Back to Journals » Patient Preference and Adherence » Volume 16
An Unsafe/Safe Typology in People with Type 2 Diabetes: Bridging Patients’ Expectations, Personality Traits, Medication Adherence, and Clinical Outcomes
Authors Reach G , Benarbia L, Benhamou PY , Delemer B, Dubois S, Gouet D, Guerci B, Jeandidier N, Lachgar K, Le Pape G , Leroy R , Masgnaux JH, Raclet P, Reznik Y, Riveline JP, Schaepelynck P, Vambergue A, Vergès B
Received 15 March 2022
Accepted for publication 5 May 2022
Published 25 May 2022 Volume 2022:16 Pages 1333—1350
DOI https://doi.org/10.2147/PPA.S365398
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Gérard Reach,1 Laurent Benarbia,2 Pierre-Yves Benhamou,3 Brigitte Delemer,4 Séverine Dubois,5 Didier Gouet,6 Bruno Guerci,7 Nathalie Jeandidier,8 Karim Lachgar,9 Gilles Le Pape,10 Rémy Leroy,11 Jean-Hugues Masgnaux,12 Philippe Raclet,13 Yves Reznik,14 Jean-Pierre Riveline,15,16 Pauline Schaepelynck,17 Anne Vambergue,18 Bruno Vergès19
1Health Education and Promotion Laboratory (LEPS EA 3412), Sorbonne Paris Nord University, Bobigny, France; 2Marketing Studio, Paris, France; 3Department of Endocrinology, Grenoble University Hospital; Grenoble Alpes University, INSERM U1055, LBFA, Grenoble, France; 4Service d’Endocrinologie – Diabète – Nutrition, CHU de Reims - Hôpital Robert Debré, and Université de Reims Champagne Ardenne, UFR Sciences Exactes Et Naturelles, Reims, France; 5Department of Diabetology and Endocrinology, CHU Angers, Angers, France; 6Department of Diabetology and Endocrinology, Saint Louis Hospital, La Rochelle, France; 7Department of Endocrinology, Diabetology and Nutrition, CHRU of Nancy, Brabois Hospital, and ILCV Lorraine University, Vandoeuvre-les-Nancy, France; 8Department of Endocrinology, Diabetes and Nutrition, Hôpitaux Universitaires de Strasbourg, and Université de Strasbourg, Strasbourg, France; 9Department of Diabetology and Endocrinology, Centre Hospitalier Simone Veil, Eaubonne, France; 10General Practice, Penmarc’h, France; 11Private Medical Practice, Endocrinology and Diabetology, Lille, France; 12M&M Conseil, Boulogne, France; 13Association Française des Diabétiques de Bourgogne Franche-Comté, Dijon, France; 14Department oEndocrinology and Diabetology, CHU Côte de Nacre, Caen, and University of Caen Basse-Normandie, Medical School, Caen, France; 15Department of Diabetology and Endocrinology, Lariboisière Hospital, Assistance Publique - Hôpitaux de Paris, Paris, France; 16Unité INSERM U1138 Immunity and Metabolism in Diabetes, ImMeDiab Team, Centre de Recherches des Cordeliers, and Université de Paris, Paris, France; 17Department of Nutrition-Endocrinology-Metabolic Diseases, Pôle ENDO, APHM-Hôpital la Conception, Marseille, France; 18Department of Diabetology, Endocrinology, Metabolism and Nutrition, CHU Lille, and University Hospital European Genomic Institute for Diabetes, Lille, France; 19Department of Endocrinology-Diabetology,CHU Dijon, and University of Burgundy, INSERM LNC UMR1231, Dijon, France
Correspondence: Gérard Reach, Health Education and Promotion Laboratory (LEPS EA 3412), Sorbonne Paris Nord University, 74 Rue Marcel Cachin, Bobigny Cedex, 93017, France, Tel + 33 (0)6 60 84 53 25, Email [email protected]
Background: Support programs are provided to people with diabetes to help them manage their disease. However, adherence to and persistence in support programs are often low, making it difficult to demonstrate their effectiveness.
Aim: To identify the determinants of patients’ perceived interest in diabetes support programs because it may be a powerful determinant of effective participation in such programs.
Patients and Methods: An online study conducted in April 2021 in metropolitan France on 600 people with diabetes recruited from a consumer panel. A 64-item psychosocial questionnaire including a question asking to evaluate the helpfulness of a support program was used. Univariate, multivariate, and multiple correspondence analyses were performed.
Results: The existence of a typology, known as Unsafe/Safe, was discovered, in which patients with type 2 diabetes respond in two distinct ways. Type U (unsafe) patients, who believe that a support program would be helpful, are more likely to be nonadherent to their treatment, have high hemoglobin A1c levels, have at least one diabetic complication, lack information regarding their disease and treatment, rate the burden of their disease and impairment of their quality of life as high, worry about their future, and are pessimistic. Type S (safe) patients have the opposite characteristics. Type U patients can be dichotomized into two broad classes: one in which they lack information regarding disease and treatment and the other in which alterations in the quality of life and burden of the disease predominate. Insulin-treated patients give more importance to the lack of information, whereas noninsulin-treated patients complain primarily about the burden of the disease and impairment of quality of life.
Conclusion: This study describes this new U/S typology, proposes a simple method based on a nine-item questionnaire to identify type U patients by calculating a Program Helpfulness Score described herein, and clarifies the nature of the intervention to be provided to them. This novel approach could be applied to other chronic diseases.
Keywords: type 2 diabetes, adherence, support programs, typology, personality traits, clinical outcomes, patients’ expectations
Introduction
People with diabetes often view their disease and its treatment as a major burden that impacts their quality of life.1–3 Support programs have been developed to help patients optimize self-care and cope with their disease over the long term.4 However, adherence to and persistence in support programs are often low. This may be due to the fact that they are sometimes provided without considering patient demand. The psychological factors that determine this demand are not yet clearly understood, as this question is rarely asked in the “Assess” phase of the patient education process,5 and, in general, studies evaluating chronic disease support programs have focused more on the determinants of effectiveness than on those of persistence.
Nonadherence is considered to be a major determinant of clinical outcomes of therapy. It concerns the three steps of implementation, correct completion, and continuation of medical prescriptions.6 There is extensive literature on the determinants of nonadherence, its frequency, and its consequences in terms of morbidity, mortality, and healthcare costs.7–11 Importantly, nonadherence can also be intentional,12 highlighting the importance of considering patients’ needs, preferences, and demands in shared medical decision making.13
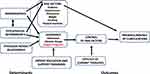
A key element influencing this study is that adherence is not only about taking medication and implementing lifestyle change measures but also about following support programs. This is shown in Figure 1, which represents the theoretical model that forms the basis for this study. Determinants of adherence to this specific aspect of care may affect clinical outcomes such as controlling risk factors and the genesis of diabetic complications. Thus, the aim of this study was to clarify the demographic and psychosocial factors involved in patients’ interest in support programs.
Materials and Methods
Type of Study and Patient Inclusion
The Timkl-1 Study, a cross-sectional online study conducted in metropolitan France, was the first step of the development of Timkl, a program aimed at developing a support program for the care optimization of people with type 2 diabetes. Patients were recruited from the Norstat panel. Norstat is one of the leading data collectors for market research in Europe. It has a panel network of over 2 million consumers across 19 countries. In France, it has access to 54,000 consumers (response rate: 40%), generally responding to marketing/media studies but also (8% of the studies) to questions concerning their health.14
The survey began with questions to identify patients suffering from type 2 diabetes. Those identified were given a login code to complete an online questionnaire. Participants were aware of NORSTAT’s extensive quality controls, including not allowing the same person to register under more than one account or email address and analysing participants’ response times.15
Responses to the questionnaire were collected between April 14 and 30, 2021, with the recruitment being stopped when 600 patients had completed the questionnaire. This sample size, decided a priori, was the one that could be expected within 15 days of the survey opening, given the size of the panel and the response rate. In a previous study using the same panel,16 it was possible to identify 3001 people with hypertension, hypercholesterolemia, or diabetes; 774 people with diabetes; and 318 people in the subpopulation of people with diabetes only. This number of participants had the power to demonstrate statistical relationships between different aspects of intentionality and adherence (unpublished results). There were no missing data. There were less than 10 implausibilities regarding BMI and age.
Questionnaire (Appendix 1)
Participants completed a 64-item questionnaire, shown in Appendix 1, including demographic information, a 6-item adherence questionnaire, used in France to screen nonadherent patients,17 such as “do you ever find you have too much medication to take,” a psychological questionnaire constructed from the literature including studies from the first author,13,16,18–21 and eight questions supposed a priori to identify patients in need of a support program. This part of the questionnaire included the question “We can offer you a diabetes support program. Do you think it would be helpful?” They were asked to rate their answer on a scale from 1 (not helpful) to 10 (extremely helpful). The entire questionnaire was discussed by the Timkl Study Group, a board of experts in diabetes care including a person with diabetes, himself being responsible for a regional section of the French association of people with diabetes. The feasibility of the questionnaire was evaluated in a pilot study of 10 patients who asked for changes to improve the intelligibility of some questions.
Answers were dichotomized as follows. In case of a Likert scale with four possible answers, eg, never, rarely, often, and always, the first two and last two answers were combined. One question concerned trust in the doctor, in which patients were dichotomized into those who answered “completely” and those who answered something else. For questions with an analog response of 0 or 1 to 10 (people were instructed to never answer 5 to avoid the tendency to choose a neutral value), the dichotomy was performed on the median. Adherence was also dichotomized on the median of the number of positive responses (0–1 vs 2–6 positive responses to the 6-item adherence questionnaire).
Construction of a Program Helpfulness Score
A univariate analysis of responses to the helpfulness question (below or above the median response) identified nine statistically significant determinants. Discriminant analysis was used to assign coefficients describing the weight of each of these determinants, the sum of which would be equal to 100. This enabled calculating a “Program Helpfulness Score” (PHS) for a given patient, ranging from 0 to 100. This procedure was performed on the whole population (n = 600) and on patients treated with insulin (n = 142) or those who were untreated (n = 458).
Statistics
Analyses were based on univariate and multivariate studies using determinants with probability P < 0.10 in the univariate study. For multivariate analyses, the question on the number of blood glucose measurements was not included in the model because several patients did not practice glucose monitoring. As a large number of patients were analysed for a large number of characteristics with different modalities, we also used multiple correspondence analysis (MCA). Briefly, this method allows the respective Euclidean coordinates of these modalities, such as being adherent or nonadherent, having complications or not having complications, and being happy or sad, to be represented on a two-axis plane. The proximity of the modalities on the graph makes it possible to visualize their associations. The MCA thus allows a holistic analysis of the characteristics of a population, allowing the discovery of relationships that would not be detected by separate classical univariate analyses. For this reason, this method, initially used in marketing studies, is now widely used in the social sciences.22 MCA was applied to the two groups of insulin-treated (n = 142) and noninsulin-treated (n = 458) patients involved in this study. A simplified MCA was also performed on the entire population of patients (n = 600) on the following four categories: adherence, control of HbA1c, presence of complications, and various ranges of the PHS, whose calculation is described earlier.
Receiver operating characteristic (ROC) curves were used to define the PHS thresholds that best distinguish between patients who find a support program useless and those who find it helpful. In patients with a score above the helpfulness threshold, an unsupervised ascending hierarchical analysis was performed on all the dimensions of the first MCA. This analysis, working “bottom-up” through a series of iterations by starting from each individual, groups together individuals that share most similarities using the Ward’s criterion that seeks to minimize the intraclass variance and maximize the interclass variance. The result is represented in the form of a tree or dendrogram. Classes are defined by cutting branches off the dendrogram where the distance between two branch formations is maximal.22 All statistical analyses were conducted using the SPAD 9.2 software (Coheris).
Ethics and Conformity to Publication Reporting Guidelines
This study complied with the Declaration of Helsinki. A priori IRB approval was obtained (Comité Local d’Éthique Avicenne-2021-180, March 5, 2021). The Norstat panel complies with the ESOMAR23 and RGDP standards that ensure data confidentiality. The following sentence was part of the document submitted to the Ethics Committee for approval: “Completing the questionnaire implies provision of consent. Indeed, as mentioned above, Norstat specifies the conditions of consent for consumers” (patients who were contacted as part of this consumer panel were included). This study conforms to STROBE guidelines.
Patient Involvement in the Study
One patient, regional director of a patient association, is the vice president of the Timkl scientific board. He was therefore involved in the development of the questionnaire used in this study. Furthermore, he submitted the questionnaire to 10 patients as a pilot study to improve its comprehensibility. He also participated in the discussion of the study results.
Results
Characteristics of Included Patients (Appendix 1)
Appendix 1 also shows all the responses to the auto-questionnaire. In particular, there were as many men as women, the median age was 61.3 years, the median body mass index was 29.5 kg/m2, the median duration of diabetes was 9.2 years, and 23% of patients were treated with insulin. In 60% of the cases, the HbA1c level was within the target, and 22% of patients had at least one diabetic complication. Regarding the last professional activity (socioprofessional category), 53.2% were or had been farmers, employees, workers, and others without activity, grouped under the term SPC.2, and 46.8% were or had been craftsmen, shopkeepers, executives, and intermediate professionals (SPC.1). Among the respondents, 35% had higher education.
In total, 366 (61%) of patients were defined as adherent. Another 12% of patients stated that they sometimes deliberately did not take their medication. Moreover, 40% of them reported experiencing adverse effects due to their medication, and 21% and 26% of patients considered that they did not have enough information about their disease and treatment, respectively. On a scale of 1–10 (intolerable), regarding tolerability in terms of burden and impairment of quality of life, the median was 6. Regarding the potential helpfulness of a support program, the median of responses ranging from useless to helpful was 6. Only 57% of patients reported they had complete trust in their doctor.
Determinants of Patients’ Views on the Helpfulness of a Support Program
In univariate analysis, the following nine determinants were found to be associated with patients finding a support program rather helpful: 1. lack of information about the treatment (P < 0.001) and 2. the disease (P < 0.001), 3. feeling a heavy disease burden (P < 0.001) and 4. a strong alteration in quality of life (P < 0.001), 5. being sometimes depressed (P < 0.001), 6. thinking that preparing the future is very important (P < 0.001), 7. that they will have physically changed a lot in 10 years (P < 0.001), 8. that they are bothered by hypoglycaemia (P = 0.003), and 9. that they engage in physical activity (P = 0.004).
In multivariate analysis (n = 535), the following five criteria emerged as independent determinants of finding a support program helpful: 1. finding the disease burden intolerable (P = 0.019), 2. being obedient (P = 0.021), 3. feeling sometimes depressed (P = 0.022), 4. finding the preparation of future important (P = 0.024), and 5. sticking to habits (P = 0.019). This logistic regression model identified patients finding a support program helpful with a sensitivity of 53.2% and a specificity of 79.3%, yielding an Area Under the Curve (AUC) of the ROC curve of 0.713.
Discovery of an Unsafe/Safe Typology Through Multiple Correspondence Analysis (MCA)
Table 1 shows the responses to the questions associated with being adherent to medication, having the HbA1c level within target, and having no complications. For adherence, the determinants are presented in the descending order of significance. In these three univariate analyses, some of these determinants are shared by the three variables. Figure 2 shows in a MCA all the elements that appeared to be significantly associated in these analyses (Box 1). Two clusters of characteristics emerged from this MCA; the first, which we propose to call Unsafe (right-hand side of the two figures), gathers the following characteristics: Q31.1: Support helpful; A2: Nonadherent; Q27.2: HbA1c level not in target; Q23.2: At least one complication; Q57.1: Deliberate nonadherence: yes; Q24.2: Intolerable burden; Q26.2: Quality of life intolerable; Q65.1: Presence of adverse effects of treatment; Q30.2: bothered by hypoglycaemia; Q58.2: not enough information about the disease; Q59.2: not enough information about treatment; BMI ≥30 kg/m2; Q50.1: depressed; Q51.2: pessimistic; Q53.2: sad; Q54.2: disobedient; Q55.2: not completely trusting the doctor; Q56.2: not sticking to habits; Age<61 years; SPC.2; Q47.2: feeling that he/she will change a lot in the next 10 years; and Q48.1: preparing the future very important. The Safe type (left-hand side of the figures) has the opposite characteristics.
 |
Table 1 Determinants of Adherence, Diabetes Control, and Absence of Diabetic Complications |
Comparison between the insulin-treated patients (left side of the figure) and noninsulin-treated patients (right side of the figure) revealed only two discordances, viz., Type U was associated with smoking (Q14.2) and absence of physical activity (Q20.2) in people not treated with insulin, whereas in patients treated with insulin, it was associated with nonsmoking (Q14.1) and physical activity (Q20.1).
 |
Box 1 Categories Used in the Multiple Correspondence Analysis |
Construction of a Program Helpfulness Score (PHS) and Relationship with Patient Characteristics
This score was constructed using a discriminant analysis of the nine determinants of the answer to the question (Q31) concerning the feeling by patients that a support program would be useful. This analysis enabled determining the respective weights of these criteria to arrive at a PHS ranging from 0 to 100 (useless to helpful). Table 2 shows these coefficients for the overall population of 600 patients and for insulin-treated and noninsulin-treated patients. In general, insulin-treated patients gave more weight to the lack of information about the treatment and the disease, while the noninsulin-treated patients gave more weight to the impairment of their quality of life and the burden of the disease. In noninsulin-treated patients, sedentary lifestyle was a determinant of the feeling that a support program could be helpful.
 |
Table 2 Respective Weights of the Nine Criteria for the Overall Population, Insulin-Treated Patients, and Noninsulin-Treated Patients |
These coefficients were used to calculate the PHS by adding their value in the case of a positive response (as shown in Table 2, in noninsulin-treated patients, the 1.555 coefficient is added if the answer to the physical activity question is sedentary). The calculation is described in Box 2.
 |
Box 2 Calculation of the Program Helpfulness Score (PHS) |
Figure 3 shows the MCA results of the entire population (n = 600), linking different ranges of PHS to the control of HbA1c level, the presence of complications, and adherence to medication. ROC curve analyses were used to determine the best PHS thresholds defining the uselessness and helpfulness of a support program. Data representing these thresholds, the percentages of patients below the uselessness and above the helpfulness thresholds, respectively, and the AUC of the ROC curves are shown in Table 3, and Table 4 shows the results of the univariate analysis of the entire population data comparing participants above and below the 58 PHS threshold, the determinants being given in the descending order of importance, shown by the test value.
 |
Table 3 Uselessness and Helpfulness PHS Thresholds |
 |
Table 4 Determinants of Program Helpfulness Score ≥ 58 in Univariate Analysis, Entire Population, n = 600 |
Typologies of Insulin- and Noninsulin-Treated Patients with High PHS
As shown in Table 3, the PHS thresholds were 50 and 54 for insulin- and noninsulin-treated patients, respectively. An ascending hierarchical analysis was performed in patients having a score above these thresholds, representing 38 (26.7%) and 112 (24.4%) patients, respectively, of the patients. The dendrograms identified two major classes in both groups of patients (Figure 4). The drop from the two-cluster to the three-cluster solution was the largest, especially in noninsulin-treated patients. The first dichotomy leading to the formation of two classes separates patients according to the importance given either to the lack of information on the disease and treatment or to the impact of the disease as a burden and impairment of the quality of life. Patients who considered the burden of disease and impact on the quality of life to be intolerable (class 1 of insulin-treated patients and class 2 of noninsulin-treated patients) believed that they would have changed a lot in 10 years (insulin-treated patients) or that preparing for the future is very important (noninsulin-treated patients) (Table 5).
 |
Table 5 Characteristics of the Two Major Classes of Type U Patients |
Discussion
Main Findings
1. The MCA of data collected from 600 people with type 2 diabetes, on the basis of a holistic approach of patients’ behaviours, revealed the presence of two clusters of characteristics linking the feeling that a support program would be useful to certain personality traits, nonadherence and two clinical outcomes: noncontrol of HbA1c level and presence of complications. Based on this discovery, it is proposed that the behaviour of individuals with a chronic disease can be described using the new typology of Unsafe/Safe (U/S).
2. We constructed a PHS from the nine determinants of the answer to a direct question of helpfulness that were found to be statistically significant in the univariate analysis of the answers. This PHS was found to be statistically related to adherence and clinical outcomes (HbA1c control, presence of diabetes complication). Importantly, the nine determinants used to construct the score did not include these variables, which gives this observation its full value.
3. We observed that patients with high PHS can be divided into two classes differing by their expectations from a support program, information on disease and treatment, or relief from burden and alteration of the quality of life. Insulin-treated patients gave more importance to the lack of information, whereas noninsulin-treated patients complained primarily about the disease burden and quality of life alteration.
Description of the U/S Typology
The complete set of characteristics associated with the U and S typologies, respectively, is shown in the MCA depicted in Figure 2, and the results of univariate analysis comparing participants below and above the PHS threshold are shown in Table 4. The ascending hierarchical analysis of type U patients (Table 5) showed that alteration in the quality of life and high disease burden were found in the same class as the importance of preparing for the future or thinking that one will have changed a lot in 10 years’ time. Their association to the similitude question can be interpreted as a fear of change in the future. In fact, univariate analysis (Table 6) indicated that patients who said that they will have changed a lot in the future also said that they were sometimes depressed (P < 0.001), naturally pessimistic (P < 0.001), sad (P=0.001), had at least one diabetic complication (P = 0.003), and their future is important to them (P = 0.001). Interestingly, these patients who gave importance to the future and were afraid of the perspective to change physically were nevertheless nonadherent to medication. This may reflect all the ambivalence of nonadherence, which has been described as a case of weakness of will (akrasia),24 one of the paradoxes of irrationality investigated by the philosopher Donald Davidson who linked them to a division of mind.25
We also observed the presence of certain personality traits in type U patients, in particular not sticking to habits, disobedience, risk-taking, pessimism, and sadness, which have been observed in earlier studies by the first author to be associated with nonadherence.19–21
There are studies on the role of habit in adherence,18,20,21,26,27 and an association between obedience and adherence has been reported elsewhere. In this study, a multivariate analysis revealed that obedience is an independent factor in finding a support program useful, confirming the role of this character trait in adherence to long-term therapies.19–21 Not fully trusting the doctor was also found in the U typology, consistently with several studies showing the relationship between trust in the doctor and adherence.13,20,21,28–32 Finally, the association between adherence and personality traits should imply that it represents a syndrome.21 As shown in Figure 2, type U patients were more often obese.
Overall, the characteristics of patients belonging to the two typologies, U and S, were identical in insulin- and noninsulin-treated patients (Figure 2), with the exception of smoking status and physical activity. To explain these discrepancies, it is speculated that type U is associated with smoking and absence of physical activity in people not treated with insulin, which would be fully consistent with the syndromic conception of nonadherence,21 as discussed previously. In patients treated with insulin, type U is associated with nonsmoking and physical activity, which may mean that individuals in this stage of the disease try to avoid these modifiable risk factors that may aggravate their condition.
The U/S Typology and Personality Traits
Among the characteristics of type U patients, there were some personality traits such as pessimism, sadness, tendency to depression, and disobedience. Other typologies have already linked personality traits to the occurrence of various morbidities,33–35 the perception of health-related quality of life (HRQOL),36 adherence to therapy, and consumption of health services,37 yielding a network consistent with associations reported herein. For instance, optimism predicts lower rates of rehospitalization after coronary artery bypass graft.38 Type D (for depressed) and pessimism personality, respectively, possess higher risk of consistently impaired HRQOL.39,40 More specific examination of personality characteristics of the Big Five Inventory personality traits (Agreeableness, Conscientiousness, Extraversion, Neuroticism, and Openness) suggested that neuroticism was the characteristic to most likely correlate with the psychosocial aspects of HRQOL.36 There is also evidence that this personality trait is negatively associated with medication adherence in elderly people41 and in people with type 2 diabetes,42 whereas both agreeableness and conscientiousness are positively related to medication adherence.43 Neuroticism was also found to be negatively associated with lifestyle (exercise and diet) in people with type 2 diabetes,44 conscientiousness being found be associated with the practice of self-monitoring of blood glucose and medication adherence in people with type 2 diabetes.45 Similar associations between these personality traits and adherence were observed in people with asthma.46 In a 2006 meta-analysis of 33 studies, extraversion (r = 0.23), neuroticism (r = −0.11), and conscientiousness (r = 0.20) were identified as correlates of physical activity.47
The existence of personality traits reflecting an invariance of the person is not incompatible with the possibility of distinct behaviours when people are faced with different situations.48 This concept is illustrated by the differences observed in this study between patients treated and not treated with insulin – for instance the difference discussed above between attitudes concerning smoking and physical activity. For type U patients, the analysis of their demands also clearly showed a difference in their priority, with insulin-treated patients asking for more information about the disease and its treatment, whereas noninsulin-treated patients complained primarily about the burden of treatment and the alteration of their quality of life. This demonstrates the importance of the event of starting insulin treatment in people with diabetes.
Strengths and Limitations of the Study
Overall, this typology is consistent with everyday medical practice and clinicians’ ability to assess which patients will do well and identify those that they are concerned with. The PHS described here can represent a novel tool helping them to confirm this impression.
However, there are some limitations in our study. Apart from the adherence questionnaire, our overall questionnaire has not been formally validated. Nonetheless, the fact that it allowed us to discover almost by serendipity a new typology suggests a posteriori that it had been grounded on a cogent theoretical foundation (Figure 1) supported by previous empirical studies.13,16,18–21,49
The major weakness is, as for all studies of this type, its self-reporting nature. Moreover, the response rate of the NORSTAT panel (40%), similar to that reported for online surveys (46%)50 may represent another source of bias. However, the fact that the respondents were part of a consumer panel rather than a patient panel and that they responded online may restrict the social desirability bias. Nevertheless, we are aware that this type of bias persists, and that it may contribute to the fact that type S patients represented the majority of participants in this study. Another factor to consider is that individuals participating in panels may have a high literacy level, which is a major determinant to good clinical outcomes in people with chronic diseases.51 Nevertheless, it can also be noted that only 39% of the patients were considered as nonadherent in this study, a proportion that is close to that reported for type 2 diabetes in a study based on drug purchase (35%).7
Interestingly, 62%, 27%, 7%, and 4% of patients answered “Yes,” “No,” “Don’t know,” or “Don’t understand,” respectively, to the question “Is your current HbA1c (glycosylated haemoglobin) in the target range?” However, 23% of patients answered “No” to the question “Do you know what HbA1c, glycated haemoglobin or glycosylated haemoglobin (%) is?” This discrepancy suggests that some patients may be able to interpret HbA1c data in terms of diabetes control without knowing “what it is,” which illustrates the semantic difficulty of constructing a questionnaire, even though particular attention was paid to the comprehensibility of the questions in a pilot test with 10 people with diabetes, which resulted in changes in the wording of the questions. Nevertheless, it is recognized that caution should be applied when interpreting the relationship between the PHS and HbA1c control.
Our study was conducted in a single country, which restricts the generalizability of the results by ignoring cultural influences. Finally, the practical value of this approach remains to be evaluated, for instance by showing that intervention programs are more effective when they include a high PHS among the inclusion criteria.
Implications for Clinicians
An important question raised by these results is whether in current practice one can transform the PHS described in this study into an eligibility score for a support program. In fact, it is easy to create a calculator that based on the answers to the nine helpfulness questions described here and on the presence of insulin in the treatment, gives a score and alerts if it is above the helpfulness threshold. This calculator could subsequently propose a typology that would lead to the personalization of the proposed interventions; there would essentially be two types, focusing either on reinforcing information about the disease and treatment or on psychological support for achieving quality of life.
However, it must be emphasized that all these data are merely statistical. On the one hand, the sensitivity and specificity of the helpfulness score to detect type U patients are far from excellent (AUC of ROC curve, 0.695). On the other hand, a score below the threshold in a patient does not automatically indicate that he or she does not individually present one or more criteria that would require long-term support. The only way to use ethically this quantitative approach is to consider that the calculation of the score should only lead to representing a criterion for inclusion in such a program, and not a criterion for exclusion. In this case, this approach would have the merit of constituting a new method for alerting health professionals to patients at risk (type U for Unsafe), for whom they should be particularly worried. This could be a method to combat clinical inertia for those patients for whom it is most harmful.52 It could be different in the context of clinical research to evaluate an intervention program. In this context, the method proposed here could represent a criterion for excluding patients who are at baseline in a safe situation, which could improve the chances of demonstrating the effectiveness of an intervention.53
Finally, in a very general manner, the question raised here is in fact that of the relationship between statistical data and individual characteristics. The results presented in this study have above all an epistemic value. They demonstrate that it is possible to define uniform behaviours with regard to a situation such as the presence of a chronic disease, which can be referred to as typology, class, etc. The fact that this is possible probably reflects the universality of human behaviours.49 Nevertheless, the statistical nature of these epistemic data represents a limitation to their applicability to practice. This need to consider individuals had been observed by Aristotle as “For it is not man that the physician cures, except incidentally, but Callias or Socrates or some other person similarly named, who is incidentally a man as well. So if a man has theory without experience, and knows the universal, but does not know the particular contained in it, he will often fail in his treatment; for it is the particular that must be treated.”54 Thus, the medical decision must consider not only the statistical data of the clinical trials but also individual patients’ preferences and the expertise of the physician.55
Further Research
The existence of a personality typology opens the pathway for research into whether it has a genetic56,57 and/or neurobiological58 basis and how it can be influenced by social context and interventions. This may apply to the U/S typology described in this study.
Conclusion
MCA of the answers to a 64-item questionnaire enabled the discovery of the existence of a typology that clearly separates patients who appear to have and do not have aspects of concern, a typology called safe (S) and unsafe (U). Type S and U patients differ in their adherence to medication and their clinical outcomes (control of diabetes and presence of complications). They also differ in personality traits, and this typology bridges these personality traits with clinical outcomes of people with type 2 diabetes. Finally, type U patients manifest a desire to participate in a support program, which is not the case for type S patients. Furthermore, a simple method was designed based on the nine questions (see Box 2) that proved to be the determinants of the answer to this question when it was directly asked to patients.
This typology could serve two purposes. Firstly, it could draw physicians’ attention to these unsafe patients (type U), and, secondly, in clinical trials designed to evaluate an intervention, it could help exclude patients who do not need this intervention (type S). Finally, this approach is in harmony with person-centred therapy that incorporates the needs and preferences of patients, which is important in an age of advancing technology.59,60 We believe that this S/U typology, described here in people with type 2 diabetes treated or not treated with insulin, can be adapted for other chronic conditions.
Acknowledgment
This work was funded by Timkl, a Roche company, and its organization was coordinated by Vivactis, a Contract Research Organization. The funder was not involved in the analysis of the results, the decision to publish the study, or the choice of journal.
Author Contributions
GR, the president of the Timkl Study Group, designed the study and analyzed the results with LB, statistician. He wrote the first draft of the article. The analysis of the data and the article were discussed in depth during several meetings of the Timkl Study Group. Thus, all the authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
GR reports receipt of personal fees as speaker at symposia organized by Novo-Nordisk, Lilly, Novartis, Sanofi-Aventis, Merck-Serono, GSK, Ipsen, Abbott-Pharma, BMS, Pfizer, Roche-Pharma, Roche-Diagnostics, Abbott-Diagnostics, Lifescan, Bayer-Diagnostics, Dexcom, BioGen, AbbVie, Servier, Novartis, Takeda, and Mylan-Viatris: lectures on patients’ adherence, doctors’ clinical inertia, patient education, and hospitality in hospital; personal fees as participants in scientific boards for Lifescan, Bayer Diagnostics, Lilly, Sanofi-Aventis, Novo-Nordisk, Abbott, Takeda, and Timkl outside the submitted work. PYB has received speaker honoraria from Abbott, Roche, Eli Lilly, Novo Nordisk, and Sanofi; has served on advisory board panels for Abbott, Dexcom, Diabeloop, Insulet, LifeScan, Eli Lilly, Medtronic, Novo Nordisk, Roche, Sanofi, and Timkl; and owns shares in Diabeloop SA outside the submitted work. BD declares having received honoraria for participation in symposia organized by Sanofi, Novo-Nordisk, Lilly, Astra-Zeneca, Abbott, Insulet, Pfizer, Recordati, Ipsen, and Novartis outside the submitted work. SD declares having received honoraria for participation in symposia organized by Sanofi, Novo-Nordisk, Lilly, Astra-Zeneca, Abbott, and Medtronic outside the submitted work. DG declares having received honoraria for participation in symposia organized by Novo-Nordisk, Lilly, Sanofi-Diabète, Astra Zeneca, and Abbott and in boards for Lilly, Sanofi-Aventis, Novo-Nordisk, Abbott, and Timkl outside the submitted work. BG reports grants, personal fees, and non-financial support from Sanofi; personal fees from Orkyn (provider), grants, personal fees, and non-financial support from Vitalaire/Air Liquide (provider); personal fees and non-financial support from Abbott; personal fees and non-financial support from Novo Nordisk; grants, personal fees, and non-financial support from Eli Lilly; personal fees and non-financial support from Boehringer Ingelheim; personal fees and non-financial support from Roche Diagnostic; personal fees and non-financial support from Astra Zeneca; personal fees and non-financial support from MSD; grants and personal fees from Medtronic; personal fees and non-financial support from Dinno Santé (provider); grants from Asten Santé; grants from Elivie; grants from Homeperf; grants from ISIS Diabète, grants from Linde Homecare France, and grants from Nestlé Home-Care outside the submitted work. NJ has received lecture fees from Eli Lilly and Sanofi-Aventis; has served on advisory boards for Defymed, Novartis, and Sanofi-Aventis; and has received investigator’s fees from Sanofi–Aventis, Eli Lilly and Company, Novartis, Novo Nordisk, and Roche Diabetes Care outside the submitted work. KL declares having received honoraria for his participation in symposia organized by Novo-Nordisk, Lilly, Sanofi-Aventis, and AstraZeneca and in the boards for Sanofi-Aventis and Timkl outside the submitted work. GLP declares advisory board activities for Sanofi and Lilly France outside the submitted work. RL declares having received honoraria for his participation in symposia or boards organized by Novo-Nordisk, Lilly, Sanofi, Merck-Serono, Abbott, Pfizer.Lifescan, Bayer, and Astra Zeneca outside the submitted work. YR has performed clinical trials as a co-investigator for Medtronic, Eli-Lilly, and Novo Nordisk; has provided advisory services to Medtronic, Abbott, Novo Nordisk, Eli-Lilly, and Air Liquide Santé; has attended conferences organized by Novo Nordisk, Eli-Lilly Sanofi, and Medtronic as a contributor; and has received investigator’s fees from Medtronic outside the submitted work. JPR is an advisory panel member for Sanofi, MSD, Eli Lilly, Novo Nordisk, Abbott, and Medtronic and has received research funding from and provided research support to Abbott, Air Liquide, Sanofi, and Novo Nordisk outside the submitted work. PS declares having received honoraria for her participation in advisory boards for Eli-Lilly, Novo-Nordisk, Ypsomed, Orkyn, Timkl, and participation in symposia organized by Novo-Nordisk, Abbott Diabetes Care, Roche, Sanofi, and Insulet outside the submitted work. AV declares having received honoraria for her participation for lectures or consultancy from Astra Zeneca, Boehringer, Lilly, Novo Nordisk, Sanofi, and Timkl outside the submitted work. BV declares having received honoraria for lectures or consultancy from Amgen, AstraZeneca, Boehringer, Lilly, MSD France, Novo Nordisk, Recordati, and Timkl outside the submitted work. The authors report no conflicts of interest in this work.
References
1. Rubin RR, Peyrot M, Siminerio LM. Health care and patient-reported outcomes: results of the cross-national Diabetes Attitudes, Wishes and Needs (Dawn) study. Diabetes Care. 2006;29(6):1249–1255. doi:10.2337/dc05-2494
2. Snoek FJ, Bremmer MA, Hermanns N. Constructs of depression and distress in diabetes: time for an appraisal. Lancet Diabetes Endocrinol. 2015;3(6):450–460. PMID: 25995123. doi:10.1016/S2213-8587(15)00135-7
3. Dennick K, Sturt J, Speight J. What is diabetes distress and how can we measure it? A narrative review and conceptual model. J Diabetes Complications. 2017;31(5):898–911. PMID: 28274681. doi:10.1016/j.jdiacomp.2016.12.018
4. Levengood TW, Peng Y, Xiong KZ, et al. Community preventive services task force. Team-based care to improve diabetes management: a community guide meta-analysis. Am J Prev Med. 2019;57(1):e17–26. PMID: 31227069; PMCID: PMC7373227. doi:10.1016/j.amepre.219.02.005
5. Glasgow RE, Emont S, Miller DC. Assessing delivery of the five ‘As’ for patient-centered counseling. Health Promot Int. 2006;21(3):245–255. PMID: 16751630. doi:10.1093/heapro/dal017
6. Vrijens B, De Geest S, Hughes DA, et al. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol. 2012;73(5):691–705. PMID 22486599. doi:10.1111/j.1365-2125.2012.04167.x
7. Briesacher BA, Andrade SE, Fouayzi H, Chan KA. Comparison of drug adherence rates among patients with seven different medical conditions. Pharmacotherapy. 2008;28(4):437–443. PMID 18363527. doi:10.1592/phco.28.4.437
8. Khunti K, Seidu S, Kunutsor S, Davies M. Association between adherence to pharmacotherapy and outcomes in type 2 diabetes: a meta-analysis. Diabetes Care. 2017;40(11):1588–1596. doi:10.2337/dc16-1925
9. Ho PM, Rumsfeld JS, Masoudi FA, et al. Effect of medication nonadherence on hospitalization and mortality among patients with diabetes mellitus. Arch Intern Med. 2006;166(17):1836–1841. PMID 17000939. doi:10.1001/archinte.166.17.1836
10. Mennini FS, Marcellusi A, von der Schulenburg JM, et al. Cost of poor adherence to anti-hypertensive therapy in five European countries. Eur J Health Econ. 2015;16(1):65–72. PMID 24390212. doi:10.1007/s10198-013-0554-4
11. Kennedy-Martin T, Boye KS, Peng X. Cost of medication adherence and persistence in type 2 diabetes mellitus: a literature review. Patient Prefer Adherence. Patient Preference and Adherence. 2017;11:1103–1117. PMID: 28721024; PMCID: PMC5501621. doi:10.2147/PPA.S136639
12. Lehane E, McCarthy G. Intentional and unintentional medication non-adherence: a comprehensive framework for clinical research and practice? A discussion paper. A discussion paper. Int J Nurs Stud. 2007;44(8):1468–1477. PMID 16973166. doi:10.1016/j.ijnurstu.2006.07.010
13. Ratanawongsa N, Karter AJ, Parker MM, et al. Communication and medication refill adherence: the Diabetes Study of Northern California. JAMA Intern Med. 2013;173(3):210–218. PMID 23277199. doi:10.1001/jamainternmed.2013.1216
14. Website Norstat [homepage on the Internet]. Available from: https://norstatgroup.com/.
15. Norstat privacy policy. Available from: https://www.norstatpanel.com/en/privacy-policy.
16. Reach G, Benarbia L, Bruckert E, et al. Intentionality in adherence to long-term therapies. Results from an online survey of 3,001 patients with cardio-metabolic pathologies in France. Patient Prefer Adherence. 2021;15:1739–1753. PMID: 34408407; PMCID: PMC8366034. doi:10.2147/PPA.S318116
17. Girerd X, Hanon O, Anagnostopoulos K, Ciupek C, Mourad JJ, Consoli S. Evaluation de l’observance du traitement antihypertenseur par un questionnaire: mise au point et utilisation dans un service spécialisé [Assessment of antihypertensive compliance using a self-administered questionnaire: development and use in a hypertension clinic]. Presse Med. 2001;30(21):1044–1048. French. PMID: 11471275.
18. Phillips LA, Cohen J, Burns E, Abrams J, Renninger S. Self-management of chronic illness: the role of ‘habit’ versus reflective factors in exercise and medication adherence. J Behav Med. 2016;39(6):1076–1091. PMID: 26980098. doi:10.1007/s10865-016-9732-z
19. Reach G. Obedience and motivation as mechanisms for adherence to medication: a study in obese type 2 diabetic patients. Patient Prefer Adherence. 2011;5:523–531. PMID: 22114466; PMCID: PMC3218113. doi:10.2147/PPA.S24518
20. Reach G, Pellan M, Crine A, Touboul C, Ciocca A, Djoudi Y. Holistic psychosocial determinants of adherence to medication in people with type 2 diabetes. Diabetes Metab. 2018;44(6):500–507. PMID: 30031714. doi:10.1016/j.diabet.2018.06.001
21. Reach G, Chenuc G, Maigret P, Elias-Billon I, Martinez L, Flipo RM. Implication of character traits in adherence to treatment in people with gout: a reason for considering nonadherence as a syndrome. Patient Prefer Adherence. 2019;13:1913–1926. PMID 31806940. doi:10.2147/PPA.S227329
22. Hjellbrekke J. Multiple Correspondence Analysis for the Social Sciences. Routledge; 2018.
23. ESOMAR. ESOMAR guideline for online research; 2011. Available from: https://www.esomar.org/uploads/public/knowledge-and-standards/codes-and-guidelines/ESOMAR_Guideline-for-online-research.pdf.
24. Reach G. A novel conceptual framework for understanding the mechanism of adherence to long term therapies. Patient Prefer Adherence. 2008;2:7–19. PMID: 19920939, PMCID: PMC2770416.
25. Davidson D. Paradoxes of irrationality. In: Wollheim R, Hopkins J editors. Philosophical Essays on Freud. Cambridge. Cambridge University Press; 1982:289–305. doi:10.1017/CBO9780511554636.018
26. Triandis HC. Values, attitudes, and interpersonal behavior. Nebr Symp Motiv. 1980;27:195–259. PMID 7242748.
27. Reach G. Role of habit in adherence to medical treatment. Diabet Med. 2005;22(4):415–420. PMID: 15787666. doi:10.1111/j.1464-5491.2004.01449.x
28. Safran DG, Taira DA, Rogers WH, Kosinski M, Ware JE, Tarlov AR. Linking primary care performance to outcomes of care. J Fam Pract. 1998;47(3):213–220. PMID: 9752374.
29. Thom DH. Stanford Trust Study Physicians. Physician behaviors that predict patient trust. J Fam Pract. 2001;50(4):323–328. PMID: 11300984.
30. Bonds DE, Camacho F, Bell RA, Duren-Winfield VT, Anderson RT, Goff DC. The association of patient trust and self-care among patients with diabetes mellitus. BMC Fam Pract. 2004;5(1):26. PMID: 15546482; PMCID: PMC535564. doi:10.1186/1471-2296-5-26
31. Nguyen GC, LaVeist TA, Harris ML, Datta LW, Bayless TM, Brant SR. Patient trust-in-physician and race are predictors of adherence to medical management in inflammatory bowel disease. Inflamm Bowel Dis. 2009;15(8):1233–1239. PMID: 19177509; PMCID: PMC2799328. doi:10.1002/ibd.20883
32. Zikmund-Fisher BJ, Hofer TP, Klamerus ML, Kerr EA. First things first: difficulty with current medications is associated with patient willingness to add new ones. Patient. 2009;2(4):221–231. PMID: 20634991; PMCID: PMC2902151. doi:10.2165/11313850-000000000-00000
33. Friedman M, Rosenman RH. Association of specific overt behavior pattern with blood and cardiovascular findings; blood cholesterol level, blood clotting time, incidence of arcus senilis, and clinical coronary artery disease. JAMA. 1959;169(12):1286–1296. PMID 13630753. doi:10.1001/jama.1959.03000290012005
34. Kupper N, Denollet J. Type D personality as a risk factor in coronary heart disease: a review of current evidence. Curr Cardiol Rep. 2018;20(11):104. PMID: 30209683; PMCID: PMC6153564. doi:10.1007/s11886-018-1048-x
35. Goodwin RD, Friedman HS. Health status and the five-factor personality traits in a nationally representative sample. J Health Psychol. 2006;11(5):643–654. PMID: 16908463. doi:10.1177/1359105306066610
36. Huang I-C, Lee JL, Ketheeswaran P, Jones CM, Revicki DA, Wu AW. Does personality affect health-related quality of life? A systematic review. PLoS One. 2017;12(3):e0173806. PMID 28355244. doi:10.1371/journal.pone.0173806
37. Taboada-Vázquez A, Gonzalez-Rodriguez R, Gandoy-Crego M, Clemente M. Personality variables as predictors of health services consumption. Int J Environ Res Public Health. 2021;18(10):5161. PMID: 34068016. doi:10.3390/ijerph18105161
38. Scheier MF, Matthews KA, Owens JF, et al. Optimism and rehospitalization after coronary artery bypass graft surgery. Arch Intern Med. 1999;159(8):829–835. PMID: 10219928. doi:10.1001/archinte.159.8.829
39. Aquarius AE, Denollet J, de Vries J, Hamming JF. Poor health-related quality of life in patients with peripheral arterial disease: type D personality and severity of peripheral arterial disease as independent predictors. J Vasc Surg. 2007;46(3):507–512. PMID: 17681714. doi:10.1016/j.jvs.2007.04.039
40. Allison PJ, Guichard C, Gilain L. A prospective investigation of dispositional optimism as a predictor of health-related quality of life in head and neck cancer patients. Qual Life Res. 2000;9(8):951–960. PMID: 11284214. doi:10.1023/A:1008931906253
41. Linkievicz NM, Sgnaolin V, Engroff P, Behr Gomes Jardim G, Cataldo A. Neto association between big five personality factors and medication adherence in the elderly. Trends Psychiatry Psychother. 2021. PMID: 33834686. doi:10.47626/2237-6089-2020-0143
42. Hazrati-Meimaneh Z, Amini-Tehrani M, Pourabbasi A, et al. The impact of personality traits on medication adherence and self-care in patients with type 2 diabetes mellitus: the moderating role of gender and age. J Psychosom Res. 2020;136:110178.PMID: 32623192. doi:10.1016/j.jpsychores.2020.110178
43. Axelsson M, Brink E, Lundgren J, Lötvall J. The influence of personality traits on reported adherence to medication in individuals with chronic disease: an epidemiological study in West Sweden. PLoS One. 2011;6(3):e18241. PMID: 21464898; PMCID: PMC3065484. doi:10.1371/journal.pone.0018241
44. Novak JR, Anderson JR, Johnson MD, et al. Does personality matter in diabetes adherence? Exploring the pathways between neuroticism and patient adherence in couples with type 2 diabetes. Appl Psychol Health Well Being. 2017;9(2):207–227. PMID: 28401663; PMCID: PMC5511078. doi:10.1111/aphw.12087
45. Skinner TC, Bruce DG, Davis TM, Davis WA. Personality traits, self-care behaviours and glycaemic control in type 2 diabetes: the Fremantle diabetes study Phase II. Diabet Med. 2014;31(4):487–492. PMID: 24147848. doi:10.1111/dme.12339
46. Cheung MM, LeMay K, Saini B, Smith L. Does personality influence how people with asthma manage their condition? J Asthma. 2014;51(7):729–736. PMID: 24690024. doi:10.3109/02770903.2014.910220
47. Rhodes RE, Smith NE. Personality correlates of physical activity: a review and meta-analysis. Br J Sports Med. 2006;40(12):958–965. PMID: 17124108; PMCID: PMC2577457. doi:10.1136/bjsm.2006.028860
48. Mischel W, Shoda Y. A cognitive-affective system theory of personality: reconceptualizing situations, dispositions, dynamics, and invariance in personality structure. Psychol Rev. 1995;102(2):246–268. PMID: 7740090. doi:10.1037/0033-295x.102.2.246
49. Reach G. The Mental Mechanisms of Patient Adherence to Long-Term Therapies, Mind and Care. Springer; 2015.
50. Meyer VM, Benjamens S, Moumni ME, Lange JFM, Pol RA. Global overview of response rates in patient and health care professional surveys in surgery: a systematic review. Ann Surg. 2022;275(1):e75–81. PMID 32649458. doi:10.1097/SLA.0000000000004078
51. Miller TA. Health literacy and adherence to medical treatment in chronic and acute illness: a meta-analysis. Patient Educ Couns. 2016;99(7):1079–1086. doi:10.1016/j.pec.2016.01.020
52. Reach G. Clinical Inertia, a Critique of Medical Reason. Springer; 2015.
53. Duke SA, Colagiuri S, Colagiuri R. Individual patient education for people with type 2 diabetes mellitus. Cochrane Database Syst Rev. 2009;2009(1):CD005268. PMID: 19160249; PMCID: PMC648631850. doi:10.1002/14651858.CD005268
54. Aristotle. Metaphysics, A, 1, 981a. 15–20.
55. Sackett DL, Rosenberg WMC, Gray JAM, Haynes RB, Richardson WS. Evidence based medicine: what it is and what it isn’t. BMJ. 1996;312(7023):71–72. PMID 8555924. doi:10.1136/bmj.312.7023.71
56. Bouchard TJ
57. Zmorzyński S, Styk W, Klinkosz W, Iskra J, Filip AA. Personality traits and polymorphisms of genes coding neurotransmitter receptors or transporters: review of single gene and genome-wide association studies. Ann Gen Psychiatry. 2021;20(1):7. PMID: 33482861; PMCID: PMC7825153. doi:10.1186/s12991-021-00328-4
58. Lu F, Huo Y, Li M, et al. Relationship between personality and gray matter volume in healthy young adults: a voxel-based morphometric study. PLoS One. 2014;9(2):e88763. PMID: 24551159; PMCID: PMC3925163. doi:10.1371/journal.pone.0088763
59. Reach G. Simplistic and complex thought in medicine: the rationale for a person-centered care model as a medical revolution. Patient Prefer Adherence. 2016;10:449–457. PMID 27103790. doi:10.2147/PPA.S103007
60. Reach G. Decisions in the psychology of glucose monitoring. J Diabetes Sci Technol. 2019;13(6):1169–1174. PMID 31200613. doi:10.1177/1932296819854109
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.