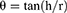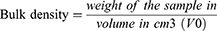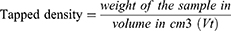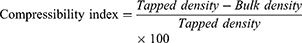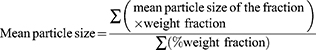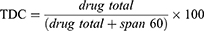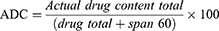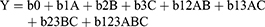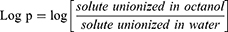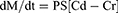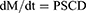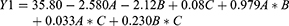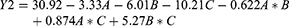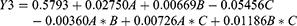Back to Journals » Drug Design, Development and Therapy » Volume 15
An Industrial Procedure for Pharmacodynamic Improvement of Metformin HCl via Granulation with Its Paracellular Pathway Enhancer Using Factorial Experimental Design
Authors Mady OY, Al-Shoubki AA , Donia AA
Received 23 July 2021
Accepted for publication 23 September 2021
Published 2 November 2021 Volume 2021:15 Pages 4469—4487
DOI https://doi.org/10.2147/DDDT.S328262
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Omar Y Mady,1 Adam A Al-Shoubki,2 Ahmed A Donia3
1Pharmaceutical Technology Department, Faculty of Pharmacy, Tanta University, Tanta, Egypt; 2Pharmaceutics and Industrial Pharmacy Department, Faculty of Pharmacy, Omar Al-Mukhtar University, Al-Bayda, Libya; 3Department of Pharmaceutical Technology, Menoufia University, Shebeen El-Kom, Egypt
Correspondence: Omar Y Mady Tel +201141819661
Email [email protected]
Adam A Al-Shoubki
Pharmaceutics and Industrial Pharmacy Department, Faculty of Pharmacy, Omar Al-Mukhtar University, Al-Bayda, Libya
Tel +218922826802
Email [email protected]
Background: Sorbitan monostearate is a surfactant used in the food industry. It was proved as a penetration enhancer to metformin HCl via a paracellular pathway. It is solid at room temperature and has a low melting point. Therefore, it was selected, as a granulating agent for metformin HCl.
Methods: Multi-level factorial design was applied to determine the optimized formula for industrial processing. The selected formulations were scanned using an electron microscope. Differential scanning calorimetry was used to ascertain the crystalline state of a drug. A modified non-everted sac technique, suggested by the authors, was used to evaluate the in vitro permeation enhancement of the drug. To simulate the emulsification effect of the bile salt, a tween 80 was added to the perfusion solution. As a pharmacodynamic marker, blood glucose levels were measured in diabetic rats.
Results: The results showed that drug permeability increases in the presence of tween 80. Drug permeability from granules increased than that of the pure drug or pure drug with tween 80. The prepared granules decreased blood glucose levels of diabetic rats than the pure drug and drug plus tween 80. There was an excellent correlation between the results of the drug permeation percent in vitro and the dropping of blood glucose level percent in vivo.
Conclusion: Improving the drug permeation and consequently, the drug pharmacodynamic effect in addition to an excellent micromeritics property of the prepared drug granules showed the dual enhancement effect of the suggested industrial procedure. Therefore, we suggest the same industrial procedure for other class III drugs.
Keywords: sorbitan monostearate, class III drugs, factorial design, modified non-everted sac
Graphical Abstract:
Introduction
Biopharmaceutical Classification System (BCS) is a scientific schematic system, which classifies the pharmaceutically active ingredients into four classes based on the solubility and the intestinal permeability parameters. That is because the leading factors that govern the rate and extent of drug absorption, are its solubility and intestinal permeability. Class I drugs have high water solubility and high intestinal permeability. The drugs of class II have high permeability but also have low solubility. In contrast to class II, class III drugs have high solubility but low permeability. Then, it could be expected that class IV drugs are poorly soluble and poorly permeable.1 There are many trials to increase the solubility of drugs. Self-micro emulsifying drug delivery system (SMEDDS) is one of the techniques used to improve the drug solubility of class two. It is a pre-concentrated mixture of surfactants, co-surfactants, and lipophilic phases. This mixture forms fine droplets of emulsion with a size range of 5–100 nm when diluted with water or the body fluids in the aqueous lumen of the gut.2 SMEDDS was also considered as an ideal carrier for the delivery of drugs class II and IV. That is due to the improvement in the in vitro and in vivo performance of poorly water-soluble drugs.3
Metformin hydrochloride, according to BCS, is a class III drug. It has high water solubility and low intestinal permeability. Accordingly, an increase in the permeability of the drug will lead to an increase in its bioavailability,4 and consequently, decreasing the drug dose. Self-emulsified drug delivery system (SEDDS) of metformin hydrochloride enhanced its intestinal permeability, which leads to increasing its oral bioavailability.5–10
Tablets are the most prevalent pharmaceutical dosage form for various reasons including self-administration with to some extent an accurate dose. The drug content and drug content uniformity would be controlled by the pharmaceutical industry within a pharmacopeia range. The filling of the die of the tablet press will determine the tablet weight and consequently the dose of the drug in mg. This means the tablet press machine uses the die volume (ml3) for dose (mg). The filling of the die of the tablet press machine will be done in most cases according to the bulk density of the powder mixture. In some filling machines, especially capsule-filling ones, there is a screwing mechanism, which creates some pressure to press the powder during the filling process as a trill to increase the amount of the powder in the capsules’ shell. This means the change of the filling process from bulk density to a certain degree of tapped density. The homogeneity of the tablet weight and consequently the drug content uniformity would depend on the powder flow, its bulk density, and its particle size range. The above technological aspects for each drug could be overcome by change the drug powder form to granules with a narrow particle size range using a suitable granulation technique. Metformin hydrochloride is one of the most popular used oral hypoglycemic drugs in a high dose (500, 850, and 1000 mg). It has poor flowability and poor compression properties, in addition to its high dose, which creates formulation problems.11
Reviewing the kinds of the literature indicated that there are a lot of trials dealing with granulation of metformin to improve only its micromeritics properties. Different granulation techniques like wet granulation technique12,13 and melt granulation technique14–17 were used. A modified wet granulation technique was also suggested by Aodah et al, 2020,18 as a trial to reduce the wet granulation steps and maybe also useful to decrease the drug degradation by heat for the drying step of the wet drug granules.18 Sorbitan monostearate (Span-60) is an ester of a sorbitol derivative and stearic acid. It is used primarily as an emulsifier and approved by the European Union to be used as a food additive emulsifier.19,20 Also, it is considered safe for human use because of long-term toxicity studies, which displayed no sign of carcinogenic activity in humans at different dose levels.21,22 Pardakhty, 2017,23 reported that
Sorbitan monostearate is the better surfactant used in noisome preparation. High-phase transition temperature, low hydrophilic-lipophilic balance, and critical packing factor of Sorbitan monostearate resulted in spherical vesicles of good size.23
Mady24 succeeded to use sorbitan monostearate as a microsphere matrix prepared by congealing technique and solvent evaporation technique.24 That is, maybe, due to its lower melting point and solid-state characteristics at room temperature. A trial to granulate paracetamol to improve its compressibility by using glyceryl monostearate or stearic acid was also studied. The results showed an improvement in the compressibility of the standard drug used for incompressibility (paracetamol). Also, the ability to the formation of spherical granules using glyceryl monostearate was shown.25 Latterly, Mady et al26 succeeded to enhance the permeability of metformin HCl by using sorbitan monostearate as a microparticle matrix.26 The drug enhancement mechanism was via the paracellular pathway absorption mechanism. Accordingly, this study aims to use the lower melting point and solid state at room temperature charactersistics of sorbitan monostearate to granulate metformin hydrochloride by using the melting congealing technique suggested by the author. The factorial experimental design would be applied to study different granulation factors using the required drug processing properties as indicators. Characterization of the drug in the prepared granules should be carried out by using some instrumental analysis. Then, the role of sorbitan monostearate as a surface-active agent on the dissolution and permeability of the drug in vitro is studied. Improving the drug permeability in vitro should be also confirmed by an in vivo test as a trial to confirm the drug permeability enhancement.
Materials and Methods
Materials
Metformin hydrochloride (El-Nasr Pharmaceutical Chemicals Co, Egypt), n-octanol (Loba Chemie, India), sorbitan monostearate and Tween 80 (Oxford, Lab Chem, Mumbai, India), disodium monohydrogen phosphate and potassium dihydrogen phosphate are purchased from El-Gomhoria Company for Chemicals (branch Tanta city, Egypt). All others reagents and chemicals used were of analytical reagent grade.
Methods
Experimental Design
Metformin HCl granulation process was optimized by using a multilevel factorial design (41, 22). The software used, is MINITAB, version 17. Table 1 shows that the selected independent variables are the concentration of granulating agent (sorbitan monostearate) at four levels, the granulation temperature, and stirring rate each at two levels. The tested dependent variables are the angle of repose, compressibility index, and mean particle size. The experimental trials were performed at all 16 possible combinations (Table 2).
 |
Table 1 Coded Unit of Variables and Their Respective Levels on the Application of (41, 22) Multi-Level Factorial Design |
 |
Table 2 The Design Matrix of the Prepared Granules Formulations |
Granulation of Metformin Hydrochloride
A physical mixture (20 g) of metformin hydrochloride and sorbitan monostearate was prepared according to Table 2. Each physical mixture was stirred at room temperature for 5 minutes using a mechanical stirrer (Heidolph, Germany) supported with a locally made shaft of a planetary mixer. The stirring rate and temperature of the prepared physical mixture are adopted according to Table 2 for 15 minutes. Then, the temperature decreased while stirring at the same stirring rate as room temperature. The prepared granules were collected and stored at room temperature for further investigation.
Characterization of the Prepared Metformin HCl Granules
Flow Properties
The flow properties of different products were carried out using the angle of repose method. The funnel was maintained at a fixed height in all experiments. The samples were passed through a funnel to form a stable cone.27 The angle of repose (θ) was calculated by using Equation 1:
where θ = angle of repose, h = height of the cone, r = radius of the cone base.
Density Measurements
A sample of 10 g was carefully introduced into a 50 mL graduated cylinder. The bulk volume (V0) was measured in cm3. The fixed cylinder was dropped from a high of 2.5 cm at one-second intervals.28 The tapping was continued until no further change in volume was noted. The tapped volume (Vt) was measured cm3.28 The different parameters of the granules were calculated according to Equations (2–4):
Mean Particle Sizes
The mean particle sizes of the granules were determined by using the sieve method. A definite weight of granules was placed on a set of standard sieves automatically shaken for 10 min using a mechanical sieve shaker at a constant speed. Granules fraction remaining on the sieves were weighed to determine the particle size distribution.25 The mean granules diameter was calculated using Equation 5.29
Determination of the Actual Drug Content
Accurate weight of metformin HCl granules (100 mg) of different particle sizes (1000 µm, 800 µm, 315 µm, and 106 µm) and prepared by using different concentrations of the granulating agent was dissolved in 100 mL of 0.1 N HCl at 60°C. The prepared solutions were measured spectrophotometry at 232 nm using 0.1 N HCl as a blank. It was also proved that the presence of sorbitan monostearate in the solution does not affect the drug absorbance. The procedure was carried out in triplicate.30 The mean actual drug content was calculated using the Equations (6 and 7):
Theoretical drug content (TDC)
Drug Release
The exact weight of the prepared sorbitan monostearate granules containing 500 mg of metformin HCl (calculated according to the determined actual drug content) and particle size range of 400–315 µm was added to the Paddle USP dissolution apparatus, Type Dis 6000 (Copley Scientific, UK). The release medium was 900 mL of 0.1N HCl with a maintained temperature at 37 ± 0.5°C. At a predetermined interval time, 5 mL samples were withdrawn for analysis, and 5 mL of the fresh release medium was added to replenish each sample withdrawn. The drug content of the samples was determined spectrophotometrically at 232 nm using a UV/visible spectrophotometer (Thermo Fisher Scientific, model EVO 300PC, software: vision pro, USA). Three replicates were conducted.31
Thermal Analysis
Thermal analysis of metformin HCl and different concentrations of sorbitan monostearate–metformin HCl granules were carried out. The heating range was between 20 to 240°C. That is to cover the melting point of the pure drug, which is reported to be at 225°C.32 For thermal analysis, differential scanning calorimeter (DSC) was conducted for each sample simultaneously using DSC (PerkinElmer simultaneous thermal analyzer SAT 6000, supplied with a software: Pyris SAT 6000, USA).32
Electron Scanning Microscope Examination
Electron scanning microscope [ESM] (JEOL-model: JSM-5200LV, Japan) was used to study the morphology and surface of the prepared sorbitan monostearate–metformin HCl granules. It was tried to elucidate the shape of the granules and the method of agglomeration by using different magnifications to obtain the best vision.
Statistical Analysis
Statistical analysis of the predetermined parameters was performed using MINITAB software, the product licensed to Adam A. Al-Shoubki, product version: (Minitab® 17.3.1). The factorial design was statistically analyzed by multiple regression analysis. A statistical model incorporating the interactive and the polynomial terms was used to evaluate the response (Equation 9):
Where Y is the dependent variable, b0 is the arithmetic mean response of the 16 runs, and bi is the estimated coefficient for the factor i. The mean effects (A, B, and C) represent the average results of changing one factor at a time from its low to a high level. The interaction terms (AB, AC, and BC) show how the response changes when two factors are changed simultaneously. The significance, validation of the chosen formula, and the contribution of each factor with different levels on response were evaluated by two-way analysis of variance at a 95% significance level (p<0.05).33
Experimental Determination of the Drug Partition Coefficients (Log P)
An amount (20 mg) of either pure drug or that of each drug granulated with sorbitan monostearate was dissolved in n-octanol. Distilled water (20mL) was added while stirring. The prepared solution was transferred into a separating funnel and allowed to equilibrate. The drug concentration in the aqueous was measured spectrophotometrically at 232 λ max. The log P was calculated according to Equation 9:
Modified Non-Everted Sac Technique to Evaluate the Intestinal Permeability
The method steps, employed to evaluate the drug permeability profile from the modified non-everted sac method, are the modified experimental procedures described by references.34–36
Preparation of the non-everted intestinal sacs: The animal used in this study was a Male albino rabbit with a weight of 2 kg obtained from the Tanta animal house. All procedures were approved and regularly controlled by the Animal Ethics Committee of Faculty of Pharmacy Tanta University (No: 2212018) and all experiments were performed in accordance with the guidelines and regulations of this committee. All the procedures were also carried out in full accordance with the ARRIVE guidelines 2020,37 and adequate care was taken to minimize pain and discomfort for animals. They are accommodated in a clean room with free access to food and water. They did not eat overnight with free access to water and were anesthetized by intramuscular injection of ketamine HCl. The animal was sacrificed upon confirmation of loss of the pain response. A midline longitudinal incision of 3–4 cm was made and the small intestine was located. A sac of 14 cm segment of the intestine was rinsed with phosphate buffer pH 6.8 to remove any solid material and the side of the segment of the small intestine was tied by using surgical thread. To check leaks, the fresh intestinal sac was filled with phosphate buffer pH 6.8, tied to the other side with surgical thread, and checked. The prepared segments after each step were placed in continuous aerated phosphate buffer pH 6.8 and used for studying the drug permeation on the same day after filling with the perfusion solution.
Preparation of perfusion solution and filling the non-everted sacs: an amount of sorbitan monostearate granules containing 50 mg of metformin HCl as an actual drug content was accurately weighed. The sample was dissolved in 3 mL of pH 6.8 phosphate buffer and then one mL of tween 80 was also added. Tween 80 was added as a simulation of the effect of the bile salts on drug absorption. After emptying the fresh intestinal sac segment from the buffer solution, the intestinal sac was then filled with the prepared perfusion solution, tied with surgical thread, and tested for leaks. For calculation, the permeability segment length and diameter were measured.
Drug permeation profile study: the prepared segment was suspended on the shaft of the USP dissolution apparatus. The outside of the sac medium [permeation medium] was 900 mL of phosphate buffer [pH 6.8] with continuous aeration and maintained the temperature at 37 ± 0.5 ° C. The stirring rate was 50 rpm. Samples of 5 mL were withdrawn at predetermined time intervals and the fresh release medium was added to replenish each sample withdrawn. The amount of drug permeated from the segment to the medium was determined spectrophotometrically at 232 λ max.19 The validation data of the method of analysis are as follows: R2 value is 0.999, linearity is between 2 and 20 μg/mL (Range used), the precision as RSD% values are less than 2%, the accuracy is confirmed by recovery percent (99.72–100.81%).
Determination permeability coefficient: the permeability coefficient [apparent permeability] was determined by using Fick’s law across the isolated rat intestine.38 The law is described mathematically as (Equation 10):
where dM/dt is moles of solute transported per unit time, P is permeability coefficient, S is the surface area of the membrane, Cd is the concentration of solute in the donor [serosal] phase and Cr is the concentration of solute in the recipient [mucosal] phase. In this case, the sink conditions prevail since the volume of the serosal fluid is much larger than the mucosal volume, then Cd is constant and much larger than Cr and Cr could be ignored. Accordingly, the equation could be written as Equation 11:
The variables M and Cd could be determined by analysis of the mucosal fluid. The surface area [S] could be calculated by considering the intestinal sac a cylinder. From these values, M/SCd could be calculated and plotted against time. The slope of the linear portion of the plot is the permeability coefficient [P], which has the units of velocity [cm/s]. The slope of the linear portion of the curve was determined by linear regression.39,40
In vivo Study
Animals
The animal used in this study was the male albino Wistar rats aged 7–8 weeks with a weight of 150–200 g. All procedures were approved and regularly controlled by the Animal Ethics Committee of Faculty of Pharmacy Tanta University (No: 2212018) and all experiments were performed in accordance with the guidelines and regulations of this committee. All the procedures were also carried out in full accordance with the ARRIVE guidelines 2020,37 and adequate care was taken to minimize pain and discomfort for animals. The housing of the animal was at an ambient temperature of 25±1°C and relative humidity of 45–55% with a 12hr each of dark and light cycle. They were fed pellet diet and water ad libitum.
Induction of Experimental Diabetes
The animals did not eat overnight. Diabetes induction was done by a single intraperitoneal injection of a freshly prepared solution of streptozotocin (50 mg/kg body weight) in 0.1 M citrate buffer (pH 4.5). To overcome the hypoglycemic effect of the drug, the animals could be able to drink a 5% glucose solution. On the third day of streptozotocin injection, the rats fasted for 6 h, and blood was withdrawn from the tail vein. The blood glucose level was measured using the Accu-Chek active using the Accu-Chek active test strips. Rats that had fasting blood glucose levels >250 mg/dl were considered to be diabetic and were used to monitor the efficacy of metformin formulations.
Determination of the Hypoglycemic Effect of Metformin HCl
On the day of experimenting, the rats for 15 minutes were given free access to the pellet. After that, the food was restricted but there was free access to water for 2 hours. That is to provide a stable glucose level in the rat’s blood. The formulations were dispersed in water at concentration of 30 mg/mL and 0.125 mL tween 80 was added for each mL. Then, 1 mL of the prepared tested dispersion was administered orally to each rat. A blood sample was withdrawn from the tail vein at time intervals of (0, 0.25, 0.5, 1, 2, 3, 4, 5, 6, 7, and 8) hours. The blood glucose was measured by using an Accu-Chek active test strip. The dropping of the blood glucose level percent was also calculated and plotted as a function of time. The area above the curve was determined and used for monitoring the efficacy of different formulations.41
Statistical Analysis
A one-way analysis of variance (ANOVA) test was performed to demonstrate statistical significance of blood glucose level as the independent factor. The GraphPad Prism version 8.0.1 for Windows (GraphPad Software, San Diego, California, USA, www.graphpad.com) was used for this purpose. P < 0.05 was considered statistically significant.
Results and Discussion
To study the effect of each factor on the response, the micro metric characteristics of the prepared granules are assigning for all formulas as shown in Table 3.
 |
Table 3 Summary of Optimization Responses |
The Angle of Repose
The values of the angle of repose are between 28.072° and 32.828° (Table 4) indicating the flow of all prepared granules is either very free-flowing or free-flowing.
 |
Table 4 In vitro Characterization of All Granule’s Formulation |
The relationship between the response Y1 (angle of repose) and the independent variables (A, B, C, AB, AC, and BC) was evaluated by using a stepwise multivariate linear regression. The result is reported in Equation 12:
Regression analyses reveal that, the only significant effect of factor A and the interaction AB on the angle of repose values (p < 0.05). The most effective factor is A with a negative coefficient value indicating that sorbitan monostearate at high concentration (20%) decreases the angle of repose significantly. The most interaction factors are AB with a positive coefficient value indicating that they are the use of high sorbitan monostearate concentration (20%) and a high temperature (80° C). Accordingly, the optimized formulation condition for the angle of repose is high sorbitan monostearate concentrations (20%) at (80° C) and 100 or 200 rpm (formulas, F15 and F16). A physical mixture between the drug and sorbitan monostearate is first prepared at room temperature to assure homogeneous mixing of the granulating agent with the drug crystals. Increasing the temperature led to molten the binder. The molten liquid is distributed onto the surfaces of fine solid drug crystals leading to the formation of the nuclei by a distribution method. The nuclei are formed by the collision between the wetted particles. The residual surface liquid of the binder promoted the successful fusion of nuclei (Figure 1B and D). The surface liquid imparts plasticity to the nuclei and is also essential for enabling the deformation of the nuclei surface for coalescence as well as promoting the rounding of granulation. This could be noticed from the ESM of the F16 (Figure 1B and D). Accordingly, the value of the angle of repose would be expected to improve by the covering of the drug crystals with sorbitan monostearate as an initiating agent for the granulation process and then the aggregation of the covered drug crystals into granules. The suggested granulation mechanism is depending on the concentration of sorbitan monostearate, which must be melted to cover the drug crystal particles while stirring.
Compressibility Index (%)
Table 4 summarizes the average compressibility index (%) values for all granule’s formulation. The regression equation for the response Y2 (compressibility index) and the independent variables (A, B, C, AB, AC, and BC) is represented in Equation 13.
Regression analyses reveal the significant effects of all tested factors and interactions at (p<0.05) on the Compressibility index (%) except AB and AC interactions. The most effective factor is negative C (stirring rate). That is, maybe, due to increasing the granules homogeneity (Figure 1A–D). The second and third effective factors are negative B (temperature) and negative A (sorbitan monostearate concentration), respectively. These results indicate that the optimized formulation conditions for the compressibility index (%) is (F16) which is formulated by high sorbitan monostearate concentration (20%) at (80 ° C) and (200 rpm).
Mean Particle Size
For tablet manufacturing, the powder should be within a particle size range of 0.2–4.0 mm. They are primarily produced as an intermediary with a size range of 0.2–0.5 mm to be either packed as a dosage form or be mixed with other excipients before tablet compaction.42 As a new application for sorbitan monostearate as a granulating agent, the mean particle size was also selected to be a response to the granulation process. Stepwise multivariate linear regression equation (Equation 14) for the response Y3 (mean particle size) is
Regression analyses reveal the significant effects of all tested factors and the interactions (p<0.05) on the mean particle size values except factor B and interaction AB. That is, maybe, due to increasing the temperature alone of powder, which could not be expected to improve its mean particle size. Also, increasing the temperature of the powder in the presence of sorbitan monostearate as a granulating agent when melted without stirring has no effect on the mean particle size as an interaction. The most effective factor is positive A (sorbitan monostearate concentration). The second effective factor is negative C (stirring rate). These results indicate that the optimized formulation condition for mean particle size increasing is high sorbitan monostearate concentrations (20%) at 200 rpm and either (60 ° C or 80 ° C) formula (F14, F16).
The result of regression analysis of the three factors (Angle of repose, compressibility index, and mean particle size) showed that the optimum formula is F15 (Table 5). Then, the optimum granulation procedure is by using a 20% granulating agent (high level), the temperature at 80 °C (high level), and 100 rpm stirring rate (low level).
 |
Table 5 The Optimum Formula for All Responses (F15) |
These results could be considered compliant with the granulation process since increasing the concentration of the granulating agent increase the efficacy of the granulation process (Figure 1A–F. High temperature maintains the granulating agent in the melt state, which enhances its effect in initiating the granulation process, a fusion of the melted droplets, and rounding the granules. Also, the low stirring rate led to an increase in the granules particle size.
Figure 1A–G shows the electron scanning microscope image of different metformin HCl granules prepared by using different sorbitan monostearate concentrations, and pure drug. Comparing the ESM of the pure drug (Figure 1G) and the ESM of the granules (Figure 1A–F), it can be concluded the aggregation of the drug crystals into granules because of using sorbitan monostearate as an aggregating agent. Also, it can be noticed that, increasing the granules particle size with increasing the granulating agent concentration. That is, maybe, due to increasing the covering effect of the molten sorbitan monostearate to the drug crystals, which aggregated to form nuclei by distribution mechanism.
Drug Content
Table 6 represents the mean actual drug content of all prepared metformin granules. From the table, it can be noticed the closest of the values of both theoretical (TDC) and mean actual drug content (ADC). Also, ACD of the four-particle size fractions of the prepared granules showed increasing the ADC by decreasing the particle size except that in the case of using 20% sorbitan monostearate as a granulating agent. Increasing the ACD with decreasing the particle size on using 5%, 10%, and 15% granulation agent indicating the insufficient amount of sorbitan monostearate to granulate the amount of the drug used.
 |
Table 6 Actual Drug Content of All Formulations |
Partition Coefficient
The partition coefficient is a parameter used to indicate the lipophilicity of the drug. Since metformin HCl is granulated by sorbitan monostearate, it is essential to measure the effect of the different concentrations of the granulating agent used on the drug granules lipophilicity. From (Table 7) we can notice increasing the values of the partition coefficient by increasing the concentration of the granulating agent. Increasing the partition coefficient of the granulated metformin, which increased by increasing the granulating agent concentration, indicates increasing the lipophilicity of the granulated hydrophilic drug with sorbitan monostearate. This led to expecting the increase in the drug permeability and hence its pharmacodynamic effect.43
 |
Table 7 Log P Values of Pure Drug and Drug Granules |
In vitro Drug Release
The drug release profiles from the same particle size range of the granules prepared by using different concentrations of sorbitan monostearate were studied (Figure 2). From the figure, it can be noticed that there are a burst effect and an incomplete drug release. These two effects are depending on the concentration of sorbitan monostearate used. Increasing the granulating agent concentration led to decreasing the burst effect and the total drug release during the dissolution process. That is, maybe, due to the coating effect of sorbitan monostearate of the drug crystals and the insolubility of sorbitan monostearate in the dissolution media. These results are in agreement with the suggested granulation mechanism, which started by melting the granulating agent which covers the drug crystals. Increasing the concentration of the sorbitan monostearate leads to a good coating of the drug crystals, which leads to decreasing the burst effect and incomplete release. The explanation could be also confirmed by the high solubility of the drug in the dissolution media, which could be also noticed from the dissolution profile of the non-granulated pure drug crystals.
 |
Figure 2 In vitro release profiles of pure metformin HCl and from drug granules (n=3). |
Differential Scanning Calorimetry (DSC)
Figure 3 shows a DSC thermogram of the pure metformin HCl and different granules prepared by using different concentrations of sorbitan monostearate (F2, F4, F14, and F16). From the figure, it can be noticed that the pure metformin HCl thermogram exhibits a clear endothermic transition stage with a maximum of 224.68 °C. This endothermic transition stage is attributed to the melting of the pure metformin HCl.32 A huge endothermic peak is also observed directly following the melting of the pure metformin HCl, which is due to the decomposition of the pure drug.44 More details about the thermal degradation of metformin HCl were also reported.45 Comparing the DSC scan of the drug in the prepared granules with that of pure drug, it can be concluded that there is no change in the drug crystallinity from the pure form as a result of the granulation process. Also, the DSC scan of (F2, F4, F14, and F16) showed an endothermic peak at around 60°C, which is increased by increasing the sorbitan monostearate concentration. This endothermic peak represents the melting point of sorbitan monostearate. These results are in agreement with the results of an electron scanning microscope of the same products, which reveals the coating of the drug crystal with the granulating agent.
 |
Figure 3 Differential scanning calorimetry (DSC) thermogram of the pure metformin HCl and (F2, F4, F14, and F16). |
Permeability Study (Modified Non-Everted Sac Technique)
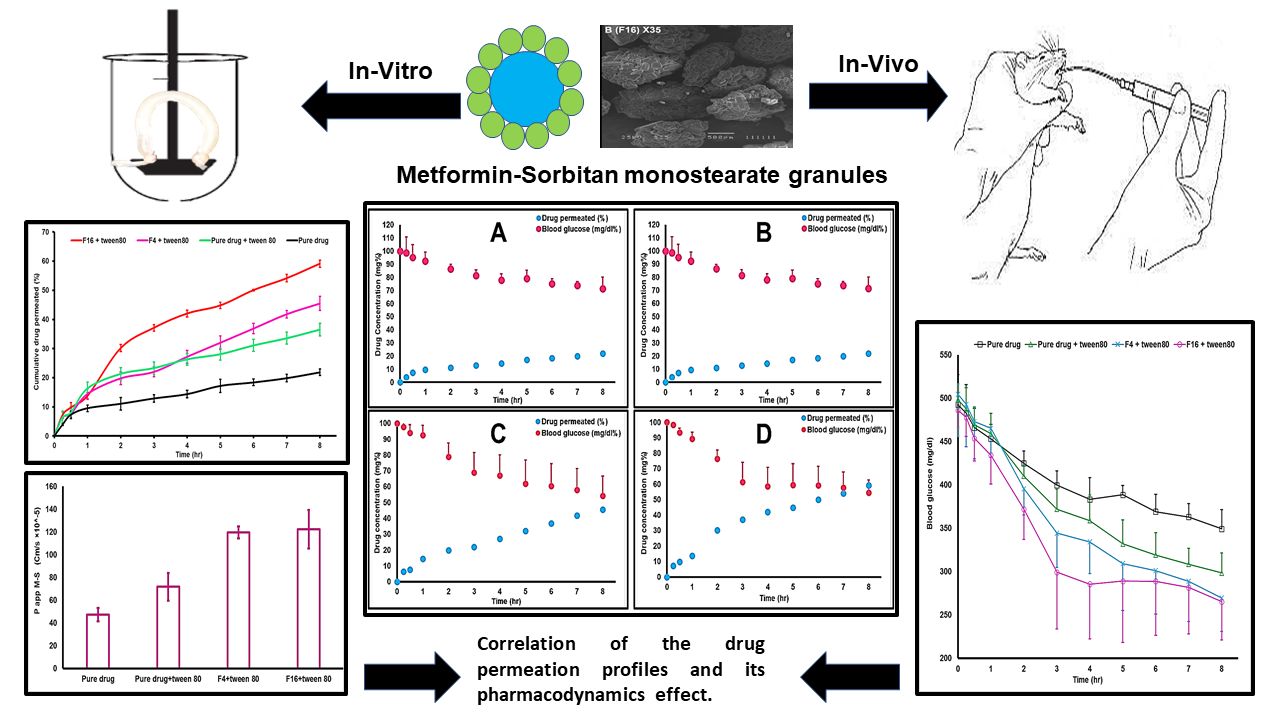
The using “intestinal sacs” for assaying the permeability is a quick and sensitive technique for determining the overall intestinal integrity or comparative transport of a specific molecule, with the added benefit of intestinal site-specificity. The apparent permeability [Papp] or permeation coefficient of a molecule through the intestinal barrier could be calculated.46
From Figure 4, can be noticed the effect of the addition of tween 80 to the drug on its permeation profile concerning the initial, the rate, and the total drug permeated during the experimental time. This effect is increased markedly from drug granules. There is a slight increase in the initial drug permeated. At the same time, the rate and total amount of drug permeated are markedly increased after 1 hr. The drug permeation profile is increased by using 20% sorbitan monostearate as a granulating agent than that of using 5%.
 |
Figure 4 The permeability profiles of the pure drug and selected formulas (n=3). |
The permeability parameters of metformin HCl, metformin-tween 80, and selected metformin-sorbitan monostearate granules across the non-everted sac were determined and the results are summarized in Table 8. From Table 8, it can be noticed that the values of R2, in each case, are high enough to consider the good fitting of the data. Also, in each case, there is no lag time and instead, there is a value for the intercept with y abscissa in concentration (Table 8). The absence of the lag time, which could be due to the high water solubility of the drug, may be responsible for the intercept values. The intercept value represents, in this case, the rapid saturation of the paracellular pathway tissues of the intestinal wall with the drug before drug transport. This result is supported by the fact that about 90% of metformin HCl is absorbed via the paracellular pathway.47–49 The addition of tween 80 to the drug led to an increase in all drug permeation parameters. That is, maybe, due to the role of nonionic surfactants on enhancing metformin absorption via the paracellular pathway. Also, the significant saturable components of the paracellular pathway may be affected by the presence of tween 80 especially the saturable paracellular pathway mediated by electrostatic interactions between the opposite charges of diffused substances (drug and tween 80) and the anionic residues of the lateral space and/or tight junctions.47 Also, from the same table, it can be noticed that emulsification of the metformin–sorbitan monostearate granules by tween 80 lead to markedly increasing the apparent permeability of the granulated drug than the drug alone or drug-tween.
 |
Table 8 Data of Metformin HCl Transferred Through Non-Everted Intestinal Sac (n=3) |
The apparent permeability of the drug, drug-tween, and drug granulated with different concentrations with sorbitan monostearate is also presented in Figure 5. From the figure, it can be noticed that the permeation enhancement of the drug can be ordered as the following, from drug-granules ˃ drug-tween ˃ drug alone. Accordingly, it can be reported that granulation of the drug by sorbitan monostearate and emulsification of the prepared granules lead to a permeation-enhancing effect of the drug. An increase in the drug permeability may lead to a decrease in the recommended drug dose and consequently its side effects, which represent a known problem specially by older patients.
 |
Figure 5 The apparent permeability of metformin (mucosal -to- serosal) from pure drug and selected formulas (n=3). |
Pharmacodynamic Evolution of the Metformin HCl Granules
Monitoring the pharmacodynamic marker parameters represents the evaluation of the in vivo performance of different classes of drugs. It measures the pharmacological effect of the drug, which is the reason for its admiration and/or application. Metformin HCl is an oral antidiabetic drug, which is used for decreasing blood glucose levels. Accordingly, measuring the blood glucose level after oral administration of the drug could be used as a pharmacodynamic marker parameter for evaluation of the in vivo performance of metformin HCl from the prepared granules compared with the pure drug.
Before the drug administration, the blood glucose level was measured which represents the blood glucose level at zero time. Then, the drug was administered and the plasma glucose level was monitored as a function of time. The blood glucose level would be expressed as dropping of the blood glucose level and the profile of dropping of the drug glucose concentration is plotted as a function of time (Figure 6A).
 |
Figure 6 (A) Profiles of the change in glucose levels (mg/dl) versus time (SD error bar), (n=6). (B) Profiles of the change in glucose levels (mg/dl) versus time (SD error bar), (n=6). |
The dropping of the blood glucose levels profile showed a slight drop in the blood glucose level for the first one hour, which is in agreement with the results of the permeation profiles. A reduction in the blood glucose level with the onset of action starting at 15 minutes after oral drug administration could be noticed. This is due to the high solubility of the drug and its main absorption mechanism (paracellular pathway). Then, the rate and maximum amount of blood glucose dropping are markable and can be arranged as the following from F16 ˃ F4 ˃ Drug-tween ˃ Drug alone. Each point represents the mean of blood glucose level measurements at that time with standard deviation as shown in Figure 6B.
Also, from Figure (6A and B), the area above the curves was calculated, tabulated in Table 9, and used for comparison. From the table, it can be concluded that the addition of tween 80 to the drug improved its pharmacodynamic effect by 20% (calculated by dividing the difference in the area above the curve between the drug-tween and drug alone). Emulsification of metformin HCl granules with tween 80 led to improving the pharmacodynamic effect of the drug by 43%, which represents an additional effect of the presence of sorbitan monostearate. Statistical analysis of the data (ANOVA test) revealed a significant enhancement of the pharmacodynamic effect of metformin HCl after oral administration with tween and from granules compared with the control (metformin HCl alone) at P ˂ 0.05.
 |
Table 9 The Blood Glucose Level of Diabetic Rats After Drug Oral Administration |
The supremacy of the drug granules over the pure drug, concerning the onset of action and area above the curve, indicates that the granulation of the drug by sorbitan monostearate increase both the rate and extent of drug absorption. The increase in the rate of drug absorption compared to the pure drug suggests an enhancement of the drug permeation, which is proved by a modified non-everted sac test. Enhancement of the drug permeation led to the enhancement of the drug bioavailability, which will be reflected in the pharmacodynamics of the drug. This theoretical concept is now also approved by the in vivo test. This finding should lead to decreasing the drug recommended doses in case applying the suggested industrial procedure and consequently decrease the drug side effects.
Metformin HCl is a class III drug that faces the permeation problem. The bioavailability of metformin HCl is 40–60%. Modified Non-everted sac study showed improvement of the total drug permeation % from 21.86 ±0.222 in case of a pure drug to 45.48 ±1.156 and 59.124 ±0.228 from F4 and F16 respectively during 8 hrs. The dropping of blood glucose level (pharmacodynamics) during the same time was improved to be 43% more than the pure drug.
Metformin is negligibly bound to plasma protein. It will be excreted unchanged in the urine and does not suffer hepatic metabolism.51 It is absorbed mainly via the paracellular pathway.50 Liquisolid system and SEDDS are reported to enhance dissolution and bioavailability of lipophilic drugs like simvastatin and famotidine with a good correlation between in vitro in vivo tests.48,49 In this study, granulation of metformin with sorbitan monostearate led to decreasing its solubility in water and increasing its partition coefficient (lipophilicity). The drug permeability and pharmacodynamic effects are more enhanced from the drug granules prepared by using sorbitan monostearate as a granulating agent. That is due to the paracellular pathway permeation enhancement of the drug by sorbitan monostearate, which improves consequently the drug pharmacodynamic effect. Accordingly, it can be reported that granulation of metformin HCl with sorbitan monostearate using melt congeal technique, suggested by the authors, led to dual effects of the food used surfactant on the drug: first; an excellent improvement of the drug micromeritic properties, which represented in a lot of trials of the literature to solve the first industrial problem facing the drug formulation, second; the using of sorbitan monostearate as a granulating agent enhanced its permeability and consequently, its bioavailability. This, maybe, lead to studying the pharmacokinetics, decreasing the recommended drug doses, and certainly, the drug side effects.
Drug Permeation–Pharmacodynamics Correlation
The term correlation is often used in the pharmaceutical and related sciences to designate the relationship that exists between variables. From the mathematic view, the term correlation means interdependence between quantitative or qualitative data or relationship between measurable variables and ranks.
FDA defined IVIVC as “a predictive mathematical model describing the relationship between an in vitro property of a dosage form and a relevant in vivo response”. In general, the in vitro characteristic is the rate or extent of drug dissolution or release. However, the in vivo response is the plasma drug concentration or amount of drug absorbed.52,53 FDA regulation described 4 levels for IVIVC which are A, B, C, and multiple C.54 Level A correlates the entire in vitro and in vivo profiles. It represents a point-to-point relationship between in vitro dissolution rate and the in vivo input rate of the drug from the dosage form.46 Accordingly, it is the highest category of correlation.
In this study, we tried to correlate data of the drug permeation profile from rabbit intestinal sac and the pharmacodynamic response of the drug on the diabetic male rats (Figure 7 and Table 10). From the figure, it can be noticed that the drug permeation profile from the rabbit intestinal sac is nearly the opposite superimposed to the pharmacodynamic profile of the drug in male rats. That is, maybe, due to the correlation is between the drug permeation percentage and the pharmacodynamic effect. The pharmacodynamic effect is the dropping of the blood glucose level %. At each time, increasing the drug permeation percent led to a drop in blood glucose level percent. Since Level A correlation is a linear relationship between two variables, it was tried to create a mathematics line correlation between the drug permeation percent and its pharmacodynamic effect (dropping of glucose level) as a point-to-point correlation. The results are summarized in (Table 10). From the table, it can be noticed the value of R2 is high enough to conclude the presence of an excellent correlation between the variables. The values of intercepts are nearly equal and nearly closed to 100%. The rate of correlation values (slope) increased by increasing the amount of sorbitan monostearate in the granules, which is in agreement with permeation enhancement of the drug by sorbitan monostearate. These results may be suggesting the use of the modified non-everted sac, suggested by the authors, as a technique to predict the drug permeation process in vitro. The intestinal non-everted sac should be suspended in the shaft of the dissolution apparatus to avoid the stagnant diffusion layer. Stirring of the dissolution media by stirring the apparatus shaft represents the circular movement of the blood.26
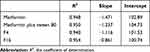 |
Table 10 Correlation Data of Drug Permeation and Pharmacodynamic Profile |
 |
Figure 7 Point to point (level A) correlation of the drug permeation profiles and its pharmacodynamic effect: (A) Metformin HCl, (B) Metformin HCl plus tween 80, (C) Formula F4, (D) Formula F16. |
Conclusion
From this study, it could be concluded that the use of sorbitan monostearate, which is normally used in the food industry, as a granulating agent led to the formation of free-flowing, good compressible, and narrow particle size granules. These physical characteristics are very essential for packaging, transport, and tablet press in the pharmaceutical industry. Also, it was found that the use of sorbitan monostearate led to improving the permeation of the model drug for class III. The suspension of the non-everted sac at the shaft of the dissolution apparatus prevents the formation of the stagnant diffusion layer. This may lead to an excellent correlation between the results of drug permeation from the modified non-everted sac and the dropping of the blood glucose level (improving the pharmacodynamic response of the drug). Accordingly, it can be reported that granulation of metformin HCl with sorbitan monostearate using melt congeal technique, suggested by the authors, led to dual effects of the food used surfactant on the drug: first; an excellent improvement of the drug micromeritic properties, which represented in many trials of the literature to solve the first industrial problem facing the drug formulation, second; the using of sorbitan monostearate as a granulating agent enhanced its permeability and consequently, its bioavailability. This, maybe, lead to, after studying the pharmacokinetics, decreasing the recommended drug doses and certainly, the drug side effects. Therefore, it is strongly recommended to apply the same technique with the same procedure to solve the permeation problem of class III drugs especially, since the granulation process is easy, reproducible, cheap, and applicable in the pharmaceutical industry.
Data Sharing Statement
The raw data supporting the conclusions of this manuscript will be made available by the author Adam A. Al-Shoubki, (E-mail: [email protected]), without undue reservation, to any qualified researcher.
Ethics Statement
All procedures of animal study were approved and regularly controlled by the Animal Ethics Committee of Faculty of Pharmacy Tanta University (No: 2212018) and all experiments were performed in accordance with the guidelines and regulations of this committee. All the procedures were also carried out in full accordance with the ARRIVE guidelines 2020.37
Acknowledgments
We have to express our appreciation to workers and faculty members, the Department of Pharmaceutical Technology, Faculty of Pharmacy, Tanta University, for sharing their pearls of wisdom with us during the course of this research. The abstract of this paper was presented at the 35th Scientific Conference of the Egyptian Pharmaceutical Society, Cairo, Egypt, as a conference talk with interim findings. The authors shared the abstract on the ResearchGate platform: https://www.researchgate.net/publication/340095954_An_industrial_procedure_for_pharmacodynamic_improvement_of_metformin_HCl_via_granulation_with_its_Paracellular_pathway_enhancer
Disclosure
The authors declare no competing interests in this work.
References
1. Sharma S, Prasad B. Prediction of negative food‐effect on drug bioavailability by mechanistic biopharmaceutics classification System (mBCS)‐guided physiologically‐based pharmacokinetic modeling. FASEB J. 2020;34(S1):1. doi:10.1096/fasebj.2020.34.s1.05705
2. Cho YD, Park YJ. In vitro and in vivo evaluation of a self-microemulsifying drug delivery system for the poorly soluble drug fenofibrate. Arch Pharm Res. 2013;37(2):193–203. doi:10.1007/s12272-013-0169-4
3. Yan B, Ma Y, Guo J, Wang Y. Self-microemulsifying delivery system for improving bioavailability of water insoluble drugs. J Nanopart Res. 2020;22(1):1–4. doi:10.1007/s11051-019-4744-6
4. Gulsun T, Akdag Y, Izat N, Cetin M, Oner L, Sahin S. Development and characterization of metformin hydrochloride- and glyburide-containing orally disintegrating tablets. Pharm Dev Technol. 2020;25(8):999–1009. doi:10.1080/10837450.2020.1772290
5. Singh AK, Chaurasiya A, Awasthi A, et al. Oral bioavailability enhancement of exemestane from Self-Microemulsifying Drug Delivery System (SMEDDS). AAPS PharmSciTech. 2009;10(3):906–916. doi:10.1208/s12249-009-9281-7
6. Cheng C. Biowaiver extension potential to BCS class III high solubility-low permeability drugs: bridging evidence for metformin immediate-release tablet. Eur J Pharm Sci. 2004;22(4):297–304. doi:10.1016/s0928-0987(04)00095-8
7. Wakerly MG, Pouton CW, Meakin BJ, Morton FS. The effect of surfactant HLB on the self-emulsifying efficiency of non-ionic surfactant-vegetable oil mixtures. J Pharm Pharmacol. 1986;38(S12):2P. doi:10.1111/j.2042-7158.1986.tb14231.x
8. Pouton CW. Lipid formulations for oral administration of drugs: non-emulsifying, self-emulsifying and “self-microemulsifying” drug delivery systems. Eur J Pharm Sci. 2000;11:S93–S98. doi:10.1016/s0928-0987(00)00167-6
9. Benita S, Lambert G, Garrigue J-S. Self-emulsifying oral lipid-based formulations for improved delivery of lipophilic drugs. Microencapsulation. 2005;2:429–480. doi:10.1201/9781420027990.pt3
10. Craig DQM, Lievens HSR, Pitt KG, Storey DE. An investigation into the physico-chemical properties of self-emulsifying systems using low frequency dielectric spectroscopy, surface tension measurements and particle size analysis. Int J Pharm. 1993;96(1–3):147–155. doi:10.1016/0378-5173(93)90222-2
11. Barot B, Parejiya P, Patel T, Parikh R, Gohel M. Development of directly compressible metformin hydrochloride by the spray-drying technique. Acta Pharm. 2010;60(2):165–175. doi:10.2478/v10007-010-0016-9
12. Takasaki H, Yonemochi E, Ito M, Wada K, Terada K. The importance of binder moisture content in Metformin HCL high-dose formulations prepared by moist aqueous granulation (MAG). Results Pharma Sci. 2015;5:1–7. doi:10.1016/j.rinphs.2015.09.001
13. Block LC, Schmeling LO, Couto AG, et al. Effect of binders on 500mg metformin hydrochloride tablets produced by wet granulation. Rev Cienc Farm Basica Apl. 2009;30(2):17–24.
14. Kelleher JF, Madi AM, Gilvary GC, et al. Metformin hydrochloride and sitagliptin phosphate fixed-dose combination product prepared using melt granulation continuous processing technology. AAPS PharmSciTech. 2019;21(1):1–4. doi:10.1208/s12249-019-1553-2
15. Vaingankar P, Amin P. Continuous melt granulation to develop high drug loaded sustained release tablet of metformin HCl. Asian J Pharm Sci. 2017;12(1):37–50. doi:10.1016/j.ajps.2016.08.005
16. Wadher KJ, Kakde RB, Umekar MJ. Formulations of sustained release metformin hydrochloride tablet using combination of lipophilic waxes by melt granulation technique. Afr J Pharm Pharmacol. 2010;4(8):555–561.
17. Kim S-H, Hwang K-M, Cho C-H, et al. Application of continuous twin screw granulation for the metformin hydrochloride extended release formulation. Int J Pharm. 2017;529(1–2):410–422. doi:10.1016/j.ijpharm.2017.07.019
18. Aodah AH, Fayed MH, Alalaiwe A, Alsulays BB, Aldawsari MF, Khafagy ES. Design, optimization, and correlation of in vitro/in vivo disintegration of novel fast orally disintegrating tablet of high dose metformin hydrochloride using moisture activated dry granulation process and quality by design approach. Pharmaceutics. 2020;12(7):598. doi:10.3390/pharmaceutics12070598
19. Tedstone A. Food Standards Agency. Nutr Food Sci. 2010;40(3). doi:10.1108/nfs.2010.01740cab.004
20. Vang Spars II F, Krog N. Food emulsifiers. Food Sci Technol. 2003. doi:10.1201/9780203913222.ch2
21. Hendy RJ, Butterworth KR, Gaunt IF, Kiss IS, Grasso P. Long-term toxicity study of sorbitan monostearate (Span 60) in mice. Food Cosmet Toxicol. 1978;16(6):527–534. doi:10.1016/s0015-6264(78)80219-3
22. Katan MB, Grundy SM, Jones P, Law M, Miettinen T, Paoletti R. Efficacy and safety of plant stanols and sterols in the management of blood cholesterol levels. Mayo Clin Proc. 2003;78(8):965–978. doi:10.1016/s0025-6196(11)63144-3
23. Pardakhty A. Non-ionic surfactant vesicles (Niosomes) as new drug delivery systems. Pharm Sci. 2017;154–184. doi:10.4018/978-1-5225-1762-7.ch007
24. Mady O. Span 60 as a microsphere matrix: preparation and in vitro characterization of novel Ibuprofen-Span 60 microspheres. J Surfactants Deterg. 2016;20(1):219–232. doi:10.1007/s11743-016-1907-7
25. Ito A, Kleinebudde P. Influence of granulation temperature on particle size distribution of granules in twin-screw granulation (TSG). Pharm Dev Technol. 2019;24(7):874–882. doi:10.1080/10837450.2019.1615089
26. Mady OY, Donia AA, Al-Shoubki AA, Qasim W. Paracellular pathway enhancement of metformin hydrochloride via molecular dispersion in span 60 microparticles. Front Pharmacol. 2019;10. doi:10.3389/fphar.2019.00713
27. Yue X, Cui Y, Yuan T, et al. Calcitriol tablets with hybrid lipid-based solid dispersions with enhanced stability and content uniformity. Pharm Dev Technol. 2020;25(7):899–907. doi:10.1080/10837450.2020.1760297
28. Saker A, Cares-Pacheco M-G, Marchal P, Falk V. Powders flowability assessment in granular compaction: What about the consistency of Hausner ratio? Powder Technol. 2019;354:52–63. doi:10.1016/j.powtec.2019.05.032
29. Behera BC, Sahoo SK, Dhal S, Barik BB, Gupta BK. Characterization of glipizide-loaded polymethacrylate microspheres prepared by an emulsion solvent evaporation method. Trop J Pharm Res. 2008;7(1):879–885. doi:10.4314/tjpr.v7i1.14672
30. Mao S, Shi Y, Li L, Xu J, Schaper A, Kissel T. Effects of process and formulation parameters on characteristics and internal morphology of poly(d,l-lactide-co-glycolide) microspheres formed by the solvent evaporation method. Eur J Pharm Biopharm. 2008;68(2):214–223. doi:10.1016/j.ejpb.2007.06.008
31. Oh TO, Kim JY, Ha JM, et al. Preparation of highly porous gastroretentive metformin tablets using a sublimation method. Eur J Pharm Biopharm. 2013;83(3):460–467. doi:10.1016/j.ejpb.2012.11.009
32. Bretnall AE, Clarke GS. Metformin hydrochloride. In: Analytical Profiles of Drug Substances and Excipients; 1998:243–293. doi:10.1016/s0099-5428(08)60757-1
33. El-Gizawy SA, El-Maghraby GM, Hedaya AA. Formulation of Acyclovir-loaded solid lipid nanoparticles: design, optimization, and in-vitro characterization. Pharm Dev Technol. 2019;24(10):1287–1298. doi:10.1080/10837450.2019.1667385
34. Zancanella P, Oliveira DML, De oliveira BH, et al. Mitotane liposomes for potential treatment of adrenal cortical carcinoma: ex vivo intestinal permeation and in vivo bioavailability. Pharm Dev Technol. 2020;25(8):949–961. doi:10.1080/10837450.2020.1762645
35. Smith PL. Methods for evaluating intestinal permeability and metabolism in vitro. In: Models for Assessing Drug Absorption and Metabolism; 1996:13–34. doi:10.1007/978-1-4899-1863-5_2
36. Jha SK, Karki R, Puttegowda VD, Harinarayana D. In vitro intestinal permeability studies and pharmacokinetic evaluation of famotidine microemulsion for oral delivery. Int Sch Res Notices. 2014;2014:1–7. doi:10.1155/2014/452051
37. Percie du Sert N, Ahluwalia A, Alam S, et al. Reporting animal research: explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 2020;18(7):e3000411. doi:10.1371/journal.pbio.3000411
38. Almoazen H. Chapter 4: dosage forms and drug delivery systems. In: The APhA Complete Review for Pharmacy.
39. Page DA, Carlson GP. Method for studying the permeability of the rat intestinal tract to carbon tetrachloride. Toxicol Methods. 1991;1(3):188–198. doi:10.3109/15376519109044569
40. Friedrich M. Membrane transport in biology. In: Herausgegeben von GG, Tosteson DC, Ussing HH, editors. Vol. 3. Transport Across Multi-Membrane Systems. XVIII und 459 Seiten. 97 Abb., 26 Tab. Berlin, Heidelberg, New York: Springer-Verlag; 1980:202. Vol. 24, No. 2. 1978. Preis: 148,— DM; 81,40 $. Food / Nahrung. doi: 10.1002/food.19800240214
41. Hemalatha S, Wahi A, Singh P, Chansouria JP. Hypoglycemic activity of Withania coagulans Dunal in streptozotocin induced diabetic rats. J Ethnopharmacol. 2004;93(2–3):261–264. doi:10.1016/j.jep.2004.03.043
42. Shanmugam S. Granulation techniques and technologies: recent progresses. BioImpacts. 2017;5(1):55–63. doi:10.15171/bi.2015.04
43. Hamdan I, Farah D, Abu-Dahab RA-D. Chromatographic behaviour and analytical method development for metformin HCl: application to permeation studies through Caco-2 cells. Acta Pol Pharm. 2020;77(1):11–21. doi:10.32383/appdr/112237
44. Plata-Vargas E, De la Cruz-hernández C, Dorazco-González A, Fuentes-Noriega I, Morales-Morales D, Germán-Acacio JM. Synthesis of metforminium succinate by melting. crystal structure, thermal, spectroscopic and dissolution properties. J Mex Chem Soc. 2017;61(3). doi:10.29356/jmcs.v61i3.345
45. Ramukutty S, Jeyasudha R, Ramachandran E. Mechanical and thermal studies of metronidazole crystals. Indian J Phys. 2013;87(10):1001–1004. doi:10.1007/s12648-013-0337-x
46. Mateer SW, Cardona J, Marks E, Goggin BJ, Hua S, Keely S. Ex vivo intestinal sacs to assess mucosal permeability in models of gastrointestinal disease. J Vis Exp. 2016;(108). doi:10.3791/53250
47. Subramanian N, Sharavanan SP, Chandrasekar P, Balakumar A, Moulik SP. Lacidipine self-nanoemulsifying drug delivery system for the enhancement of oral bioavailability. Arch Pharm Res. 2015;39(4):481–491. doi:10.1007/s12272-015-0657-9
48. Deli MA. Potential use of tight junction modulators to reversibly open membranous barriers and improve drug delivery. Biochim Biophys Acta. 2009;1788(4):892–910. doi:10.1016/j.bbamem.2008.09.016
49. Dimitrijevic D, Shaw AJ, Florence AT. Effects of some non-ionic surfactants on transepithelial permeability in Caco-2 cells. J Pharm Pharmacol. 2000;52(2):157–162. doi:10.1211/0022357001773805
50. Proctor WR
51. Scheen AJ. Clinical pharmacokinetics of metformin. Clin Pharmacokinet. 1996;30(5):359–371. doi:10.2165/00003088-199630050-00003
52. Metzler CM. Pharmaceutical statistics: practical and clinical applications. Second edition. By Sanford Bolton. Marcel Dekker: new York. 1990. xvii + 646pp. 16 × 23cm. ISBN 0-8247-8267-4, $ 99.75. J Pharm Sci. 1991;80(6):614. doi:10.1002/jps.2600800624
53. Al-Dossary BN. In-vitro and in-vivo availability of mebeverine hydrochloride suppositories. Sci Pharm. 2006;74(1):31–51. doi:10.3797/scipharm.2006.74.31
54. Uppoor VRS. Regulatory perspectives on in vitro (dissolution)/in vivo (bioavailability) correlations. J Control Release. 2001;72(1–3):127–132. doi:10.1016/s0168-3659(01)00268-1
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.