Back to Journals » Journal of Pain Research » Volume 12
An integrated safety analysis of combined acetaminophen and ibuprofen (Maxigesic®/ Combogesic®) in adults
Authors Aitken P , Stanescu I, Playne R , Zhang J, Frampton CMA, Atkinson HC
Received 9 October 2018
Accepted for publication 2 January 2019
Published 8 February 2019 Volume 2019:12 Pages 621—634
DOI https://doi.org/10.2147/JPR.S189605
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Michael A Ueberall
Phillip Aitken,1 Ioana Stanescu,1 Rebecca Playne,1 Jennifer Zhang,1 Christopher MA Frampton,2 Hartley C Atkinson1
1Drug Development, AFT Pharmaceuticals Ltd, Auckland, New Zealand; 2Department of Medicine, University of Otago, Christchurch, New Zealand
Introduction: Acetaminophen (APAP) and ibuprofen (IBP) are two analgesic compounds with a long history of use. Both are considered safe at recommended over-the-counter daily doses. Chronic use, high doses, or concomitant medication can produce safety risks for both drugs. APAP is associated with increased risk of hepatic injury, while IBP can produce gastric bleeding and thromboembolic events. Using a combination of APAP and IBP provides superior analgesia without transgressing daily dose limits of each individual drug.
Methods: The present study aimed to determine if treatment with a fixed-dose combination (FDC) containing APAP and IBP results in any unexpected adverse events (AEs) and/or changes in the safety profiles of its two ingredients compared to monotherapy. The analysis will examine clinical safety data obtained from either single dose trials, multiple dose trials, a long-term exposure trial, and post-marketing surveillance data of APAP/IBP FDC tablets (Maxigesic®/Combogesic®, AFT Pharmaceuticals Ltd). The largest dataset was obtained by pooling the four randomized-controlled, multiple-dose clinical studies with either APAP 325 mg + IBP 97.5 mg (FDC 325/97.5, three tablets per dose) or APAP 500 mg + IBP 150 mg (FDC 500/150, two tablets per dose). At maximum doses, the two FDCs are bioequivalent, permitting the pooling of data for the analysis of safety.
Results: A safety population of 922 patients who received full doses of either FDC, APAP alone, IBP alone, or placebo was compiled from the four studies. A total of 521 AEs were experienced with the incidence of FDC AEs similar to or below either monotherapy group or placebo. The FDC did not alter the incidence and percentage of the most common AEs, including gastrointestinal events and postoperative bleeding.
Conclusion: Overall, the FDC is well tolerated and has a strong safety profile at single and multiple doses with improved efficacy over monotherapy.
Keywords: paracetamol, nonsteroidal anti-inflammatory drugs, surgical pain, postoperative analgesia, multimodal pain management
Plain language summary
The combined use of acetaminophen (APAP) and ibuprofen (IBP) for pain management has many potential benefits over other pharmaceutical treatments such as opioids (codeine, tramadol, oxycodone, morphine, etc). It has been shown previously that a combination of APAP and IBP fixed dose product provides increased pain relief compared with the individual components. There remains uncertainty over the safety of combining these products, in particular the use of IBP following surgery, due to the increased risk of postoperative bleeding which is common in drugs of this type (nonsteroidal anti-inflammatory drugs).
This paper examines the safety profile of a combination of APAP and IBP product using pooled clinical trial data. The clinical trials examined multiple doses in tablet form of the combination therapy. Patients were provided with the maximum recommended dose of either APAP alone, IBP alone, the combination, or a placebo tablet during the 24–48 hours following either dental or knee surgery.
Overall, the combination tablets are well tolerated. The safety profile was found to be the same as APAP or IBP when taken alone. Together, with the additional pain relief provided by the combination, this shows an improved and beneficial treatment that can be well tolerated following surgery.
Introduction
Multimodal analgesia has the potential to provide safe and effective management of postoperative pain.1 Combining oral analgesics into a single product potentially increases compliance, safety, and efficacy.2 The use of acetaminophen (APAP; N-acetyl-para-aminophenol or paracetamol) and/or ibuprofen (IBP) in postoperative pain management is well established internationally, often provided as an adjunct to opioid analgesia.3–5 The current opioid crisis in the United States illustrates the immediate need for alternative or adjunct nonopioid analgesics with improved safety profiles.6 Opioid use can lead to addiction, with users developing a compulsive need to continue taking opioids despite negative consequences.7 Many opioid abusers begin their addiction through prescriptions for the treatment of postoperative pain.8 More than 40% of all opioid deaths in the United States involve prescription opioids.9 More than 75% of people misusing prescription opioids receive excess medication prescribed to friends or relatives.10 Other countries, such as Australia,11,12 New Zealand,13,14 Canada,15 and the UK16,17 have also seen increases in the number opioid prescriptions and opioid-related deaths in recent years.
The combination of APAP and IBP is an effective alternative to opioid-based analgesia.18–27 IBP is a nonsteroidal anti-inflammatory drug (NSAID) with analgesic, antipyretic, and anti-inflammatory activities. A good tolerability profile along with extensive clinical experience has resulted in IBP being sold over-the-counter (OTC) in pharmacies worldwide, as well as in supermarkets and other general retailers. Approved indications include fever reduction and relief of minor aches and pains associated with the common cold, headache, toothache, muscular aches, backache, arthritis, and menstrual cramps.
Like other NSAIDs, IBP is believed to work by inhibiting cyclooxygenase (COX), thus inhibiting prostaglandin synthesis.28 The COX family of enzymes is responsible for the metabolism of arachidonic acid to prostaglandin H2, an unstable molecule, which is in turn converted to numerous other pro-inflammatory compounds. There are at least two COX isoforms, designated as COX-1 and COX-2. IBP inhibits both isoforms nonselectively. Its desired activities are principally due to COX-2 inhibition, while its unwanted side effects on platelet aggregation and the gastrointestinal (GI) mucosa are due to COX-1 inhibition.29 The activity of the COX enzyme relies on being in the oxidized form.30,31 It has been shown that APAP can reduce the oxidized form of the COX enzyme in low peroxide environments such as the central nervous system, but not in peripheral immune cells.32
APAP is an analgesic and antipyretic agent that has little anti-inflammatory activity. It has been used clinically for more than a century, having been introduced into use in 1893 and is now the most widely prescribed analgesic in the world.33 APAP is commonly used for the relief of fever, headaches, and other minor aches and pains. Unlike other common OTC analgesics such as aspirin and IBP, APAP has a relatively little anti-inflammatory activity, and so it is not considered to be an NSAID. Used in combination with NSAIDs, APAP is useful in the management of more severe pain, where it allows lower dosages of NSAIDs to be used, thereby minimizing the risk of class-related adverse effects.18,19,34,35
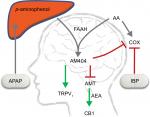
APAP undergoes a two-step metabolic process to reach the active metabolite (Figure 1).36 APAP is first metabolized in the liver to p-aminophenol which is then metabolized in the brain by fatty acid amide hydrolase (FAAH) containing cells into N-(4-Hydroxyphenyl) arachidonylamide (AM404).33,36–40 AM404 is a potent agonist of the transient receptor potential vanilloid type 1 (TRPV1),39 a low-affinity ligand of the cannabinoid receptor type 1 (CB1),36,41 an anandamide membrane transporter blocker,42 and a COX inhibitor.36 Furthermore, AM404 inhibits sodium channels in a similar manner to neuroprotectants and anesthetics.43 All the above actions have individually been shown to reduce pain and are considered possible mechanisms of action for APAP. It has been demonstrated that after either genetically or pharmacologically blocking cannabinoid receptors,41,44 or knocking out FAAH and TRPV1 in mice,37 APAP no longer has any analgesic effect, suggesting the pain-relieving action is mediated by the activation of both TRPV1 and CB1 in the central nervous system.
APAP has a recommended maximum daily dose of 4,000 mg in adults. Exceeding this dose increases the risk of hepatic injury from the toxic metabolite NAPQI (N-Acetyl-4-benzoquinoneimine).45 IBP, while having one of the best safety profiles of any COX inhibitor, may still cause GI and cardiovascular adverse effects.46,47 In the elderly population, reduced doses of both APAP and IBP are recommended due to poor clearance.48–50 Clinical trial data in oral surgery found that a combination of APAP and IBP provides greater pain relief than either drug alone.18,27,35,51
Combination treatment with APAP and IBP has the potential to provide greater analgesia while using lower doses of each individual drug, reducing the likelihood/severity of adverse effects.18,19,27 There is also evidence of combination APAP and IBP treatment providing synergistic efficacy.52 Safety profiles of APAP and IBP alone are well understood as they have been used extensively in many countries for decades. Therefore, the present study aimed to determine if treatment with a fixed-dose combination (FDC) containing APAP and IBP results in any unexpected adverse events (AEs) and/or changes in the safety profiles of its two ingredients compared with monotherapy. This review specifically examines the clinical safety data collected during clinical studies of FDCs containing APAP and IBP at a 3:1 ratio (Maxigesic®/Combogesic®).
Methods
Study selection for safety population
The data in this cumulative safety analysis are derived from all published and unpublished, Phase II/III sponsored (AFT Pharmaceuticals Ltd) clinical studies investigating oral dosing of APAP/IBP fixed-dose tablets for the treatment of pain. Four randomized controlled, multiple-dose clinical studies have been conducted.18,19,27,53–56 All studies were approved by the sites’ local independent ethics committees. All participants were at least 16 years of age, and written informed consent was obtained either directly from the participant or from his/her legally authorized representative. The clinical trials were conducted with two different strengths, the FDC 325/97.5 (APAP 325 mg + IBP 97.5 mg tablets, three tablets per dose every 6 hours, Study: AFT-MX-6)53 and the related higher strength combination FDC 500/150, (APAP 500 mg + IBP 150 mg, two tablets per dose every 6 hours, Studies: AFT-MX-1, AFT-MX-3, and AFT-MX-6E).54–56 Studies concerning both FDCs constitute the primary source of safety information in this review. Pooling data from both combinations is justified by the fact that the cumulative dose of both active ingredients from the lower strength product (FDC 325/97.5) is 97.5% of that of the related product (FDC 500/150) at full doses (three and two tablets, respectively). The products have been demonstrated to fall within 80%–125% bioequivalence in Phase I pharmacokinetic studies under fed and fasting conditions.57–60 Therefore, for the purposes of safety, these two products can be considered similar.
Study characteristics
Phase I single-dose studies
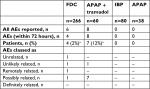
A total of ten Phase I pharmacokinetic trials have been performed for the FDCs, all of which were of single-dose crossover design with washout periods of 3–7 days (Table 1).57–59,61–67 AEs occurring within 72 hours of drug administration were pooled across trials and their relationship to the study drug reported. AEs occurring either prior to drug administration or >72 hours following administration were not included in the pooled data.
Phase II/III repeated-dose studies
Each of the Phase II/III repeated-dose clinical studies were of double-blind, randomized, parallel group design. The duration of the double-blind treatment periods of AFT-MX-6 and AFT-MX-1 were 48 hours, while AFT-MX-3 and AFT-MX-6E were 24 hours (Table 1). Only AEs that occurred during the double-blind treatment period were included in the pooled safety analysis. AFT-MX-6E included a 24 hour double-blind phase after which subjects were offered additional doses of either the FDC 500/150 or APAP 500 mg as part of an open-label phase extension. Inclusion criteria for each study varied, but were based on age and surgical type (Table 1). Exclusion criteria included patients unable/unwilling to provide consent, women of childbearing potential, recent NSAID, APAP, or opioid use, and contraindications to IBP or APAP. Double-blinding was performed in all trials. Blinding was achieved by the use of tablets that were identical in appearance, packaging, and administration route. Each participant was allocated a unique randomization number. This unique randomization number was presented on the label of the study medication. Both participants and investigators were blinded to the treatment allocation. In AFT-MX-3, 80 subjects were administered quarter or half doses of the combination product (FDC 250/75 and FDC 125/37.5) and were therefore omitted from the safety population to maintain the comparison of FDCs with full-dose active comparators or placebo.
Long-term exposure studies
The safety profile of long-term exposure to the FDC was investigated in an exploratory Phase II study (AFT-MX-4).68 Patients undergoing treatment for osteoarthritis of the knee were recruited (Table 1). Subject inclusion criteria included at least 6 months of previous treatment requiring analgesic medication with an average pain score of at least 40 mm and no more than 80 mm on the WOMAC Visual Analog Scale pain scales following a 3–7 days washout of existing analgesics. Subjects received double-blind treatment of either FDC, APAP, or IBP during the first 4 weeks. This was followed by an open-label phase for 48 weeks with the FDC only. Patients received the maximum dose approved for OTC use of two tablets every 6 hours (APAP 500 + IBP 150 mg, 2q6h).
Postmarketing surveillance
Reports of adverse drug reactions (ADRs) have been collected since 2009. The postmarketing surveillance ADRs are received through mandatory reporting by local distributors as part of their distribution agreement. The ADRs reported in the present study consist of all individual safety case reports from the global market between October 2009 and August 2018. An ADR was considered unexpected if it was not consistent with the nature or severity of ADRs listed in the product labeling.
Safety outcome measures
An AE was defined using the International Conference on Harmonization (ICH) guidelines as any unintended, unfavorable clinical sign or symptom, any new illness or disease or deterioration of existing illness or disease, or any clinically relevant deterioration in laboratory variables (eg, hematological, biochemical, and hormonal) or other clinical tests (eg, ECG and X-ray), whether or not considered treatment related.69 Planned hospital admissions and/or surgical operations for an illness or disease that existed before the drug was given or the participant was randomized in the clinical study were not considered AEs. Serious adverse events (SAEs) were defined according to ICH guidelines as one that resulted in death; was life-threatening; resulted in persistent or significant disability; resulted in hospitalization or prolongation of hospitalization; was a congenital anomaly/birth defect; was a medically significant event that, on the basis of appropriate medical judgement, may require medical or surgical intervention to prevent one of the outcomes listed above.69 All AEs were standardized to the Medical Dictionary for Regulatory Affairs version 9.1 or above and summarized by treatment group.
The term “study drug” here refers to either APAP, IBP, FDCs, or placebo. The term “baseline” is defined as the value obtained immediately prior to administration of the study drug. Any change from baseline is defined as the value at the named assessment time minus the baseline value. No transformations or imputations were applied to any safety data for the purpose of this analysis. Patients treated for pain had access to rescue medication regardless of treatment allocation in all trials. Rescue was either oxycodone (5–10 mg PO as required every 4–6 hours) during MX-3, MX-6, and MX-6E; or fentanyl (10 µg IV) and/or codeine (30–60 mg PO) during AFT-MX-1. All subjects who received at least one full dose of any study drug are included in the safety analysis, and all subjects were analyzed according to the actual drug received.
AEs were classified by the investigator in terms of severity and relationship to the study drug. The relationship of the study drug to each AE was deemed as either Unrelated, Unlikely Related, Possibly Related, or Definitely Related, considering the temporal relationship of the AE to administration of the study product and the likelihood that another cause could reasonably explain the event. In AFT-MX-1, the relationship of the AE with the study medication could be classified as Probable in addition to Possible. These terms were combined into the Possibly Related group in the pooled data. AEs could be rated as mild, moderate, or severe, depending on the level of discomfort and the effect of the AE on daily activity.
Statistical analysis
Data from four Phase II/III repeated-dose studies (AFT-MX-1, AFT-MX-3, AFT-MX-6, and AFT-MX-6E) were compiled to form the safety population. The AEs within the 24–48 hour double-blind periods were pooled for the integrated analysis. This included the majority (79%) of AEs recorded across all four studies (521/659). Descriptive and analytic statistics were calculated using IBM SPSS version 25.0 and GraphPad Prism version 7.04. The odds ratio for experiencing one or more AEs was calculated with GraphPad Prism. The number of subjects requiring rescue was analyzed with Fisher’s exact tests comparing the FDC to either APAP alone, IBP alone, or placebo with GraphPad Prism. The purpose of this integrated summary of safety is to compile data from all studies of the FDC 325/97.5 and FDC 500/150 to assess the safety profile of the combination relative to the comparators in a higher number of subjects across different pain models (third molar removal and arthroscopic knee surgery).
Ethics approval and informed consent
All studies were conducted in accordance with the ethical principles that have their origins in the Declaration of Helsinki, and in conformance with the ICH GCP guidelines (1997) and regulations of the sites’ local independent ethics committees (New Zealand, USA, and India). All enrollees were at least 16 years of age, and written informed consent was obtained either directly from the participant or from his/her legally authorized representative.
Results
Single-dose safety data
Across the ten Phase I trials, 266 subjects received a dose of the FDC 500/150 (two tablets) or FDC 325/97.5 (three tablets), 80 subjects received IBP (800 mg), 38 subjects received APAP (1,000 mg), and 60 subjects received Ultracet® (two tablets, APAP 325 mg + tramadol HCl 37.5 mg). Throughout all of the Phase I clinical trials, 14 AEs were reported within 72 hours of drug administration amongst 10 patients (Table 2). Five Phase I clinical trials had no reported AEs. During the other five Phase I studies, the majority of AEs (8/14 AEs, 57%) occurred within 24 hours of administration of combination APAP + tramadol. The AEs reported following APAP + tramadol use consisted of either abdominal pain, dyspnea, vomiting, headache, or dizziness. Among the four AEs reported within 72 hours of administration of the FDC, three were reported within 24 hours: catheter-site bruising (unrelated), dyspnea (remotely related), and dizziness (possibly related). A single AE, flank pain, was reported >24 hours after FDC 325/97.5; the same patient also reported flank pain during the APAP + tramadol treatment period. Both AEs were considered unlikely to be related to the study drugs.
Repeated-dose safety data
Patient population
A total of 1,002 patients were enrolled in the Phase II/III repeated-dose clinical efficacy studies of FDC 325/97.5 and FDC 500/150, of whom 922 patients were allocated to full-dose treatment groups or placebo. Of these, 408 were enrolled in AFT-MX-6 (FDC 325/97.5) and 514 were enrolled in clinical efficacy studies of FDC 500/150: 135 subjects in AFT-MX-1, 79 subjects in AFT-MX-3, and 300 subjects in AFT-MX-6E. Consequently, the safety population comprises a total of 922 patients: 261 received full doses of either FDC 325/97.5 or FDC 500/150, 231 received APAP alone (975 or 1,000 mg), 231 received IBP alone (292.5 or 300 mg), and 199 received placebo. A total of 18 patients (2%) across all studies were >65 years old. The demographics of the subjects that make up each pooled treatment group in the safety population (FDC, APAP, IBP, and placebo) are similar in terms of age, gender, and racial distribution (Table 3).
  | Table 3 Demographic characteristics and dosing of patients by treatment group Abbreviations: APAP, acetaminophen; FDC, fixed-dose combination; IBP, ibuprofen. |
Study medication exposure
Across the four clinical studies, 261 subjects were administered either FDC (325/97.5 or 500/150) over 24 or 48 hours (Table 3). Of the 261 subjects receiving the FDC, 243 received the maximum dose. There was no difference in the percentage of subjects receiving the full dose of medication between treatment groups (92%–93%).
Safety analysis
Overall, 314/922 (34%) patients in the safety population experienced at least one AE (Table 4). The pooled FDC and the pooled IBP monotherapy groups had comparable proportions of patients who experienced at least one AE (~30%). The pooled APAP monotherapy and placebo groups had comparable proportions that were higher than the other two groups (~38%). The odds of experiencing at least one AE during the double-blind period if exposed to full doses of FDC relative to each of the comparators was evaluated (Figure 2). In each of the comparisons, the odds of experiencing at least one AE after exposure to the FDC is not significantly different to odds for placebo (OR =0.73, 95% CI: 0.49–1.08). Furthermore, the odds of experiencing at least one AE following an FDC is not significantly different from APAP monotherapy (OR =0.72, 95% CI: 0.50–1.05) or IBP monotherapy (OR =1.08, 95% CI: 0.73–1.60).
  | Figure 2 Odds ratio of experiencing at least one adverse event during the double-blind treatment period. Abbreviations: APAP, acetaminophen; FDC, fixed-dose combination; IBP, ibuprofen. |
Common AEs
The incidence of the most common AEs was largely comparable between FDC, APAP, IBP, and placebo (Table 4). Nausea was the most common AE, accounting for 30% of all AEs and affecting 16% of patients. Vomiting and headache accounted for ~10% of all AEs each. The ten most common AEs (accounting for 74% of all AEs) were nausea, vomiting, headache, dizziness, facial swelling, postprocedural hemorrhage, constipation, dyspepsia, pruritus, and somnolence (Table 4). The distribution of these AEs was similar across studies; nausea, headache, and vomiting occurring at similar rates across all studies, with some procedure-related AEs occurring in each study (eg, facial swelling and alveolar osteitis in the wisdom teeth extraction studies).
Gastrointestinal effects
GI disorders such as abdominal pain, nausea, vomiting, dyspepsia, diarrhea, and constipation accounted for 54% of all pooled AEs (281/521) and affected 22.7% of patients (209/922). Eighty percent of these AEs were nausea and vomiting (223/281). IBP had the lowest incidence of GI AEs across pooled treatment groups, although this was not significantly different from the FDC group (Table 4).
Postoperative bleeding
Of the 521 pooled AEs, a total of 17 bleeding events were recorded by 17 separate subjects: 14 postprocedural hemorrhages and three instances of hemarthrosis. The incidence of bleeding events was 2.3% in the FDC group (6/261), 0.4% in the APAP group (1/231), 1.7% in the IBP group (4/231), and 3.0% in the placebo group (6/199). All subjects made a full recovery from bleeding events, with 14 events lasting 3 or fewer days. Three events were SAEs, and occurred in the placebo and IBP groups (Table 5).
Serious adverse events
A total of seven nonfatal SAEs were reported in six participants in the safety population (n=922). Of the seven nonfatal SAEs, only one was in a patient randomized to the FDC group (deep vein thrombosis in a patient treated with the FDC 500/150 in AFT-MX-6E), and this was not considered related to the study drug (Table 5). In AFT-MX-6E, subjects had the option to continue treatment in the open-label phase with the FDC or APAP 500 mg. Three of the six participants experiencing SAEs in AFT-MX-6E took the FDC during the open-label phase; however, only one of these was still taking the FDC when the SAE occurred.
One death occurred across all clinical trials. The death occurred in AFT-MX-6 and was due to a gunshot wound (homicide) during the trial follow-up period. The SAE was unrelated to the study medication.
Discontinuations due to AEs
Across the four Phase II/III repeated-dose clinical trials of the FDC, there were 11 discontinuations due to AEs (Table 4; 1.2%). Of the eleven participants who discontinued the study due to AEs, four (0.4%) were randomized to the placebo group, three (0.3%) were randomized to the APAP group, two (0.2%) were randomized to the IBP group, and two (0.2%) were in the FDC group. The majority of AEs that led to discontinuations from the study were either due to nausea/vomiting (n=4) or hemarthrosis associated with the surgical procedure (n=4).
Relationship of AEs with study medication
Overall, 16% of AEs were considered possibly related to the study medication (85/520). By treatment group, this was 22% for the FDC, 18% for APAP, 17% for IBP, and 8% for placebo (Table 6). Related AEs were largely considered either mild or moderate (Table 6), with only six considered severe, three of which were in the placebo group. The proportion of patients experiencing AEs that were considered possibly related to the study medication was comparable between the FDC and APAP groups (10%) and lowest in the IBP and placebo groups (5%–6%). No AEs were considered definitely related to the study medication.
Rescue medication
All pooled studies examined the use of rescue medication following surgery as a secondary efficacy end point. The percentage of patients requiring rescue medication was pooled and assessed. The percentage of subjects that required at least one dose of rescue medication was highest in the placebo group (70%) and lowest in the FDC group (34%). In the active comparator groups, over 39% (IBP) and 50% (APAP) of subjects required at least one dose of rescue medication. The difference between the FDC group with APAP and placebo was statistically significant, but there was no significant difference between the FDC and IBP (FDC vs APAP P<0.001; IBP P=0.348; Placebo P<0.001).
Long-term exposure safety data
An exploratory long-term exposure study (AFT-MX-4) recruited a total of 33 subjects (aged 45–73 years old), of whom 29 continued through to the open-label phase and 24 completed the full 52-week long-term exposure trial (Table 7).68 During the 52-week treatment period, 42 AEs were reported among the 33 subjects. Seven of these AEs occurred during the double-blind phase, while 35 occurred during the open-label phase. The majority of AEs in the 48-week open-label phase were GI disturbances (75%), such as hyperchlorhydria, gastritis, dyspepsia, vomiting, and abdominal discomfort. Four SAEs were reported in two patients during the open-label phase (lower limb fracture, palpitations, vertigo, and hypoglycemia), with each event considered unrelated to the study medication. No significant changes associated with the study medication were observed in hematology or biochemistry parameters between screening and week 52.
Postmarketing surveillance data
FDC 500/150 has been available in New Zealand, the country of origin, since 2009, and in Australia since 2013. As of August 2018, it has been approved in over 32 countries worldwide. Between October 2009 and August 2018, more than 186 million tablets were sold worldwide. Since 2009, 15 ADRs have been reported worldwide, including five serious ADRs (Table 8). None of the serious ADRs were considered unexpected.
  | Table 8 Postmarketing surveillance adverse events Abbreviations: APAP, acetaminophen; F, Female; IBP, ibuprofen; M, Male; NSAID, nonsteroidal anti-inflammatory drug. |
Discussion
Analysis of the safety data pooled from multiple repeated-dose Phase II/III clinical studies illustrates the excellent tolerability and safety of multiple FDC APAP/IBP oral tablet doses over 24–48 hours. By pooling the four studies, a safety population which was demographically broader than any individual study was able to be examined. The results show comparable safety to both the individual components of the FDC and placebo (with opioid rescue available in all groups) during treatment of postsurgical pain. The FDC did not increase the incidence of AEs, the proportion of subjects that experienced at least one AE, or the proportion of AEs that were deemed possibly or probably related to the study medication, when compared to the monotherapy groups. The proportion of subjects that experienced severe AEs and the number of severe AEs were also no different between treatment groups. The ten most common AEs, which accounted for 74% of all AEs (nausea, vomiting, headache, dizziness, swelling face, postprocedural hemorrhage, constipation, dyspepsia, pruritus, and somnolence), are consistent with what can be expected in the postoperative setting. Nausea and vomiting are common AEs in postoperative circumstances and may relate to the use of local or general anesthetics or rescue medication, for example. The FDC did not result in an increase in the number of postoperative bleeding events compared to IBP monotherapy or placebo (2%–3%). NSAIDs such as IBP can interfere with bleeding due to their potential inhibition of platelet COX. Consequently, there is often concern that the use of NSAIDs will increase bleeding in the immediate postoperative period. Examination of combined administration of APAP and IBP has found that the efficacy of the combination is improved compared with APAP or IBP monotherapy, while the tolerability remains similar.18–20,22–25,34
A dose comparison of the FDC has been reported previously.19 There was no difference (P>0.8) in the incidence of AEs reported between the placebo group, FDC (500/150, two tablets) standard dose group, FDC half dose group, and FDC quarter dose group. The incidence of AEs among groups was similar to the pooled safety analysis, with GI (56%) and nervous system (20%) AEs making up the majority of complaints.
A small exploratory study during which patients received the maximum OTC doses of the FDC (500/150 2q6h) for 48 weeks demonstrated good tolerability with chronic use.68 Over the 48-week study, 35 AEs were reported (0.025/week/patient). The majority of these were mild GI disorders (75%), such as hyperchlorhydria, gastritis, and abdominal discomfort.
The pooling of single-dose Phase I clinical trials also found a strong safety profile, with four AEs reported between 266 subjects within 72 hours following the FDC phase of the cross-over trials. Only one AE, dizziness, was considered possibly related to the FDC. While AEs following APAP and IBP are more likely to occur at high and repeated doses, the single-dose studies further support the strong safety profile of the pooled Phase II/III safety population.
APAP, IBP, and other NSAIDs have been shown to provide a significant opioid sparing effect.70–73 A previous study has found that combined administration of APAP and IBP provides similar efficacy to low-dose opioid analgesia such as codeine (30 mg), hydrocodone (5 mg), and oxycodone (5 mg), each provided in combination with 300–325 mg APAP.21 Other studies have consistently found that the efficacy of codeine (30–60 mg) is not superior to the combination of APAP and IBP.22–26 In support of the data obtained during the Phase I studies of the FDC, other studies suggest that combined administration of APAP and IBP provides increased tolerability and safety during the treatment of acute mild–moderate postoperative pain over opioids.22,23,25,34,64,65 The additional adjunct pain relief and separate mechanism of action of opioids means that they will still be a useful clinical analgesic tool in acute moderate–severe pain relief. However, use of the FDC may reduce the dose of opioids required, which may limit the potential for opioid-related AEs and addiction.6,27 In the studies of the FDC, monotherapy with APAP or IBP provided a significant opioid sparing effect compared with placebo by decreasing the proportion of patients requiring rescue medication. The FDC provided an even greater opioid sparing effect, with significantly fewer patients requiring rescue medication compared with APAP monotherapy (P<0.001). The morphine milligram equivalent units for the exact amount of rescue were not pooled in the current paper as the rescue protocols and surgery types varied between studies. The individual studies AFT-MX-3 and AFT-MX-6 have previously published the amount of rescue required.18,19,27 Briefly, in each study, patients receiving the FDC required significantly less oxycodone rescue than the placebo group. In AFT-MX-6, where APAP and IBP monotherapy groups were also studied, patients receiving the FDC required significantly less oxycodone rescue than either monotherapy group.27
Conclusion
APAP and IBP are two analgesic compounds with a long history of use, and both are considered safe and well tolerated at maximum recommended OTC daily doses. The major safety risks of APAP (hepatic injury) and IBP (gastric bleeding and thromboembolic) are largely related to dose. Using a combination addresses these risks by providing superior analgesia without transgressing daily OTC dose limits. Combinations containing APAP and IBP are beneficial as there is less opportunity for drug interactions due to their distinct metabolic pathways.57–59,61–67 Providing an FDC without an opioid also limits the risk of overdosing, as patients attempt to increase their opioid intake due to tolerance and dependence.74,75
Clinical safety data obtained from clinical trials with both the FDC 325/97.5 and the FDC 500/150 demonstrate the safety and tolerability of an FDC of APAP and IBP at or below maximum OTC daily doses of 4,000 mg, and in the case of IBP, well below maximum daily dose of 2,400 mg. This is supported by safety data from a variety of FDC formulations reported previously, including a large meta-analysis of the literature.18–20,22–25,34 When taken at full doses, the cumulative daily consumption of APAP and IBP from the FDC 325/97.5 is 97.5% of that from the FDC 500/150. This permitted the pooling of data for the analysis of safety. Among a pooled safety population of 922 patients, 521 AEs were reported. Data from these 922 subjects demonstrated that the superior analgesic efficacy obtained by combining APAP and IBP did not come at the expense of safety or tolerability. During the double-blind period (24–48 hours), treatment with the FDC did not increase the incidence of AEs, discontinuations due to AEs, or the proportion of subjects experiencing at least one AE relative to either monotherapy group or placebo. There was no increase in the percentage of AEs deemed possibly or probably related to the study medication. The FDC did not differ in the percentage of AEs rated as severe. The incidence of common AEs was also unchanged. The FDC did not alter the rates of GI AEs or postoperative bleeding compared with either monotherapy or placebo. There were also no appreciable changes to hemoglobin and liver biochemistry levels compared to the APAP monotherapy during the open-label phase of one study (AFT-MX-6E). The additional analgesia provided by the FDC does not come at the cost of a reduction in tolerability.18,19 The FDC provides a well tolerated and effective means of analgesia for mild–moderate pain, which can be used as an alternative or adjunct to opioid use post surgery.
Acknowledgments
We are most grateful to the participants who volunteered to take part in this research. We wish to thank Charles Beasley, Irenee Stewart, Prof Alan Merry, Dr John Currie, Dr Ashish Mungantiwar, Dr John Moodie, Dr Simon Carson, Dr Amanda Potts, and Dr Stephen E Daniels for their roles in conducting and monitoring the various clinical trials. We would also like to thank all patients who participated in the clinical trials. The clinical trials were funded by AFT Pharmaceuticals Ltd., Auckland, New Zealand. AFT Pharmaceuticals Ltd. performed the analysis and prepared the manuscript.
Author contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
HA is a shareholder and Managing Director of AFT Pharmaceuticals Ltd. IS and JZ are employees and shareholders of AFT Pharmaceuticals Ltd. PA and RP are employees of AFT Pharmaceuticals Ltd. CMAF provides consultancy services to AFT Pharmaceuticals Ltd. The authors report no other conflicts of interest in this work.
References
Altman RD. A rationale for combining acetaminophen and NSAIDs for mild-to-moderate pain. Clin Exp Rheumatol. 2004;22(1):110. | ||
Raffa RB. Pharmacology of oral combination analgesics: rational therapy for pain. J Clin Pharm Ther. 2001;26(4):257–264. | ||
McQuay HJ, Moore RA. An Evidence-Based Resource for Pain Relief. USA: Oxford University Press; 1998. | ||
Stockler M, Vardy J, Pillai A, Warr D. Acetaminophen (paracetamol) improves pain and well-being in people with advanced cancer already receiving a strong opioid regimen: a randomized, double-blind, placebo-controlled cross-over trial. J Clin Oncol. 2004;22(16):3389–3394. | ||
Edwards JE, McQuay HJ, Moore RA. Combination analgesic efficacy: individual patient data meta-analysis of single-dose oral tramadol plus acetaminophen in acute postoperative pain. J Pain Symptom Manage. 2002;23(2):121–130. | ||
Goldstein FJ. Adjuncts to opioid therapy. J Am Osteopath Assoc. 2002;102(9 Suppl 3):S15–S21. | ||
Kolodny A, Courtwright DT, Hwang CS, et al. The prescription opioid and heroin crisis: a public health approach to an epidemic of addiction. Annu Rev Public Health. 2015;36(1):559–574. | ||
Vadivelu N, Kai AM, Kodumudi V, Sramcik J, Kaye AD. The opioid crisis: a comprehensive overview. Curr Pain Headache Rep. 2018;22(3):16. | ||
Seth P, Rudd RA, Noonan RK, Haegerich TM. Quantifying the epidemic of prescription opioid overdose deaths. Am J Public Health. 2018;108(4):500–502. . | ||
Jones CM, Paulozzi LJ, Mack KA. Sources of prescription opioid pain relievers by frequency of past-year nonmedical use United States, 2008-2011. JAMA Intern Med. 2014;174(5):802. | ||
Monheit B, Pietrzak D, Hocking S. Prescription drug abuse – a timely update. Aust Fam Physician. 2016;45(12):862–866. | ||
Ryan J. Australia’s annual overdose report 2017; 2017. Available from: www.penington.org.au/australias-annual-overdose-report-2017/. Accessed October 1, 2018. | ||
Davis A, Davis K, Gerard C, et al. Opioid rain: opioid prescribing is growing and practice is diverging. NZ Med J. 2016;129(1440):7. | ||
Morrow PL. The American opioid death epidemic—lessons for New Zealand? NZ Med J. 2018;131(1469):5. | ||
Special Advisory Committee on the epidemic of opioid overdoses. National report: apparent opioid-related deaths in Canada; 2018. Available from: https://www.canada.ca/en/public-health/services/publications/healthy-living/national-report-apparent-opioid-related-deaths-released-september-2018.html. Accessed January 10, 2018. | ||
Office for National Statistics [webpage on the Internet]. Opioid drug deaths by cause, 1993 to 2015. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/drugusealcoholandsmoking/adhocs/006149opioiddrugdeathsbycause1993to2015. Accessed August 15, 2018. | ||
Mordecai L, Reynolds C, Donaldson LJ, de C Williams AC. Patterns of regional variation of opioid prescribing in primary care in England: a retrospective observational study. Br J Gen Pract. 2018;68(668):e225–e233. | ||
Merry AF, Gibbs RD, Edwards J, et al. Combined acetaminophen and ibuprofen for pain relief after oral surgery in adults: a randomized controlled trial. Br J Anaesth. 2010;104(1):80–88. | ||
Atkinson HC, Currie J, Moodie J, et al. Combination paracetamol and ibuprofen for pain relief after oral surgery: a dose ranging study. Eur J Clin Pharmacol. 2015;71(5):579–587. | ||
Moore RA, Derry S, Aldington D, Wiffen PJ. Single dose oral analgesics for acute postoperative pain in adults - an overview of Cochrane reviews. Cochrane Database Syst Rev. 2015;107(3). | ||
Chang AK, Bijur PE, Esses D, Barnaby DP, Baer J. Effect of a single dose of oral opioid and nonopioid analgesics on acute extremity pain in the emergency department: a randomized clinical trial. JAMA. 2017;318(17):1661. | ||
Mitchell A, McCrea P, Inglis K, Porter G. A randomized, controlled trial comparing acetaminophen plus ibuprofen versus acetaminophen plus codeine plus caffeine (Tylenol 3) after outpatient breast surgery. Ann Surg Oncol. 2012;19(12):3792–3800. | ||
Mitchell A, van Zanten SV, Inglis K, Porter G. A randomized controlled trial comparing acetaminophen plus ibuprofen versus acetaminophen plus codeine plus caffeine after outpatient general surgery. J Am Coll Surg. 2008;206(3):472–479. | ||
Daniels SE, Goulder MA, Aspley S, Reader S. A randomised, five-parallel-group, placebo-controlled trial comparing the efficacy and tolerability of analgesic combinations including a novel single-tablet combination of ibuprofen/paracetamol for postoperative dental pain. Pain. 2011;152(3):632–642. | ||
Sniezek PJ, Brodland DG, Zitelli JA. A randomized controlled trial comparing acetaminophen, acetaminophen and ibuprofen, and acetaminophen and codeine for postoperative pain relief after Mohs surgery and cutaneous reconstruction. Dermatol Surg. 2011;37(7):1007–1013. | ||
Graudins A, Meek R, Parkinson J, Egerton-Warburton D, Meyer A. A randomised controlled trial of paracetamol and ibuprofen with or without codeine or oxycodone as initial analgesia for adults with moderate pain from limb injury. Emerg Med Australas. 2016;28(6):666–672. | ||
Daniels SE, Atkinson HC, Stanescu I, Frampton C. Analgesic efficacy of an acetaminophen/ibuprofen fixed-dose combination in moderate to severe postoperative dental pain: a randomized, double-blind, parallel-group, placebo-controlled trial. Clin Ther. 2018;40(10):1765–1776. | ||
Bushra R, Aslam N. An overview of clinical pharmacology of ibuprofen. Oman Med J. 2010;25(3):155–161. | ||
Kakuta H, Zheng X, Oda H, et al. Cyclooxygenase-1-selective inhibitors are attractive candidates for analgesics that do not cause gastric damage. Design and in vitro/in vivo evaluation of a benzamide-type cyclooxygenase-1 selective inhibitor. J Med Chem. 2008;51(8):2400–2411. | ||
Harvison PJ, Egan R, Gale P, Nelson SD. Acetaminophen as a cosubstrate and inhibitor of prostaglandin H synthase. In: Kocsis JJ, Jollow DJ, Witmer CM, Nelson JO, Snyder RR, editors. Biological Reactive Intermediates III: Mechanisms of Action in Animal Models and Human Disease. New York: Springer; 1986:739–747. | ||
Ohki S, Ogino N, Yamamoto S, Hayaishi O. Prostaglandin hydroperoxidase, an integral part of prostaglandin endoperoxide synthetase from bovine vesicular gland microsomes. J Biol Chem. 1979;254(3):829–836. | ||
Lucas R, Warner TD, Vojnovic I, Mitchell JA. Cellular mechanisms of acetaminophen: role of cyclo-oxygenase. FASEB J. 2005;19(6):635–637. | ||
Mallet C, Eschalier A, Daulhac L. Paracetamol: update on its analgesic mechanism of action. In: Maldonado C, editor. Pain Relief – From Analgesics to Alternative Therapies. London: IntechOpen; 2017. | ||
Moore RA, Derry S, Aldington D, Wiffen PJ. Adverse events associated with single dose oral analgesics for acute postoperative pain in adults - an overview of Cochrane reviews. Cochrane Database Syst Rev. 2015;(10):CD011407. | ||
Derry CJ, Derry S, Moore RA. Single dose oral ibuprofen plus paracetamol (acetaminophen) for acute postoperative pain. Cochrane Database Syst Rev. 2013:(6):CD010210. | ||
Högestätt ED, Jönsson BA, Ermund A, et al. Conversion of acetaminophen to the bioactive N-acylphenolamine AM404 via fatty acid amide hydrolase-dependent arachidonic acid conjugation in the nervous system. J Biol Chem. 2005;280(36):31405–31412. | ||
Mallet C, Barrière DA, Ermund A, et al. TRPV1 in brain is involved in acetaminophen-induced antinociception. PLoS One. 2010;5(9): e12748. | ||
Costa B, Siniscalco D, Trovato AE, et al. AM404, an inhibitor of anandamide uptake, prevents pain behaviour and modulates cytokine and apoptotic pathways in a rat model of neuropathic pain. Br J Pharmacol. 2006;148(7):1022–1032. | ||
Zygmunt PM, Chuang H-Hu, Movahed P, Julius D, Högestätt ED. The anandamide transport inhibitor AM404 activates vanilloid receptors. Eur J Pharmacol. 2000;396(1):39–42. | ||
Slattery WT, Klegeris A. Acetaminophen metabolites p-aminophenol and AM404 inhibit microglial activation. Neuroimmunol Neuroinflamm. 2018;5(4):11. | ||
Ottani A, Leone S, Sandrini M, Ferrari A, Bertolini A. The analgesic activity of paracetamol is prevented by the blockade of cannabinoid CB1 receptors. Eur J Pharmacol. 2006;531(1–3):280–281. | ||
Giuffrida A, Rodriguez de Fonseca F, Nava F, Loubet-Lescoulié P, Piomelli D. Elevated circulating levels of anandamide after administration of the transport inhibitor, AM404. Eur J Pharmacol. 2000;408(2):161–168. | ||
Nicholson RA, Liao C, Zheng J, et al. Sodium channel inhibition by anandamide and synthetic cannabimimetics in brain. Brain Res. 2003;978(1–2):194–204. | ||
Mallet C, Daulhac L, Bonnefont J, et al. Endocannabinoid and serotonergic systems are needed for acetaminophen-induced analgesia. Pain. 2008;139(1):190–200. | ||
Lee WM. Acetaminophen and the U.S. acute liver failure Study group: lowering the risks of hepatic failure. Hepatology. 2004;40(1):6–9. | ||
Castellsague J, Riera-Guardia N, Calingaert B, et al. Individual NSAIDs and upper gastrointestinal complications: a systematic review and meta-analysis of observational studies (the SOS project). Drug Saf. 2012;35(12):1127–1146. | ||
Southworth SR, Woodward EJ, Peng A, Rock AD. An integrated safety analysis of intravenous ibuprofen (Caldolor(®)) in adults. J Pain Res. 2015;8:753. | ||
Barkin RL, Beckerman M, Blum SL, Clark FM, Koh EK, Wu DS. Should nonsteroidal anti-inflammatory drugs (NSAIDs) be prescribed to the older adult? Drugs Aging. 2010;27(10):775–789. | ||
Liukas A, Kuusniemi K, Aantaa R, et al. Pharmacokinetics of intravenous paracetamol in elderly patients. Clin Pharmacokinet. 2011;50(2):121–129. | ||
Divoll M, Abernethy DR, Ameer B, Greenblatt DJ. Acetaminophen kinetics in the elderly. Clin Pharmacol Ther. 1982;31(2):151–156. | ||
Bailey E, Worthington HV, van Wijk A, Yates JM, Coulthard P, Afzal Z. Ibuprofen and/or paracetamol (acetaminophen) for pain relief after surgical removal of lower wisdom teeth. Cochrane Database Syst Rev. 2013;(12):CD004624. | ||
Miranda HF, Puig MM, Prieto JC, Pinardi G. Synergism between paracetamol and nonsteroidal anti-inflammatory drugs in experimental acute pain. Pain. 2006;121(1–2):22–28. | ||
AFT Pharmaceuticals, Ltd. Maxigesic 325 acute dental pain study. Available from: https://clinicaltrials.gov/ct2/show/NCT01420653. NLM identifier: NCT01420653. Accessed August 20, 2018. | ||
AFT Pharmaceuticals, Ltd. A study comparing the effects of different paracetamol and ibuprofen combination doses and placebo. Available from: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=336805&isReview=true. Accessed August 20, 2018. | ||
AFT Pharmaceuticals, Ltd. A study comparing the effects of a combination of paracetamol and ibuprofen with paracetamol alone or ibuprofen alone or placebo. Available from: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=343438&isReview=true. Accessed August 20, 2018. | ||
AFT Pharmaceuticals, Ltd. Maxi-Analgesic study. Available from: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=1443&isReview=true. Accessed August 20, 2018. | ||
AFT Pharmaceuticals, Ltd. A study to compare the bio-equivalence between single dose of ibuprofen tablet versus a combination of acetaminophen and ibuprofen. Available from: http://www.ANZCTR.org.au/ACTRN12618001465246.aspx. Accessed August 31, 2018. | ||
AFT Pharmaceuticals, Ltd. Pharmacokinetic study of four orally formulated combinations of acetaminophen and ibuprofen in healthy volunteers under fasting conditions. Available from: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=370407&isReview=true. Accessed August 20, 2018. | ||
AFT Pharmaceuticals, Ltd. Pharmacokinetic study of four orally formulated combinations of acetaminophen and ibuprofen in healthy volunteers under fed conditions. Available from: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=370429&isReview=true. Accessed August 20, 2018. | ||
Aitken P, Salem II, Stanescu I, Playne R, Atkinson HC. A single dose, four-way, open-label bioavailability study of oral acetaminophen and ibuprofen combinations (Maxigesic) under both fasting and fed conditions. J Bioequiv Availab. 2018;10(5):84–91. | ||
AFT Pharmaceuticals, Ltd. Maxigesic metabolism study: to describe the metabolism of paracetamol when combined with ibuprofen in healthy adult volunteers. Available from: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=362903&isReview=true. Accessed August 20, 2018. | ||
AFT Pharmaceuticals, Ltd. Pharmacokinetic study of acetaminophen and ibuprofen, solution for infusion and the oral tablet of acetaminophen and ibuprofen in healthy volunteers. Available from: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=369555&isReview=true. Accessed August 20, 2018. | ||
AFT Pharmaceuticals, Ltd. Exploratory phase 1 pharmacokinetic study of different doses of paracetamol plus ibuprofen under fasting and fed conditions. Available from: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=364727&isReview=true. Accessed August 20, 2018. | ||
AFT Pharmaceuticals, Ltd. Pharmacokinetic study of acetaminophen and ibuprofen oral formulation, in healthy volunteers, fasting conditions. Available from: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=364728&isReview=true. Accessed August 20, 2018. | ||
AFT Pharmaceuticals, Ltd. Pharmacokinetic study of acetaminophen and ibuprofen, oral formulations, in healthy volunteers under fed conditions. Available from: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=370285&isReview=true. Accessed August 20, 2018. | ||
AFT Pharmaceuticals, Ltd. Pharmacokinetic study of paracetamol and ibuprofen, solution for infusion, in healthy volunteers. Available from: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=363704&isReview=true. Accessed August 20, 2018. | ||
AFT Pharmaceuticals, Ltd. Phase I pharmacokinetic study evaluating the pharmacokinetic profile of a fixed dose combination product (1000 Mg paracetamol + 300 Mg ibuprofen) under fasting and fed conditions. Available from: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=365149&isReview=true. Accessed August 20, 2018. | ||
AFT Pharmaceuticals, Ltd. A clinical trial to study the effects of Maxigesic (paracetamol 500mg + ibuprofen 150 mg) with the other 3 treatment groups (paracetamol 500 mg; low dose ibuprofen 150 mg; high dose ibuprofen 300 mg) in patients who have painful osteoarthritis of the knee. 2009. Available from: http://ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=362&EncHid=&userName=CTRI/2009/091/000096. Accessed August 20, 2018. | ||
International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH E2A: clinical safety data management: definitions and standards for Expedited reporting; 1994. Available from: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E2A/Step4/E2A_Guideline.pdf. Accessed October 1, 2018. | ||
Cobby TF, Crighton IM, Kyriakides K, Hobbs GJ. Rectal paracetamol has a significant morphine-sparing effect after hysterectomy. Br J Anaesth. 1999;83(2):253–256. | ||
Wong I, St John-Green C, Walker SM. Opioid-sparing effects of perioperative paracetamol and nonsteroidal anti-inflammatory drugs (NSAIDs) in children. Paediatr Anaesth. 2013;23(6):475–495. | ||
Maund E, McDaid C, Rice S, Wright K, Jenkins B, Woolacott N. Paracetamol and selective and non-selective non-steroidal anti-inflammatory drugs for the reduction in morphine-related side-effects after major surgery: a systematic review. Br J Anaesth. 2011;106(3):292–297. | ||
Dahl JB, Nielsen RV, Wetterslev J, et al. Post-operative analgesic effects of paracetamol, NSAIDs, glucocorticoids, gabapentinoids and their combinations: a topical review. Acta Anaesthesiol Scand. 2014;58(10):1165–1181. | ||
Lanier RK, Lofwall MR, Mintzer MZ, Bigelow GE, Strain EC. Physical dependence potential of daily tramadol dosing in humans. Psychopharmacology. 2010;211(4):457–466. | ||
Chu LF, D’Arcy N, Brady C, et al. Analgesic tolerance without demonstrable opioid-induced hyperalgesia: a double-blinded, randomized, placebo-controlled trial of sustained-release morphine for treatment of chronic nonradicular low-back pain. Pain. 2012;153(8):1583–1592. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.