Back to Journals » Journal of Pain Research » Volume 15
An Algorithm Approach to Phantom Limb Pain
Authors Boomgaardt J, Dastan K, Chan T, Shilling A, Abd-Elsayed A, Kohan L
Received 22 December 2021
Accepted for publication 14 October 2022
Published 26 October 2022 Volume 2022:15 Pages 3349—3367
DOI https://doi.org/10.2147/JPR.S355278
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Dawood Sayed
Jacob Boomgaardt,1 Kovosh Dastan,1 Tiffany Chan,2 Ashley Shilling,2 Alaa Abd-Elsayed,3 Lynn Kohan2
1Department of Physical Medicine and Rehabilitation, University of Virginia, Charlottesville, VA, USA; 2Department of Anesthesiology, University of Virginia, Charlottesville, VA, USA; 3Department of Anesthesiology, University of Wisconsin-Madison, Madison, WI, USA
Correspondence: Lynn Kohan, Department of Anesthesiology, University of Virginia, 545 Ray C Hunt Suite 3168, Charlottesville, VA, 22903, USA, Tel +1-434-243-5676, Fax +1-434-243-5689, Email [email protected]
Abstract: Phantom limb pain (PLP) is a common condition that occurs following both upper and lower limb amputation. First recognized and described in 1551 by Ambroise Pare, research into its underlying pathology and effective treatments remains a very active and growing field. To date, however, there is little consensus regarding the optimal management of phantom limb pain. With few large well-designed clinical trials of which to make treatment recommendations, as well as significant heterogeneity in clinical response to available treatments, the management of PLP remains challenging. Below we summarize the current state of knowledge in the field, as well as propose an algorithm for the approach to the treatment of PLP.
Keywords: phantom pain, phantom sensations, stump pain, residual limb pain
History of Phantom Pain
Phantom pain was first described by Ambroise Paré in 1551. Paré, a surgeon with the French army, earned the rank of master barber-surgeon in 1536, and served four French kings over a period of 30 years. He had extensive experience with amputees in his practice and was reported to have amputated as many as 200 limbs in a day.
In his experience with soldiers following amputations, he wrote,
For patients, long after the amputation is made, say they still feel pain in the amputated part. Of this they complain strongly, a thing worthy of wonder and almost incredible to people who have not experienced this.
His description of phantom pain foreshadowed current thoughts, with suggestions of a peripheral model (noting contractions of muscles, nerves and tendons), as well as postulations that “pain memory” in the central nervous system could account for pain without contractures.1 His astute observations, however, seem to have been ignored for the next 300 years. The difficulty of understanding the phenomenon of continued sensations and pain in a limb that has been amputated seems to have rendered the matter “indelicate.”
In 1871, Silas Weir Mitchell published the first modern description of phantom pain and reestablished interest in the topic. Mitchell was a physician in Philadelphia during the Civil War and was responsible for the treatment of nervous injuries and problems at Turners Lane Hospital. He described some of the unusual symptoms from wounds to peripheral nerves, including both causalgia and phantom pain, in his book entitled Injuries of Nerves and Their Consequences.
In his writing about the consequences of amputation, he writes:
Sensory hallucination.—No history of physiology of stumps would be complete without some account of the sensorial delusions to which persons are subject in connection with their lost limbs…Nearly every man who loses a limb carries about with him a constant or inconstant phantom of the missing member, a sensory ghost of that much of himself, and sometimes a most inconvenient presence, faintly felt at times, but ready to be called up to his perception by a blow, a touch, or a change of wind.6
In more recent years, phantom experiences with other organs including tongue, breast, testicle, bladder, gallbladder and penis have been described.16 Similar sensations and pain have also been noted after spinal cord injury, thalamic lesions, and nerve avulsion injuries.
Today, we continue to struggle to explain phantom sensations and pain. Unfortunately, the rate of phantom pain after amputation remains high and effective treatment remains elusive. This paper will review the current literature on phantom pain and discuss a stepwise approach to treatment based on the currently available evidence.
Definitions, Epidemiology, and Risk Factors of Phantom Pain
Phantom sensations are defined as any sensation, excluding pain, of the missing limb and are experienced by almost all patients who undergo amputation. Young children, however, seem to be much less likely to experience phantom sensations. Simmel studied this phenomenon and found that phantom sensations were reported to occur in 20% of amputees <2 years old, in 25% of children between 2 and 4 years old, in 61% between 4 and 6 years old, in 75% of children 6 to 8 years old, and in 100% in children greater than 8 years old.2
Phantom sensations are described using terms such as tingling, tightness, touch, temperature, and itching. The position of the limb can be relaxed, distorted or fixed. Immediately after the amputation, the limb is generally described as resembling the pre-amputation limb in volume, shape, length, and movement. With time, telescoping may occur.3 This describes a sensation that the proximal portion of the limb shrinks or shortens, leaving the distal part attached or approaching the residual limb. For example, the patient will describe feeling as though their missing hand is attached to their shoulder.
Phantom limb pain (PLP), defined as pain in the missing limb, is thought to occur in 60–85% of adult amputees. It is estimated that there are approximately 1.6 million amputees in the United States, with approximately 30–40,000 amputations performed yearly, most commonly due to peripheral vascular disease.4,5
Historically, the incidence and prevalence have been drastically underestimated. Factors that are likely to have contributed to this underestimation are healthcare providers failing to ask the patients about phantom pain and patients being reluctant to report such symptoms for fear that they would be considered psychologically unstable.14
Phantom pain typically starts quickly after the amputation, sometimes immediately after the anesthetic wears off, and usually within the first few days following the procedure. A recent study by Flahaut et al found that PLP developed in 50% of patients within the first 24 hours post-amputation and in 85% of patients within 1 week.7 This timing of onset, however, is variable with research by Schley et al suggesting there may be a second peak in onset at approximately 12 months post amputation.12
Phantom pain, like phantom sensation, is usually concentrated in the distal aspect of the limb. It is commonly described as shooting, throbbing, squeezing, stabbing, boring, pricking, cramping or burning.10 Initially, Simmel claimed that children born with missing limbs could not experience phantom pain.2 This concept that those with congenital amputations could not experience PLP has since been refuted. Wilkins et al studied 60 child and adolescent amputees, 27 born with a missing limb and 33 who underwent surgical amputation. They found an incidence of phantom pain in 3.7% in the congenital amputees and 48.5% in the surgical group.8 Further research by Weinstein et al found that amongst 30 congenital amputees, five experienced phantom limb pain.9 This suggests that although rare compared to acquired amputations, those with congenital amputations may experience PLP.
Although it has been suggested that pre-amputation pain increases the risk of phantom pain, the relationship is not entirely clear. Traumatic amputations seem to produce about the same percentage of patients with phantom pain as those with chronic painful conditions prior to the amputation.13 Jensen et al studied 58 amputees, comparing the rate of phantom pain between a group with and without pre-amputation pain. Phantom pain was significantly more frequent in the group with pre-amputation pain at 8 days and 6 months, but not after 2 years.10 Additionally, several recent studies by Nikolajsen et al have found that pre-operative pain, as well as preoperative limb sensitivity measured using the limb pain-pressure threshold, predict early phantom limb pain but not late phantom limb pain assessed at 6 months.11,15 More recent work by Noguchi et al suggests that inadequate pre-operative pain control and the presence of diabetes mellitus are major risk factors for the subsequent development of PLP.17 Overall, the evidence supports inadequate pre-operative pain control as an important risk factor in the subsequent development of PLP, but that this relationship appears to be more significant in predicting early PLP development.
While the association between pre-amputation pain and phantom pain remains unclear, studies consistently demonstrate an association between phantom pain, phantom sensations and residual limb pain.11,12,14,15 Residual limb pain, or stump pain, is pain at the amputation site, typically near the incision site. This type of pain is common in the immediate postoperative period but for some patients, can persist for years after the wound has healed.3 The pain is often described as stabbing, shocking or burning at the end of the residual limb. Examination of the limb may reveal infection, neuromas or bone spurs that account for at least some of the pain.
Kooijman et al reviewed data collected from 124 upper extremity amputees. They found a significant association between phantom pain and phantom sensation (relative risk 11.3) and between phantom pain and residual limb pain (relative risk 1.9).18 While some patients might have difficulty distinguishing between these phenomena, it is also possible that residual limb pain triggers phantom pain, making separation difficult. Still, multiple studies have reported an interrelationship between phantom pain, residual limb pain and phantom sensations for both upper and lower extremity amputations.18,19
In Kooijman’s report, there was a trend toward increased risk of phantom pain with more proximal amputation, but it did not reach significance. This increased incidence of phantom pain with more proximal amputation has also been reported by others.19,24
Understanding the duration and natural history of PLP remains elusive. Studies by authors such as Parks3 have suggested that phantom pain often decreases or ceases after two years. Kooijman,18 however, failed to find this pattern of diminishing pain. Nikolajsen et al15 analyzed data from 56 patients 1 week, 3 months and 6 months after amputation. The incidence and intensity of pain remained constant but the frequency and duration of attacks decreased significantly. Further work by Flor et al did not note a clear relationship between time and PLP severity, though their work using functional neuroimaging suggests that it is the degree of cortical reorganization that helps to predict PLP.22 Further work in this area is needed to elucidate this relationship. Like other aspects of understanding phantom pain, meaningful conclusions are complicated by differences in study populations, study designs and outcomes measured.
Pathophysiology of Phantom Pain
The pathophysiology of phantom pain is not well understood, but many theories have been suggested. The hypotheses are detailed below, divided into peripheral factors, central factors, and psychological factors.
Peripheral Factors
- When nerves are injured or severed, the neuron undergoes retrograde degeneration and shrinkage. The nerve terminal swells, and the axon then sprouts in an attempt to regenerate and reestablish the previous connection. Unfortunately, as reconnection is impossible in the case of amputation, this often results in the development of a disorganized mass of nerve fibers, termed a neuroma. These fibers have pathological spontaneous activity as well as low mechanical and chemical thresholds for stimulation and firing. Much of the abnormal behavior is generated along the axon or in the soma, and such discharges are termed ectopic. The sodium channels along the axon appear to be upregulated or novel. Local upregulation of sodium channels has been found to correlate with more frequent bouts of pain.20 Altered transduction molecules for mechano-, heat and cold sensitivity also appear to play a role.21,22 Ectopic discharges from myelinated axons appear to start earlier and to be rhythmic, while C-fibers tend to demonstrate slow, irregular patterns.22 Non-functional connections between axons are also observed and are thought to contribute to abnormal nerve firing patterns.22
Neuroma formation is thought to contribute to residual limb pain and can sometimes be felt during examination of the residual limb. Neuromas may also be a source of abnormal impulses to the central nervous system, where such input may contribute to central reorganization, which may contribute to the later development of PLP.21 Residual limb pain, as noted above, is linked to higher rates of phantom pain. Neuromas, however, are not formed at the time when most patients start to experience phantom sensation and pain in the immediate postoperative period. Additionally, pain is often reported in the absence of a neuroma, and the surgical resection of neuromas has limited success in the alleviation of PLP. Furthermore, the injection of local anesthetic into the neuroma site does not eliminate phantom pain consistently, and sometimes produces pain relief that far exceeds the active life of the local anesthetic.3 Thus, while neuroma development may contribute, many other factors are involved in the development of PLP.
- Another site that could contribute to ectopic discharge is the dorsal root ganglia. Abnormal discharges at this location can amplify and potentially distort input from the periphery. Cross-excitation and depolarization of nearby neurons may also occur.22
- The sympathetic system may also contribute to phantom pain through pathological coupling of sensory and sympathetic nerves. In animal models, increased postsynaptic norepinephrine produced in emotionally stressful situations was associated with hyperalgesia and increased spinal nociception.20 The purported mechanisms by which the sympathetic system contributes to phantom pain include sympathetically triggered ephaptic transmission as well as direct sympathetic activation of nociceptors.23 Sympathetic sprouting into the DRGs has been described, but its role in phantom pain is unclear at this time.20,26
Clinically, this could help explain why some patients report increased pain during stressful or emotional events that are likely to be associated with increased sympathetic output. Further supporting the role of the sympathetic system are the observations that some patients with phantom pain report at least partial relief with the use of adrenergic blocking agents, while others have reported exacerbation of the pain with the injection of epinephrine.25
While peripheral factors likely play a role in the development of PLP, the lack of uniform and complete response to techniques such as regional anesthetic blocks suggests that central factors play an important role in both the development and maintenance of PLP.
Central Factors
- Altered activity of the peripheral nervous system can lead to changes in the central nervous system through central sensitization. Nerve injury causes increased firing of dorsal horn neurons, leading to structural changes in primary sensory neurons and reduction of inhibitory processes in the spinal cord. This may directly lead to alterations in function of inhibitory GABAeric and glycinergic interneurons. These interneurons functions may also be altered by the release of brain-derived neurotrophic factor from microglia.23
- Nerve injury can result in a “rewiring” of the nervous system so that previously low-threshold afferents become connected with the transmission of nociceptive information. A-beta fibers from lamina III and IV sprout into lamina II to form functional connections with second-order nociceptors.27,28
- Sustained C-fiber input recruits N-methyl-D-aspartate (NMDA) receptors on second-order neurons. This upregulation of NMDA receptors and glutamate at the molecular level correlates to increased sensitivity, which may contribute to the development of allodynia and hyperalgesia.29
- After major nerve damage, opioid receptors are downregulated on both primary afferent endings and interneurons.33
- Wiesenfeld-Hallin et al also demonstrated a role for cholecystokinin in chronic pain. While normally an endogenous inhibitor of the opiate receptor, it is upregulated in injured tissue, resulting in the experience of increased pain.30
- Harris proposed that pain is caused, in part, by a discrepancy between senses. For example, when a patient with an amputation tries to move the missing arm, the motor commands are met with missing visual and proprioceptive input.31 This idea will be further explored in more details below.
- Reorganization of the cerebral cortex, described in more detail below, is another proposed central mechanism.
- Finally, recent theories of stochastic entanglement propose that in the setting of sensorimotor incongruence following amputation, there is crossover connection between cortical and subcortical centers charged with sensorimotor and pain processing. This may explain the development of PLP in patients without findings of cortical reorganization, and helps underlie the theory behind phantom motor exercises in the treatment of PLP, which is discussed further below.132
One of the most intriguing aspects of the study of phantom pain is that it has encouraged medical specialties to reexamine traditional teaching about the brain. The brain has been studied and found to be highly organized and specialized. These connections, once established in fetal and early life, were previously assumed to be static. In the 1980s, however, evidence from animal studies began to emerge that demonstrated changes in the somatosensory map in animals following amputation or denervation, challenging this conventional wisdom.32,33
Building on this work in animal studies, Ramachandran demonstrated that following an upper extremity amputation, he could elicit phantom sensations in a patient’s missing hand by touching his face. He could do so in a reproducible fashion, mapping out a topographic representation of the patient’s hand on his ipsilateral face.34 The Penfield homunculus provided a vital clue as to the explanation. The area representing the hand neighbors the area representing the face. It appeared that the sensory input from the face “invades” the area formerly occupied by the hand. Thus, touching the face stimulates not only the face but the phantom hand as well. While these findings have not consistently been replicated, it has lead to considerable investigation into changes in functional cortical reorganization, as discussed in more detail below, and has helped inform several therapeutic modalities such as mirror therapy.
Functional imaging, particularly combined magnetoencephalogram (MEG) and 3D surface-rendered MRI, has shown that cortical reorganization can occur following amputation. As above, such studies demonstrate that the area that previously received input from the face expands to the area that previously received information from the upper extremity.34,35 Work by Flor et al demonstrated that the degree of cortical reorganization correlates with the phantom limb pain in an essentially linear fashion (r = 0.93). Residual limb pain and phantom sensation were not found to correlate with cortical reorganization.22
Psychological Factors
- Psychological factors have long been thought to contribute to both the development of and maintenance of phantom pain. As noted above, the prior belief that PLP was predominantly a psychological phenomenon likely contributed to patients’ reluctance to report their symptoms and to seek treatment. Sherman studied the psychological factors of patients with phantom pain and found them to have normal psychological profiles.14 Arena et al, however, published data supporting a significant relationship between stress and the onset of phantom pain, as well as exacerbation of pain episodes.36 The sympathetic nervous system and increased muscle tension could be the physiologic link to explain these findings. Psychological factors are also important in the ability of patients to cope and likely play a significant role in overall function. For example, work by Fuchs et al has found that emotional factors seem to modulate PLP, though this link appears less strong when compared to other causes of chronic pain.37 The reality of phantom pain as a complication from amputation is widely accepted and is not thought to be primarily a psychological phenomenon.
Furthermore, there is no reason to believe that cortical reorganization is limited to sensory-discrimination. Other areas important for emotional and motivational aspects of pain may change in the setting of amputation. For example, Wei et al found potentiation of sensory responses in the anterior cortex following amputation in rats.38
Full understanding of the pathophysiology of PLP remains elusive and is likely multifactorial and may vary with each patient. As with other chronic pain conditions, clarification of the etiology of the problem will likely lead to more effective and targeted treatment strategies.
Traditional Treatment
When treating PLP, it is best to establish a multimodal treatment team.44 Members of this team could include a neurologist, pain specialist, physical therapist, neurosurgeon, rehabilitation specialist, anesthesiologist, and psychologist. A multimodal regimen can be established as early as the perioperative period, as early pain is a strong predictive factor of chronic and severe PLP.39,40 In clinical practice, this is challenging as there are few controlled clinical studies on treatment, many of which have equivocal outcomes. Additionally, many studies do not differentiate between residual limb pain and phantom limb pain, thus confounding results.
Below, we will summarize the literature with regard to current available treatment options. These treatments have been divided into different categories including pharmacological, surgical, interventional, physical, and psychological modalities.
Pharmacological Therapies
Non-opioid medications are the most commonly used medications in all types of pain conditions. Numerous types of non-opioid medications are frequently used in the treatment of PLS, despite a paucity of data reflecting their efficacy. In a cross-sectional study of 255 individuals with PLS, the most common pain medications used were acetaminophen, opioids, nonsteroidal anti-inflammatories (NSAIDs), amitriptyline and nortriptyline, respectively.41 Given that non-opioid medications are generally considered safer, with less long-term adverse effects, they are considered first-line options in pharmacologic management of PPS.
Anti-Inflammatories Including NSAIDs and Acetaminophen
NSAIDs can be helpful in the treatment of PLP in that they can act as adjuvants, thereby reducing the need for narcotics. In addition, they may be used to treat local irritations that can precipitate phantom pain flare-ups. Acetaminophen’s analgesic mechanisms are not clear but thought to work on the central nervous system pathways including inhibiting serotonergic descending pathways.42 NSAIDs work on both the central and peripheral nervous system, and their analgesic effects result from inhibiting COX enzymes that produce prostaglandins.43
Antidepressants
Despite limited data, antidepressants are often used for PLP because of their efficacy in the treatment of other types of neuropathic pain.45 Tricyclic antidepressants (TCA) and serotonin norepinephrine reuptake inhibitors (SNRI) have both been studied in PLP, however strong evidence is lacking, and most studies are limited by small sample sizes. A Cochrane review found two reports on studies using amitriptyline with conflicting results. A small randomized control study by Wilder-Smith demonstrated reduction of pain in 86% of subjects at 1 month using an average dose of 56 mg nightly.46 By comparison, a study by Robinson et al demonstrated no benefit when comparing amitriptyline to an active placebo, benztropine mesylate, when comparing pain scores at 6 weeks.45
Another small case series of 4 patients demonstrated that mirtazapine, an alpha-2 antagonist, was efficacious with a greater than 50% pain reduction and fewer side effects compared to TCAs. This suggests that mirtazapine is an agent worth consideration and warrants further investigation in its use of PLP.47 There are also case reports regarding the efficacy of duloxetine, a norepinephrine and serotonin reuptake inhibitor (SNRI), in the treatment of PLP.49 Even though there may be a role for the use of SSRI and SNRI in the treatment of neuropathic pain, the evidence is very limited for PLP and further research is needed.48,49
TCAs are known to have side effects such as sedation, orthostatic hypotension, arrhythmia, dry mouth and dizziness and should be used with caution. In a study done by Jefferies, nortriptyline and desipramine were equally effective and with fewer side effects compared to amitriptyline.130
SSRIs are overall well tolerated and in general are better tolerated when compared to TCAs. Common adverse effects include sexual dysfunction, weight gain, drowsiness and insomnia.
Examples of these medications and their typical dosing can be found in the chart below.
Anticonvulsants
Anticonvulsants are also frequently used in the treatment of neuropathic pain including PLP. Gabapentin has an inhibitory action on voltage-gated calcium channels via action on the alpha-2 G-subunit, resulting in inhibition of neurotransmitters and reduction in nerve excitability.
A few studies specifically evaluate gabapentin in the treatment of PLP. There have been several well-constructed, blinded trials by Bone, Nikolajsen, and Smith examining the utility of gabapentin. All noted studies failed to demonstrate a statistically significant treatment response to gabapentin.51–53 All three trials were, however, limited by small sample sizes and a Cochrane review combining the results of the studies by Bone and Smith found a statistically significant improvement in pain scores. Overall research appears to favor the use of gabapentin, but further research is needed. Other anticonvulsants including pregabalin, topiramate and zonisamide have been used, but there has been very little overall evidence to support their use in the treatment of PLP.48–50,54,55 The most commonly reported adverse effects include somnolence, dizziness, headache or nausea.
Opioids
The use of opioids in the treatment of PLP is controversial. Initial studies appeared to suggest efficacy, however these findings do not appear to be supported by more recent studies. An early case series by Bergmans et al suggested that oral methadone might be of value in the treatment of PLP. There was a reduction in pain in four patients with refractory PLP from 50% to 90% while on low-dose methadone, ranging from 10 to 20 mg daily, in divided doses.67 A double-blind crossover study of 12 patients by Huse et al comparing oral morphine with placebo found that opioids can be effective in reducing PLP, possibly by reducing cortical reorganization.68 More recent work by Mishra et al suggested that high doses of morphine may be necessary to achieve effective reduction of PLP.69 In addition, work by Wu et al found that while morphine may be effective in decreasing PLP, it was associated with significant side effects and did not improve self-reported function or pain-related interference in ADLs.70
In summary, while opioids may have some utility in the treatment of PLP, the significant side effects associated with their use, as well as limited demonstrated functional improvement, suggest that the risks may outweigh the benefit. Therefore, data does not support their routine use in PLP and their use should be limited to carefully selected cases.
Overall, there is a paucity of well-conducted clinical studies on the pharmacologic management of PLP with most studies limited by small sample sizes. The NMDA antagonist class in particular appears to have promise in the treatment of PLP, especially if used early in the treatment course to prevent the development of central sensitization, but further studies are needed.
Others
Other non-narcotic analgesics that have been used in the treatment of PLP have shown limited success. While limited, the data on the efficacy of propranolol are mixed, with a small case series by Ahmad demonstrating efficacy56 while a small trial by Scadding et al did not find any significant efficacy.57 Clonazepam is generally not used for pain but one study published in 1996 found it beneficial in two patients.58 Additionally, a small study by Su et al demonstrated midazolam as a potential treatment of severe PLP associated with spinal anesthesia.66
Botulinum toxin A has also been evaluated as a possible agent for the treatment of PLP. Kollewe et al successfully injected botulinum toxin type A into areas of strong fasciculations in the residual limbs of 3 patients with PLP. All 3 patients reported alleviation of their pain.59 In 2012, Wu et al conducted a randomized double-blinded pilot study to compare effects of botulinum toxin A versus lidocaine/methylprednisolone injection in 14 individuals who had intractable residual limb and phantom limb pain. They used 50 units of botulinum toxin A in up to 6 painful sites (total units ranging 250–300 units). The study concluded that although there was some improvement in residual limb pain, there was no improvement in PLP when followed monthly for 6 months.60 A Cochrane review did not report enough evidence to recommend Botulinum Toxin A as first-line therapy for PLP.50
Additionally, calcitonin has been studied with conflicting results. A study by Jaeger et al found calcitonin effective in treating phantom pain, but another study by Eichenberger did not find any efficacy in treating PLP.62,63
Dextromethorphan has also shown some efficacy in the treatment of phantom pain in a small study, most likely through inhibition of NMDA receptor hyperexcitability.61
Intravenous ketamine infusions have shown some potential in treating PLP. Eichenberger reported positive results in a randomized double-blind crossover trial where they compared ketamine, ketamine + calcitonin and placebo. They concluded that ketamine may be helpful, while calcitonin did not demonstrate efficacy when compared to placebo in the treatment of PLP.63 A 2018 review of single and combined pharmacologic management of PLP by Hall and Eldabe found that only NMDA antagonists, namely ketamine, produced consistent positive results. Most notably, ketamine infusions reduced pain pressure thresholds and windup, presumably by limiting central sensitization.64 A systematic review evaluating memantine, another NMDA antagonist, found evidence of efficacy in acute, but not in chronic (greater than one year post amputation) PLP.65
Interventional Therapies
Epidurals
Until recently, there was great hope that preoperative epidural use could prevent the development of PLP. Preemptive analgesia is thought to reduce the occurrence of postoperative pain by inhibiting the transmission of peripheral nociceptive afferent input to the central nervous system or spinal cord. Otherwise, prolonged states of central sensitization and hyperexcitability can occur, amplifying future nociception from the amputated site.71 Bach et al showed a persistent decrease in PLP in patients who had undergone a lumbar epidural with 0.25% bupivacaine and/or morphine started 72 hours prior to their amputation surgery compared to those that did not receive an epidural. These results persisted at least 12 months postoperatively.72 In addition, a study by Jahangiri et al further supported these results. Jahangiri studied the effects of epidural infusions containing bupivacaine, clonidine, and diamorphine in 24 patients undergoing lower limb amputations. The epidurals were started 24–48 hours preoperatively and continued for 3 days postoperatively. PLP and sensations, but not residual limb pain, were found to be significantly lower in those who received the epidural infusion compared to the control group. These results also persisted for one year postoperatively.73 Unfortunately, these initial studies by Bach and Jahangiri have not been consistently reproduced. Nikolajsen et al showed that preoperative epidurals had no effect on PLP at 6 and 12 months postoperatively.74 In addition, Ong et al showed no long-term decrease in phantom pain following preoperative epidurals or spinals. He did find better analgesia in the immediate postoperative period (1 week) in those receiving epidurals or spinals compared to those that did not.75 In a recent review focusing on preventive measures, Ahuja et al agree that robust multicenter randomized controlled trials are needed to establish the preventative role of epidural analgesia.76 Due to the lack of evidence substantiating the earlier findings, the use of epidurals in the prevention of PLP has largely been abandoned.
Regional/Peripheral Nerve Blocks
There has been emerging interest in the use of peripheral nerve blocks in the prevention or treatment of PLP. Evidence supporting the efficacy of perineural infusions has been demonstrated in several recent studies. A study by Madabhushi et al showed no PLP 12 months after surgery in which the participants had received sciatic nerve infiltration with clonidine and bupivacaine intraoperatively.77 A case report by Borghi et al further supported these results. A patient underwent an infusion of 0.5% ropivacaine for 28 days following a BKA. The infusion was temporarily discontinued every week to assess for PLP. After 7 days of the infusion, PLP had decreased by 30%. After 14 days, PLP had decreased 60%. There was no pain reported after 21 days of the infusion. After 28 days, phantom limb sensations had disappeared as well. There was no PLP reported after 6, 12, 24, and 36 months of follow-up.78 Another case report by Granville-Chapman et al also suggest a role for regional anesthesia (direct placement of a brachial plexus perineural catheter—0.25% bupivacaine 20 mL bolus followed by a 10 mL per hour continuous infusion) in the prevention of PLP following traumatic upper extremity amputation. The results of this study may be somewhat confounded, however, as the patient was also treated with IV ketamine intraoperatively as well as numerous medications postoperatively, such as pregabalin, amitriptyline, oxycodone, diclofenac, acetaminophen, and mirror therapy.79
Furthermore, a retrospective study by Grant et al examined 64 patients who underwent major lower limb amputations between 1998 and 2001. Thirty-one patients received the usual standard of care, while 33 patients had an intraneural anesthetic catheter placed. In the intraneural catheter group, there was a significant decrease in the postoperative analgesic requirement (median postoperative opioid analgesia of 10mg in the treatment group vs 74mg in the control group) as well as a significant decrease in postoperative prescriptions for amitriptyline (4 patients in the treatment group, 11 in the control group).80 While the use of perineural infusions shows promise, more research is needed to determine standardized protocols for clinical use as well replicate clinical efficacy in large rigorously designed clinical trials.
Surgery
Surgical interventions have been attempted in the treatment of PLP, largely without overwhelming success, as summarized in Table 1. Targeted muscle reinnervation has been recently evaluated as a potentially promising surgical treatment option for PLP and is discussed in more detail (Table 1).
 |
Table 1 Summary of Surgical Interventions for PLP |
Targeted Muscle Reinnervation
As noted above, no one surgical approach has thus far been determined to be superior in the management of PLP. One area of potential promise is targeted muscle reinnervation. Introduced clinically in 2004, TMR was originally designed to improve functional utility of myoelectric prosthesis.134 During this initial work, it was noted that many patients additionally had improvement in pain, and thus more recent work has attempted to evaluate its efficacy in the treatment of post-amputation pain. In traditional surgical techniques, nerves are severed with no distal target available, leaving them at high risk for neuroma formation. As described above, this could lead to pain, which could subsequently lead to central sensitization, and may secondarily contribute to the later development of PLP. In TMR, the severed nerves are coapted to end motor targets of innervated muscles, giving the regenerating fascicles “somewhere to go and something to do”. A recent prospective RCT by Dumanian et al evaluated 28 patients with chronic RLP and PLP and compared conventional neuroma excision to TMR. They found at one-year follow-up statistically significant improvements in PLP and a trend towards significant improvement in RLP in those treated with TMR.129 While potentially promising, work in this area remains in its infancy. In addition to confirming potential clinical efficacy of TMR, many additional questions such as the ideal timing of TMR, as well as differences in primary versus secondary TMR remain to be answered and further study is warranted.135
Local Injections
A recent study by Casale et al showed relief of PLP by contralateral myofascial injection with local anesthetic. They showed that contralateral injections of 1 mL of 0.25% bupivacaine in hyperalgesic myofascial areas on the existing limb attenuated PLP and affected phantom limb sensation.82
Pulsed Radiofrequency Ablation of Stump Neuromas
In a study by West et al, four patients underwent pulsed radiofrequency ablation (RFA) after failing conservative treatment for their PLP and stump pain. All four had at least 80% reduction in their pain for over 6 months. All of the participants reported improved overall function, improved prosthetic tolerance, and a decrease in oral pain medications.83 In addition, Wilkes et al performed pulsed RF to the sciatic nerve in a patient with refractory PLP after a revision to her lower extremity amputation for progressive peripheral vascular disease with good results.84
Intrathecal Pumps
There are limited data regarding the use of intrathecal pump therapy for the treatment of PLP. A recent case series by Carvajal et al demonstrated its use in a limited study, but further research overall is needed to evaluate its utility as a possible treatment modality of PLP.85
Neurostimulation Techniques
Neurostimulation techniques for the treatment of PLP include peripheral nerve stimulation, spinal cord stimulation, deep brain stimulation and motor cortex stimulation. Corbett et al published a systematic review of various randomized controlled trials, noncomparative group studies, case reports, and epidemiological research of neurostimulation modalities for chronic PLP.86 While repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS) appeared to have modest short-term effect, the benefit did not seem to last. Spinal cord stimulation (SCS) was the most prevalent therapy used, followed by dorsal root ganglion (DRG) stimulation. According to a questionnaire distributed to 37 clinicians at National Health Services in the UK, deep brain stimulation (DBS) is more highly regarded to be effective than motor cortex stimulation (MCS) in treating chronic PLP. Still, multiple authors agree that the literature lacks interventional trials using invasive neurostimulation for both treatment of chronic PLP and for prevention of PLP in the immediate postoperative period after amputation.76,86
Peripheral Nerve Stimulation
Peripheral nerve stimulation involves the direct placement of implanted electrodes to peripheral nerves. A few published studies examine the effectiveness of this therapy. Cohen et al utilized a percutaneously implanted lead system to deliver femoral or sciatic nerve stimulation, and 75% of patients reported 50% or greater relief of chronic post-amputation pain associated with associated reductions in disability. The authors defined post-amputation pain to include both nociceptive residual limb pain and sensitized phantom limb pain. The implanted open coil leads were removed after up to 60 days of PNS therapy.87
Spinal Cord Stimulation
Spinal cord stimulation does hold some promise in the treatment of PLP. Broggi et al found a decrease in PLP in patients with spinal cord stimulators. Twenty-six patients with PLP underwent a spinal cord stimulator trial. Of the 26 patients who underwent trials, 23 participants went on to receive implantations after verbally indicating decrease in their phantom pain and improved quality of life after trial.88 Katayama et al performed spinal cord stimulator trials on 19 patients with PLP as a first-line intervention. Successful outcome was defined as a greater than 80% reduction in their Visual Analog Scale (VAS) score. Using these criteria, 6 of the 19 patients (32%) had long-term pain relief with SCS. The remaining patients underwent either motor cortex stimulation or deep brain stimulation as adjuvant treatments.89 More recently, Viswanathan et al retrospectively studied four patients who had undergone spinal cord stimulation for intractable PLP. Postoperatively, all patients had reported a greater than 80% reduction in their pain. Three of the four patients reported that they would choose to undergo SCS implantation again.90 While the use of spinal cord stimulation shows promise in the treatment of PLP, more research in this area is needed.
Deep Brain Stimulation and Motor Cortex Stimulation
Deep brain stimulation (DBS) involves electrical stimulation of two deep target sites in the brain, the periaqueductal gray and the lateral thalamus-internal capsule. Phantom pain appears more likely to respond to thalamic stimulation than periaqueductal gray stimulation. Katayama et al showed some success with the use of deep brain stimulation in the treatment of PLP. Six out of ten patients who underwent deep brain stimulation had relief of their PLP.89
There is growing evidence to support the use of motor cortex stimulation in the treatment of chronic neuropathic pain, including PLP. Carroll et al showed two out of 3 patients with PLP exhibited a 50% decrease in their pain following motor cortex stimulation.91 Work by Katayama et al evaluated the effects of spinal cord stimulation (SCS), DBS of the thalamic nucleus ventralis caudalis (VC), and motor cortex stimulation (MCS) in 19 patients with PLP. All of the patients underwent SCS and, if SCS failed to reduce their pain, they were considered for DBS and/or MCS. Satisfactory long-term pain control was achieved in 6/19 (32%) by SCS, 6/10 (60%) by DBS, and 1/5 (20%) by MCS. DBS of the VC produced dramatic effects on pain, leading to a long-term pain-free interval and infrequent use of stimulation.89 Lefaucheur et al performed a study investigating the effects of motor cortex stimulation for the treatment of peripheral nerve lesions, including PLP, which support Katayama’s findings.92 While these results are encouraging, it is difficult to predict which patients may respond to treatment prior to implantation, and thus more studies are warranted.
Dorsal Root Entry Zone Lesion
Literature has shown that lesions in the dorsal root entry zone (DREZ) can relieve pain from brachial plexus avulsions even when most other interventions including SCS, ablations and motor cortex stimulators have proven unsuccessful. This had led some researchers to believe a large component of pain from brachial plexus avulsions originated at least partially in the dorsal horn. DREZ is a surgical procedure where a laminectomy is performed along the spine with opening of the dura and arachnoid to reach targeted dorsal roots and ultimately the dorsal horn with the goal of creating a lesion by coagulating. While DREZ has been reported to show satisfactory relief for patients with avulsed dorsal roots, Saris et al reported only 40% (2 of 5) patients with PLP and intact roots had satisfactory results after DREZ.93 Zheng et al conducted a study of 14 individuals with amputations and brachial plexus avulsions that developed PLP, and they were treated with DREZ for relief of PLP. Of the fourteen patients who underwent DREZ, 90% reported satisfactory pain relief after two weeks of surgery and at the next follow-up (15 ± 6.6 months) and nine of the fourteen (64.3%) reported significant pain relief. All the patients in the study were found to have at least 2 avulsions at 2 dorsal roots.81 At this time, there is limited evidence to support DREZ as a treatment option in those with PLP as well as evidence of root avulsions and evidence to suggest that this may be a poor treatment option in those with intact dorsal roots. Given the small available body of literature, however, further research is warranted.
Physical and Psychological Modalities
Acupuncture
Acupuncture involves inserting sterilized needles into the skin at specific parts of the body. The therapy is thought to stimulate the central nervous system to release neurotransmitters, hormones, or endorphins. It may also alter how blood pressure, blood flow, and body temperature are regulated and the body’s response to pain.94 Much of the work on treatment of PLP, while demonstrating promise, is limited to case reports and limited case series.95,96 A recent RCT comparing traditional Chinese acupuncture with standard of care demonstrated statistically significant improvement in pain scores in patients with PLP.97 While this demonstrates promise, it is limited by both a small sample size and difficulty with blinding participants to their treatment arm, limiting conclusions regarding efficacy. Although large-scale studies are needed to prove efficacy, acupuncture may be considered as an adjunctive treatment option for PLP.
Transcutaneous Electrical Nerve Stimulation (TENS)
TENS has been a recommended adjunctive treatment for PLP; however, most of the evidence supporting its use is limited to case studies.98,99 There are several small trials on the utility of TENS, with earlier work by Katz et al using auricular TENS and a pilot study of 10 patients by Mulvey et al which applied TENS to the affected limb. Both of these studies demonstrated improvement in pain scores.100,101 More robust data is lacking, and a 2015 Cochrane review noted that there was insufficient evidence to judge the efficacy of TENS in the management of PLP.102
Biofeedback
Electromyography (EMG) biofeedback and combined EMG thermal biofeedback have also been used in controlling phantom pain. Belleggia et al performed six sessions of EMG biofeedback and six sessions of temperature biofeedback on a patient with extreme PLP after an upper extremity amputation. The patient had complete relief of his PLP following treatment, which was maintained at 3 and 12 months follow-up. They hypothesized that biofeedback may have a lasting effect on cortical reorganization in the somatosensory cortex thus alleviating PLP.103 A subsequent pilot study by Harden et al examined the effectiveness of biofeedback in the treatment of nine individuals with PLP who received up to seven thermal/autogenic biofeedback sessions over the course of 4 to 6 weeks. Results showed a 20% reduction in VAS scores in five of the nine patients after sessions 4, and at least a 30% reduction in 6 patients after session 6.104 While these studies show preliminary support for the use of biofeedback in the treatment of PLP, further investigation is warranted.
Eye Movement Desensitization and Reprocessing (EMDR)
It is thought that long-lasting pre-amputation pain or pain flashbacks secondary to a traumatic injury are in part responsible for PLP. De Roos et al investigated whether a psychological treatment directed at processing emotional and somatosensory memories that are associated with amputation decreases PLP. Ten subjects with PLP were treated with EMDR. Eight of the ten patients improved and four indicated that they were pain free at 3 months follow-up. Six participants were available at long-term follow-up. Three of these patients were pain free and two had reduced pain intensity.105 This is further supported by a case series by Schneider et al, who evaluated EMDR as a treatment in 5 patients with PLP. These cases demonstrated not only an improvement in PLP but additionally in severity of depression and PTSD. The authors suggest that the physiological storage of nociceptive pain experience at the time of the trauma contributes to PLP and that these memories may be reprocessed, thus decreasing pain.106 It is unclear which patients may benefit most from EMDR treatment. De Roos et al questioned whether it was necessary for patients to have an explicit amputation-related memory to benefit from EMDR. He hypothesized this notion based on the findings that the 2 non-responders in his study had regarded their amputation operation and related events as positive because it was life-saving.105 Additionally, it is unclear whether the duration of pre-amputation pain or time since amputation contributes significantly to treatment response. Further studies should address these questions in hopes of identifying which patients with PLP may benefit the most from EMDR.
Electroconvulsive Therapy
There are limited data evaluating the potential use of electroconvulsive therapy (ECT) in the treatment of PLP. Rasmussen et al performed ECT on two patients with PLP who did not have any comorbid psychiatric diagnosis. Both patients reported substantial pain relief following the ECT sessions. One of the two had persistent relief of his phantom pain 3.5 years after the treatment.107 Once again, further research is indicated.
Mirror Therapy
The mechanism of PLP is thought to be related to changes in cortical representation of areas adjacent to the amputated limb. It is thought that this reorganization of the cortical map might be responsible for PLP and that its restitution may relieve it.20 Work by Diers et al (2010) evaluated fMRI data in those with PLP compared to controls. They found that the presence and magnitude of PLP correlated negatively with activation during the mirror movement condition in the contralateral primary somatosensory and motor cortex.21
This therapy is typically performed by placing a mirror in front of the amputated limb where the patient will see a reflection of their intact limb. This gives the patient a perception of an intact limb. Limitations include having one intact limb to use as a mirror image. First demonstrated by Ramachandran in 1995, there have since been numerous small studies supporting mirror therapy as a potentially promising treatment modality.108–114 A systematic review by Barbin et al reviewed 20 studies, including 5 RCTs and found that the overall quality of evidence is poor and there is insufficient evidence to support mirror therapy as a first-line treatment for PLP.115 There were no significant side effects noted with mirror therapy in the above noted studies. Thus, while potentially promising, further research is needed to confirm mirror therapy as an effective treatment of PLP and to standardize treatment protocols.
Phantom Exercises
Phantom exercises, also referred to as phantom motor execution, involve the patient performing the same movement with both their intact limb and the amputated limb. In the case of a patient with a left arm amputation and phantom pain in his left hand, the patient would move his right hand, and imagine moving his left (amputated) hand in the same way. The mechanism by which this may improve PLP has not been clearly elucidated. Work by MacIver et al has demonstrated reductions in cortical reorganization in those treated with phantom exercises using fMRI data with associated reductions in PLP intensity.131 This suggests that this therapy may exert its therapeutic effects by ameliorating maladaptive cortical reorganization. Work by Ortiz-Catalan suggests that independent of cortical reorganization, improving sensorimotor control in the phantom limb, may allow this circuit to be operated outside of the pain circuit, functionally disentangling these two circuits and thus improving PLP.132 Studies by Ulger et al and Anaforoglu et al have demonstrated phantom limb exercise as a potentially beneficial treatment for improving PLP.113,116 Additionally, recent work by Munger et al found that phantom limb movement was a protective factor against the development of both PLP and residual limb pain.117 This therapy remains limited in patients with bilateral amputations. To overcome this limitation, Gaggioli developed a myoelectric-controlled virtual reality system for the treatment of PLP in patients with bilateral transradial upper extremity amputations. The system allows the patient to directly control a virtual limb by recognizing stump muscle patterns recorded with EMG sensors.128
Multimodal Sensorimotor Training
Work by Gagne et al demonstrated a positive correlation between the amount of EMG activity acquired in the residual limb during phantom limb movement and the intensity of PLP.118 This prompted a study by deNunzio et al where 10 upper limb amputees were enrolled in a 16-day intensive training program whereby, through the use of visual and tactile feedback driven by muscular activity at the residual limb, the patient was trained to better control phantom limb motor output. Patients achieved an initial 21.6% average reduction in pain intensity at the end of the training period, which was increased to 32.1% at 6-week follow-up.119 While this represents a potentially promising treatment option for PLP, this study is limited by the lack of a control group. Further studies are needed to evaluate this as a potential therapeutic modality in the treatment of PLP.
Immersive Virtual Reality
While mirror box therapy has shown promise as a potential safe and effective therapy for PLP, there are certain limitations that make effective application challenging. The patient is required to focus on the reflection of their intact arm in order to receive illusory visual feedback of their phantom limb; however, if they briefly lose focus and actually look at their intact limb, this visual illusion is broken. In addition, the patient must remain in a fixed position with their head oriented towards the mirror so as to not alter the reflection of their limb. Finally, each individual has their own perceptions of the phantom limb, which may differ from their unaffected limb, and may help to explain why mirror box therapy is very effective for some, whilst for other patients it is completely ineffective.120 Thus, using immersive virtual reality has been studied as a potential treatment modality that can overcome some of the above noted limitations of traditional mirror therapy. Additionally, it has been suggested that as VR systems allow more interactive games, this can challenge the patient and keep them motivated, potentially leading to improved compliance and better therapeutic outcomes.133
Early work in this field largely consisted of various case reports and case series demonstrating generally positive treatment response though demonstrating considerable individual variability in treatment response as has been consistently documented in the literature for conventional mirror box therapy.120–124 Recently, a 2016 trial by Ortiz-Catalan et al enrolled 14 patients with intractable PLP with failed conventional therapy. They found that after 12 sessions, pain was significantly reduced in 12 of 14 patients with a mean pain reduction of 47%.125 Work by Rutledge et al evaluated the use of VR technology as a novel method of augmented mirror therapy. This was a feasibility trial using fourteen veterans and found improvements in PLP symptoms, phantom sensations, as well as high to very high satisfaction with no adverse events.126 A 2017 literature review by Dunn et al noted that while a potentially promising treatment option, given that the data is predominantly limited to case reports and limited case series, there is insufficient data to confirm its efficacy and further research is warranted.127
Treatment Considerations and Algorithm
While research into the underlying pathophysiology of PLP and its treatment is a growing field, to date, large, well-controlled prospective clinical trials directly comparing different therapeutic modalities have been lacking. Without clear level Ia consensus data to drive clinical decision-making, we are required to utilize our clinical acumen to best tailor the treatment regimen to meet the individual needs of patients. Thus, careful consideration of the patient’s comorbidities and the severity of the patient presentation will help to drive medical management.
The treatment that offers optimal clinical improvement is an interdisciplinary approach that combines both pharmacologic and non-pharmacologic interventions. Additionally, optimizing peri-procedural pain control appears to be a crucial step in preventing sensitization and the cortical changes that appear to be the key pathologic mechanism by which PLP develops. With regard to pharmacologic treatment of phantom limb pain, conservative therapies with good safety profiles should be considered as a first line before instituting opioids, infusion therapies and surgical management for recalcitrant cases. One caveat to the above approach, however, is the importance of addressing the process of central sensitization and cortical reorganization that underlies the development and persistence of PLP.20,22,78
Finally, when possible, it is preferable to treat the PLP as well as associated comorbidities using as few agents as possible. For example, a patient presenting with both an underlying mood disorder and PLP may benefit specifically from a serotonin norepinephrine reuptake inhibitor. A patient presenting with sleep disturbance and PLP may benefit from nightly dosing of a tricyclic antidepressant.
There are many additional treatment modalities that are discussed, such as augmented virtual reality, for which the research remains in its infancy. Thus, there are inadequate data to date to recommend for or against these modalities in the treatment of PLP. We would recommend reserving these treatment options as adjunctive therapies in recalcitrant cases.
Below we have created a general guideline (Figure 1) to consider when treating PLP.
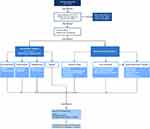 |
Figure 1 Management algorithm for phantom limb pain. |
Conclusion
Post-amputation pain can be disabling, limiting a patient’s ability to function. It is imperative that a treatment plan be established in order to decrease pain and improve function. An interdisciplinary approach to the treatment of PLP offers the best chance for improvement in function and reduction of PLP. This approach includes pharmacological treatment, physical rehabilitation, and psychological support. Unfortunately, many traditional treatments for PLP have failed to result in decreased pain and increased functionality. Therefore, theories involving a more central cause of this pain syndrome are beginning to gain support and are leading to creative therapy. It is important to understand that evidence is emerging indicating that our brains are more plastic than once thought. Therefore, there is hope that we can focus on this plasticity of the brain to find new ways to treat certain pain conditions including PLP. It is here where the use of mirror therapy and virtual reality shows great promise and, therefore, more research in these areas is strongly encouraged. Novel therapies need to be developed and older ones validated; post-amputation complications continue to devastate affected patient populations and warrant significant attention.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
No funding was received.
Disclosure
Alaa Abd-Elsayed serves as a consultant for Medtronic, Avanos and Averitas. The authors report no other conflicts of interest in this work.
References
1. Pare A, Linker RW, Womack N. Ten Books of Surgery with the Magazine of the Instruments Necessary for It. University of Georgia Press; 2010.
2. Simmel ML. Phantom experiences following amputation in childhood. J Neurol Neurosurg Psychiatry. 1962;25(1):69–78. doi:10.1136/jnnp.25.1.69
3. Hill A. Phantom Limb Pain. J Pain Symptom Manage. 1999;17(2):125–142. doi:10.1016/s0885-3924(98)00136-5
4. Ramachandran VS, Rogers-Ramachandran D. Phantom limbs and neural plasticity. Arch Neurol. 2000;57(3):317. doi:10.1001/archneur.57.3.317
5. Hanyu-Deutmeyer AA, Cascella M, Varacallo M. Phantom limb pain. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021. PMID: 28846343. Available from. https://www.ncbi.nlm.nih.gov/books/NBK448188/.
6. Mitchell SW. Injuries of Nerves and Their Consequences. JB Lippincott & Co; 1965.
7. Flahaut M, Laurent NL, Michetti M, et al. Patient care for postamputation pain and the complexity of therapies: living experiences. Pain Manag. 2018;8(6):441–453. doi:10.2217/pmt-2018-0033
8. Wilkins KL, McGrath PJ, Finley AG, Katz J. Phantom limb sensations and phantom limb pain in child and adolescent amputees. Pain. 1998;78(1):7–12. doi:10.1016/S0304-3959(98)00109-2
9. Weinstein S, Sersen EA. Phantoms in cases of congenital absence of limbs. Neurology. 1961;11(10):905. doi:10.1212/wnl.11.10.905
10. Jensen TS, Krebs B, Nielsen J, Rasmussen P. Immediate and long-term phantom limb pain in amputees: incidence, clinical characteristics and relationship to pre-amputation limb pain. Pain. 1985;21(3):267–278. doi:10.1016/0304-3959(85)90090-9
11. Nikolajsen L, Ilkjaer S, Jensen TS. Relationship between mechanical sensitivity and postamputation pain: a prospective study. Eur J Pain. 2000;4(4):327–334. doi:10.1053/eujp.2000.0194
12. Schley MT, Wilms P, Toepfner S, et al. Painful and Nonpainful phantom and stump sensations in acute traumatic amputees. J Trauma. 2008;65(4):858–864. doi:10.1097/ta.0b013e31812eed9e
13. Houghton AD, Nicholls G, Houghton AL, Saadah E, McColl L. Phantom pain: natural history and association with rehabilitation. Ann R Coll Surg Engl. 1994;76(1):22–25.
14. Sherman RA, Sherman CJ, Parker L. Chronic phantom and stump pain among American veterans: results of a survey. Pain. 1984;18(1):83–95. doi:10.1016/0304-3959(84)90128-3
15. Nikolajsen L, Ilkjær S, Krøner K, Christensen JH, Jensen TS. The influence of preamputation pain on postamputation stump and phantom pain. Pain. 1997;72(3):393–405. doi:10.1016/s0304-3959(97)00061-4
16. Postone N. Phantom limb pain. A review. Int J Psychiatry Med. 1987;17(1):57–70. doi:10.2190/pkg8-mduw-urcq-h2q2
17. Noguchi S, Saito J, Nakai K, Kitayama M, Hirota K. Factors affecting phantom limb pain in patients undergoing amputation: retrospective study. J Anesth. 2019;33(2):216–220. doi:10.1007/s00540-018-2599-0
18. Kooijman CM, Dijkstra PU, Geertzen JH, Elzinga A, Van der Schans CP. Phantom pain and phantom sensations in upper limb amputees: an epidemiological study. Pain. 2000;87(1):33–41. doi:10.1016/s0304-3959(00)00264-5
19. Dijkstra PU, Geertzen JH, Stewart R, van der Schans CP. Phantom pain and risk factors. J Pain Symptom Manage. 2002;24(6):578–585. doi:10.1016/s0885-3924(02)00538-9
20. Flor H, Nikolajsen L, Staehelin Jensen T. Phantom limb pain: a case of maladaptive CNS plasticity? Nat Rev Neurosci. 2006;7(11):873–881. doi:10.1038/nrn1991
21. Diers M, Christmann C, Koeppe C, Ruf M, Flor H. Mirrored, imagined and executed movements differentially activate sensorimotor cortex in amputees with and without phantom limb pain. Pain. 2010;149(2):296–304. doi:10.1016/j.pain.2010.02.020
22. Flor H, Elbert T, Knecht S, et al. Phantom-limb pain as a perceptual correlate of cortical reorganization following arm amputation. Nature. 1995;375(6531):482–484. doi:10.1038/375482a0
23. Flor H. Phantom-limb pain: characteristics, causes, and treatment. Lancet Neurol. 2002;1(3):182–189. doi:10.1016/s1474-4422(02)00074-1
24. Limakatso K, Bedwell GJ, Madden VJ, Parker R. The prevalence and risk factors for phantom limb pain in people with amputations: a systematic review and meta-analysis. PLoS One. 2020;15(10):e0240431. doi:10.1371/journal.pone.0240431
25. Torebjörk E, Wahren L, Wallin G, Hallin R, Koltzenburg M. Noradrenaline-evoked pain in neuralgia. Pain. 1995;63(1):11–20. doi:10.1016/0304-3959(95)00140-N
26. Collins KL, Russell HG, Schumacher PJ, et al. A review of current theories and treatments for phantom limb pain. J Clin Invest. 2018;128(6):2168–2176. doi:10.1172/jci94003
27. Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32(1):1–32. doi:10.1146/annurev.neuro.051508.135531
28. Woolf CJ, Shortland P, Coggeshall RE. Peripheral nerve injury triggers central sprouting of myelinated afferents. Nature. 1992;355(6355):75–78. doi:10.1038/355075a0
29. Woolf CJ, Thompson SW. The induction and maintenance of central sensitization is dependent on N -methyl-d-aspartic acid receptor activation; implications for the treatment of post-injury pain hypersensitivity states. Pain. 1991;44(3):293–299. doi:10.1016/0304-3959(91)90100-c
30. Wiesenfeld-Hallin Z, Xu XJ, Hökfelt T. The role of spinal cholecystokinin in chronic pain states. Pharmacol Toxicol. 2002;91(6):398–403. doi:10.1034/j.1600-0773.2002.910619.x
31. Harris AJ. Cortical origin of pathological pain. Lancet. 1999;354(9188):1464–1466. doi:10.1016/s0140-6736(99)05003-5
32. Merzenich MM, Nelson RJ, Stryker MP, Cynader MS, Schoppmann A, Zook JM. Somatosensory cortical map changes following digit amputation in adult monkeys. J Comp Neurol. 1984;224(4):591–605. doi:10.1002/cne.902240408
33. Ramachandran VS, Hirstein W. The perception of phantom limbs. The D. O. Hebb lecture. Brain. 1998;121(Pt 9):1603. doi:10.1093/brain/121.9.1603
34. Ramachandran VS. Behavioral and magnetoencephalographic correlates of plasticity in the adult human brain. Proc Nat Acad Sci. 1993;90(22):10413–10420. doi:10.1073/pnas.90.22.10413
35. Mogilner A, Grossman JA, Ribary U, et al. Somatosensory cortical plasticity in adult humans revealed by magnetoencephalography. Proc Natl Acad Sci USA. 1993;90(8):3593–3597. doi:10.1073/pnas.90.8.3593
36. Arena JG, Sherman RA, Bruno GM, Smith JD. The relationship between situational stress and phantom limb pain: cross-lagged correlational data from six month pain logs. J Psychosom Res. 1990;34(1):71–77. doi:10.1016/0022-3999(90)90009-s
37. Fuchs X, Flor H, Bekrater-Bodmann R. Psychological factors associated with phantom limb pain: a review of recent findings. Pain Res Manag. 2018;2018:5080123. doi:10.1155/2018/5080123
38. Wei F, Zhuo M. Potentiation of sensory responses in the anterior cingulate cortex following digit amputation in the anaesthetised rat. J Physiol. 2001;532(Pt 3):823–833. doi:10.1111/j.1469-7793.2001.0823e.x
39. Subedi B, Grossberg GT. Phantom limb pain: mechanisms and treatment approaches. Pain Res Treat. 2011;2011:1–8. doi:10.1155/2011/864605
40. Karanikolas M, Aretha D, Tsolakis I, et al. Optimized perioperative analgesia reduces chronic phantom limb pain intensity, prevalence, and frequency: a prospective, randomized, clinical trial. Anesthesiology. 2011;114(5):1144–1154. doi:10.1097/ALN.0b013e31820fc7d2
41. Hanley MA, Ehde DM, Campbell KM, Osborn B, Smith DG. Self-reported treatments used for lower-limb phantom pain: descriptive findings. Arch Phys Med Rehabil. 2006;87(2):270–277. doi:10.1016/j.apmr.2005.04.025
42. Smith H. Potential analgesic mechanisms ofAcetaminophen. Pain Physician. 2009;12(1):269–280. doi:10.36076/ppj.2009/12/269
43. Cashman JN. The mechanisms of action of NSAIDs in analgesia. Drugs. 1996;52(Supplement 5):13–23. doi:10.2165/00003495-199600525-00004
44. Department of Veterans Affairs/Department of Defense. VA/DOD clinical practice guideline for rehabilitation of lower amputation. Washington, DC; 2007: 1–55.
45. Robinson LR, Czerniecki JM, Ehde DM, et al. Trial of amitriptyline for relief of pain in amputees: results of a randomized controlled study. Arch Phys Med Rehabil. 2004;85(1):1–6. doi:10.1016/s0003-9993(03)00476-3
46. Wilder-Smith CH, Hill LT, Laurent S. Postamputation pain and sensory changes in treatment-naive patients: characteristics and responses to treatment with tramadol, amitriptyline, and placebo. Anesthesiology. 2005;103(3):619–628. doi:10.1097/00000542-200509000-00027
47. Kuiken TA, Schechtman L, Harden RN. Phantom limb pain treatment with mirtazapine: a case series. Pain Pract. 2005;5(4):356–360. doi:10.1111/j.1533-2500.2005.00038.x
48. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162–173. doi:10.1016/S1474-4422(14)70251-0
49. Spiegel DR, Lappinen E, Gottlieb M. A presumed case of phantom limb pain treated successfully with duloxetine and pregabalin. Gen Hosp Psychiatry. 2010;32(2):228.e5–228.e7. doi:10.1016/j.genhosppsych.2009.05.012
50. Alviar MJM, Hale T, Lim-Dungca M. Pharmacologic interventions for treating phantom limb pain. Cochrane Database Syst Rev. 2016. 10. doi:10.1002/14651858.CD006380.pub3
51. Bone M, Critchley P, Buggy D. Gabapentin in postamputation phantom limb pain: a randomized, double-blind, placebo-controlled, cross-over study. Reg Anesth Pain Med. 2002;27(5):481–486. doi:10.1053/rapm.2002.35169
52. Nikolajsen L, Finnerup N, Kramp S, Vimtrup AS, Keller J, Jensen T. A randomized study of the effects of gabapentin on postamputation pain. Anesthesiology. 2006;105(5):1008–1015. doi:10.1097/00000542-200611000-00023
53. Smith DG, Ehde DM, Hanley MA, et al. Efficacy of gabapentin in treating chronic phantom limb and residual limb pain. J Rehabil Res Dev. 2005;42(5):645. doi:10.1682/jrrd.2005.05.0082
54. Hu AM, Harden RN, Kuiken T. Topiramate for phantom limb pain in an upper limb amputee: a case report. J Back Musculoskelet Rehabil. 2002;16(4):141–143. doi:10.3233/bmr-2002-16404
55. Moore RA, Wiffen PJ, Derry S, Lunn MP. Zonisamide for neuropathic pain in adults. Cochrane Database Syst Rev. 2015. doi:10.1002/14651858.cd011241.pub2
56. Ahmad S. Phantom limb pain and propranolol. Br Med J. 1979;1(6160):415. doi:10.1136/bmj.1.6160.415-a
57. Scadding JW, Wall PD, Parry WC, Brooks DM. Clinical trial of propranolol in post-traumatic neuralgia. Pain. 1982;14(3):283–292. doi:10.1016/0304-3959(82)90135-x
58. Bartusch SL, Sanders JB, D’Alessio JG, Jernigan JR. Clonazepam for the treatment of lancinating phantom limb pain. Clin J Pain. 1996;12(1):59–62. doi:10.1097/00002508-199603000-00011
59. Kollewe K, Jin L, Krampfl K, Dengler R, Mohammadi B. Treatment of phantom limb pain with botulinum toxin type A. Pain Med. 2009;10(2):300–303. doi:10.1111/j.1526-4637.2008.00554.x
60. Wu H, Sultana R, Taylor KB, Szabo A. A prospective randomized double-blinded pilot study to examine the effect of botulinum toxin type A injection versus Lidocaine/Depomedrol injection on residual and phantom limb pain: initial report. Clin J Pain. 2012;28(2):108–112. doi:10.1097/AJP.0b013e3182264fe9
61. Abraham RB, Marouani N, Weinbroum AA. Dextromethorphan mitigates phantom pain in cancer amputees. Ann Surg Oncol. 2003;10(3):268–274. doi:10.1245/aso.2003.08.007
62. Jaeger H, Maier C. Calcitonin in phantom limb pain: a double-blind study. Pain. 1992;48(1):21–27. doi:10.1016/0304-3959(92)90127-w
63. Eichenberger U, Neff F, Sveticic G, et al. Chronic phantom limb pain: the effects of calcitonin, ketamine, and their combination on pain and sensory thresholds. Anesth Analg. 2008;106(4):1265–1273. doi:10.1213/ane.0b013e3181685014
64. Hall N, Eldabe S. Phantom limb pain: a review of pharmacological management. Br J Pain. 2018;12(4):202–207. doi:10.1177/2049463717747307
65. Loy BM, Britt RB, Brown JN. Memantine for the treatment of phantom limb pain: a systematic review. J Pain Palliat Care Pharmacother. 2016;30(4):276–283. doi:10.1080/15360288.2016.1241334
66. Su CJ, Liu K, Wang YM. Midazolam as an effective drug for severe phantom limb pain in a patient after undergoing spinal anesthesia for two consecutive surgeries in the contralateral lower limb. Acta Anaesthesiol Taiwan. 2009;47(1):32–35. doi:10.1016/s1875-4597(09)60018-7
67. Bergmans L, Snijdelaar DG, Katz J, Crul BJ. Methadone for phantom limb pain. Clin J Pain. 2002;18(3):203–205. doi:10.1097/00002508-200205000-00012
68. Huse E, Larbig W, Flor H, Birbaumer N. The effect of opioids on phantom limb pain and cortical reorganization. Pain. 2001;90(1):47–55. doi:10.1016/s0304-3959(00)00385-7
69. Mishra S, Bhatnagar S, Singhal AK. High-dose morphine for intractable phantom limb pain. Clin J Pain. 2007;23(1):99–101. doi:10.1097/01.ajp.0000210944.88933.3d
70. Wu C, Agarwal S, Tella P, et al. Morphine versus mexiletine for treatment of postamputation pain. Anesthesiology. 2008;109(2):289–296. doi:10.1097/aln.0b013e31817f4523
71. Tessler MJ, Kleiman SJ. Spinal anaesthesia for patients with previous lower limb amputations. Anaesthesia. 2007;49(5):439–441. doi:10.1111/j.1365-2044.1994.tb03483.x
72. Bach S, Noreng MF, Tjéllden NU. Phantom limb pain in amputees during the first 12 months following limb amputation, after preoperative lumbar epidural blockade. Pain. 1988;33(3):297–301. doi:10.1016/0304-3959(88)90288-6
73. Jahangiri M, Jayatunga AP, Bradley JW, Dark CH. Prevention of phantom pain after major lower limb amputation by epidural infusion of diamorphine, clonidine, and bupivacaine. Ann R Coll Surg Engl. 1994;76:324–326.
74. Nikolajsen L, Ilkjaer S, Christensen JH, Krøner K, Jensen TS. Randomised trial of epidural bupivacaine and morphine in prevention of stump and phantom pain in lower-limb amputation. Lancet. 1997;350(9088):1353–1357. doi:10.1016/s0140-6736(97)06315-0
75. Ong BY, Arneja A, Ong EW. Effects of anesthesia on pain after lower-limb amputation. J Clin Anesth. 2006;18(8):600–604. doi:10.1016/j.jclinane.2006.03.021
76. Ahuja V, Thapa D, Ghai B. Strategies for prevention of lower limb post-amputation pain: a clinical narrative review. J Anaesthesiol Clin Pharmacol. 2018;34(4):439–449. doi:10.4103/joacp.JOACP_126_17
77. Madabhushi L, Reuben SS, Steinberg RB, Adesioye J. The efficacy of postoperative perineural infusion of bupivacaine and clonidine after lower extremity amputation in preventing phantom limb and stump pain. J Clin Anesth. 2007;19(3):226–229. doi:10.1016/j.jclinane.2006.07.008
78. Borghi B, Bugamelli S, Stagni G, Missiroli M, Genco R, Colizza MT. Perineural infusion of 0.5% ropivacaine for successful treatment of phantom limb syndrome: a case report. Minerva Anestesiol. 2009;75(11):661–664.
79. Granville-Chapman J, Tennant M, Aldington D, Smith SR, Nott DM. Direct placement of a brachial plexus neural catheter for analgesia after traumatic upper limb amputation. Pain Med. 2009;10(6):1132–1135. doi:10.1111/j.1526-4637.2009.00638.x
80. Grant A, Wood C. The effect of intra-neural local anaesthetic infusion on pain following major lower limb amputation. Scott Med J. 2008;53(1):4–6. doi:10.1258/rsmsmj.53.1.4
81. Zheng Z, Hu Y, Tao W, Zhang X, Li Y. Dorsal root entry zone lesions for phantom limb pain with brachial plexus avulsion: a study of pain and phantom limb sensation. Stereotact Funct Neurosurg. 2009;87(4):249–255. doi:10.1159/000225978
82. Casale R, Ceccherelli F, Labeeb A, Biella G. Phantom limb pain relief by contralateral myofascial injection with local anaesthetic in a placebo-controlled study: preliminary results. Eur J Pain. 2009;41(6):418–422. doi:10.2340/16501977-0353
83. West M, Wu H. Pulsed radiofrequency ablation for residual and phantom limb pain: a case series. Pain Pract. 2010;10(5):485–491. doi:10.1111/j.1533-2500.2009.00353.x
84. Wilkes D, Ganceres N, Solanki D, Hayes M. Pulsed radiofrequency treatment of lower extremity phantom limb pain. Clin J Pain. 2008;24(8):736–739. doi:10.1097/ajp.0b013e318170d758
85. Carvajal G, Rocha A, Dupoiron D. Multimodal intrathecal therapy for phantom limb pain. J Trauma. 2019. doi:10.1097/CJ9.0000000000000106
86. Corbett M, South E, Harden M, et al. Brain and spinal stimulation therapies for phantom limb pain: a systematic review. Health Technol Assess. 2018;22(62):1–94. doi:10.3310/hta22620
87. Cohen SP, Gilmore CA, Rauck RL, et al. Percutaneous peripheral nerve stimulation for the treatment of chronic pain following amputation. Mil Med. 2019;184(7–8):e267–e274. doi:10.1093/milmed/usz114
88. Broggi G, Servello D, Dones I, Carbone G. Italian multicentric study on pain treatment with epidural spinal cord stimulation. Stereotact Funct Neurosurg. 1994;62(1–4):273–278. doi:10.1159/000098632
89. Katayama Y, Yamamoto T, Kobayashi K, Kasai M, Oshima H, Fukaya C. Motor cortex stimulation for phantom limb pain: comprehensive therapy with spinal cord and thalamic stimulation. Stereotact Funct Neurosurg. 2001;77(1–4):159–162. doi:10.1159/000064593
90. Viswanathan A, Phan PC, Burton AW. Use of spinal cord stimulation in the treatment of phantom limb pain: case series and review of the literature. Pain Prac. 2010;10(5):479–484. doi:10.1111/j.1533-2500.2010.00374.x
91. Carroll D, Joint C, Maartens N, Shlugman D, Stein J, Aziz TZ. Motor cortex stimulation for chronic neuropathic pain: a preliminary study of 10 cases. Pain. 2000;84(2):431–437. doi:10.1016/s0304-3959(99)00245-6
92. Lefaucheur JP, Drouot X, Cunin P, et al. Motor cortex stimulation for the treatment of refractory peripheral neuropathic pain. Brain. 2009;132(6):1463–1471. doi:10.1093/brain/awp035
93. Saris SC, Iacono RP, Nashold BS
94. Freed S. Acupuncture as therapy of traumatic affective disorders and of phantom limb pain syndrome. Acupunct Electrother Res. 1989;14(2):121–129. doi:10.3727/036012989816358452
95. Monga TN, Jaksic T. Acupuncture in phantom limb pain. Arch Phys Med Rehabil. 1981;62(5):225–231.
96. Bradbrook D. Acupuncture treatment of phantom limb pain and phantom limb sensation in amputees. J Rehabilitat Med. 2004;22(2):93–97. doi:10.1136/aim.22.2.93
97. Trevelyan EG, Turner WA, Summerfield-Mann L, Robinson N. Acupuncture for the treatment of phantom limb syndrome in lower limb amputees: a randomised controlled feasibility study. Trials. 2016;17(1):519. doi:10.1186/s13063-016-1639-z
98. Black LM, Persons RK, Jamieson B. What is the best way to manage phantom limb pain? J Fam Pract. 2009;58(3):155–158.
99. Giuffrida O, Simpson L, Halligan PW. Contralateral stimulation, using TENS, of phantom limb pain: two confirmatory cases. Pain Med. 2010;11(1):133–141. doi:10.1111/j.1526-4637.2009.00705.x
100. Katz J, Melzack R. Auricular transcutaneous electrical nerve stimulation (TENS) reduces phantom limb pain. J Pain Symptom Manage. 1991;6(2):73–83. doi:10.1016/0885-3924(91)90521-5
101. Mulvey MR, Radford HE, Fawkner HJ, Hirst L, Neumann V, Johnson MI. Transcutaneous electrical nerve stimulation for phantom pain and stump pain in adult amputees. Pain Pract. 2013;13(4):289–296. doi:10.1111/j.1533-2500.2012.00593.x
102. Johnson MI, Mulvey MR, Bagnall A-M. Transcutaneous electrical nerve stimulation (TENS) for phantom pain and stump pain following amputation in adults. Cochrane Database Syst Rev. 2015. 8. doi:10.1002/14651858.CD007264.pub3
103. Belleggia G, Birbaumer N. Treatment of phantom limb pain with combined EMG and thermal biofeedback: a case report. Appl Psychophysiol Biofeedback. 2001;26(2):141–146. doi:10.1023/a:1011391223713
104. Harden RN, Houle TT, Green S, et al. Biofeedback in the treatment of phantom limb pain: a time-series analysis. Appl Psychophysiol Biofeedback. 2005;30(1):83–93. doi:10.1007/s10484-005-2177-8
105. de Roos C, Veenstra AC, de Jongh A, et al. Treatment of chronic phantom limb pain using a trauma-focused psychological approach. Pain Res Manag. 2010;15(2):65–71. doi:10.1155/2010/981634
106. Schneider J, Hofmann A, Rost C, Shapiro F. EMDR in the treatment of chronic phantom limb pain. Pain Med. 2008;9(1):76–82. doi:10.1111/j.1526-4637.2007.00299.x
107. Rasmussen KG, Rummans TA. Electroconvulsive therapy for phantom limb pain. Pain. 2000;85(1):297–299. doi:10.1016/s0304-3959(99)00288-2
108. Ramachandran VS, Rogers-Ramachandran D. Synaesthesia in phantom limbs induced with mirrors. J Rehabilitat Med. 1996;263(1369):377–386. doi:10.1098/rspb.1996.0058
109. Brodie EE, Whyte A, Niven CA. Analgesia through the looking-glass? A randomized controlled trial investigating the effect of viewing a ‘virtual’ limb upon phantom limb pain, sensation and movement. Eur J Pain. 2007;11(4):428–436. doi:10.1016/j.ejpain.2006.06.002
110. Chan BL, Witt R, Charrow AP, et al. Mirror therapy for phantom limb pain. N Engl J Med. 2007;357(21):2206–2207. doi:10.1056/nejmc071927
111. Sumitani M, Miyauchi S, McCabe CS, et al. Mirror visual feedback alleviates deafferentation pain, depending on qualitative aspects of the pain: a preliminary report. Rheumatology. 2008;47(7):1038–1043. doi:10.1093/rheumatology/ken170
112. Darnall BD. Self-delivered home-based mirror therapy for lower limb phantom pain. Am J Phys Med Rehabil. 2009;88(1):78–81. doi:10.1097/PHM.0b013e318191105b
113. Anaforoğlu Külünkoğlu B, Erbahçeci F, Alkan A. A comparison of the effects of mirror therapy and phantom exercises on phantom limb pain. Eur J Pain. 2019;49(1):101–109. doi:10.3906/sag-1712-166
114. Rothgangel A, Braun S, Smeets R, Beurskens A. Feasibility of a traditional and teletreatment approach to mirror therapy in patients with phantom limb pain: a process evaluation performed alongside a randomized controlled trial. Clin Rehabil. 2019;33(10):1649–1660. doi:10.1177/0269215519846539
115. Barbin J, Seetha V, Casillas JM, Paysant J, Pérennou D. The effects of mirror therapy on pain and motor control of phantom limb in amputees: a systematic review. Ann Phys Rehabil Med. 2016;59(4):270–275. doi:10.1016/j.rehab.2016.04.001
116. Ülger O, Topuz S, Bayramlar K, Şener G, Erbahçeci F. Effectiveness of phantom exercises for phantom limb pain: a pilot study. Eur J Pain. 2009;41(7):582–584. doi:10.2340/16501977-0380
117. Münger M, Pinto CB, Pacheco‐Barrios K, et al. Protective and risk factors for phantom limb pain and residual limb pain severity. Pain Pract. 2020;20(6):578–587. doi:10.1111/papr.12881
118. Gagné M, Reilly K, Hétu S, Mercier C. Motor control over the phantom limb in above-elbow amputees and its relationship with phantom limb pain. Neuroscience. 2009;162(1):78–86. doi:10.1016/j.neuroscience.2009.04.061
119. de Nunzio AM, Schweisfurth MA, Ge N, et al. Relieving phantom limb pain with multimodal sensory-motor training. J Neural Eng. 2018;15(6):066022. doi:10.1088/1741-2552/aae271
120. O’Neill K, dePaor A, MacLachlan M, et al. An investigation into the performance of augmented reality for use in the treatment of phantom limb pain in amputees.
121. Desmond DM, O’Neill K, de Paor A, McDarby G, MacLachlan M. Augmenting the reality of phantom limbs: three case studies using an augmented mirror box procedure. J Prosthet Orthot. 2006;18(3):74–79. doi:10.1097/00008526-200607000-00005
122. Murray CD, Patchick E, Pettifer S, Caillette F, Howard T. Immersive virtual reality as a rehabilitative technology for phantom limb experience: a protocol. Cyberpsychol Behav. 2006;9(2):167–170. doi:10.1089/cpb.2006.9.167
123. Mercier C, Sirigu A. Training with virtual visual feedback to alleviate phantom limb pain. Neurorehabil Neural Repair. 2009;23(6):587–594. doi:10.1177/1545968308328717
124. Cole J, Crowle S, Austwick G, Henderson Slater D. Exploratory findings with virtual reality for phantom limb pain; from stump motion to agency and analgesia. Disabil Rehabil. 2009;31(10):846–854. doi:10.1080/09638280802355197
125. Ortiz-Catalan M, Guðmundsdóttir RA, Kristoffersen MB, et al. Phantom motor execution facilitated by machine learning and augmented reality as treatment for phantom limb pain: a single group, clinical trial in patients with chronic intractable phantom limb pain. Lancet. 2016;388(10062):2885–2894. doi:10.1016/s0140-6736(16)31598-7
126. Rutledge T, Velez D, Depp C, et al. A virtual reality intervention for the treatment of phantom limb pain: development and feasibility results. Pain Med. 2019;20(10):2051–2059. doi:10.1093/pm/pnz121
127. Dunn J, Yeo E, Moghaddampour P, Chau B, Humbert S. Virtual and augmented reality in the treatment of phantom limb pain: a literature review. NeuroRehabilitation. 2017;40(4):595–601. doi:10.3233/nre-171447
128. Gaggioli A, Amoresano A, Gruppioni E, Verni G, Riva G. A myoelectric-controlled virtual hand for the assessment and treatment of phantom limb pain in trans-radial per extremity amputees: a research protocol. Stud Health Technol Inform. 2010;154:220–222.
129. Dumanian GA, Potter BK, Mioton LM, et al. Targeted muscle reinnervation treats neuroma and phantom pain in major limb amputees: a randomized clinical trial. Ann Surg. 2019;270(2):238–246. doi:10.1097/SLA.0000000000003088
130. Jefferies K. Treatment of neuropathic pain. Semin Neurol. 2010;30(4):425–432. doi:10.1055/s-0030-1267286
131. MacIver K, Lloyd DM, Kelly S, Roberts N, Nurmikko T. Phantom limb pain, cortical reorganization and the therapeutic effect of mental imagery. Brain. 2008;131(Pt 8):2181–2191. Epub 2008 Jun 20. PMID: 18567624; PMCID: PMC2494616. doi:10.1093/brain/awn124
132. Ortiz-Catalan M. The stochastic entanglement and phantom motor execution hypotheses: a theoretical framework for the origin and treatment of phantom limb pain. Front Neurol. 2018;9:748. PMID: 30237784; PMCID: PMC6135916. doi:10.3389/fneur.2018.00748
133. Sveistrup H. Motor rehabilitation using virtual reality. J Neuroeng Rehabil. 2004;10:1–8. doi:10.1186/1743-0003-1-10
134. Oh C, Carlsen BT. New innovations in targeted muscle reinnervation: a critical analysis review. JBJS Rev. 2019;7(6):e3. PMID: 31188155. doi:10.2106/JBJS.RVW.18.00138
135. Peters BR, Russo SA, West JM, Moore AM, Schulz SA. Targeted muscle reinnervation for the management of pain in the setting of major limb amputation. SAGE Open Med. 2020;8:2050312120959180. PMID: 32974021; PMCID: PMC7495925. doi:10.1177/2050312120959180
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
