Back to Journals » Open Access Emergency Medicine » Volume 13
Alveolar Hemorrhage Following Thrombolytic Therapy for Acute Myocardial Infarction: Two Case Reports and Literature Review
Authors Ben Mrad I , Ben Mrad M , Oumaya Z, Zairi I , Besbes B, Ouaghlani K, kamoun S, Mleyhi S, Miri R, Mzoughi K, Kraiem S
Received 22 June 2021
Accepted for publication 4 August 2021
Published 27 August 2021 Volume 2021:13 Pages 399—405
DOI https://doi.org/10.2147/OAEM.S324366
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Hans-Christoph Pape
Imtinene Ben Mrad,1 Melek Ben Mrad,2 Zeineb Oumaya,1 Ihsen Zairi,1 Boutheina Besbes,1 Khalil Ouaghlani,1 Sofien kamoun,1 Sobhi Mleyhi,2 Rim Miri,2 khadija Mzoughi,1 Sondos Kraiem1
1Cardiology Department, Habib Thameur Hospital, Tunis, Tunisia; 2Cardiovascular Surgery Department, Rabta Hospital, Tunis, Tunisia
Correspondence: Imtinene Ben Mrad
Cardiology Department, Habib Thameur Hospital, App 2b, Bloc 12, cite Olympique, Tunis, 1003, Tunisia
Tel +011 216 20156852
Email [email protected]
Abstract: Alveolar hemorrhage (AH) is a heterogeneous clinical syndrome with a high mortality rate, characterized by extensive bleeding into the alveolar spaces. AH secondary to systemic thrombolysis treatment in the setting of acute myocardial infarction is an uncommon complication, but potentially fatal and can lead to acute respiratory failure. This entity is rarely reported in the literature. We report two cases of acute AH after intravenous thrombolysis for acute myocardial infarction, which could contribute to the literature on the subject, and discuss the risk factors as well as the clinical and radiological findings supporting the diagnosis. We overview also the rare previous published case reports in this context, and we contrast our findings with those reported in the literature.
Keywords: hemoptysis, alveolar hemorrhage, myocardial infarction, fibrinolytic therapy, streptokinase
Introduction
Alveolar hemorrhage is a clinical syndrome resulting from bleeding into the alveolar spaces secondary to disruption of the alveolar-capillary membrane. It is a rare and serious medical emergency potentially leading to fatal acute respiratory failure (ARF).1
Systemic thrombolysis in the setting of acute myocardial infarction (AMI) remains, in the absence of contraindications, an effective treatment.2 However, it exposes to a non-negligible bleeding risk that remains its major adverse event. Most of the latter occur at sites of vascular access and are mild, though patients may also present with other locations such as gastrointestinal, retroperitoneal, genitourinary, and cerebral bleeding.3,4
Pulmonary alveolar hemorrhage is an extremely uncommon and potentially fatal complication of intravenous thrombolytic therapy. So far, only few cases have been reported in the literature.5–21
We hereby report two cases of acute alveolar hemorrhage complicating thrombolytic therapy in myocardial infarction, hoping to contribute to expand the literature data on this subject.
We discuss in this article, through our cases and a literature review, the risk factors, the clinical and the radiographic findings that suggest and support the diagnosis, as well as the management issues related to this unusual and life-threatening situation.
Cases Presentation
Patient 1
A 61-year-old male patient, heavy smoker, with coronary heart disease history, presented to the emergency department of our hospital with ST segment elevation myocardial infarction (STEMI). He received, immediately, loading doses of aspirin and clopidogrel, and then had successful thrombolysis with Tenecteplase (Metalyse).
Two days later, he developed a moderate hemoptysis. On physical examination, he was apyretic at 37°C, he was hemodynamically stable with a blood pressure at 100/60 mmHg, a heart rate at 60 beats/minutes and no peripheral signs of shock.
However, he was polypneic with a respiratory rate at 25 cycles/minute and his oxygen saturation (SaO2) on room air had dropped from an initial value of 96% to 89%.
Pulmonary auscultation revealed crackles limited to the lung bases. There was no other sign of heart failure.
Arterial blood gas analysis revealed normal pH of 7.45, normocapnia of 32 mmHg, but hypoxia 54 mmHg and low oxygen saturation (SaO2) of 89%.
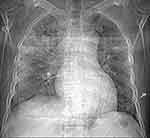
The chest X-ray revealed bilateral alveolar infiltrates (Figure 1).
The transthoracic echocardiography showed an ejection fraction of about 50% and a posterior wall hypokinesia.
Given the context, the first diagnosis evoked was acute pulmonary oedema, and on this presumption, we started intravenous (IV) furosemide that was ineffective.
Meanwhile, the urgent biologic workup carried out showed a significant drop of hemoglobin level from 15.6 g/dL to 12.8 g/dl, with normal platelet and leukocyte count. A second oriented physical examination did not reveal any other bleeding manifestations.
In front of hemoptysis, acute drop in hemoglobin level and bilateral alveolar infiltrates on chest X-ray, the diagnosis of AH was considered.
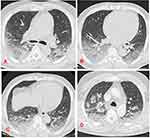
High-resolution computed tomography (HRCT) of the chest (Figure 2) was performed and revealed bilateral alveolar patchy condensations predominating posteriorly especially at the basal area associated with diffuse micro nodules, strongly suggestive of AH.
We decided to withdraw anticoagulation (Intravenous continuous heparin sodium infusion) and maintain the double antiplatelet therapy.
The patient experienced progressive improvement of his respiratory parameters over the next 48 hours under oxygen administered via facial mask, without any recurrence of hemoptysis.
A coronary angiogram performed six days after myocardial infarction revealed a significant thrombotic stenosis of the distal right coronary artery, successfully treated with the implantation of a drug-eluting stent.
He was discharged, after adequate monitoring, on dual antiplatelet therapy (100 mg of aspirin and 75 mg of clopidogrel) and had an uneventful 12-month follow-up.
Patient 2
A 63-year-old male without any comorbidities presented to the emergency department one hour after the onset of acute constricting chest pain. Shortly thereafter, he developed an episode of sustained ventricular tachycardia, which was converted to sinus rhythm by electrical cardioversion. He soon recovered his full consciousness, with good vitals. An electrocardiogram revealed ST segment elevation in the inferior leads. After receiving loading doses of aspirin and clopidogrel, thrombolysis with Streptokinase (1.5 million units) was initiated according to standard protocol. The chest pain disappeared, and the electrocardiogram performed 90 minutes after thrombolysis showed ST segment elevation resolution.
Within the first 24 hours of monitoring in the cardiology intensive care unit, the patient experienced acute dyspnea with hemoptysis. Oxygen saturation level dropped from 97% to 89% and pulmonary auscultation revealed bilateral diffuse crackles. There were no other signs of heart failure otherwise.
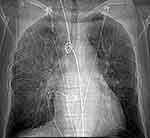
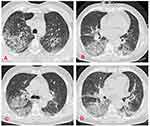
A bedside chest X-ray revealed alveolar opacities (Figure 3), and the thoracic CT scan (Figure 4) showed bilateral multiple alveolar lesions strongly suggesting a diffuse DAH. The blood workup results, revealed a severe drop of hemoglobin level from 13 to 9 g/dl.
The transthoracic echocardiography showed an ejection fraction of about 40% as well as posterior wall hypokinesia and pulmonary arterial hypertension.
We proceeded to coronary angiography (CAG) performed through the right radial artery, revealing a critical sub-occlusion of the left circumflex artery with TIMI grade 3 flow.
The indication of a transluminal angioplasty was evident; however, we decided to delay the angioplasty because of the severity of the hemorrhage.
Oxygen therapy and blood transfusion were implemented. We maintained the double antiplatelet therapy but decided on anticoagulation withdrawal (Intravenous continuous heparin sodium infusion).
After two weeks of hospitalization and close monitoring, the hemoptysis started to improve gradually, his oxygen saturation on room air increased to 95% and his lung fields were clear. He had a steady hemoglobin level. A chest X-ray taken after 3 weeks showed resolution of lesions. Before being discharged, the patient underwent a successful angioplasty of the circumflex coronary lesion. One year later, the patient is asymptomatic at follow-up.
For our two patients, we conducted an etiological investigation, looking for arguments in favour of vasculitis: the screening for anti-glomerular basal membrane antibodies, antinuclear antibodies, anti-double stranded deoxyribonucleic acid antibodies, peripheral anti-neutrophil cytoplasmic antibodies (p-ANCAs) and cytoplasmic ANCAs was negative. The retroviral screening was negative, as well.
Discussion
Thrombolytic therapy is a well proven strategy for reperfusion in acute myocardial infarction and has been proven to decrease the morbidity and mortality related to this condition. However, it can lead to hemorrhagic complications. The most common types of thrombolytic-related major bleeding complications are gastrointestinal tract and intracranial hemorrhages.3,4
Diffuse alveolar hemorrhage (DAH) syndrome is a very unusual complication of intravenous thrombolytic treatment for acute myocardial infarction with a high mortality rate and can go unnoticed. This pathology is defined by bleeding within the alveoli, which is due to dislocation of the alveolar-capillary membrane caused by injury or acute inflammation of the arterioles, venules, or alveolar capillaries.1 The available literature does not cover the exact incidence of this complication, but it rather consists of few case reports.
These two cases highlight a serious hemorrhagic complication of fibrinolytic treatment for AMI that can go unnoticed because it can be confused with other diagnoses. According to our knowledge, no case of AH associated with Tenecteplase (Metalyse) has been previously reported.
Table 1 summarizes data from all cases reported in the literature to date. All reported cases were of males aged between 24 and 75 years old, suggesting that male sex might be a risk factor for DAH secondary to intravenous thrombolytic therapy in the setting of AMI.
 |
Table 1 Published Reports of Diffuse Alveolar Hemorrhage Following Thrombolytic Therapy for Myocardial Infarction (1990–2020) |
Three fibrinolytic agents have been used in these cases: streptokinase, urokinase and Actilyse® (Alteplase-rtPA). Most of these patients received streptokinase, similarly to our second case.
We can see that DAH usually occurs within a few hours to 5 days after thrombolysis.
The pathogenesis of DAH attributable to thrombolytic therapy remains uncertain and may be explained by the pre-existing fibrinolytic states, the presence of parenchymal abnormalities or an immune reaction to streptokinase causing pulmonary capillaritis as it has been proposed.20 It has been mentioned that certain potential cofactors may predispose to this complication such as underlying lung diseases (chronic obstructive pulmonary disease, prior emphysema), recent pneumonia, cardiac catheterization, arrhythmias requiring defibrillation shock or cardiopulmonary resuscitation, heart failure, and substances abuse as cocaine and tobacco.21 Green et al22 described that some patients with alveolar hemorrhage had capillaritis, suggesting an immune reaction since streptokinase is associated with a wide spectrum of allergic reactions, such as anaphylaxis, bronchospasm, and type III immune reactions. Tio et al7 described the finding of antibodies against streptokinase in patients with HA treated with thrombolytics, which would support this theory. However, in some cases, no predisposing factor has been identified.
For our second patient, defibrillation prior to thrombolytic administration may have contributed to the occurrence of DAH. The potential role of defibrillation in DAH should be further clarified.
The clinical presentation is often characterized by the acute onset of a classical diagnostic triad: hemoptysis, anemia, and radiographic infiltrates. In addition, this triad is associated with acute respiratory failure. In our first case, heart failure was suspected, which led to a delay in diagnosis.
On chest X-ray, the radiological findings, consist, classically, in diffuse patchy infiltrates, and alveolar opacities involving, primarily, the central portion of the lung, particularly the middle and lower lobes. Rarely, areas of consolidation were observed. On Chest CT scan as well, the bilateral ground-glass opacities predominate in central area of the lungs with relative sparing of the lung periphery. It is also worth noting that the infiltrates were unilateral in two of the reported cases.9,10,12
In myocardial infarction, acute pulmonary edema is the first diagnosis to be suspected in the case of acute respiratory distress with alveolar opacities on chest X-ray which can lead to inappropriate treatment with diuretic. Therefore, we should also consider the diagnosis of DAH especially in the presence of hemoptysis and/or a sudden drop in hemoglobin levels with no apparent bleeding site.
When the diagnosis is suggested, bronchial endoscopy with bronchoalveolar lavage (BAL) is the gold standard exam to confirm the diagnosis.18 However, it is not usually possible, mainly depending on the patient’s condition. In our cases, these exams were not performed.
The management of DAH is based on two pillars: the treatment of respiratory failure and the correction of the anemia with packed red cell transfusions. Nasal oxygen therapy should suffice in correcting hypoxemia in moderate forms. Otherwise, artificial ventilation with the use of positive pressure would be in order.21
Discontinuing antiplatelet and anticoagulation medication is necessary.22 Antifibrinolytic agents, such as tranexamic acid was used in one case.22
The clinical course was good in about 80% of cases with full recovery within one to two weeks. The prognosis depends on the extent of the myocardial infarction, the volume of the hemorrhage and the degree of the cardiorespiratory compromise. Before our publication, only 20 cases were reported in the English medical literature, with five deaths; therefore, the mortality rate was 25%.
It should be considered that when diagnosing DAH, systemic vasculitis or connective tissue disease should be ruled out. The analytical results of our cases were negative, so that fibrinolytic drug was the cause.
Conclusion
Diffuse alveolar hemorrhage is an unusual complication of fibrinolytic therapy for MI, especially with streptokinase. The diagnosis of diffuse alveolar hemorrhage requires a high degree of suspicion, as signs and symptoms are nonspecific and may delay diagnosis. Diagnosis should be considered in patients with acute pulmonary distress associated to hemoptysis, acute anemia, and pulmonary infiltrates after thrombolysis. Early diagnosis and therapeutic management are critical to avoid acute respiratory failure and death. Risk factors and a probable etiology of this complication should be investigated.
Institutional Approval
We have the approval of our institution (Habib Thameur hospital of Tunis) to publish the case details.
Consent
Written informed consent was obtained from the two patients for the publication of this report and accompanying images.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Parrot A, Voiriot G, Canellas A, et al. Pulmonary alveolar hemorrhage. Med Intensive Reanim. 2018;27(4):331–343. doi:10.3166/rea-2018-0060
2. Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2018;39(2):119–177. doi:10.1093/eurheartj/ehx393
3. Califf RM, Fortin DF, Tenaglia AN. Clinical risks of thrombolytic therapy. Am J Cardiol. 1992;68(2):12–20. doi:10.1016/0002-9149(92)91168-4
4. Disler LJRA. Pulmonary hemorrhage following intravenous streptokinase for acute myocardial infarction. Int J Cardiol. 1990;29(3):387–390. doi:10.1378/chest.101.4.1150
5. Nathan PE, Torres AV, Smith AJ, Gagliardi AJRK, Rapeport KB. Spontaneous pulmonary hemorrhage following coronary thrombolysis. Chest. 1992;101(4):1150–1152. doi:10.1378/chest.101.4.1150
6. Cuéllar Obispo E, Torrado González E, Alvarez Bueno M, et al. A diffuse pulmonary hemorrhage following thrombolytic therapy in an acute myocardial infarct. Rev Esp Cardiol. 1992;45(6):421–424.
7. Tio RA, Voorbij RH, Enthoven R. Adult respiratory distress syndrome after streptokinase. Am J Cardiol. 1992;70(20):1632–1633. doi:10.1016/0002-9149(92)90477-g
8. Awadh N, Ronco JJ, Bernstein V, Gilks BWP, Wilcox P. Spontaneous pulmonary hemorrhage after thrombolytic therapy for acute myocardial infarction. Chest. 1994;106(5):1622–1624. doi:10.1378/chest.106.5.1622
9. Hammoudeh AJ, Haft JI, Eichman GT. Hemoptysis and unilateral intra- alveolar hemorrhage complicating intravenous thrombolysis for myocardial infarction. Clin Cardiol. 1996;19(7):595–596. doi:10.1002/clc.4960190714
10. Basher A, Oduwole A, Bhalodkar N, et al. Fatal hemoptysis during coronary thrombolysis. J Thromb Thrombolysis. 1996;3(1):87–89. doi:10.1007/bf00226416
11. Chang YC, Patz EF
12. Swanson GA, Kaeley GGS. Diffuse pulmonary hemorrhage after streptokinase administration for acute myocardial infarction. Pharmacotherapy. 1997;17(2):390–394.
13. Gopalakrishnan D, Tioran T, Emanuel C, Clark VL. Diffuse pulmonary hemorrhage complicating thrombolytic therapy for acute myocardial infarction. Clin Cardiol. 1997;20(3):298–300. doi:10.1002/clc.4960200321
14. Masip J, Vecilla FPJ. Diffuse pulmonary hemorrhage after fibrinolytic therapy for acute myocardial infarction. Int J Cardiol. 1998;63(1):95–97. doi:10.1016/s0167-5273(97)00285-4
15. Yigla M, Sprecher E, Azzam Z, Guralnik L, Kapeliovich MKN, Krivoy N. Diffuse alveolar hemorrhage following thrombolytic therapy for acute myocardial infarction. Respiration. 2000;67(4):445–448. doi:10.1159/000029546
16. Ayyub M, Barlas S, Iqbal M, Khurshid SM. Diffuse alveolar hemorrhages and hemorrhagic pleural effusion after thrombolytic therapy with streptokinase for acute myocardial infarction. Saudi Med J. 2003;24(2):217–220.
17. González A, Sagardía J, Redondo A, Villaverde M, Monteverde A. Hemorragia alveolar como complicación del uso de trombolíticos. Medicina (B Aires). 2011;71(6):547–549.
18. Kinsara A, Abuosa A, EL-Sheikh A. Abnormal chest x-ray postmyocardial infarction. Hear Views. 2014;15(4):121. doi:10.4103/1995-705x.151086
19. Khaheshi I, Paydary K, Mahjoob M. Diffuse pulmonary hemorrhage after fibrinolytic therapy for acute myocardial infarction in a cocaine abuser patient. Hear Views. 2014;15(3):83. doi:10.4103/1995-705x.144797
20. Narayanan S, Thulaseedharan NK, Subramaniam G, Panarkandy G, Arathi N. Pulmonary alveolar hemorrhage following thrombolytic therapy. Int Med Case Rep J. 2017;10:123–125. doi:10.2147/imcrj.s129087
21. Prasad K, Singh P, Kanabar KVR. Pulmonary haemorrhage following thrombolysis with streptokinase in myocardial infarction. BMJ Case Rep. 2020;13(1):232–308. doi:10.1136/bcr-2019-232308
22. Green RJ, Ruoss SJ, Kraft SA, Berry GJ, Raffan TA. Pulmonary capillaritis and alveolar hemorrhage. Chest. 1996;110:1305–1316. doi:10.1378/chest.110.5.1305
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.