Back to Journals » Cancer Management and Research » Volume 14
An Ovarian Large-Cell Neuroendocrine Carcinoma Accompanied by Clear Cell Carcinoma with Specific High Level of AFP: Case Report and Review of the Literature
Authors Qiu J, Xu J, Yao G, Zhu F, Wang Y, Fu Y
Received 2 April 2022
Accepted for publication 22 June 2022
Published 22 July 2022 Volume 2022:14 Pages 2235—2241
DOI https://doi.org/10.2147/CMAR.S366771
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Jian Qiu,1 Jiewei Xu,2 Guorong Yao,1 Fengjia Zhu,1 Yanyan Wang,3 Yunfeng Fu4
1Department of Obstetrics and Gynaecology, Huzhou Central Hospital, Affiliated Central Hospital Huzhou University, Huzhou, 313000, People’s Republic of China; 2Department of General Surgery, Huzhou Central Hospital, Affiliated Central Hospital Huzhou University, Huzhou, 313000, People’s Republic of China; 3Department of Pathology, Huzhou Central Hospital, Affiliated Central Hospital Huzhou University, Huzhou, 313000, People’s Republic of China; 4Department of Gynecologic Oncology, Women’s Hospital, School of Medicine, Zhejiang University, Hangzhou, 310006, People’s Republic of China
Correspondence: Yunfeng Fu, Department of Gynecologic Oncology, Women’s Hospital, School of Medicine, Zhejiang University, Xueshi Road #2, Hangzhou, 310006, People’s Republic of China, Tel +86-571-87061501, Fax +86-571-87061878, Email [email protected]
Abstract: Large cell neuroendocrine carcinoma (LCNEC) is a rare histological subtype of ovarian cancer. A few cases have been reported in the literature with extreme invasiveness and a poor prognosis. However, there still have not been accepted criteria for diagnosis and treatment of LCNEC. Here we report an unmarried 37 year-old woman who was diagnosed with LCNEC associated with clear cell carcinoma and the tumor index was manifested with a specific increase of AFP. The case received six courses of etoposide and carboplatin chemotherapy as an adjuvant therapy after primary curative surgery. However, she relapsed within 6 months after surgery and metastasized rapidly to distant organs despite combined chemotherapy of paclitaxel, cisplatin, and bevacizumab, and died 18 months after primary surgery. This is the first reported case of LCNEC manifested with a specific increase of AFP and characteristically metastasized to the spine as recurrence. Reviewing our case as well as previously reported cases, LCNEC present with aggressive malignancy and vulnerable to distant metastasis through a hematogenous approach, we conjectured that adding Bevacizumab in primary chemotherapy may be beneficial to extend disease-free survival. But so far there is no recommendation of this regimen for treatment of LCNEC in current guidelines. Further research is needed to confirm this view so as to find the best treatment of LCNEC and improve the prognosis of these patients.
Keywords: large cell neuroendocrine carcinoma, ovarian tumors
Background
Large cell neuroendocrine carcinoma (LCNEC) is a rare histological subtype of ovarian cancer which is characterized by the presence of large cells with ample cytoplasm, highly malignant nuclei, abundant mitotic activity, and necrosis, and has a tendency to neuroendocrine differentiation.1 However, up till now, there exist no generally accepted criteria for neuroendocrine tumor differentiation, which usually depends on histological and immunohistochemical analysis. Trabecular architecture, insular architecture with or without central necrosis, and cytoplasmic granularity usually suggest the neuroendocrine differentiation.2 Additionally, immunohistochemical markers such as chromogranin A, synaptophysin and CD56 are required to confirm the diagnosis of LCNEC.3,4 LCNEC is usually accompanied by other epithelial and germ tumors, pure LCNEC is extremely rare.5–8 Despite extensive surgery and adjuvant chemotherapy performed for this type of tumor, the prognosis is commonly poor in the published literature, even when the diagnosis is made at an early stage.3,9,10 The survival period ranges from 3–120 months, while median overall survival was only 10 months. Here, we present a case of a 37 year old woman who was diagnosed with stage IIIA1 LCNEC of the ovary. She was characterized by presentation of combined clear cell carcinoma and elevated AFP level, which were different from other reported LCNEC cases. We present the clinical details and imaging findings, followed by discussions of treatment and prognostic factors.
Case Presentation
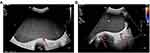
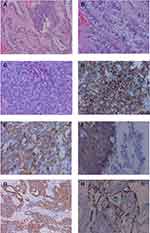
A 37-year-old unmarried woman without sexual life who complained of abdominal distension with frequent urination for a month attended our hospital. Physical examination revealed that the abdomen was obviously swollen and a mass was detected in the abdominal cavity. Ultrasonographic tomography and magnetic resonance imaging (MRI) demonstrated a monolocular cyst measuring 25 cm in diameter in the abdominal cavity (Figures 1 and 2). Preoperative serum level of Alpha-fetoprotein (AFP) was 962.23 ng/mL, carbohydrate antigen (CA)125 was 40.30 U/mL, and CA19-9 was 421.46 U/mL, while other tumor indexes like squamous cell carcinoma antigen (SCC), carcinoembryonic antigen (CEA), and lactate dehydrogenase were within normal ranges. Chest computed tomography (CT), gastroscopy, and colonoscopy also showed no abnormalities. After discussion about the possible risk factors of each treatment, she chose radical surgery. A huge cystic mass with a solid component in the pelvic and abdominal cavity was found in laparotomy. We suctioned the sac fluid to shrink the mass under the protection of the incision and explored to find that the tumor originated from the right ovary. The uterus, tubes, left ovary, and omentum were normal and no other tumor was evident in the abdominal cavity. Pelvic and para-aortic lymph nodes were not swollen upon palpation. Total abdominal hysterectomy, bilateral salpingo-oophorectomy, omentectomy, pelvic lymphadenectomy, abdominal aortic lymph node resection, and multiple peritoneal biopsy were performed. Peritoneal washing cytology was negative. On pathological examination, the right attachment contained ovarian clear cell carcinoma (approximately 10%) and poorly differentiated carcinoma (consistent with large cell neuroendocrine carcinoma, accounting for approximately 90%) (Figure 3A–C). Most of the tumor had a characteristic nest formation with central and peripheral coagulative necrosis. A metastasis lesion was found in one of the right internal iliac lymph nodes under the microscope. Immunohistochemistry was performed to assess the features of the large cells. The neuroendocrine component proved positive for CD56, chromogranin, and synaptophysin (Figure 3D–F). Besides, the tumor cells were also intensely positive for CK and CK7 (Figure 3G and H), while immunostaining for ER and PR was negative.
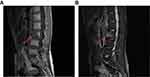
According to FIGO staging of ovarian cancer, she was diagnosed as LCNEC for stage IIIA1. Since LCNEC occupied 90% of the tumor tissue, based on the experience reported in the literature, six courses of postoperative EP [120 mg/m 2 VP-16 and carboplatin at AUC 5.0] chemotherapy were carried out after surgery. AFP level decreased to normal after the second course of chemotherapy while other abnormal tumor indicators were decreased to normal after the first course of chemotherapy, indicating that chemotherapy was effective and achieves complete clinical remission. Unfortunately, the tumor index of AFP increased again in the 6 months after surgery and showed a progressive upward trend in the next 6 months (28.39 ng/mL~7,346 ng/mL). PET-CT scan showed that there were multiple enlarged lymph nodes in the upper and lower left clavicle, left pectoral muscle space, left axillary, left hilum, bilateral diaphragm feet, and retroperitoneum. Meanwhile, the third lumbar spine vertebrae (L3 vertebra) showed osteolytic bone destruction, FDG metabolism is abnormally increased, considering metastasis, which was further proved by lumbar MRI (Figure 4). She received TCA (Paclitaxel 200 mg d1, Cisplatin 30 mg d1–d3, and Bevacizumab 280 mg d1 q3w) as the first-line chemotherapy. However, a clinical response to this regimen was not observed and the cancer progressed rapidly. She died of tumor progression 18 months after her primary operation. The timeline of the process of diagnosis and treatment for this case is shown in Figure 5.
 |
Figure 5 Timeline of the process of diagnosis and treatments of this case. |
Discussion and Conclusions
Ovarian LCNEC is a rare, aggressive, poorly-differential neuroendocrine tumor that is often with poor outcome. The histogenesis of neuroendocrine tumors remains unclear. Several hypotheses suggested that LCNEC may derive from ovarian neuroendocrine cells, non-neuroendocrine cells, teratomatous cells, or primitive cells,11 so LCNEC is usually accompanied by other epithelial and germ cell tumors, and pure LCNEC is extremely rare. According to previous cases, associated ovarian cancer is mainly mucinous carcinoma, and endometrioid carcinoma, serous carcinoma, or teratoma are less common.12,13 The tumor in our case was lined with a small part of clear cell carcinoma, while most of the tumor consisted of a poorly differentiated component of large tumor cells with large nuclei and prominent nucleoli. Immunohistochemistry revealed that the cells of the poorly differentiated component were diffusely positive for CD56, chromogranin A, and synaptophysin, which fulfilled the histopathological criteria for a neuroendocrine carcinoma. To our knowledge, this case presented for the first time that LCNEC can also be associated with clear cell carcinoma.
The initial symptoms presented by ovarian LCNEC with early stage are not typical, and most of them are found accidentally during surgery from rapid pathological examination. The clinical manifestations of ovarian LCNEC with advanced stage are identical to that of epithelial ovarian cancer (EOC), such as the presence of an abdominal mass, pain, or distention.9 In addition, endocrine-related symptoms may occur among some patients with ovarian LCNEC, such as hypercalcemia caused by abnormal secretion of parathyroid hormone, which was reported by Ohira.15 Based on previousky reported cases, more than half of the patients were diagnosed at an advanced stage (III–IV), indicating that it is difficult to diagnose ovarian LCNEC at an early stage.
There is no specific tumor index for ovarian LCNEC. Because it is often associated with ovarian epithelial tumors and germ cell tumors, various tumor markers can be elevated,11 such as carbohydrate antigen 125 (CAl25), carbohydrate antigen 199 (CA199), carbohydrate antigen 724 (CA724), carcinoembryonic antigen (CEA), and neuronspecincenolase (NSE). Among them, CA125 elevation occurs most frequently, occupying 83.87%. In our presented case, tumor index was specified by abnormally elevated level of AFP while the level of CA125 was increased slightly. Moreover, it is only manifested as a continual increase of AFP level in the later course of recurrence. To our knowledge, this is the first reported case of LCNEC characterized by specific elevation of AFP level which is further to be used as a monitor for disease recurrence and progression in the follow-up.
It has been reported that ovarian LCNEC is extremely aggressive and its prognosis appears to be very poor despite extensive surgery and adjuvant chemotherapy. Yang et al11 conducted a statistical analysis of previous cases, and found that the median overall survival of LCNEC is only 10.0 months. The total 5-year survival was 34.9%, and still only 35.3% for stage I cases, even though over 95% of these cases accepted complete or optimal surgery.11 These results may be mainly due to the high recurrence rate of LCNEC, and once recurrence occurs, it is not only limited to the abdominal cavity, but also to specific sites that differed from the usual ovarian cancer distribution. For example, the reported case by Kayo Asada showed multiple liver metastases 4 months after her primary operation even diagnosed at stage Ia (pT1aN0M0),11 brain metastasis was observed 17 months after initial treatment of LCNEC in a case reported by Oshita et al.3 It can also metastasize to the lung, mediastinal lymph nodes, bone, and so on.2 These results suggest that LCNEC may have strongly lymph-vascular space invasion, which contributes to its poor prognosis.11
In our case, although the primary chemotherapy was effective and achieved complete clinical remission, the recurrence rapidly occurred 6 months after primary surgery, no obvious recurrence lesion was found at the beginning, when the patient complained about the pain in her lumbar spine, further examination was taken which found the destruction of the vertebra L3, indicating that LCNEC is prone to hematogenous metastasis. Therefore, for patients with LCNEC, once recurrence occurs, a systemic assessment should be performed to detect metastatic lesions as soon as possible, which may benefit from early treatment decisions and prognosis.
Until now, there is no accepted consensus treatment for patients with LCNEC, the clinical management of most reported cases still follows the conventional regimens for epithelial ovarian cancer, which is the cytoreductive surgery combined with platinum-based chemotherapy. There were also a few case reports using other chemotherapy options, including cyclophosphamide combined with cisplatin, irinotecan combined with nedaplatin, liposomes doxorubicin monotherapy, and BEP programs.2,8,9,14–16 But all these reported chemotherapeutic regimens seem to not be very effective for LCNEC. In our case, the patient was sensitive to initial EP regimen chemotherapy, achieving complete clinical remission, but she relapsed soon after initial treatment, and migrated to spine through blood approach. So we add Bevacizumab for chemotherapy. But the response seems to not be satisfied as we expected. By retrospective analysis of this case, we are also thinking about whether Bevacizumab could reduce the probability of recurrence and improve its prognosis if it is used in primary treatment since LCNEC is easily migrating to distant places through blood approach.
In summary, LCNEC is a very rare historical subtype of ovarian cancer with extremely aggressive behavior. For the first time, We reported a case of LCNEC coexisted with clear cell carcinoma and characterized by elevation of AFP. According to previously reported cases and our case, LENEC can easily metastasize to distant place through blood approach, we fiercely recommend that adding Bevacizumab in primary chemotherapy in treatment of LCNEC, which may be beneficial to extend disease-free survival since it has been proved that Bevacizumab can significantly extend PFS for advanced epithelial ovarian cancer with high risk of recurrence according to previous data. However, the use of bevacizumab in treating patients with LCNEC is no recommendation in the current guidelines, and this regimen is also not supported by our case’s disease course. It is urgent to appeal a global medical database of ovarian LCNEC be collected and much more research should be investigated to confirm this proposal and to establish optimal adjuvant chemotherapy.
Ethics Approval and Consent
This report was approved by the Ethics Committee of Huzhou Central Hospital (No. 202110016-02). Signed informed consent for publication of her clinical data was obtained from the patient.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the project of Zhejiang Medical and Health Science and Technology (grant number: 2022KY357).
Disclosure
The authors declare that they have no conflicts of interest in relation to this work.
References
1. Eichhorn JH, Young RH. Neuroendocrine tumors of the genital tract. Am J Clin Pathol. 2001;115:S94–S112. doi:10.1309/64CW-WKGK-49EF-BYD1
2. Chenevert J, Bessette P, Plante M, Tetu B, Dube V. Mixed ovarian large cell neuroendocrine carcinoma, mucinous adenocarcinoma, and teratoma: a report of two cases and review of the literature. Pathol Res Pract. 2009;205(9):657–661. doi:10.1016/j.prp.2009.01.013
3. Oshita T, Yamazaki T, Akimoto Y, et al. Clinical features of ovarian large-cell neuroendocrine carcinoma: four case reports and review of the literature. Exp Ther Med. 2011;2(6):1083–1090. doi:10.3892/etm.2011.325
4. Gardner GJ, Reidy-Lagunes D, Gehrig PA. Neuroendocrine tumors of the gynecologic tract: a Society of Gynecologic Oncology (SGO) clinical document. Gynecol Oncol. 2011;122(1):190–198. doi:10.1016/j.ygyno.2011.04.011
5. Hirasawa T. Ovarian neuroendocrine carcinoma associated with mucinous carcinoma and teratoma. Nihon Rinsho. 2004;62(5):973–978.
6. Choi YD, Lee JS, Choi C, Park CS, Nam JH. Ovarian neuroendocrine carcinoma, non-small cell type, associated with serous carcinoma. Gynecol Oncol. 2007;104(3):747–752. doi:10.1016/j.ygyno.2006.11.008
7. Chen KT. Composite large-cell neuroendocrine carcinoma and surface epithelial-stromal neoplasm of the ovary. Int J Surg Pathol. 2000;8(2):169–174. doi:10.1177/106689690000800214
8. Collins RJ, Cheung A, Ngan HY, Wong LC, Chan SY, Ma HK. Primary mixed neuroendocrine and mucinous carcinoma of the ovary. Arch Gynecol Obstet. 1991;248(3):139–143. doi:10.1007/BF02390091
9. Veras E, Deavers MT, Silva EG, Malpica A. Ovarian nonsmall cell neuroendocrine carcinoma: a clinicopathologic and immunohistochemical study of 11 cases. Am J Surg Pathol. 2007;31(5):774–782. doi:10.1097/01.pas.0000213422.53750.d1
10. Asada K, Kawana K, Teshima S, Saito A, Kawabata M, Fujii T. Poor prognosis of ovarian cancer with large cell neuroendocrine carcinoma: case report and review of published works. J Obstet Gynaecol Res. 2014;40(3):869–872. doi:10.1111/jog.12235
11. Yang X, Chen J, Dong R. Pathological features, clinical presentations and prognostic factors of ovarian large cell neuroendocrine carcinoma: a case report and review of published literature. J Ovarian Res. 2019;12(1):69. doi:10.1186/s13048-019-0543-z
12. Eichhorn JH, Lawrence WD, Young RH, Scully RE. Ovarian neuroendocrine carcinomas of non-small-cell type associated with surface epithelial adenocarcinomas. A study of five cases and review of the literature. Int J Gynecol Pathol. 1996;15(4):303–314. doi:10.1097/00004347-199610000-00002
13. Mhawech-Fauceglia P, Odunsi K, Dim D, et al. Array-comparative genomic hybridization analysis of primary endometrial and ovarian high-grade neuroendocrine carcinoma associated with adenocarcinoma: mystery resolved? Int J Gynecol Pathol. 2008;27(4):539–546. doi:10.1097/PGP.0b013e31816bcda4
14. Ohira S, Itoh K, Shiozawa T, et al. Ovarian non-small cell neuroendocrine carcinoma with paraneoplastic parathyroid hormone-related hypercalcemia. Int J Gynecol Pathol. 2004;23(4):393–397. doi:10.1097/01.pgp.0000139655.18062.12
15. Draganova-Tacheva RA, Khurana JS, Huang Y, Hernandez E, Zhang X. Large cell neuroendocrine carcinoma of the ovary associated with serous carcinoma with mucin production: a case report and literature review. Int J Clin Exp Pathol. 2009;2(3):304–309.
16. Clark OH, Benson AR, Berlin JD, et al. NCCN clinical practice guidelines in oncology: neuroendocrine tumors. J Natl Compr Canc Netw. 2009;7(7):712–747.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.