Back to Journals » Research and Reports in Tropical Medicine » Volume 15
An Update on the Pathogenesis of Fascioliasis: What Do We Know?
Authors Tanabe MB , Caravedo MA, White AC Jr, Cabada MM
Received 15 October 2023
Accepted for publication 27 January 2024
Published 13 February 2024 Volume 2024:15 Pages 13—24
DOI https://doi.org/10.2147/RRTM.S397138
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Mario Rodriguez-Perez
Melinda B Tanabe,1 Maria A Caravedo,1 A Clinton White Jr,1,2 Miguel M Cabada1,2
1Division of Infectious Disease, Department of Internal Medicine, University of Texas Medical Branch, Galveston, TX, USA; 2Cusco Branch – Alexander von Humboldt Tropical Medicine Institute, Universidad Peruana Cayetano Heredia, Cusco, Peru
Correspondence: Miguel M Cabada, University of Texas Medical Branch, 301 University Blvd RT 0435, Galveston, TX, 77555, USA, Tel +1 409-747-0236, Fax +1 409-772-6527, Email [email protected]
Abstract: Fasciola hepatica is a trematode parasite distributed worldwide. It is known to cause disease in mammals, producing significant economic loses to livestock industry and burden to human health. After ingestion, the parasites migrate through the liver and mature in the bile ducts. A better understanding of the parasite’s immunopathogenesis would help to develop efficacious therapeutics and vaccines. Currently, much of our knowledge comes from in vitro and in vivo studies in animal models. Relatively little is known about the host-parasite interactions in humans. Here, we provide a narrative review of what is currently know about the pathogenesis and host immune responses to F. hepatica summarizing the evidence available from the multiple hosts that this parasite infects.
Keywords: Fasciola spp, pathogenesis, infection, immunological response
Introduction
Fasciola hepatica and Fasciola gigantica are foodborne trematodes that infect a wide range of mammals including human, livestock, and wild animals. Fascioliasis has been reported in all inhabited continents, but the host range varies geographically and according to income and development.1 Fasciola infection causes economic losses exceeding 3 billion US Dollars a year in the livestock industry and severely affects production and income among small farmers in resource poor countries.1–3 F. hepatica causes acute and chronic symptoms in human and is associated with weight loss, stunting, and anemia in children.4,5 In addition, infections have been associated with liver hematomas, abscesses, cholangitis, and fibrosis with Fasciola likely contributing to the burden of chronic liver disease in endemic areas.6–8
Fasciola has a complex life cycle that includes infection of snails where asexual reproduction and amplification of the infecting stages occur and infection of mammals (the definitive hosts) where sexual reproduction occurs. F. hepatica has adapted to the widest host range of all foodborne trematodes infecting human, ruminants, equids, lagomorphs, macropods, and rodents. There are over 30 Lymnaeid snail species that can be infected naturally or in vitro.9 Some snail species have adapted to harsh conditions such as the high altitude of the South American Andes Mountain range.10
The host-parasite interface in fascioliasis is complex and incompletely understood. Current understanding stems from studies in animal models but few studies in humans.4,5 The parasite has developed mechanisms to escape the host’s responses during the different phases of the infection.4 The invasive phase of Fasciola sp. infection starts when metacercariae release the juvenile parasites in the duodenum of the definitive host. These juveniles penetrate the intestinal wall and migrate through the liver tissues for several weeks until reaching the biliary tree. The biliary phase starts with the invasion of the biliary tree and the establishment of a chronic infection by mature parasites.1,6,7 The tissue damage in fascioliasis may be mediated by the action of proteolytic enzymes excreted by the parasites, the immune responses to the parasites excretory/secretory products and tegument, and the mechanical action of the parasites in the tissue. In this manuscript we review the current knowledge on the pathogenesis of Fasciola infection including the immune responses and pathogenic processes describe in different hosts with emphasis on human. Fasciola will refer to F. hepatica unless indicated otherwise in the text.
Immunopathogenesis
Fascioliasis has two distinct clinical and diagnostic phases: the acute phase in which the fluke larvae migrate from the intestines through the liver parenchyma and the chronic phase in which the adults establish the infection in the bile ducts where they produce eggs (Figure 1). 11
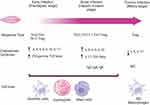 |
Figure 1 Immune response to fascioliasis depending on the phase of the infection. (Illustration created with BioRender.com).4,12 |
Acute Phase
Humans and animals acquire the infection via ingestion of metacercariae either through contaminated water or leafy plants growing in contact with water. The metacercariae are activated in the duodenum by CO2, temperature, bile salts, and reducing conditions.12,13 In vitro studies have demonstrated that increased temperature has a significant impact on the invasive capability and glycogen metabolism of metacercariae.13 The activated metacercariae excyst releasing newly excysted juveniles (NEJ). The NEJ attach to the gut via surface glycans and penetrate the intestinal epithelium with the aid of secreted cysteine proteases.5,12 This intestinal penetration is not thought to cause clinical disease. In lambs, intestinal penetration occurs within 72 hours post infection.14 In Wistar rats, experimental studies demonstrated a rapid intestinal penetration within 6 hours associated with little inflammation.15 Similarly, in Malaguena goats no inflammatory responses were noticed in the intestinal wall.16 The permeability of the intestinal barrier changes after reinfection. Ex vivo studies with Wistar rat intestines demonstrated infiltrates of eosinophils in the intestinal mucosa causing partial protection to subsequent reinfection.17 Rodent models have demonstrated protection via eosinophil-mediated responses involving IgG antibodies in the gastrointestinal tract. In Wistar rats immunized for Fasciola and rechallenged with metacercariae, NEJs were coated by IgG1 and IgG2 antibodies, mast cells, and eosinophils.15 Likewise, in Simmental and Red Holstein bulls, a high density of mastocytes and eosinophils were found in biopsies of small intestines and their numbers increased after each reinfection.18
After penetrating the intestine wall, the NEJs migrate through the peritoneum and invade through the liver capsule. Upon entry to the peritoneum, the host begins to respond to the invasive larvae, setting up a struggle between the host and the parasite.19 The NEJs tegumental surface that sloughs off and the excretory and secretory antigens (ES) modulate the host response.5 In vitro experiments using serum from sheep and rats, demonstrated that sloughed antigens of the Fasciola tegument decreased as the flukes matures. The ES are released from the parasite intestine, excretory pores, and tegumental surface and are the primary mediators of host evasion (Figure 2). The secretome analysis of newly excysted juveniles demonstrates expression of proteins related to the host parasite interactions such as cathepsin L, cathepsin B, cystatin −1, and thioredoxin.12 Thioredoxin is one of the most abundant proteins in the secretome, which protects the parasite against reactive oxygen and nitrogen generated by the host phagocytes.13,20 Other important proteins expressed include antioxidants such as fatty acid binding proteins (FABP) and glutathione S transferases (GST).21 FABP reduces the production of pro-inflammatory cytokines and aids in the activation of M2 macrophages.22 Helminth defense molecules (FhHDM-1) inhibit inflammation by blocking lysosomal acidification and antigen presentation.23,24 Further studies demonstrated heme-binding characteristics with potential role in nutrient acquisition.25
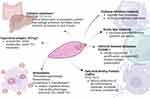 |
Figure 2 Immunomodulatory effects of the excretory/secretory and tegumental antigens in Fasciola hepatica (Illustration created with BioRender.com).12,26,27 Notes: #Only main functions are included in the figure There are many functions of the E/S antigens that are discussed in the manuscript. The list of E/S products is not comprehensive, only most prominent are presented. *Vaccine candidates. |
The glycocalix tegumental coat (FhTeg) protects the parasite from host enzymes and bile.28 In vitro studies demonstrated the role of FhTeg in induction of M2 macrophages and suppression on dendritic cells (DC) by impairing a Th1 response.29,30 Cameron et al reported that serum of sheep collected 4 weeks after Fasciola infection contained IgG antibodies that recognized FhTeg antigens and extracellular vesicles. The authors suggested that these can trigger an antibody-dependent cell-mediated cytotoxic response and could be vaccine candidates.31 Studies in humans sera demonstrated the production of IgG against enolase, aldolase, GST, and FABP as the main immunoreactive components against FhTeg.32 FhTeg is also responsible for secreting extracellular vesicles (EV). Proteomic analysis of excretory vesicles from NEJ demonstrated 29 different proteins some related to structural activity, metabolism, and locomotion, but interestingly no cathepsins were found.33
Mouse models with fascioliasis demonstrate recruitment of DC, macrophages, eosinophils, neutrophils, and CD4 + T cells into the peritoneal cavity.34,35 However, Fasciola ES components such as thioredoxin peroxidase shift the host response by stimulating alternatively activated macrophages (also called M2 macrophages). M2 cells are anti-inflammatory and characterized by production of high levels of interleukin-10 (IL-10), prostaglandin E2, leading to low levels of interleukin-12 (IL-12).36 Donnelly et al demonstrated that peroxiredoxin (Prx) secreted by the tegument of Fasciola, induced a Th2 response increasing IL-4, IL-5, and IL-13 secretion.37 After Fasciola infection, the upregulation of transcription factors aids in the development of M2 and plasma cells and leads to Th2 differentiation via DC.38 The M2 cells blunt the Th17 inflammatory response involved in chemotaxis of myeloid cells.
Rodrigues et al showed that different glycans from the F. hepatica tegument promote maturation of the DC in mice.39 Glycans induce a partially mature phenotype change in DC causing them to secrete anti-inflammatory cytokines such as IL-10 and express low levels of MHCII.39 In addition, in vitro experiments showed that the use of recombinant forms of ES antigens (cysteine protease enzyme/CL1 and GST) partially activates DC and suppress the development of Th17 but could not differentiate Th2 cells.40
The NEJ reach the liver in a period of days where they tunnel a path facilitated by secreted cathepsin peptidases (FhCL2, and FhC3).12 The parasite migrates through the liver for 8–10 weeks.41 Liver damage is limited by rapid wound healing via immune cells and induction of fibrosis. Once in the liver parenchyma, a predominant Th2 response takes over and is characterized by the production of IL-4, IL-5, as well as mastocytosis, hypereosinophilia, and IgE production and the absence of IFN-gamma and IL-2.26,42 Increased IL-4, IL-5, and IL-10 was associated with liver damage in mice.39 Aron-Said et al demonstrated an increase in IL-5 and IL-17 during human acute fascioliasis compared to levels of this cytokines in controls and humans with chronic infection.43
Eosinophils are crucial for the response against helminth infections, as they have been shown to cause the demise of parasites in vitro.11 In contrast, during fascioliasis, the hypereosinophilia has been shown to be a protective mechanism to decrease liver damage and modulate the immune response. Experiments by Frigerio et al demonstrated that depletion of eosinophils caused more liver damage in mice.44 In the same study, eosinophils were shown to limit the production of IL-10 and promote the production of Th2 cytokines, in addition to induce more granulation as compared to eosinophil depleted mice.44 ES antigens released by Fasciola have been shown to induced apoptosis of eosinophils around 21 days post infection in rats via tyrosine kinases and caspases.45 Additionally, ES products such as cathepsins prevent the attachment of antibody mediated eosinophils to the parasite.46 Mast cells assist in the expulsion of parasites from the intestines and the secretions of proinflammatory cytokines via a Th1 immunological response.47 Mice experiments have demonstrated the suppressive effect of FhTeg on mast cells’ capacity to induce a Th1 response via the suppression of TNF-alpha, INF-gamma, and IL-10 secretion and ICAM1 expression.30 Subsequent experiments supported these findings, Vukman et al injected mice with FhTeg and exposed them to Bordetella pertussis, demonstrating that FhTeg inhibited the production TNF-alpha and other proinflammatory mast cells.30 These experiments suggested the early recruitment of mastocytes to induce a Th1 response that is later inhibited by FhTeg.48
The inflammatory response to F. hepatica increases the release of IgE by plasma cells. IgE is thought to play a role in protective immunity evidenced by increased morbidity in parasitic infections after anti-IgE treatment.49 Silva et al reported elevated levels of total IgE and specific IgE antibodies in humans infected with Fasciola.50 IgE-dependent killing involves the attachment of mast cells and eosinophils to the helminth via the Fc receptor of IgE.51 However, there was a decrease expression of the high affinity IgE receptor in Fasciola infected sheep livers, suggesting that IgE-dependent processes are inhibited during infection.38
There are other mechanisms that partially explain why Fasciola is able to survive despite a strong Th2 immune response that normally would allow for elimination of helminths. The parasite has an evolving FhTeg with changing antigenic capacities while invading different tissues. The constant shedding of this changing FhTeg avoids the effect of immune complex attachment and potentially explain the poor efficacy of monovalent vaccines.52–55 Another mechanism of immune evasion is the secretion of serine protease inhibitors (serpins) that regulate proteolytic events.41 Genomic and transcriptomic data have demonstrated the presence of seven serpins from the invasive NEJ stage in the ES products.12 In vitro, serpins inhibit the activity of human neutrophils cathepsin G, which protects the reproductive organs assuring the parasite’s survival.56 In vitro experiments have demonstrated the secretion of cysteine proteinase by Fasciola which cleaves the host IgG as another attempt to evade the host immune system.57 Recently, De Marco Verissimo et al reported that NEJ are resistant to complement activation. The serine protease inhibitors secreted by NEJ block complement activation by the inhibition of lectin complement pathway.58
Chronic Phase
The chronic phase of F. hepatica infection occurs after the parasite reaches the biliary ducts and completes its maturation. Clinically, this stage is associated with production of eggs that are shed in the stool.12,59 During this phase, the parasite continues to modulate the immune system to survive without causing damage to the host. In human infection, this phase is characterized by modulation of the immune response, such that eosinophilia largely resolves, and cytokine levels are suppressed.43 Other helminths, such as Necator spp., Schistosoma spp., and Taenia spp., exert an immunomodulatory effect via activation of regulatory T cells (Treg), release of anti-inflammatory cytokines (IL-10, TGFβ), activation of M2 macrophages, suppression of DC maturation, and inhibition of Th1/Th17 cell differentiation.26,34,59,60 In chronic infection, the ES products activate Treg and DC and stimulate the conversion of macrophages to M2 phenotype.61 Lund et al showed that intraperitoneal administration of ES products to NOD (non-obese) mice protected them from developing type I diabetes, by production of macrophage dependent IL-10.61 Co-culture of M2 peritoneal macrophages with naïve splenocytes led to an increase in Foxp3+ Treg cells. In contrast, ES did not modulate splenocytes when applied directly, suggesting that activation is mediated via M2 macrophages.61 Costa et al demonstrated that depletion of M2 macrophages was associated with partial resistance to infection and increase levels of Foxp3+ Treg.62 In a subsequent study, this group demonstrated that splenic Tregs and production of heme-oxygenase-1 (HO-1) by peritoneal antigen presenting cells depends on IL-10 activity in Fasciola infected mice. Upregulation of HO-1 decreased the production of reactive oxygen and nitrogen species and increased the number of Treg. Thus, decreasing oxidative damage to the parasite and decreasing the host capacity to avoid infection.63 This overall lack of immune response was also noted in infected Wistar rats. Studies demonstrated a profound unresponsiveness to mitogens during chronic Fasciola infection, with no significant differences between eosinophil count and minimal to no cytokine production.64
Pathology Associated with Fascioliasis
Fasciola interactions with its mammal hosts are mediated by contact with tegumental and excretory/secretory antigens, secretory vesicles, and the mechanical action of the parasites in tissue. These trigger a cascade of cellular responses leading to different disease manifestations ranging from local such as erosion into different tissues (eg blood vessels and liver parenchyma) or fibrosis to systemic such as anemia and weight loss. These interactions vary significantly by parasite stage, host species, and intensity of the infection. Our knowledge of the different pathologic processes associated with Fasciola infection is fragmented and stems from animal model studies and descriptions of natural infection in livestock and humans.
Liver Parenchyma Pathology
One of the hallmarks of fascioliasis is inflammation leading to deposition of extracellular matrix and scarring in liver and biliary tree tissues. Although, some reversibility of the lesions caused by the infection has been documented, the contribution of untreated fascioliasis to chronic liver disease among different hosts in endemic areas is not well known.65 A description of histopathologic changes caused by F. hepatica natural infection in the European hare (Lepus europaeus) reported hyperplasia of bile ducts with inflammatory cells and chronic fibroblastic response and portal fibrosis.66 Similar findings were described in a different study including Sika deer from Hokkaido Japan. In this study, a higher burden of infection (45% prevalence) was documented in deer slaughtered for human consumption. The histopathologic examination of the livers demonstrated chronic inflammatory and hyperplastic changes in the biliary epithelium with papillary hyperplasia, goblet cells metaplasia, pyloric glands metaplasia, and periductal fibrosis in all the animals with adult parasites in the bile ducts.67 More severe changes were described in naturally infected pigs which included the presence of fibrotic tracts in the liver surface associated with biliary tree and gallbladder mucosal thickening and dilation. On histopathology, perilobular chronic hepatitis with marked increased in connective tissue deposition, thickening of the biliary tree mucosa and submucosa, granulomas and diffuse fibrosis were observed.68 Studies from abattoirs in naturally infected cattle showed similar hyperplastic and fibrotic changes in and around the biliary tree suggesting similar pathogenic processes in this species.69–71 In addition, one study in South Africa showed a significant correlation between the severity of the fibrotic changes in the liver, the physical condition of the animals, and the final carcass weight suggesting that the severity of the inflammatory changes and liver disease have a systemic effect and may be behind the high impact on production parameters observed with fascioliasis.69 However, natural infection studies might be difficult to interpret as most fail to account for the influence of other helminth infections and other concomitant conditions, such as rearing practices.
Animal models of experimental infection isolate the effects of Fasciola infection on liver pathology. Mice experimentally infected with F. gigantica metacercariae displayed progressive and significant deposition of collagen in the liver as early as 5 days post infection.72 In experimentally infected rats, myofibroblast and deposits of collagen appeared within infiltrates of inflammatory cells in and around the migratory tracks left by juvenile flukes as early as 4 weeks post infection and persisted up to the 7th week post infection. Of note, fibrotic changes associated with proliferation of bile ducts and inflammatory infiltrates were also described in areas of the liver that did not undergo mechanical disruption by the migrating juveniles. In the same study, adult parasites during the chronic phase caused epithelial and glandular hyperplasia with intense inflammatory infiltrates in the biliary ducts. The liver parenchyma showed cirrhotic changes with axial and non-axial nodule formation as well as significant expression of cytokeratin in ductules and the common bile duct suggesting severe fibrosis in this model of infection.73 Evaluation of METAVIR fibrosis scores in groups of rats experimentally infected with increasing doses of F. hepatica metacercariae revealed severe fibrosis (METAVIR F3–F4 scores) in 60% of the rats infected with 10 metacercariae (harboring 1–3 adult parasites) and in 100% of the rats infected with ≥20 metacercariae (harboring 3 to 7 adult parasites). In this study, the expression of collagen I, TGF-BR1 receptor, TIMP1 and TIMP 2 fibrogenic genes in the liver was associated with the infectious dose and the time elapsed since infection. The authors suggested that liver stellate cell and the parasite’s cathepsin L played a paramount role in F. hepatica induced fibrogenesis.74 Similarly, the evaluation of experimentally infected sheep showed increased transcription, as high as 35-fold of genes associated with extra cellular matrix deposition and fibrosis during acute infection.75
The liver pathology associated with fibrosis in human with acute and chronic fascioliasis is poorly characterized and limited to case reports. Almendras-Jaramillo et al reported cases of children presenting with advanced liver disease such as liver cirrhosis and concomitant Fasciola infections in Peru.76 Another case series from Peru describes severe fibrosis in the liver biopsy of a child presenting with hepatosplenomegaly, anasarca, and hypergammaglobulinemia diagnosed with chronic Fasciola infection but without further evaluations for other potential causes of liver disease.77 Sanchez-Sosa reported the cases of two alcoholic patients presenting with advanced liver cirrhosis associated with significant dilation and fibrosis of the biliary tree and massive infection with F. hepatica. Although, establishing causality for fascioliasis in the development of cirrhosis in these patients is not possible, the presence of advanced biliary tree disease and massive infections suggest that the parasite could have played a contributing role in chronic liver disease.78
Biliary Tree Pathology
Biliary tree disease associated with obstruction of the biliary ducts may be caused by the mechanical action of the parasites and/or chronic inflammation causing mucosal thickening, stenosis, and strictures. Human fascioliasis associated with acute or chronic cholecystitis and obstructive jaundice with dilation of the common bile duct is common in endemic countries.79–82 These patients present with abdominal pain and different combinations of eosinophilia, elevation of transaminases, alkaline phosphatase, gamma-glutamyl transferase, and/or total bilirubin. Findings upon laparoscopic evaluation of patients admitted for acute or chronic cholecystitis caused by Fasciola, often without lithiasis, in a major referral center in Lima, Peru, showed significant inflammation around the common bile duct and gallbladder, thickening of their walls, and formation of adhesions.6,7 In the presence of dilation of the common bile duct, endoscopic extraction of the liver flukes makes the diagnosis and treats the obstruction.82,83 Complications arising from biliary tree obstruction in fascioliasis include pancreatitis, cholangitis, and liver abscesses.83–86 Chronic infection and inflammation of the biliary tree can cause focal dilation associated with biliary epithelial injury, hyperplasia causing narrowing, and cellular atypia as well as eosinophilic granulomas surrounding Fasciola eggs in the liver parenchyma.87–89 Some of these lesions may be attributed to malignancy and lead to invasive procedures increasing the morbidity and the probability of complications.
Other liver flukes such as Opisthorchis sp. and Clonorchis sp. are recognized as precursors of intrahepatic and extrahepatic gallstones in human.90 In contrast, this association has not been well characterized in fascioliasis. The co-occurrence of gallstones and fascioliasis in human is not uncommon in endemic countries and evidence from naturally infected animals and animal model suggest that Fasciola may be associated with stone formation.91,92 Brahmbhatt et al demonstrated higher levels of alkaline phosphatase and gamma-glutamyl transferase in cattle and buffaloes infected with Fasciola compared to uninfected animals suggesting ongoing cholestasis.93 In addition, the formation of biliary calculi has been described in cattle naturally infected with Fasciola.94 Isolating the effects of fascioliasis on gallstone formation in naturally infected animals is difficult as they may have concomitant parasite infections and comorbid conditions. However, in the rat model of infection, the appearance of gallstones was associated with chronic Fasciola infection with fully mature flukes and was more common at the time when flukes reached their maximal size. Gallstone formation was more common with higher burdens of infection, days post experimental infection, and certain lipid profiles suggesting that obstruction and stasis may play an important role on gallstone formation in the presence of comorbidities.95 Obstruction and bile stasis are associated with bacterial overgrowth and colonization of the biliary tree with gut bacteria. A study using the rat model showed that from 157 animals experimentally infected with Fasciola, 18 (11%) developed lithiasis in the intra and extrahepatic bile ducts 100 to 400 days after the infection compared to none of the uninfected control rats. No stones formed in the gallbladder and formation was associated with longer time since experimental infection. In these animals, stones were predominantly composed of calcium (82–94%) and palmitic acid forming calcium salts.96 Early studies of gallstone composition suggested that calcium palmitate was associated with pigmented stones and that these formed in the setting of chronic biliary infections.97 The paramount role of bacteria in cholesterol and pigmented stone formation in the biliary tree is now being recognized.98 Different types of bacteria are associated with the specific composition of stones. It has been proposed that bile and gut microbiome are similar and that dysbiosis of these environments may predispose to stone formation.98–100 Fascioliasis is associated with gut dysbiosis in cattle with reduction in Bacteroidetes and Ascomycota abundance and increase in commensals such as Peptostreptococcaceae which may impair the cattle’s ability to digest food.101 Although, a direct parallel between cattle and human gut microbiome changes cannot be made, similar decreases in Bacteroidetes in the bile microbiota are associated with gallstone formation in human.100 Studies directed at elucidating the epidemiologic associations of biliary tree stones with fascioliasis in human and the potential underlying pathologic mechanisms are needed.
Cholangiocarcinoma has been associated with chronic bacterial and parasitic infections of the biliary tree including Salmonella and liver fluke infections. O. viverrini and C. sinensis are recognized as class I carcinogens associated with gallbladder cancer.102 The pathologic mechanisms that contribute to cholangiocarcinoma associated with Opisthorchis/Clonorchis may include mechanical epithelial injury and secretion of cathepsins that cause chronic inflammation, oxidative DNA damage, and secretion of granulin-like growth factor and thioredoxins with mitogenic and antiapoptotic effects.103 Despite the overlap of highly endemic areas for cholangiocarcinoma and fascioliasis in South America and the potential for similar parasite host interactions, no association between these illnesses has been systematically reported.104–107 Case reports of concomitant cholangiocarcinoma and Fasciola infection are not common and do not constitute evidence to claim an association.108
Systemic Manifestations
Fascioliasis is associated with manifestations beyond the gastrointestinal system. Acute and chronic fascioliasis has been associated with anemia in animals and humans. Asymptomatic children with fascioliasis were 3 times more likely than children without the infection to have anemia in the highlands of Peru.109 The pathologic processes underlying the development of anemia may depend on the phase of the infection and the host, but their study is confounded by difficulties in separating the multiple other factors affecting hemoglobin levels in Fasciola endemic areas.
Changes in the liver parenchyma during the acute phase and the biliary tree mucosa during the chronic phase of the infection suggest that blood loss and iron deficiency, although not always apparent, may explain in part the pathogenesis of anemia. In sheep, necrotic and hemorrhagic tracts with fibrin deposits are evident upon macroscopic and histologic examination of the liver during the migratory phase of fascioliasis. The severity of these findings depends on the intensity of the infection and may lead to massive hemorrhage in sheep.110 Massive bleeding associated with fascioliasis is also described in humans which may account for the few lethal cases of Fasciola infection. Although, the incidence of acute blood loss caused by Fasciola in humans is not known, haemobilia, upper gastrointestinal bleeding, liver subcapsular hematomas, and hemoperitoneum cases have been described with acute and chronic infections.76,111–116
Acute blood loss is not the only mechanism associated with iron deficiency anemia in fascioliasis. Ulceration of the biliary tree mucosa, blood loss, and haemobilia have been reported in animals. A study evaluating the feeding behavior of adult Fasciola parasites in the rabbit model of infection showed diffuse necrotic ulceration of the biliary tree mucosa with contained hemorrhage in the areas where parasites fed.117 In the rat model, chronic infections with Fasciola at weeks 20 and 60 were associated with detectable blood in the stool, microcytic anemia, and iron deficiency in the majority of the animals.118 In naturally infected cattle, hemoglobin and iron concentrations were lower in animals with chronic fascioliasis compared to animals without infection suggesting iron deficiency and chronic blood loss as the cause of anemia according to the authors.119 Other mechanisms have been proposed for anemia associated with fascioliasis. A study in naturally infected goats evaluating the efficacy of different drugs against fascioliasis showed that hemoglobin levels started recovering 7 days after treatment in all groups compared to the untreated controls with normalization by day 30 after drug administration.120 Such a fast response of hemoglobin levels after treatment for Fasciola is unlikely in iron deficiency anemia. In a study by El-Shazly among humans with chronic fascioliasis in Egypt, only 30% of those with anemia had hypochromia and microcytosis suggestive of iron deficiency while more than 60% had normochromic normocytic anemia suggestive of other underlying processes such chronic immune activation.121 An association between the type of host immune response to Fasciola infection and anemia and its severity has been proposed in the rat model.122 However, comprehensive human studies evaluating the different potential mechanisms of anemia associated with fascioliasis controlling for other variables such as chronic liver disease and socioeconomic factors are needed.
Weight loss has been reported in Fasciola infections in animals and treating fascioliasis in livestock increases milk production and weight gain.123 Weight loss and chronic malnutrition has been associated with fascioliasis in humans with a particular impact on school aged children.11,124 However, the mechanisms underlying weight loss in acute and chronic fascioliasis have not been well characterized.
Conclusions
In conclusion, Fasciola sp. causes disease via multiple mechanisms that include modulating inflammation, enzymatic action of the ES, and mechanical disruption depending on the phase of the infection. Most of what we know about the parasite host interactions stems from animal model and natural infections, but the specific pathogenic mechanisms in human need further characterization. The description of multiple Fasciola sp. secretory and excretory proteins have helped elucidate some pathologic mechanisms including the modulation of the immune response. Some of these proteins offer potential targets for therapeutics, diagnostics, and vaccine development.
Acknowledgments
Drs. Tanabe, White, and Cabada are supported by grant U01AI168622 and Dr. Cabada is supported by grants R01AI146353 and U01AI155323. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute for Allergy and Infectious Diseases.
Disclosure
The authors have no financial or conflicts of interest to disclose for this work.
References
1. Caravedo MA, Cabada MM. Human Fascioliasis: current Epidemiological Status and Strategies for Diagnosis, Treatment, and Control. Res Rep Trop Med. 2020;11:149–158.
2. May K, Bohlsen E, Konig S, Strube C. Fasciola hepatica seroprevalence in Northern German dairy herds and associations with milk production parameters and milk ketone bodies. Vet Parasitol. 2020;277:109016.
3. Mehmood K, Zhang H, Sabir AJ, et al. A review on epidemiology, global prevalence and economical losses of fasciolosis in ruminants. Microb Pathog. 2017;109:253–262.
4. Donnelly S, Flynn RJ, Mulcahy G, O’Neill SM Chapter Immunological interaction between Fasciola and its host. In Fascioliasis II. 2022. Available from: http://hdl.handle.net/10453/166688.
5. Lalor R, Cwiklinski K, Calvani NED, et al. Pathogenicity and virulence of the liver flukes Fasciola hepatica and Fasciola gigantica that cause the zoonosis Fasciolosis. Virulence. 2021;12(1):2839–2867.
6. Chang Wong MR, Pinto Elera JO, Guzman Rojas P, Terashima Iwashita A, Samalvides cuba F. Caracterizacion clinica y epidemiologica de la infeccion por Fasciola hepatica entre los años 2003-2010 en el Hospital Nacional Cayetano Heredia, Lima, Peru. [Demographic and clinical aspects of Fasciola hepatica between 2013-2010 in National Hospital Cayetano Heredia, Lima, Peru] Spanish. Rev Gastroenterol Peru. 2016;36(1):23–28.
7. Machicado C, Machicado JD, Maco V, Terashima A, Marcos LA. Association of Fasciola hepatica Infection with Liver Fibrosis, Cirrhosis, and Cancer: a Systematic Review. PLoS Negl Trop Dis. 2016;10(9):e0004962.
8. Cabada MM, Goodrich MR, Graham B, et al. Prevalence of intestinal helminths, anemia, and malnutrition in Paucartambo, Peru. Rev Panam Salud Publica. 2015;37(2):69–75.
9. Vázquez AA, Alda P, Lounnas M, et al. Lymnaeid snails hosts of Fasciola hepatica and Fasciola gigantica (Trematoda: digenea): a worldwide review. CABI Reviews. 2018;1–15.
10. Mas-Coma S, Bargues MD, Valero MA. Human fascioliasis infection sources, their diversity, incidence factors, analytical methods and prevention measures. Parasitology. 2018;145(13):1665–1699.
11. Klion AD, Nutman TB. The role of eosinophils in host defense against helminth parasites. J Allergy Clin Immunol. 2004;113(1):30–37.
12. Cwiklinski K, Jewhurst H, McVeigh P, et al. Infection by the Helminth Parasite Fasciola hepatica Requires Rapid Regulation of Metabolic, Virulence, and Invasive Factors to Adjust to Its Mammalian Host. Mol Cell Proteomics. 2018;17(4):792–809.
13. Andreyanov ON, Postevoy AN, Sidor EA. The effect of ambient temperature on biological properties and energy metabolism of Fasciola hepatica metacercariae. Vet Parasitol. 2021;299:109576.
14. Dow C, Ross JG, Todd JR. The histopathology of Fasciola hepatica infections in sheep. Parasitology. 1968;58(1):129–135.
15. Van Milligen FJ, Cornelissen JB, Hendriks IM, Gaasenbeek CP, Bokhout BA. Protection of Fasciola hepatica in the gut mucosa of immune rats is associated with infiltrates of eosinophils, IgG1 and IgG2a antibodies around the parasites. Parasite Immunol. 1998;20(6):285–292.
16. Zafra R, Perez-Ecija RA, Buffoni L, et al. Early hepatic and peritoneal changes and immune response in goats vaccinated with a recombinant glutathione transferase sigma class and challenged with Fasciola hepatica. Res Vet Sci. 2013;94(3):602–609.
17. van Milligen FJ, Cornelissen JB, Gaasenbeek CP, Bokhout BA. A novel ex vivo rat infection model to study protective immunity against Fasciola hepatica at the gut level. J Immunol Methods. 1998;213(2):183–190.
18. Wicki P, Schwalbach B, Charbon JL, et al. Réactions cellulaires intestinales du bovin après infection par Fasciola hepatica [Intestinal cellular reaction of cattle after infection by Fasciola hepatica]. Schweiz Arch Tierheilkd. 1991;133(9):429–437.
19. Ruiz-Campillo MT, Molina Hernandez V, Escamilla A, et al. Immune signatures of pathogenesis in the peritoneal compartment during early infection of sheep with Fasciola hepatica. Sci Rep. 2017;7:2782.
20. Robinson MW, Hutchinson AT, Dalton JP, Donnelly S. Peroxiredoxin: a central player in immune modulation. Parasite Immunol. 2010;32(5):305–313.
21. Di Maggio LS, Tirloni L, Pinto AF, et al. Across intra-mammalian stages of the liver fluke Fasciola hepatica: a proteomic study. Sci Rep. 2016;6:32796.
22. Figueroa-Santiago O, Espino AM. Fasciola hepatica fatty acid binding protein induces the alternative activation of human macrophages. Infect Immun. 2014;82(12):5005–5012.
23. Ryan S, Shiels J, Taggart CC, Dalton JP, Weldon S. Fasciola hepatica-Derived Molecules as Regulators of the Host Immune Response. Front Immunol. 2020;11:2182.
24. Robinson MW, Alvarado R, To J, et al. A helminth cathelicidin-like protein suppresses antigen processing and presentation in macrophages via inhibition of lysosomal vATPase. FASEB J. 2012;26(11):4614–4627.
25. Martinez-Sernandez V, Mezo M, Gonzalez-Warleta M, et al. The MF6p/FhHDM-1 major antigen secreted by the trematode parasite Fasciola hepatica is a heme-binding protein. J Biol Chem. 2014;289(3):1441–1456.
26. O’Neill S, Donnelly S. Mechanisms of Immune Modulation by Fasciola hepatica: the Impact of Innate Immune Cells on the Developing Adaptive Immune Response. In: Jirillo E, Magrone T, Miragliotta G, editors. In Immune Response to Parasitic Infections: Immunity to Helminths and Novel Therapeutic. Approaches. Bentham Books; 2014.
27. Flynn RJ, Mulcahy G, Elsheikha HM. Coordinating innate and adaptive immunity in Fasciola hepatica infection: implications for control. Vet Parasitol. 2010;169(3–4):235–240.
28. Ravida A, Cwiklinski K, Aldridge AM, et al. Fasciola hepatica Surface Tegument: glycoproteins at the Interface of Parasite and Host. Mol Cell Proteomics. 2016;15(10):3139–3153.
29. Adams PN, Aldridge A, Vukman KV, Donnelly S, O’Neill SM. Fasciola hepatica tegumental antigens indirectly induce an M2 macrophage-like phenotype in vivo. Parasite Immunol. 2014;36(10):531–539.
30. Vukman KV, Adams PN, Metz M, Maurer M, O’Neill SM. Fasciola hepatica tegumental coat impairs mast cells’ ability to drive Th1 immune responses. J Immunol. 2013;190(6):2873–2879.
31. Cameron TC, Cooke I, Faou P, et al. A novel ex vivo immunoproteomic approach characterizing Fasciola hepatica tegumental antigens identified using immune antibody from resistant sheep. Int J Parasitol. 2017;47(9):555–567.
32. Morales A, Espino AM. Evaluation and characterization of Fasciola hepatica tegument protein extract for serodiagnosis of human fascioliasis. Clin Vaccine Immunol. 2012;19(11):1870–1878.
33. Trelis M, Sanchez-Lopez CM, Sanchez-Palencia LF, Ramirez-Toledo V, Marcilla A, Bernal D. Proteomic Analysis of Extracellular Vesicles from Fasciola hepatica Hatching Eggs and Juveniles in Culture. Front Cell Infect Microbiol. 2022;12:903602.
34. Walsh KP, Brady MT, Finlay CM, Boon L, Mills KH. Infection with a helminth parasite attenuates autoimmunity through TGF-beta-mediated suppression of Th17 and Th1 responses. J Immunol. 2009;183(3):1577–1586.
35. Flynn RJ, Mulcahy G. The roles of IL-10 and TGF-beta in controlling IL-4 and IFN-gamma production during experimental Fasciola hepatica infection. Int J Parasitol. 2008;38(14):1673–1680.
36. Donnelly S, O’Neill SM, Sekiya M, Mulcahy G, Dalton JP. Thioredoxin peroxidase secreted by Fasciola hepatica induces the alternative activation of macrophages. Infect Immun. 2005;73(1):166–173.
37. Donnelly S, Stack CM, O’Neill SM, Sayed AA, Williams DL, Dalton JP. Helminth 2-Cys peroxiredoxin drives Th2 responses through a mechanism involving alternatively activated macrophages. FASEB J. 2008;22(11):4022–4032.
38. Naranjo-Lucena A, Correia CN, Molina-Hernandez V, et al. Transcriptomic Analysis of Ovine Hepatic Lymph Node Following Fasciola hepatica Infection - Inhibition of NK Cell and IgE-Mediated Signaling. Front Immunol. 2021;12:687579.
39. Rodriguez E, Noya V, Cervi L, et al. Glycans from Fasciola hepatica Modulate the Host Immune Response and TLR-Induced Maturation of Dendritic Cells. PLoS Negl Trop Dis. 2015;9(12):e0004234.
40. Dowling DJ, Hamilton CM, Donnelly S, et al. Major secretory antigens of the helminth Fasciola hepatica activate a suppressive dendritic cell phenotype that attenuates Th17 cells but fails to activate Th2 immune responses. Infect Immun. 2010;78(2):793–801.
41. De Marco Verissimo C, Jewhurst HL, Tikhonova IG, et al. Fasciola hepatica serine protease inhibitor family (serpins): purposely crafted for regulating host proteases. PLoS Negl Trop Dis. 2020;14(8):e0008510.
42. Donnelly S, Stack CM, O’Neill SM, Sayed AA, Williams DL, Dalton JP. Helminth 2-Cys peroxiredoxin drives responses through a mechanism involving alternatively activated macrophages. FASEB J. 2008;22(11):4022.
43. Aron-Said C, Montes M, White AC, Cabada MM. Plasma cytokines during acute human fascioliasis. Parasitol Res. 2021;120(8):2965–2968.
44. Frigerio S, da Costa V, Costa M, et al. Eosinophils Control Liver Damage by Modulating Immune Responses Against Fasciola hepatica. Front Immunol. 2020;11:579801.
45. Serradell MC, Guasconi L, Cervi L, Chiapello LS, Masih DT. Excretory-secretory products from Fasciola hepatica induce eosinophil apoptosis by a caspase-dependent mechanism. Vet Immunol Immunopathol. 2007;117(3–4):197–208.
46. Carmona C, Dowd AJ, Smith AM, Dalton JP. Cathepsin L proteinase secreted by Fasciola hepatica in vitro prevents antibody-mediated eosinophil attachment to newly excysted juveniles. Mol Biochem Parasitol. 1993;62(1):9–17.
47. Bramhall M, Zaph C. Mastering gut permeability: new roles for old friends. Eur J Immunol. 2017;47(2):236–239. doi:10.1002/eji.201646842
48. Robinson MW, Dalton JP, O’Neill SM, Donnelly SM. Chapter 27: mechanisms of Immune Modulation by Fasciola hepatica: importance for Vaccine Development and for Novel Immunotherapeutics. In: Cr C, editor. Parasitic Helminths: Targets, Screens, Drugs and Vaccines. Wiley Online Library; 2012.
49. Cruz AA, Lima F, Sarinho E, et al. Safety of anti-immunoglobulin E therapy with omalizumab in allergic patients at risk of geohelminth infection. Clin Exp Allergy. 2007;37(2):197–207.
50. Silva SML, Vindimian M, Wattre P, Capron A. Etude des anticorps IgE dans la distomatose humaine a Fasciola hepatica. [IgE antibodies in human Fasciola hepatica distomiasis] French. Pathol Biol. 1985;33(7):746–750.
51. Kita H, Kaneko M, Bartemes KR, et al. Does IgE bind to and activate eosinophils from patients with allergy? J Immunol. 1999;162(11):6901–6911.
52. Hamilton CM, Dowling DJ, Loscher CE, Morphew RM, Brophy PM, O’Neill SM. The Fasciola hepatica tegumental antigen suppresses dendritic cell maturation and function. Infect Immun. 2009;77(6):2488–2498.
53. Morrison CA, Colin T, Sexton JL, et al. Protection of cattle against Fasciola hepatica infection by vaccination with glutathione S-transferase. Vaccine. 1996;14(17–18):1603–1612.
54. Golden O, Flynn RJ, Read C, et al. Protection of cattle against a natural infection of Fasciola hepatica by vaccination with recombinant cathepsin L1 (rFhCL1). Vaccine. 2010;28(34):5551–5557.
55. Zafra R, Buffoni L, Perez-Caballero R, et al. Efficacy of a multivalent vaccine against Fasciola hepatica infection in sheep. Vet Res. 2021;52(1):13.
56. Maggio L, Tirloni L, Uhl M, et al. Serpins in Fasciola hepatica: insights into host-parasite interactions. Int J Parasitol. 2020;50(12):931–943.
57. Smith AM, Dowd AJ, Heffernan M, Robertson CD, Dalton JP. Fasciola hepatica: a secreted cathepsin L-like proteinase cleaves host immunoglobulin. Int J Parasitol. 1993;23(8):977–983.
58. De Marco Verissimo C, Jewhurst HL, Dobo J, Gal P, Dalton JP, Cwiklinski K. Fasciola hepatica is refractory to complement killing by preventing attachment of mannose binding lectin (MBL) and inhibiting MBL-associated serine proteases (MASPs) with serpins. PLoS Pathog. 2022;18(1):e1010226.
59. Guasconi L, Serradell MC, Masih DT, Chiapello LS. Immunomodulatory Effect of Fasciola hepatica Excretory–Secretory Products on Macrophages. In: Fasciola Hepatica: Methods and Protocols. Springer; 2020.
60. White MPJ, McManus CM, Maizels RM. Regulatory T-cells in helminth infection: induction, function and therapeutic potential. Immunology. 2020;160(3):248–260.
61. Lund ME, O’Brien BA, Hutchinson AT, et al. Secreted proteins from the helminth Fasciola hepatica inhibit the initiation of autoreactive T cell responses and prevent diabetes in the NOD mouse. PLoS One. 2014;9(1):e86289.
62. Costa M, da Costa V, Lores P, et al. Macrophage Gal/GalNAc lectin 2 (MGL2)(+) peritoneal antigen presenting cells during Fasciola hepatica infection are essential for regulatory T cell induction. Sci Rep. 2022;12(1):17661.
63. Costa M, da Costa V, Frigerio S, et al. Heme-Oxygenase-1 Attenuates Oxidative Functions of Antigen Presenting Cells and Promotes Regulatory T Cell Differentiation during Fasciola hepatica Infection. Antioxidants. 2021;10(12):1322.
64. Gironènes N, Valero AM, García-Bodelón MA, et al. Immune Suppression in Advanced Chronic Fascioliasis: an Experimental Study in a Rat Model. J Infectious Dis. 2007;195(10):1504–1512.
65. Richter J, Freise S, Mull R, Millan JC. Fascioliasis: sonographic abnormalities of the biliary tract and evolution after treatment with triclabendazole. Trop Med Int Health. 1999;4(11):774–781.
66. Oyarzun-Ruiz P, Alvelo C, Vera F, Moroni M. Histopathological findings of Fasciola hepatica infection in non-native European hare (Lepus europaeus) in Southern Chile. Rev Bras Parasitol Vet. 2019;28(1):145–150.
67. Matsuda K, Kogame S, Niki H, Saito M, Ishiguro Y, Sano Y. Gross and histological lesions in the livers of sika deer with particular emphasis on fascioliasis. J Vet Med Sci. 2020;82(2):125–134.
68. Capucchio MT, Catalano D, Di Marco V, et al. Natural trematode infestation in feral Nebrodi Black pigs: pathological investigations. Vet Parasitol. 2009;159(1):37–42.
69. Mpisana Z, Jaja IF, Byaruhanga C, Marufu MC. Body condition scores, fluke intensity, liver pathology, and carcass quality of different dairy cattle genotypes infected with Fasciola species at high throughput abattoirs in South Africa. Parasitol Res. 2022;121(6):1671–1682.
70. Winaya IBO, Oka IBM, Adnyana IBW, Sudipa PH. Fibrosis and Collagen-I Accumulation in Bali Cattle Liver Tissue Infected with Fasciola gigantica. Inter J Vet Sc. 2023;12(2):224–229.
71. Phiri AM, Phiri IK, Sikasunge CS, Chembensofu M, Monrad J. Comparative fluke burden and pathology in condemned and non-condemned cattle livers from selected abattoirs in Zambia. Onderstepoort J Vet Res. 2006;73(4):275–281.
72. Zhang Y, Mei X, Liang Y, et al. Newly excysted juveniles (NEJs) of Fasciola gigantica induce mice liver fibrosis and M2 macrophage-like phenotype in vivo. Microb Pathog. 2020;139:103909.
73. Kolodziejczyk L, Laszczynska M, Masiuk M, Grabowska M, Skrzydlewska E. Immunoexpression of intermediate filaments and morphological changes in the liver and bile duct of rats infected with Fasciola hepatica. Biotech Histochem. 2015;90(7):477–485.
74. Marcos LA, Terashima A, Yi P, et al. Mechanisms of liver fibrosis associated with experimental Fasciola hepatica infection: roles of Fas2 proteinase and hepatic stellate cell activation. J Parasitol. 2011;97(1):82–87.
75. Alvarez Rojas CA, Ansell BR, Hall RS, et al. Transcriptional analysis identifies key genes involved in metabolism, fibrosis/tissue repair and the immune response against Fasciola hepatica in sheep liver. Parasit Vectors. 2015;8:124.
76. Almendras-Jaramillo M, Rivera-Medina J, Seijas-Mogrovejo J, Almendras-Jaramillo K. Fascioliasis Hepatica En niños: manifestaciones clinicas poco frecuentes. [Hepatic fascioliasis in children: uncommon clinical manifestations] Spanish. Arq Gastroenterol. 1997;34(4):241–247.
77. Marcos LA, Maco V, Castillo M, Terashima A, Zerpa R, Gotuzzo E. Reporte de casos de Fasciolosis humana en el Instituto Especializado de Salud del Nino, Lima, Peru (1988-2003). [Report of cases of human fascioliasis in the Specialized Children’s Health Institute, Lima, Peru (1988’-2003)] Spanish. Rev Gastroenterol Peru. 2005;25(2):198–205.
78. Sanchez-Sosa S, Rojas-Ortega S, Reed-San Roman G, Torres-Santana MA. Fasciolosis hepatobiliar masiva. [Massive hepatobiliary fascioliasis] Spanish. Rev Gastroenterol Mex. 2000;65(4):179–183.
79. Bestas R, Yalcin K, Cicek M. Cholestasis caused by Fasciola gigantica. Turkiye Parazitol Derg. 2014;38(3):201–204.
80. Hosamirudsari H JRY. Fasciola hepatica an Unusual Cause of Chronic Cholecystitis. Int J Infect. 2015;2(2):e23108.
81. Guzman Calderon E, Vera Calderon A, Diaz Rios R, Arcana Lopez R, Alva Alva E. Fasciola hepatica in the common bile duct: spyglass visualization and endoscopic extraction. Rev Esp Enferm Dig. 2018;110(10):671–673.
82. Gulsen MT, Savas MC, Koruk M, Kadayifci A, Demirci F. Fascioliasis: a report of five cases presenting with common bile duct obstruction. Neth J Med. 2006;64(1):17–19.
83. Dolay K, Hasbahceci M, Hatipoglu E, Umit Malya F, Akcakaya A. Endoscopic diagnosis and treatment of biliary obstruction due to acute cholangitis and acute pancreatitis secondary to Fasciola hepatica infection. Ulus Travma Acil Cerrahi Derg. 2018;24(1):71–73.
84. Ha JS, Choi HJ, Moon JH, et al. Endoscopic Extraction of Biliary Fascioliasis Diagnosed Using Intraductal Ultrasonography in a Patient with Acute Cholangitis. Clin Endosc. 2015;48(6):579–582.
85. Veerappan A, Siegel JH, Podany J, Prudente R, Gelb A. Fasciola hepatica pancreatitis: endoscopic extraction of live parasites. Gastrointest Endosc. 1991;37(4):473–475.
86. Dzib Calan EA, Larracilla Salazar I, Morales Perez JI. A giant liver abscess due to Fasciola hepatica infection. Rev Esp Enferm Dig. 2019;111(10):815–816.
87. Losada H, Hirsch M, Guzman P, Fonseca F, Hofmann E, Alanis M. Fascioliasis simulating an intrahepatic cholangiocarcinoma-Case report with imaging and pathology correlation. Hepatobiliary Surg Nutr. 2015;4(1).
88. Wang H, Itoh S, Matsumoto Y, et al. Surgically resected hepatic mass caused by fascioliasis. Clin J Gastroenterol. 2021;14(2):662–667.
89. Yen TJ, Hsiao CH, Hu RH, Liu KL, Chen CH. Education and imaging: hepatobiliary and pancreatic: chronic hepatic abscess associated with fascioliasis. J Gastroenterol Hepatol. 2011;26(3):611.
90. Sripa B, Kanla P, Sinawat P, Haswell-Elkins MR. Opisthorchiasis-associated biliary stones: light and scanning electron microscopic study. World J Gastroenterol. 2004;10(22):3318–3321.
91. Pfeifer CM, Bourm KS, Brandt MR, Ali K. Liver fluke-induced choledocholithiasis with biliary ductal obstruction. Radiol Case Rep. 2019;14(12):1483–1486.
92. Lefryekh R, Bensaad A, Bensardi F, et al. Hepatic fascioliasis presenting with bile duct obstruction: a case report. Pan Afr Med J. 2017;28:44.
93. Brahmbhatt NN, Kumar B, Thakre BJ, Bilwal AK. Haemato-biochemical characterization of fasciolosis in Gir cattle and Jaffrabadi buffaloes. J Parasit Dis. 2021;45(3):683–688.
94. Javaregowda AK, Rani KB. Chronic bovine fasciolosis associated cholangiolithiasis: a case report. J Parasit Dis. 2017;41(1):90–92.
95. Valero MA, Santana M, Morales M, Hernandez JL, Mas-Coma S. Risk of gallstone disease in advanced chronic phase of fascioliasis: an experimental study in a rat model. J Infect Dis. 2003;188(5):787–793.
96. Valero MA, Varea MT, Marin R. Fasciola hepatica: lithogenic capacity in experimentally infested rats and chemical determination of the main stone components. Parasitol Res. 2000;86(7):558–562.
97. Cetta F. The role of bacteria in pigment gallstone disease. Ann Surg. 1991;213(4):315–326.
98. Binda C, Gibiino G, Coluccio C, et al. Biliary Diseases from the Microbiome Perspective: how Microorganisms Could Change the Approach to Benign and Malignant Diseases. Microorganisms. 2022;10(2):312.
99. Stewart L, Grifiss JM, Jarvis GA, Way LW. Biliary bacterial factors determine the path of gallstone formation. Am J Surg. 2006;192(5):598–603.
100. Dan WY, Yang YS, Peng LH, Sun G, Wang ZK. Gastrointestinal microbiome and cholelithiasis: current status and perspectives. World J Gastroenterol. 2023;29(10):1589–1601.
101. Ramirez AL, Herrera G, Munoz M, et al. Describing the intestinal microbiota of Holstein Fasciola-positive and -negative cattle from a hyperendemic area of fascioliasis in central Colombia. PLoS Negl Trop Dis. 2021;15(8):45.
102. Humans IWGotEoCRt. Biological agents. IARC Monogr Eval Carcinog Risks Hum. 2012;100(Pt B).
103. Liau MYQ, Toh EQ, Shelat VG. Opisthorchis viverrini-Current Understanding of the Neglected Hepatobiliary Parasite. Pathogens. 2023;12(6):56.
104. Salazar M, Ituarte C, Abriata MG, Santoro F, Arroyo G. Gallbladder cancer in South America: epidemiology and prevention. Chin Clin Oncol. 2019;8(4):32.
105. Machicado C, Marcos LA, Zimic M. Hypothetical granulin-like molecule from Fasciola hepatica identified by bioinformatics analysis. Springerplus. 2016;5(1):773.
106. Vale N, Gouveia MJ, Gartner F, Brindley PJ. Oxysterols of helminth parasites and pathogenesis of foodborne hepatic trematodiasis caused by Opisthorchis and Fasciola species. Parasitol Res. 2020;119(5):1443–1453.
107. Bottari NB, Mendes RE, Baldissera MD, et al. Relation between iron metabolism and antioxidants enzymes and delta-ALA-D activity in rats experimentally infected by Fasciola hepatica. Exp Parasitol. 2016;165:58–63.
108. Swain B, Otta S, Sahu MK, Uthansingh K. Fasciola hepatica association with gallbladder malignancy: a rare case report. Trop Parasitol. 2021;11(1):42–45.
109. Lopez M, White AC Jr, Cabada MM. Burden of Fasciola hepatica Infection among children from Paucartambo in Cusco, Peru. Am J Trop Med Hyg. 2012;86(3):481–485.
110. Stuen S, Ersdal C. Fasciolosis-An Increasing Challenge in the Sheep Industry. Animals. 2022;12(12).
111. Wong RK, Peura DA, Mutter ML, Heit HA, Birns MT, Johnson LF. Hemobilia and liver flukes in a patient from Thailand. Gastroenterology. 1985;88(6):1958–1963.
112. Bahcecioglu IH, Ataseven H, Aygen E, Coskun S, Kuzu N, Ilhan F. Fasciola hepatica case with hemobilia. Acta Medica. 2007;50(2):155–156.
113. Acuna-Soto R, Braun-Roth G. Bleeding ulcer in the common bile duct due to Fasciola hepatica. Am J Gastroenterol. 1987;82(6):560–562.
114. Leon M, Alave J, Alvarado R, Gotuzzo E, Terashima A, Seas C. A 52-year-old woman with a subcapsular liver hematoma. Clin Infect Dis. 2011;52(9):1137, 1195–6.
115. Loja Oropeza D, Alvizuri Escobedo J, Vilca Vasquez M, Aviles Gonzaga R, Sanchez Mercado M. Hematoma hepatico subcapsular por Fasciola. [Hepatic subcapsular hematoma caused by fascioliasis] Spanish. Rev Gastroenterol Peru. 2003;23(2):142–148.
116. Montembault S, Serfaty L, Poirot JL, Wendum D, Penna C, Poupon R. Ascite hemorragique revelant une infection massive a Fasciola hepatica. [Hemorrhagic ascites disclosing massive Fasciola hepatica infection] French. Gastroenterol Clin Biol. 1997;21(10):785–788.
117. Sukhdeo MV, Sangster NC, Mettrick DF. Permanent feeding sites of adult Fasciola hepatica in rabbits? Int J Parasitol. 1988;18(4):509–512.
118. Valero MA, Girones N, Garcia-Bodelon MA, et al. Anaemia in advanced chronic fasciolosis. Acta Trop. 2008;108(1):35–43.
119. Lotfollahzadeh S, Mohri M, Bahadori Sh R, Dezfouly MR, Tajik P. The relationship between normocytic, hypochromic anaemia and iron concentration together with hepatic enzyme activities in cattle infected with Fasciola hepatica. J Helminthol. 2008;82(1):85–88.
120. Shrimali RG, Patel MD, Patel RM. Comparative efficacy of anthelmintics and their effects on hemato-biochemical changes in fasciolosis of goats of South Gujarat. Vet World. 2016;9(5):524–529.
121. El-Shazly AM, El-Nahas HA, Abdel-Mageed AA, et al. Human fascioliasis and anaemia in Dakahlia Governorate, Egypt. J Egypt Soc Parasitol. 2005;35(2):421–432.
122. Valero MA, Perez-Crespo I, Chillon-Marinas C, et al. Fasciola hepatica reinfection potentiates a mixed Th1/Th2/Th17/Treg response and correlates with the clinical phenotypes of anemia. PLoS One. 2017;12(3).
123. Rashid M, Zahra N, Chudhary A, et al. Cost-benefit ratio of anthelmintic treatment and its comparative efficacy in commercial dairy farms. Front Vet Sci. 2022;9:1047497.
124. Karahocagil MK, Akdeniz H, Sunnetcioglu M, et al. A familial outbreak of fascioliasis in Eastern Anatolia: a report with review of literature. Acta Trop. 2011;118(3):177–183.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
