Back to Journals » Clinical Ophthalmology » Volume 14
Analysis and Causation of All Inaccurate Outcomes After WaveLight Contoura LASIK with LYRA Protocol
Authors Motwani M
Received 19 June 2020
Accepted for publication 3 September 2020
Published 13 November 2020 Volume 2020:14 Pages 3841—3854
DOI https://doi.org/10.2147/OPTH.S267091
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Manoj Motwani
Motwani LASIK Institute, San Diego, CA 92121, USA
Correspondence: Manoj Motwani
Motwani LASIK Institute, 4520 Executive Dr., Suite 230, San Diego, CA 92121, USA
Tel +1 (858) 554-0008
Email [email protected]
Purpose: This study analyzes every eye that had an outcome greater than 0.25D of sphere or astigmatism from planned goal after treatment with WaveLight Contoura with LYRA Protocol.
Methods: The study included 266 consecutive eyes treated with LASIK Contoura using the LYRA Protocol. All LASIK procedures were performed on the WaveLight EX500 excimer laser. Flaps were created with either the Alcon WaveLight FS200 femtosecond laser or the Moria M2 microkeratome. Eyes that were off by > 0.25 diopters (D) sphere or cylinder from the targeted goal within 3 months after surgery were identified and analyzed for cause. Topographical, higher-order aberration, and epithelial maps were created.
Results: Causes for inaccurate outcomes were biomechanical corneal change from LASK flap creation (9.78% of total eyes), pre-operative epithelial compensation of corneal higher-order aberration (4.1% of total eyes), changes to lamellar corneal tension from laser ablation causing a hyperopic shift (1.9% of total eyes), epithelial thickening over the ablation area post-operatively causing a refractive change (1.5% of total eyes), and posterior astigmatism (0.75%).
Conclusion: The causes of the majority of inaccurate outcomes have not been properly defined and must be incorporated into further improving outcomes. Current and planned advances in technology do not address the majority of these causes.
Keywords: astigmatism, corneal epithelium, femtosecond laser, higher-order aberration, LASIK, refractive error; Contoura; topography guided ablation; LYRA protocol
Introduction
Although for some years now leading refractive surgeons have been discussing and encouraging the need for better refractive outcomes, there has been little published trying to discern the exact causes of inaccurate outcomes. The prevailing thought in the refractive research and device manufacturing community is to use the Gullstrand model, in other words to treat the eye as a fixed entity wherever more technology and refinement can lead to hyper-accurate outcomes. Nomogram analysis software systems such as IBRA and Surgivision Datalink attempt to refine the procedure with the underlying assumption that the vast majority, if not all, corneas will react essentially the same to a procedure. The admitted goal of Alcon clinical experts is a system that has under a 2% enhancement rate, and well-respected surgeons who are clinical consultants of Alcon quote enhancement rates of under 1% with Wavefront Optimized Ablation (WFO) on the WaveLight EX500 laser.
Enhancement decisions vary from center to center. Some are based on the vision, some based on the refraction and patient complaints, and the fact that patient populations/types of corrections (myopic vs hyperopic, etc.) performed vary from center to center. There is no set standard for reporting for enhancements, except perhaps achievement of a certain level of vision. This is highly inexact, as patients with significant refractive errors can still manage to get 20/20 or 20/25 on the chart but have poor quality of vision. Patients will still complain of blurred vision, doubled vision, night glare, halos, and other visual artifacts.
Furthermore, this issue is exacerbated by surgeons not performing topographies or refractions post-operatively, and only taking visions as a standard of success. Visions themselves are inaccurate, as many centers have uncalibrated chart projectors, and are subject to issues such as dry eyes, allergic inflammation, patient fatigue, etc. At our center, we have never achieved an enhancement rate anywhere as low as 1 or 2% with any system or procedure used, or even with any particular patient population studied. For example, at our center when we looked at a large population of patients treated with WFO (Wavefront Optimized) via LASIK performed on the WaveLight EX500 we had an enhancement rate of about 10%. This enhancement rate is far greater than the 2% or less reported by some surgeons, but we perform enhancements for small corrections based on patient complaints and measurable deviation from goal of 0.50D or more from target and not just achieved vision.
Historically, most attempts to improve refractive outcomes have mainly been through technology. Software systems such as the Phorcides Analytic Engine and new technologies such as Ray Tracing also assume a fixed model that simply does not change, ie, the eye at the time of scanning and analysis will remain the same fixed shape after a refractive procedure, and only affected by the procedure itself.1 Since there is no way to predict further changes over time due to aging processes, those factors are simply not included.
When we embarked on studying epithelial compensation for higher-order aberration utilizing the new OCT epithelial thickness mapping technology by Optovue, we were attempting to confirm changes we saw on post-operative topographies over the first few days to weeks after topographic guided ablation with the LYRA Protocol. This attempt to understand inaccurate outcomes led to further study of a large group of consecutive eyes that had such a treatment performed and fit within the FDA approved treatment range of Contoura. To remove the subjective nature to the refractive outcomes and enhancement rates, we created a different standard based on the fact that no patients reported visual complaints with refractions less than or equal to 0.25D of sphere or astigmatism. We noted that although Snellen vision was important, we had patients with refractive errors and 20/15 vision that would complain, while patients with 20/20 vision and no refractive error that were completely happy. Thus, we retrospectively analyzed every single eye of a large cohort that had LASIK performed with Contoura with LYRA Protocol, and an outcome that was off by greater than 0.25D of sphere or astigmatism, essentially greater than or equal to 0.5D, in an attempt to discern the reasons for the inaccurate refractive outcomes in an attempt to guide attempts to improve accuracy.
Stemming from the results of this analysis are two separate manuscripts, where the authors describe epithelial compensation for corneal higher-order aberrations, as well as a biomechanical change upon flap creation in certain types of corneas. These manuscripts have been submitted separately for publication to examine these topics in greater detail. This retrospective study reports on the overall analysis and different reasons for inaccurate outcomes at 3 or more months post-operatively in the overall cohort of 266 eyes.
Patients and Methods
We undertook a retrospective study examining 266 consecutive primary eyes treated with primary LASIK via Contoura using the LYRA Protocol which utilizes the Contoura Measured Astigmatism and treats the anterior elevation of the cornea.2–4 All eyes were within the FDA parameters of 8 diopters of myopia and 3 diopters of astigmatism (9 diopters spherical equivalent total) of measured total correction after using the LYRA Protocol. LYRA Protocol treats the full magnitude of the Contoura measured astigmatism with the sphere adjusted for the spherical equivalence of the difference between the manifest refraction and the Contoura measured astigmatism. In other words, each treatment was required to fit on one Contoura procedure card. This is the preferred and almost exclusive methodology of treatment for myopic/myopic astigmatism eyes in our center.
Every outcome that deviated from plano by greater than 0.25D (equal to 0.50D or more) of astigmatism or sphere either initially, or via “regression” over the ensuing 3 months, was analyzed to determine the cause. Each patient was required to have at least 3 months of post-operative follow up. Visions and manifest refractions were obtained at all post-operative follow up visits, and topographies and OCT maps were also performed on all visits except the initial post-op day 1 visits. All pre-operative manifest refraction were performed by the surgeon with tropicamide dilation to rule out accommodative inaccuracy. All visions were taken on projected Snellen charts utilizing Topcon ACP-8 projectors that had been calibrated utilizing the included chart. Manifest refractions were performed on every post-operative visit by one of 3 refracting technicians, and any refractions that showed significant deviation from planned goal or in cases of patient visual complaints were confirmed by the operating surgeon (Manoj Motwani, MD; MM) Final pre-operative cycloplegic manifest refractions were performed by the operating surgeon, and all Contoura surgical planning was performed by the surgeon as well. Treatment of ancillary issues such as dry eye and allergic inflammation/conjunctivitis was also performed to prevent incorrect diagnosis of inaccurate outcome. The 3-month time minimum post-operative follow up was used to ensure a reasonable period of time for the epithelium to re-normalize to the new anterior corneal shape. All patients had follow up visits 1 day, 1 week, 1 month, 3 months, 6 months, and 1 year post-operatively.
All LASIK procedures were performed on the WaveLight EX500 excimer laser with flaps being made with either the Alcon WaveLight FS200 femtosecond laser or the Moria M2 microkeratome with Microspecialties blades. As we have noted in prior studies, we have found no correlation in outcomes between the two flap making devices. In our experience, they do not affect the visual or refractive outcome of the patient.
All corneal elevation maps were obtained on the Contoura planning stations by zeroing the sphere and inputting the Contoura measured astigmatism and axis. All topographies were obtained utilizing the Topolyzer Vario (Alcon Surgical, Fort Worth, TX). All epithelial maps (ETM) were obtained with the Optovue iVue (6 mm ETM) or Avanti (9 mm ETM) devices (Optovue, Fremont, CA). The Pentacam (Oculus, Wetzlar, Germany) was used to analyze pachymetry maps and rule out corneal ectasia, and examine corneal elevations.
Patients were excluded if they could not achieve 20/20 vision before surgery, had prior refractive surgery, or were not within the approved treatment parameters for the instrument. Anterior segment abnormalities such as corneal ectasia or keratoconus, recurring eye disease such as severe dry eye, uncontrolled diabetes or hypertension, and pregnancy also disqualified patients from participating in the study. One patient was excluded due to corneal flap striae in both eyes due to patient non-compliance post-operatively. We also excluded one patient with very uncommon scenario of epithelial ingrowth after primary LASIK procedure.
All patients signed written informed consent forms allowing their data to be used in this study. This study falls under the exemption of the Health and Human Services (HHS) Policy for the Protection of Human Research Subjects 45 CFR 46.101 (b) for retrospective studies, and thus, no Institutional Review Board approval was required. This study also conforms to the Declaration of Helsinki guidelines. There were no safety-related incidents that occurred or were reported to Alcon Inc. or WaveLight during this trial.
Results
This study retrospectively examined a consecutive group of eyes of which 266 eyes had at least 3 month post-operative data for vision outcomes, topographic analysis, epithelial thickness analysis, and refractive error. We analyzed eyes that had >0.25D of refractive error, in other words equal to or greater than 0.5D to determine the cause of inaccuracy from targeted outcome. Table 1 gives a breakdown of the demographics and range of corrections in the 266 eye cohort.
 |
Table 1 Demographics |
Average laser treatment (utilizing LYRA Protocol) was: Sphere: −3.12D, range 0–8D; Cylinder: −1.06D, range 0–3D at average axis 88.14D.
Deviation from manifest to Contoura measured astigmatism was −0.53D, range 0 to −1.69DD. Average deviation of axis was 6 degrees, range of 0–89 degrees.
Analysis showed inaccurate outcomes of greater than or equal to 0.5D (>0.25D) of sphere or astigmatism or sphere in 48 eyes of the 266 (18%). Analysis of the data determined 5 main causes of inaccuracy, whether immediately after the procedure or “regression” within the ensuing 3 or more months after the procedure: Biomechanical corneal change from LASK flap creation, pre-operative epithelial compensation of corneal higher-order aberration changes to lamellar corneal tension from laser ablation causing a hyperopic shift, epithelial thickening over the ablation area post-operatively causing a refractive change, and posterior astigmatism. These causes were determined through extensive analysis of manifest refraction of refractive error post-operatively and change over time, topographic changes, epithelial thickness changes via OCT mapping, and also visual complaints which we attempted to correlate to finding from the objective data. The 5 groups were not pre-determined prior to analysis, we were able to place the inaccurate outcomes into these 5 groups after extensive analysis of all the objective data. Table 2 shows the type and magnitude of residual refractive errors for each of the 5 categories.
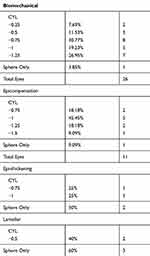 |
Table 2 The Type and Magnitude of Residual Refractive Errors for Each of the 5 Categories |
The average age of patients of these patients was 37.3 years, range 21–56 years.
Biomechanical Corneal Change from LASIK Flap Creation
Twenty-six eyes of 17 patients, 9.78% of total eyes and 54.2% of inaccurate outcomes
Average amount of inaccurate cylinder magnitude: −0.81D; range −0.75 to −1.25
Average Axis of cylinder: 85.7 degrees; range 64 to 120 degrees
Three eyes with spherical inaccurate outcomes of +0.75, +0.75, and −0.50
This occurred in relatively thick (>550 micron at thinnest point) corneas with a central thinnest point that was significantly lateral to the corneal apex (usually 2 mm or more). This biomechanical change caused a one time and immediate shift to the corneal shape resulting in a change in refractive efficiency at different ablation points in comparison to the pre-operative Topolyzer scans and Contoura planning. This would result in an irregular, elliptical ablation with epithelial thickening occurring on the OCT ETM map at the periphery of the ellipse. All of these patients, except one, had resulting astigmatism on manifest refraction along the axis of the ellipse. These patients tended to report visual complaints as early as 1 week post-operatively. There was one patient with a severe displacement of the elliptical ablation with the edge of the ellipse in the central visual area. This patient had a +0.75 refraction in one eye with no astigmatism, and had astigmatism in the other eye. The patients irregularity as well as her complaints of poor night vision, glare and doubling were severe enough that she was treated with repeat Contoura and ended up with a very uniform, round ablation as well amelioration of all of her symptoms. All other patients had secondary treatment with Wavefront Optimized ablation (WFO) with successful outcomes.
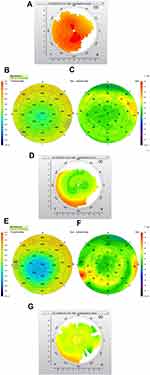
Pre-op topography and Pentacam pachymetry (Figure 1A and B):
Pre-op OCT pachymetry and epithelial thickness map (ETM) (Figure 1C and D).
Post-op topography at 5 months (Figure 1E):
5-month post-op pachymetry and epithelial thickness map (Figure 1F and G). Note the irregular epithelial thickening at the edge of the elliptical ablation.
Pre-Operative Epithelial Compensation of Corneal Higher-Order Aberration
Eleven eyes of 8 patients, 4.1% of total eyes and 22.9% of patient with inaccurate outcomes
Average amount of inaccurate cylinder magnitude: −0.79D; range −0.75 to −1.25
Average Axis of cylinder: 80.8 degrees; range 35 to 123 degrees
One eyes with spherical inaccurate outcomes of +0.75
These patients had central visual axis epithelial irregularity of greater than 2 microns, usually in an irregular pattern that was due to compensation of corneal higher-order aberration. Determination of this was via comparison of the pre-operative epithelial thickness map to the post-operative corneal elevation map on the Contoura surgical planning system (zeroing out sphere, and entering the measured astigmatism and axis to find the resulting elevation). Although there is no tracking system on the Optovue OCT device, it was still possible to see the pattern of epithelial irregularity matching residual corneal elevation to demonstrate the presence of this issue. This was also subject to re-compensation of the epithelium to the residual corneal aberration post-operatively, but it was still possible to see the patterns to determine that this was the cause.
These patients would end up with astigmatism as well as residual significant higher-order corneal aberration. Treatment for correction consisted of WFO treatment of the manifest refraction with initial targeted outcome in all cases.
Pre-op topography and post-op anterior elevation (HOA plus cylinder) on WaveLight Contoura planning demonstrating the epithelial compensation that led to the residual corneal anterior elevation (Figure 2A and B):
 |
Figure 2 (A) Pre-op pachymetry showing corneal thickness.(B) Pre-op epithelium map. – 9 mm ETM (Avanti XR). |
Lamellar Corneal Tension Change During Laser Ablation Causing a Hyperopic Shift
Five eyes of 3 patients, 1.9% of total eyes and 10.4% of inaccurate outcomes
Average amount of hyperopic shift: +1.4D; range +1.00 to +1.50
Pre-op topography (Figure 3A):
These eyes had a resultant immediate hyperopic outcome post-operatively with no evidence of incorrect ablation shape/biomechanical change, epithelial compensation of aberration, or irregular corneal thickening on ETM. In other words, these patients appear to have a normal outcome in all respects except end up with post-operative hyperopia. These patients did not show any significant astigmatism, just spherical hyperopia and an round, regular ablation pattern on post-operative topography. Each patient did not change significantly after correction over time, and were treated successfully with WFO hyperopic treatment.
Epithelial Thickening Over the Ablation Area Post-Operatively Causing Refractive Change
Four eyes of 4 patients, 1.5% of total eyes and 8.3% of inaccurate outcomes
Two eyes with hyperopic astigmatism of +0.75 and +1.00D of cylinder
One eye with −0.75D myopic shift
One eye with +0.75D hyperopic shift
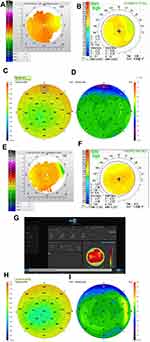
Pre-op topography (Figure 4A).
 |
Figure 4A Continued. |
Pre-op OCT pachymetry and epithelial thickness map (ETM) (Figure 4B and C).
Three-month post-op topography (Figure 4D).
Three-month post-op OCT pachymetry and epithelial thickness map (ETM) (Figure 4E and F).
Four-month post-ENH Topography (Figure 4G).
Four-month post-ENH OCT pachymetry and epithelial thickness map (ETM) (Figure 4H and I).
These patients would have regular, round, regular ablation patterns and a fairly accurate initial outcome post-operatively. Over time they displayed changes on the OCT ETM mapping showing a thickening that could be regular or irregular, and could have any form of refractive change. In the small number of cases we have seen at our center, these patients usually have irregular epithelial thickening over time and end up with some level of myopic astigmatism. This peripheral thickening can be differentiated from the biomechanical corneal change group as these patients will have normal round, regular post-operative topographies and the refractive error develops over time.
Posterior Astigmatism
Two eyes of 1 patient- 0.75% of total eyes and 4.1% of inaccurate outcomes
Manifest Cyclopleged Refraction: −0.50, −0.75 x 23
Contoura Measured Treatment (LYRA Protocol): plano, −2.01 x 14 (note this is similar to the cylinder on topo)
Residual post-operative primary correction: −0.25, −1.50 x 110
Pre-op topography and pre-op OPD (Figure 5A and 5B).
Pre-op OCT pachymetry and epithelial thickness map (ETM) (Figure 5C and D).
Post-op 4 Month Topography and OPD-note the lack of corneal astigmatism (Figure 5E and F).
Four-month post-op HOA Contoura Planning: no corneal astigmatism present (Figure 5G).
Post-op 4 month OCT pachymetry and epithelial thickness map (ETM) (Figure 5H and I).
Two eyes of 1 patient, 0.75% of total outcomes, 4.17% of the inaccurate outcomes
One patient with 2 eyes has posterior astigmatism determined by Wavefront analysis demonstrating astigmatism matching manifest astigmatism without significant corneal astigmatism on topography or Contoura processing, and no significant elevation causing astigmatism present as well. This patient was corrected with Wavefront Optimized correction which resulted in a new regular astigmatism on the cornea that cancelled out the posterior astigmatism. As an extra note, this is only one of 2 patients we have ever seen with significant posterior astigmatism at our center since 2016.
The overall group of 266 eyes had 218 yes that were on target goal or within 0.25D and without subjective complaints. Of this group of eyes, 22 were monovision eyes, leaving 196 distance eyes. Of those 196 distance eyes, 146 were 20/15 or better or 74.5%. All 22 of the monovision eyes achieved visions of J2 or better, and distance vision in the monovision eyes ranged from 20/20 to 20/50.
Discussion
Our results demonstrate that the majority of inaccurate outcome are not predictable by technology to result in more accurate outcomes. Most appear to be caused by biomechanical factors in the cornea, or by changes to the epithelium before or after the procedure. It is notable that many of the patients with inaccurate outcomes had visions that would be counted as a successful treatment- 20/15, 20/20, 20/25, and 20/30 visions were common in this group. In fact only 4 eyes had visions of 20/40 or worse in the primary outcomes resulting in a 1.5% enhancement rate if the standard of correction was 20/40 or worse. This appears to match the 2% or less goal, and the stated enhancement rates of the Alcon expert surgeons. This particular standard is an old one, dating back to the 90’s when achieving a vision that allowed one to pass a driving test was the goal. Today’s patients in our experience demand much more: they want 20/20 or better, good day and night vision without visual abnormalities or anomalies such as night glare/significant halos/starbursts/blurry or non-clear vision/ghosting/doubling, and vision that is at least as good as their contacts or glasses, or perhaps better. This is also complicated by the fact that making a more uniform anterior corneal surface as with Contoura with LYRA Protocol decreases light scatter, more easily exposing any visual to the patient.
Refractive surgery has long relied on the Gullstrand model as the basis for refractive surgery.5 The Gullstrand model is essentially fixed, and assumes that a normal cornea, absent disease or pre-existing conditions such as corneal ectasia, will maintain its shape after flap construction, and will also not react or change after laser correction. Further, strategies that try to improve accuracy of outcomes such as the Phorcides Analytic Engine or the Ray Tracing technology also rely on this lack of change and lack of any reaction such as epithelial compensation for residual aberration on a non-uniform anterior corneal surface. The main goal of these technologies is to incorporate posterior astigmatism, ie, astigmatism that is posterior to the anterior cornea, into an overall laser correction for the eye performed on the anterior corneal surface. This has been based on long standing assumptions and theories that inaccurate outcomes or differences in between manifest astigmatism and topographic astigmatism were due to posterior astigmatic causes.
In our center, we have only documented two patients with significant posterior astigmatism since 2016, and one is in this group of 266 eyes. Treatment of posterior astigmatism would not appear to increase outcome accuracy in any significant manner, and due to aging changes in the eye such as lens changes, may actually lead to inaccurate corrections over time. Furthermore, the attempt to treat posterior astigmatism/aberration may lead to an incorrect corneal higher-order aberration/elevation removal, with a resulting new anterior corneal aberration and non-uniform anterior corneal surface. This will likely also result in epithelial compensation changing the outcome, with a seemingly good outcome changing to an inaccurate outcome over time. Analyzing the results of this study, patients in the first 4 types of inaccurate outcomes had changes that were not predictable by any software or hardware technologies that are currently being utilized today.
In fact, LYRA Protocol was more accurate than expected by the authors. In the 218 eyes that had primary correction and an accurate outcome, 200 of them were for distance while 18 were for monovision. Out of these eyes in this group, 74.5% achieved 20/15 vision, with the rest achieving 20/20 vision. This is a significant number as we have noted more patients become 20/15 over 6 months plus of healing time, and we have also noticed that many patients who are doing well simply do not return for post-ops so there is a bias in any large scale study of consecutive patients towards patients that are having issues, as many of those with excellent outcomes do not take the time to return for the entire post-operative appointment schedule. Thus, the actual 20/15 number is likely a bit higher than the 74.5%.
It should be noted that the largest subgroup of inaccurate outcomes representing more than 50% could not be predicted in any way or time, except for recognizing the lateralized central thin point on pachymetry. These patients could be treated more accurately by first performing the flap creation procedure, waiting for a period of time, re-scanning the cornea for Contoura planning and then performing the ablation. An accurate way of treating these patients with one procedure would be PRK, or better yet transepithelial PRK (Streamlight, Alcon Surgical, Forth Worth, TX) which would also eliminate the chance of inaccurate outcome because of epithelial compensation of corneal higher-order aberration. This would eliminate 77% of the inaccurate outcomes, and as transepithelial PRK likely makes the most uniform anterior corneal surface possible, it may well decrease irregular epithelial thickening as well.
Epithelial thickening in the ablation bed leading to refractive change (Figure 4) can lead to different types of refractive change dependent on the shape and amount of the thickening. This represented relatively few cases, and a much larger series of patients with this issue would be able to better define the pattern of thickening with the particular refractive change. Although we have attempted to remove the epithelium to attempt to reset it back to a normal epithelial thickness in 3 patients, this works temporarily and eventually, the epithelium seems to return to the thickness and pattern that leads to a similar refractive error as compared to pre-epithelial removal. It is as of yet unknown what factors play into this, whether they are mechanical factors or other reasons to cause this thickening.
Lamellar tension change was also postulated by Dr. Cynthia Roberts.6,7 She theorized that altered postoperative from central tissue removal would a change in corneal structure.
The relaxed peripheral lamellar segments expand due to the negative fluid pressure and lack of constraining intact lamellar structure. This causes an outward force, which is transmitted to underlying layers via corneal crosslinking, causing central flattening.
This would cause the hyperopic shift, and since this is a symmetrical change, the topography does not show an irregular ablation pattern or change, nor would the epithelial thickness show a compensation for irregularity. This is what we see in the results of our eyes with a hyperopic shift.
Posterior astigmatism has been cited as a significant factor by many authors and there is a common belief by respected surgeons such as Dr. Wallerstein that treatments that focus on treating only the corneal higher and lower order aberrations to make a more uniform anterior cornea cannot possibly be correct.8 After years of constant searching, we have only found 2 patients since 2016 that had significant posterior astigmatism. One of these patients was in this group of eyes, and the results are shown in figures in the results section. Posterior astigmatism can only be a causation factor if the cornea shows no significant anterior higher-order aberrations or astigmatism on Contoura analysis, and Wavefront analysis and manifest refraction reveal significant astigmatism. Treatment by WFO will correct the patient and show a regular astigmatism on corneal topography that cancels out the posterior astigmatism.
A note on secondary treatments for inaccurate outcomes. We attempted initially in 2016 to perform secondary corrections with repeat Contoura, with very mixed results as epithelial compensation to residual corneal aberration was occurring and the outcome after secondary correction was not always accurate. We began to perform secondary treatments with WFO which works extremely well for most patients, as the corneal higher-order aberrations are already significantly reduced and the final correction adjusts the refraction with WFO. In the case of biomechanical change during flap construction, it could be argued that repeat Contoura is a better procedure, and this is a viable and perhaps even better option as the anterior corneal shape is normalized and more regular.
It has been theorized in the past that creating a uniform anterior corneal surface may well be the preferred and best method of vision correction. Jankov et al in 20069 also questioned the wisdom of
Trying to correct the anterior corneal surface using the ablation profile based on the whole eye aberrations when the anterior corneal surface aberrations are clearly preponderant.
Furthermore, it also pointed out that posterior aberrations can change with age, and postulated that recreating the natural aspheric shape and a uniform cornea might well be the ideal. Manns et al in 200210 noted that because the corneal reshaping alters the path of rays propagating in the eye, and although the lens and posterior corneal surface are unchanged after surgery, their contributions to the ocular aberrations will likely be different from pre-operatively.
Furthermore, the authors have noted that if the anterior elevation is not treated on a cornea, residual aberration may be left behind. In other words, anterior corneal aberrations may be left behind that cancel out posterior astigmatism/aberrations. Unfortunately, as we are learning via the use of OCT epithelial thickness mapping, this may create a reaction via epithelial hyperplasia that may affect the refractive outcome. We have seen even small amounts of epithelial compensation to corneal HOA (2–3 microns) can cause residual astigmatism.
In past studies, the authors have shown the high level of satisfaction with Contoura with LYRA Protocol with subjective patient surveys. In our center, such problems as halos, night glare, blurry vision, doubling of vision are no longer a major problem, but if they are present occur mainly in the first few weeks or months of healing time as the epithelium becomes more uniform after the procedure. This may also be affected by problems such as allergies or dry eyes, as any epithelial degradation seems to affect the high quality of vision with these uniform corneas.
It is unknown if the Ray Tracing procedure will end up being subject to the same issues, as it also attempts to treat aberrations at that specific time to make each incoming light ray focus on the corresponding retina. It does not account for internal structures changing with age, nor does it account for the variability of the epithelial layer. If utilized in a LASIK procedure, it also cannot compensate for the biomechanical corneal changes that occur in some patients after flap creation that lead to an inaccurate outcome.
We believe further study of these reasons for inaccurate outcomes is warranted, and solutions such as transepithelial PRK will hopefully be approved for use in the United States. We have the unique opportunity at this time to use technology to not only correct patients and eliminate the need for glasses or contacts, but to make vision better than could be obtained with these implements. These results demonstrate that it is necessary to further understand the cause of inaccurate outcomes to further develop solutions to better outcomes. Furthermore, with the technological ability to increase the quality of vision using only the post-operative vision as a measure of success does not tell the complete story. The refraction, topographic analysis an subjective patient complaints must also be considered. It is notable that the majority of patients that we defined as having an inaccurate outcome had 20/15, 20/20, and 20/25 visual results. These would be considered a successful result by vision, but these patients are not happy patients until the residual refraction is treated.
Acknowledgment
The author would like to thank Julie Crider, PhD for editorial contributions, Sissimos Lemonis of Alcon, Inc for data analysis and conceptual inspiration, as well as Guillermo Lizano and Brigitte Ordway for data collection and organization.
Disclosure
Dr. Motwani has received a grant from Alcon for a previous unrelated study in 2017. Dr. Motwani received non-financial support from Optovue, Inc. (loan of Optovue Avanti, clinical support in understanding analysis of data). Dr. Motwani has a patent pending on a theoretical device that could combine corneal HOA data and epithelial thickness data to achieve a more accurate refractive outcome. The author reports no other potential conflicts of interest for this work.
References
1. Cummings AB, Kelly GE. Optical ray tracing-guided myopic laser in situ keratomileusis: 1-year clinical outcomes. Clin Ophthalmol. 2013;7:1181–1191. doi:10.2147/OPTH.S44720
2. Motwani M. The use of WaveLight® contoura to create a uniform cornea: the LYRA protocol. Part 3: the results of 50 treated eyes. Clin Ophthalmol. 2017;11:915–921. doi:10.2147/OPTH.S133841
3. Motwani M. The use of WaveLight® contoura to create a uniform cornea: the LYRA protocol. Part 2: the consequences of treating astigmatism on an incorrect axis via excimer laser. Clin Ophthalmol. 2017;11:907–913. doi:10.2147/OPTH.S133840
4. Motwani M. The use of WaveLight® contoura to create a uniform cornea: the LYRA protocol. Part 1: the effect of higher-order corneal aberrations on refractive astigmatism. Clin Ophthalmol. 2017;11:897–905. doi:10.2147/OPTH.S133839
5. Atchison DA. Optical models for human myopic eyes. Vision Res. 2006;46(14):2236–2250. doi:10.1016/j.visres.2006.01.004
6. Roberts C. Biomechanics of the cornea and wavefront-guided laser refractive surgery. J Refract Surg. 2002;18(5):S589–592.
7. Roberts C. Biomechanical customization: the next generation of laser refractive surgery. J Cataract Refract Surg. 2005;31(1):2–5.
8. Wallerstein A, Gauvin M, Cohen M. WaveLight((R)) contoura topography-guided planning: contribution of anterior corneal higher-order aberrations and posterior corneal astigmatism to manifest refractive astigmatism. Clin Ophthalmol. 2018;12:1423–1426. doi:10.2147/OPTH.S169812
9. Jankov MR
10. Manns F, Ho A, Parel JM, Culbertson W. Ablation profiles for wavefront-guided correction of myopia and primary spherical aberration. J Cataract Refract Surg. 2002;28(5):766–774. doi:10.1016/S0886-3350(01)01322-0
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.