR monitoring platform categories: general, severe, new general, and new severe. The severity of adverse drug reactions are defined as follows:
- General: mild reactions or illnesses with symptoms that do not require treatment;
- Severe: obvious adverse reaction symptoms, serious damage to organs and system functions in the body;
- New general: mild adverse reaction symptoms, and no significant impact on vital organs or system function;
- New Severe: severe damage to vital organs or system function, resulting in disability or shortening or life-threatening.4
Preventability of the ADR/ADE was also evaluated based on the criteria: preventable, possibly preventable, probably preventable, and not preventable.
Drug Categories
The National ADR monitoring platform predetermined the drug categories, which include anti-infective drugs, antineoplastic drugs, cardiovascular drugs, central nervous system drugs, respiratory drugs, endocrine system drugs, blood and hematopoietic system drugs, and others.
Route of Administration/Pharmaceutical Formulation
Routes of administration include oral administration, intravenous drip, intravenous injection, subcutaneous injection, intramuscular injection and intrapump injection. The pharmaceutical formulation include injection, tablet, capsule, granule, atomized solution, suppository, etc.
System/Organ Involved
Affected systems and organs include the skin and appendages, hematologic, respiratory, gastrointestinal, hepatobiliary, systemic reactions, cardiovascular, metabolic and endocrine systems, nervous system, circulatory and urinary systems, psychiatric disorders, and visual impairment.
ADR/ADE Causality Determination
The causal relationship between drugs and clinical adverse events (including laboratory abnormalities, or “events”) was assessed according to the National ADR monitoring platform’s levels: definite correlation, likely correlation, possible correlation, possible uncorrelation, to be evaluated, and inability to evaluate.5 The same causality categories were used throughout the study to ensure consistency.
Results
Overview of ADR/ADE Reports
This study analyzed 1221 ADR/ADE cases. The majority were general (1048 cases, 85.83%), while 144 cases (11.79%) were serious, and 29 cases (2.38%) were newly general. Reports were primarily submitted by certified doctors (890 cases, 72.89%), with a smaller number submitted by pharmacists (320 cases, 26.21%), and only 11 cases (0.9%) reported by primary nurses (Table 1).
 |
Table 1 Overview of ADR/ADE Reports |
Age and Gender Distribution of ADR/ADE
Gender distribution of ADR/ADE reports showed near parity between females (573 cases, 46.93%) and males (648 cases, 53.07%). The most affected age group was between 51–70 years (49.22%) (Table 2).
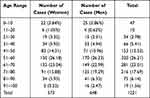 |
Table 2 Age and Gender Distribution of ADRs/ADEs |
Route of Administration of ADr/ADE and Main Drug Species
Among total ADR/ADE reports, 16 routes of administration were involved, with the drugs that were most commonly related to intravenous infusion (52.09%) and oral administration (33.25%). The distribution of the routes of administration that triggered the ADR/ADE are presented in Table 3. Among the 17 pharmaceutical dosage forms involved, The dosage forms that more often were involved in serious ADRs were injection (62.73%) and tablet (26.86%), as shown in Table 4.
 |
Table 3 ADR/ADE Route of Administration |
 |
Table 4 Pharmaceutical Dosage Forms |
327 drugs were covered in the report. The top five drugs were anti-infective drugs (359 cases, 29.40%), anti-tumor drugs (336 cases, 27.52%), cardiovascular drugs (79 cases, 6.47%), central nervous system drugs (75 cases, 6.14%) and respiratory system drugs (57 cases, 4.67%), as shown in Figure 1.
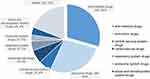 |
Figure 1 Relate to classification of drug categories. |
Major System/Organ Involvement and Clinical Manifestations of ADR/ADE
In the 1221 reports, the cumulative number of ADRs/ADEs (1274) was higher than the actual number of ADR/ADE reports (1221) because some ADRs/ADEs involve multiple organs/systems. Among them, the organs/systems affected by ADR/ADE were mainly the skin and its accessories (24.96%), with the main clinical manifestations of rash, pruritus, and drug rash. The hematological system was next (21.35%), with clinical manifestations of bone marrow suppression, leukopenia, and thrombocytopenia, as shown in Table 5.
 |
Table 5 Major System, Organ and Clinical Manifestations of ADR/ADE |
Causality Evaluation
Causality of adverse drug reactions (ADR/ADE) is a routine procedure for pharmacovigilance, in that it is used to evaluate drug safety parameters and the correlation and possibility between drug use and the occurrence of ADR/ADE. The causal relationship evaluation results of the reporters in this hospital were “likely correlation” (858 cases, 70.27%), “possible correlation” (253 cases, 20.72%) and “definite correlation” (110 cases, 9.01%), as shown in Table 6.
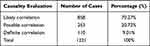 |
Table 6 Causality Evaluation |
Management and Outcomes
ADR/ADE outcomes generally include recovery, improvement, no improvement, sequelae, and death.Out of 1221 patients, 166 (13.6%) fully recovered, 893 (73.14%) showed symptom improvement, the condition of 160 patients (13.1%) remained unknown, and two patients (0.16%) experienced sequelae following dose reduction, discontinuation of the suspected drug, or specific clinical treatments, as shown in Figure 2.
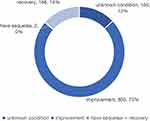 |
Figure 2 Outcome evaluation. |
Analysis of Difference in Severity and Outcome of ADR/ADE
There was no significant difference in ADR/ADE outcomes across different age groups (p>0.05). However, age appeared to significantly influence the severity of the adverse reaction (p<0.05) (Table 7).
 |
Table 7 Age for Adverse Reaction Status, Difference Analysis of Adverse Reaction Results |
Discussion
As the listing rate of new drugs increases year by year, it is important to strengthen drug safety management and improve the level of rational administration of drug. The Notice on Further Strengthening Drug Safety Management and Promoting Rational Use of Drugs published by the National Health and Wellness Commission emphasizes that ADR/ADE monitoring reports and analysis should be strengthened, and actively respond to ADR/ADE.6 Medical institutions carefully analyze ADR/ADE reports and monitoring data will be beneficial for further safeguarding and safeguarding medical quality and safety, as well as people’s health rights and interests.Prior literature has also stressed the importance of improving the ADR/ADE monitoring and reporting systems, which aligns with our study and reflects the general consensus in the field.7
Severity Analysis of ADR/ADE
In this study, more than 70% of the ADR/ADE reports from medical institutions were reported by physicians. It might be related to the fact that physicians had more contact with patients and were easy to obtain the first time feedback information about adverse reactions from patients. Our results are in accordance with previous literature that found physicians to be the major reporters of ADR/ADE.8
In 85.83% of total patients, the severity level was average. It indicated that the adverse reactions caused by drug application in this medical institution were mild and not to need therapy. However, 11.79% of the patients still experienced serious reactions. This aligns with previous studies highlighting a significant portion of patients who encounter severe ADR/ADE.9 It indicated that the adverse reactions in some patients seriously damaged their organ functions. In these patients, we found that the main drug categories included anti-infection, anti-tumor, cardiovascular system treatment and central nervous system treatment, indicating that the monitoring of these drug categories should be continuously strengthened in clinical practice.
Gender and Age
ADR/ADE is a significant and increasingly serious global healthcare issue. Older age and female gender are significant predictors of ADR/ADE.10 In this study, the reported proportion of women to men was almost the same, indicating there was no significant correlation between ADR/ADE and the gender. It is inconsistent with that reported in the literature, a published research found more women with ADR/ADE than men.11,12 From the age distribution, the incidence of ADR/ADE in patients over 50 years old reached 74.61%. Nearly half of the patients are over 60 years old, which is significantly higher than that in patients of other age groups. However, age also affected the severity of ADR/ADE, and 79.86% severe ADR/ADE occurred in patients over the age of 50. It may be related to the organ dysfunction in elderly patients, especially the liver and kidney dysfunction, which affects the metabolism and excretion of drugs.13 In addition, elderly patients often suffer from multiple chronic diseases and need concomitant medications, further increasing the incidence and severity of ADR/ADE.14
Previous studies have shown that the implementation of medication reconciliation15 and comprehensive assessment of older age16 can reduce potential ADR/ADE. Therefore, the characteristics of elderly patients should be fully considered in the clinical medication, and they need reasonable drug formulation and dosage to reduce the occurrence of adverse reactions.
Route of Administration and Formulation
According to the data of this study, injection was the main formulation causing ADR/ADE (62.73%), and intravenous administration was also the main route causing ADR/ADE (55.28%), which was also consistent with previous reports.17 The reasons for this result may include: there was no first-pass effect when the drugs were directly injected into the blood by intravenous administration; the drugs had a rapid onset; the pH value; osmotic pressure and endotoxin of the injection were induced; the concentration of the drug was high by intravenous administration; the drug was given quickly; large injection administration base for hospitalized patients.17,18 Therefore, the WHO principle of “being able to take orally without injection and intramuscular without intravenous injection” should be advocated in clinic to reduce unnecessary intravenous administration. At the same time, hospitals should provide relevant education on intravenous medication, and strictly control the infusion speed to reduce ADR/ADE.
Drug Category
The results of this study showed that among the 1221 patients, there were more ADR/ADE cases caused by anti-infective drugs and anti-tumor drugs. It is consistent with that reported in the literature, the main drug category is anti-infective drugs.19–21 There are many reports of anti-infective drugs, which may be related to the large number of patients and the non-standardized drugs use (such as no correction of medication, excessive preventive medication, combination therapy, and long-term treatment).22 The most common clinical symptom caused by anti-infective drugs is allergic reaction due to skin and accessory damage, and the incidence of serious adverse reactions is low. The application of anti-tumor drugs often leads to severe bone marrow suppression. Therefore, active and reasonable prevention and intervention measures should be taken clinically to avoid the severe ADR/ADE.
Systems/Organs and Clinical Manifestations
According to data analysis, the top three systems/organs affected of total patients were skin and its accessories damage, blood system damage, and gastrointestinal tract damage. The blood system is mainly characterized by severe ADR/ADE such as bone marrow suppression. Due to the lack of external symptoms, blood system damage should be judged based on clinical experience and examination results. Therefore, blood system damage occurred for a long time and had a high severity. Skin and its accessories damage was mainly caused by rash and pruritus. Moreover, the common clinical manifestations of skin and its accessories damage are easy to observe and judge, and it is not easy to fail to report. After timely drug withdrawal or symptomatic treatment, it is not easy to cause serious consequences. Gastrointestinal tract injury is mainly characterized by nausea, vomiting and diarrhea, with average severity. It is easy to handle in clinical practice and usually not causing serious consequences.
Outcomes and Causality Analysis
Among the 1221 ADR/ADE reports, 1059 cases (86.74%) were cured or improved, indicating that most of the ADR/ADE could be cured or improved after drug discontinuation or treatment. From the evaluation of causal relationship, the proportion of drug safety positively or possibly related to ADR/ADE accounted for 79.28%, and the rest were possibly related without “unrelated” patients. Therefore, we should pay more attention to the reporting of ADR/ADE, and strengthen the monitoring and early warning of serious adverse reactions to promote clinical rational use of drugs.
While the limitations of this study are primarily rooted in the single-center study design, which may not reflect the situation in other medical institutions, our study has also its own unique strengths. The strength of our study lies in its comprehensive analysis of ADR/ADE reports from a well-established medical institution, providing valuable insights into the local landscape of drug administration. It underscores the need for further multicenter, cross-regional studies to expand on our findings and better understand the severity and occurrence of ADR/ADE in different populations and under varying circumstances.
Conclusion
The occurrence of ADR/ADE is related to many influencing factors such as age, drug categories, and route of administration. Older patients aged >50 years are the population with high incidence of ADR/ADE, and intravenous administration is the most important route of drug administration causing ADR/ADE. Attention should be payed to anti-infective drugs and anti-tumor drugs. Previous investigations have shown that establish an ADR/ADE management system and to regularly monitor indicators then maintain the quality of ADR/ADE processes may improve patient medication safety.23 Strengthening supervision and management to promote safe and rational drug use.
Ethics Approval
This study was conducted in accordance with the declaration of Helsinki. This study was conducted with approval from the Ethics Committee of Yangquan Hospital of Shanxi Medical University. Written informed consent was obtained from all participants/local guardians. No identifiable participant information (such as patients’ images, faces, or names) was disclosed in the study.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by Scientific and Technologial Innovation Programs of Higher Education Institutions in Shanxi and Shanxi Provincial Education Department (No. 2022L239).
Disclosure
All of the authors had no any personal, financial, commercial, or academic conflicts of interest separately.
References
1. Morales-Ríos O, Cicero-Oneto C, García-Ruiz C, et al. Descriptive study of adverse drug reactions in a tertiary care pediatric hospital in México from 2014 to 2017. PLoS One. 2020;15(3):e0230576. doi:10.1371/journal.pone.0230576
2. Bailey C, Peddie D, Wickham ME, et al. Adverse drug event reporting systems: a systematic review. Br J Clin Pharmacol. 2016;82(1):17–29. doi:10.1111/bcp.12944
3. National Center for Adverse Drug Reactions, National Food and Drug Administration Drug Evaluation Center. WHO Glossary of Adverse Drug Reaction Terminology [M]. Beijing: China Medical Science and Technology Press; 2002:1–172.
4. Ju YF. Analysis of 186 adverse drug reaction reports in our hospital. Strait Pharma J. 2021;33(6):205–207. doi:10.3969/j.issn.1006-3765.2021.06.084
5. Huang Q, Wen ZH. An initiative to establish coordinated and consistent causality assessment criteria for adverse drug reaction in clinical trials. Chin J New Drugs. 2021;30(12):1132–1136. doi:10.3969/j.issn.1003-3734.2021.12.013
6. Dong C. National Health Commission: strengthen monitoring and strictly prevent adverse drug reactions [N]. Baojianshibao. 2022.
7. Khan LM. Comparative epidemiology of hospital-acquired adverse drug reactions in adults and children and their impact on cost and hospital stay--a systematic review. Eur J Clin Pharmacol. 2013;69(12):1985–1996. doi:10.1007/s00228-013-1563-z
8. van Grootheest AC, de Jong-van den Berg LT. The role of hospital and community pharmacists in pharmacovigilance. Res Social Adm Pharm. 2005;1(1):126–133. doi:10.1016/j.sapharm.2004.12.009
9. Li Q, Zhang SM, Chen HT, et al. Awareness and attitudes of healthcare professionals in Wuhan, China to the reporting of adverse drug reactions. Chin Med J. 2004;117(6):856–861.
10. Lavan AH, O’Mahony D, Gallagher P, et al. The effect of SENATOR (Software ENgine for the Assessment and optimisation of drug and non-drug Therapy in Older peRsons) on incident adverse drug reactions (ADRs) in an older hospital cohort - Trial Protocol. BMC Geriatr. 2019;19(1):40. doi:10.1186/s12877-019-1047-9
11. Scripcaru G, Mateus C, Nunes C. Adverse drug events-analysis of a decade. A Portuguese case-study, from 2004 to 2013 using hospital database. PLoS One. 2017;12(6):e0178626. doi:10.1371/journal.pone.0178626
12. Miguel A, Azevedo LF, Araújo M, et al. Frequency of adverse drug reactions in hospitalized patients: a systematic review and meta-analysis. Pharmacoepidemiol Drug Saf. 2012;21(11):1139–1154. doi:10.1002/pds.3309
13. Ding X, Sun MZ, Wen W. Physiological characteristics of the elderly and issues that should be noted in medication. Nei Mongol J Trad Chin Med. 2012;31(22):47. doi:10.3969/j.issn.1006-0979.2012.22.051
14. Lei X. Reflections on the issue of multiple medication use in the elderly and suggestions for medication strategies for the elderly. Electron J Clin Med Literature. 2019;6(69):197.
15. Lavan A, Eustace J, Dahly D, et al. Incident adverse drug reactions in geriatric inpatients: a multicentred observational study. Ther Adv Drug Saf. 2018;9(1):13–23. doi:10.1177/2042098617736191
16. Yourman L, Concato J, Agostini JV. Use of computer decision support interventions to improve medication prescribing in older adults: a systematic review. Am J Geriatr Pharmacother. 2008;6(2):119–129. doi:10.1016/j.amjopharm.2008.06.001
17. Xiao XC. Analysis of adverse drug reaction reports of a hospital from 2018 to 2021. Chin J Clin Rational Drug Use. 2022;15(30):6–9. doi:10.15887/j.cnki.13-1389/r.2022.30.002
18. Du XL, He KG. Analysis of rules and characteristics of 1716 adverse drug reactions from 2019 to 2021. China Prac Med. 2022;17(23):179–184. doi:10.14163/j.cnki.11-5547/r.2022.23.056
19. Poudel DR, Acharya P, Ghimire S, et al. Burden of hospitalizations related to adverse drug events in the USA: a retrospective analysis from large inpatient database. Pharmacoepidemiol Drug Saf. 2017;26(6):635–641. doi:10.1002/pds.4184
20. Parikh S, Christensen D, Stuchbery P, et al. Exploring in-hospital adverse drug events using ICD-10 codes. Aust Health Rev. 2014;38(4):454–460. doi:10.1071/AH13166
21. Silva LT, Modesto ACF, Oliveira RA, et al. Hospitalizations and adverse drug events in the Brazilian unified health system: a ten-year retrospective analysis of routine data. Rev Saude Publica. 2022;56:86. doi:10.11606/s1518-8787.2022056003913
22. Liu Y, Zhu JX, Yu ML, et al. Analysis of 1728 ADR reports in the first affiliated hospital of Bengbu Medical College. China Pharma Affairs. 2013;27(12):1333–1336.
23. Aung AK, Walker S, Khu YL, et al. Adverse drug reaction management in hospital settings: review on practice variations, quality indicators and education focus. Eur J Clin Pharmacol. 2022;78(5):781–791. doi:10.1007/s00228-022-03287-1
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
