Back to Journals » International Journal of Nanomedicine » Volume 18
Alterations of Gut-Derived Melatonin in Neurobehavioral Impairments Caused by Zinc Oxide Nanoparticles
Authors Yang C, Lu Z, Xia Y, Zhang J , Zou Z , Chen C , Wang X, Tian X, Cheng S, Jiang X
Received 14 August 2022
Accepted for publication 24 December 2022
Published 7 April 2023 Volume 2023:18 Pages 1899—1914
DOI https://doi.org/10.2147/IJN.S386240
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Yan Shen
Cantao Yang,1,* Zhaohong Lu,1,* Yinyin Xia,1 Jun Zhang,2,3 Zhen Zou,2,3 Chengzhi Chen,1,3 Xiaoliang Wang,4 Xin Tian,5 Shuqun Cheng,1,3 Xuejun Jiang3,6
1Department of Occupational and Environmental Health, School of Public Health, Chongqing Medical University, Chongqing, 400016, People’s Republic of China; 2Molecular Biology Laboratory of Respiratory Diseases, Institute of Life Sciences, Chongqing Medical University, Chongqing, 400016, People’s Republic of China; 3Research Center for Environment and Human Health, School of Public Health, Chongqing Medical University, Chongqing, 400016, People’s Republic of China; 4Medical Sciences Research Center, University-Town Hospital of Chongqing Medical University, Chongqing, 401331, People’s Republic of China; 5Department of Neurology, The First Affiliated Hospital of Chongqing Medical University, Chongqing, People’s Republic of China; 6Center of Experimental Teaching for Public Health, Experimental Teaching and Management Center, Chongqing Medical University, Chongqing, 400016, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Shuqun Cheng; Xuejun Jiang, Research Center for Environment and Human Health, School of Public Health, Chongqing Medical University, Number 1, Yixueyuan Road, Yuzhong District, Chongqing, 400016, People’s Republic of China, Tel +86-23-68485008, Fax +86-23-68485207, Email [email protected]; [email protected]
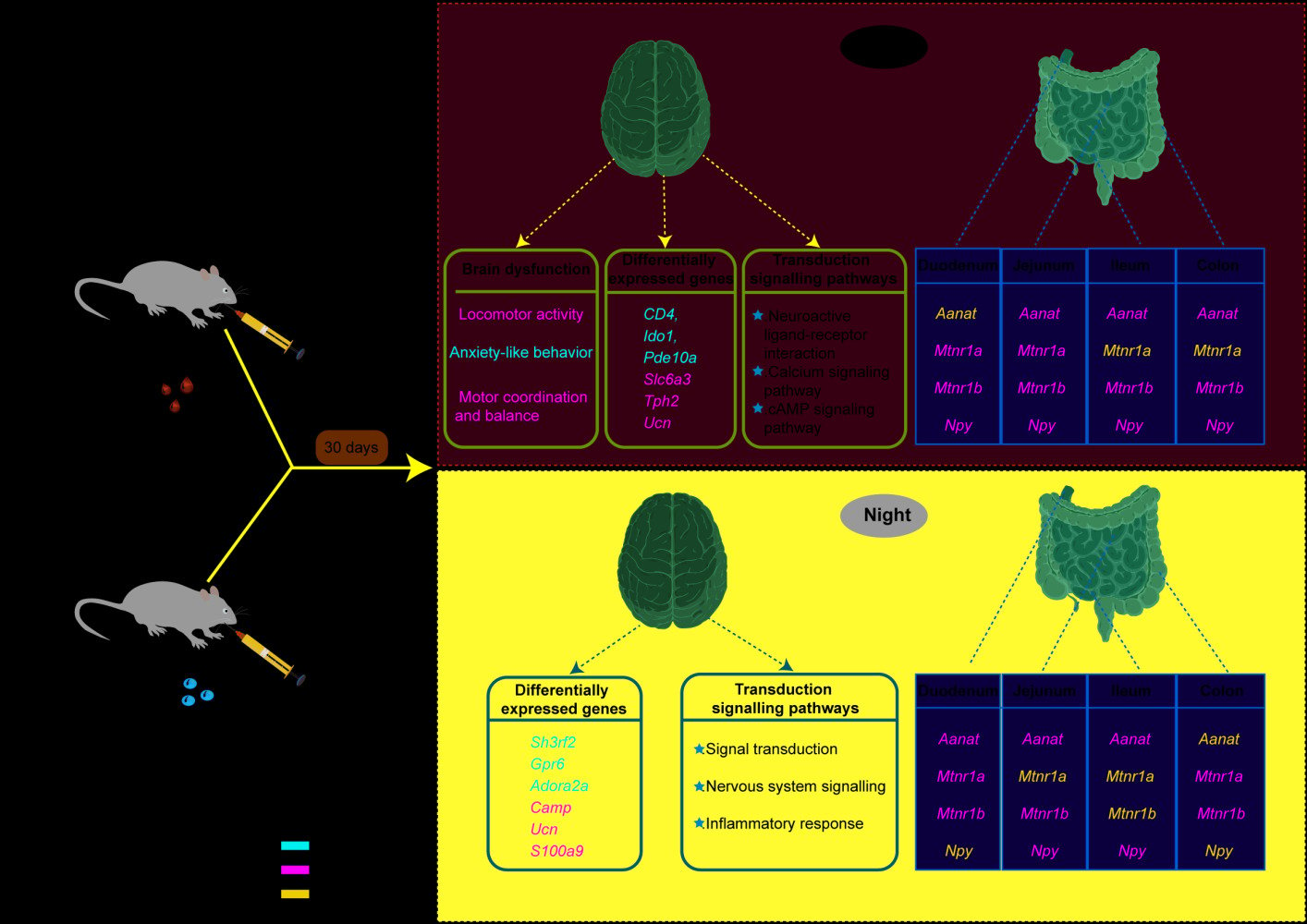
Purpose: The widespread use of zinc oxide nanoparticles (ZnONPs) has raised concerns about its potential toxicity. Melatonin is a neurohormone with tremendous anti-toxic effects. The enterochromaffin cells are an essential source of melatonin in vivo. However, studies on the effects of ZnONPs on endogenous melatonin are minimal. In the present study, we aimed to investigate the effects of ZnONPs exposure on gut-derived melatonin.
Methods: In the present study, 64 adult male mice were randomly and equally divided into four groups, and each group was exposed to ZnONPs (0, 6.5, 13, 26 mg/kg/day) for 30 days. Subsequently, the neurobehavioral changes were observed. The effects of ZnONPs on the expression of melatonin-related genes arylalkylamine N-acetyltransferase (Aanat), melatonin receptor1A (Mt1/Mtnr1a), melatonin receptor1B (Mt2/Mtnr1b), and neuropeptide Y (Npy) on melatonin synthesis and secretion in duodenum, jejunum, ileum and colon during day and night were also assessed.
Results: The results revealed that oral exposure to ZnONPs induced impairments of locomotor activity and anxiety-like behavior in adult mice during the day. The transcriptional analysis of brain tissues revealed that exposure to ZnONPs caused profound effects on genes and transcriptional signaling pathways associated with melatonin synthesis and metabolic processes during the day and night. We also observed that, in the duodenum, jejunum, ileum and colon sites, ZnONPs resulted in a significant reduction in the expression of the gut-derived melatonin rate-limiting enzyme Aanat, the membrane receptors Mt1 and Mt2 and Npy during the day and night.
Conclusion: Taken together, this is the first study shows that oral exposure to ZnONPs interferes with melatonin synthesis and secretion in different intestinal segments of adult mice. These findings will provide novelty insights into the neurotoxic mechanisms of ZnONPs and suggest an alternative strategy for the prevention of ZnONP neurotoxicity.
Keywords: gut-derived melatonin, neurobehavioral impairment, transcriptome sequencing, zinc oxide nanoparticle exposure
Graphical Abstract:
Introduction
Nowadays, nanomaterials have received increasing attention due to their wide applications in agriculture, biomedicine, electronic communications, etc.1 Zinc oxide nanoparticles (ZnONPs) are metal oxides with a diameter of less than 100 nm and possess selective toxicity to bacteria. ZnONPs have high catalytic activity and stability and are widely used in sunscreen, biosensor, food additive, drug carrier and electronic material, etc.2 Effects of ZnONPs on the biological functions depend on their morphology, particle size, exposure duration, concentration, pH value and biocompatibility.3 The significant anti-bacterial effect of ZnONPs has been extensively reported.4 Individuals are primarily exposed to ZnONPs through inhalation, ingestion, and dermal contact in the workplace and food. Traditionally, ZnONPs are considered to be one type of less toxic nanoparticles. A previous study showed that acute exposure to ZnONPs did not affect cognitive function and neurotransmitter levels in adult rats.5 However, with the wide use of ZnONPs in various fields, serious concerns have been raised about their impacts on human health and the environment. One recent study reported that ZnONPs exhibited adverse health effects on the human male reproductive system.6 A concentration of 100 ppm of 50 nm ZnONPs resulted in the death of 50% of mouse neuroblastoma cells in vitro, they were also able to induce apoptosis of neural stem cells at 12 ppm.7 It may be related to the fact that ZnONPs cause cell death by disrupting lipids and proteins in cell membranes and generating reactive oxygen species.8
In recent years, the influences of ZnONPs on neurobehavior have attracted much attention.9 It has been shown that ZnONPs can enter the brain through the blood–brain barrier and induce oxidative stress and inflammatory responses, therefore leading to neurological disorders.10 For example, in an epidemiological investigation, the workers exposed to ZnONPs exhibited significantly decreased levels of antioxidant enzymes, superoxide dismutase, and correct rate of 7-digit backward memory.11 In addition, acute exposure to ZnONPs also triggered oxidative stress, microglia activation and tau protein expression in the brain.12 Results from a cellular assay also revealed that ZnONPs reduced cell viability in neuroblastoma and altered the structure of tau protein, contributing to the development of Alzheimer’s disease.13
Melatonin is thought initially to be a hormone secreted at night from the pineal gland. It plays a crucial role in the adjustment of the sleep-wake cycle, pubertal development and seasonal adaptation.14 It is synthesized from tryptophan and produces 5-hydroxytryptamine as an intermediate product.15 Recent available studies have demonstrated that melatonin can also be produced in other organs. For instance, melatonin is found in chromophores of the human intestinal mucosa at concentrations more than 400 times that of melatonin in the pineal gland and 10–100 times that of melatonin in plasma.16 Melatonin has antidepressant, anti-anxiety, anti-phobic, motor-modulatory, neuroprotective, anti-inflammatory and antioxidant effects.14 Among these, the neurological effects of melatonin are particularly significant. One study observed that melatonin could modulate memory formation by directly affecting hippocampal neurons.17 Meanwhile, melatonin can also regulate addictive behavior disorders by inhibiting dopamine release.18 In addition, melatonin has been demonstrated to decelerate the progression of neurodegenerative diseases such as Alzheimer’s disease via the enhancement of anti-fibrinogen (inhibition of amyloidosis).19
Recently, one study revealed that exposure of rice to ZnONPs remarkably increased melatonin biosynthesis, thereby mitigating the toxicity of ZnONPs to rice.20 Another study showed that treatment of maize with ZnONPs significantly increased the content of tryptophan, a precursor of melatonin, which in turn promoted maize growth and improved its drought tolerance.21 However, in mammals, the effect of ZnONPs on gut-derived melatonin and the exact mechanism of melatonin in ZnONPs-induced neurotoxicity are currently unknown.
Therefore, in the present study, we hypothesized that ZnONPs might induce changes in gut-derived melatonin, thereby affecting neurobehaviors. The adult C57BL/6J male mice were herein exposed to ZnONPs by oral gavage at doses of 0, 6.5, 13, and 26 mg/kg for 30 days, followed by neurobehavioral assessment, transcriptomic analysis of brain tissue and detection of the expression of melatonin receptors Mt1 and Mt2, the melatonin synthesis limiting enzyme Aanat, and the neuromodulator gene Npy in the intestine, respectively. The results showed that oral exposure to ZnONPs caused significant neurobehavioral impairments, with significant changes in many metabolic and biosynthetic processes and melatonin-related genes. This study initially explores the role of gut-derived melatonin in the neurological damage induced by ZnONPs, which is expected to provide a new approach to prevention of neurological damage attributed to ZnONPs.
Materials and Methods
Preparation of ZnONPs Solution
In this study, ZnONPs were obtained from Sigma Aldrich Chemical Co. (Cat. #MKCH9705, Sigma-Aldrich, MO, USA) and primary particle sizes were less than 50 nm. The characteristics of ZnONPs were described previously in our lab.22 Briefly, ZnONPs were diluted in MilliQ water and sonicated with 20% of the maximum amplitude for 20 min in an ice water bath by an ultrasonic cleaner (SB-5200DT; Ningbo Scientz Biotechnology Co., Ltd, China). The vehicle solution was prepared with MilliQ water, and the ZnONPs suspension solution was fresh each time.
Animals and Treatment
Eight-week-old healthy male C57BL/6J mice were purchased from the Animal Center of Chongqing Medical University [Chongqing, China, license numbers: SCXK(Yu)2018-0003]. The animals were kept in an environmentally controlled room under a 12-h light/dark cycle, a temperature of 23 ± 1 °C, and humidity of 55% ± 10%. They were free to access to sterilized rodent food and tap water. A total of 64 mice were divided into four groups according to the simple randomized allocation method. Each group included 16 animals. All the mice were treated with concentrations of ZnONPs (6.5, 13, 26 mg/kg/day)23 or vehicle solution. The mice received water or ZnONPs by oral gavage tube according to body weight every morning at 9 A.M. for 30 days. Eight mice in each group were immediately euthanized by cervical dislocation under anesthesia,24 and the brain, duodenum, jejunum, ileum and colon were collected in the day-time, then stored at −80 °C immediately. Tissues from the same parts of the remaining eight mice in each group were collected in the dark-time. All experimental participants were blinded to the treatments and data analysis. The experimental protocols involving animals were scrutinized and approved by the Institutional Animal Care and Use Committee of Chongqing Medical University. All procedures were conducted following the guidelines contained in the guide for the care and use of laboratory animals. All efforts were made to minimize the suffering of mice.
Open-Field Test
The open-field test was used to detect the locomotor activity of mice.25 The open field apparatus consisted of four square areas that were painted gray. Briefly, the mice were initially placed in the central area of the open field test apparatus facing the same direction and explored for 5 min. The movement of mouse was recorded by a tracking system with video camera installed above the apparatus. The total distance, central square duration, and distance moved in center were measured, and the apparatus was wiped with 75% ethanol before performing each new test.
Elevated Plus Maze
The elevated plus maze (EPM) test was commonly used to assess anxiety-like behavior.26 The EPM apparatus consisted of two open arms, two closing arms, and a central square. In short, the mouse was placed in the center square of the maze facing one of the open arms and observed continuously for 5 min. A video-tracking system with a camera was used to record the distance moved in the open arm, number of entries into open arm, duration of the open arm, number of head-dipping in open arm. The apparatus was cleaned using 75% alcohol before each test to eliminate possible odors left by the previous animal.
Rotarod Test
The rotarod test was used to assess motor coordination and balance in rodents. The protocol procedure was performed according to the previous study.27 The rotarod apparatus consisted of a rotating cylinder (3 cm in diameter) with compartments for simultaneously testing of up to five mice. All animals were placed on the cylinder at a constant speed of 25 rpm for a maximum duration of 25 min. The mice moved in the same direction to keep balance on cylinder rod. The latency of time to fall was recorded from the start to the end of the rotarod test. The apparatus was cleaned carefully with 75% ethanol between each test.
Quantitative PCR Assay
Total mRNA was isolated from the intestine with the EastepTM Total RNA Super Extraction Kit (Promega, Cat#LS1040, MA, USA), carried out according to the manufacturer’s instruction. Each sample was homogenized with 300 µL of lysate and was mixed with 300 µL of RNA dilution and placed for 5 min at room temperature. The supernatant was added to 0.5 times the volume of anhydrous ethanol with mixing. After washing with RNA Wash, each sample was added to 50 µL of DNase and placed at room temperature for 15 min, followed by two washes with RNA Wash. Total RNA was obtained with 50 µL of nuclease-free water. Then, the total RNA quantity and purity was detected with a NanoDrop Technologies and the acceptable purity range was an A260/A280 ratio of 1.8 to 2.1. The reverse transcription to the cDNA was performed Perfect Real Time Prime-Script™ RT Master Mix (Cat. #RR036A, TaKaRa, Beijing, China). The real-time PCR was performed by TBGREEN Premix Ex Taq™ II (TliR-NaseH Plus) (Cat. #RR820A, TaKaRa, Beijing, China) on CFXConnect™ Real-Time System (Bio-Rad, Hercules, CA, USA). The initial temperature was set up at 95 °C for 2 min, then 40 amplification cycles for 5 sec of 95 °C, 15 sec of 60 °C and 20 sec of 72 °C, and finally, the melt curve for 5 sec from 65 °C to 95 °C. Negative control using the Tbp gene was normalized and calculated with the 2−ΔΔCt method. The primer sequences were synthesized by Sangon Biotech, Co., Ltd. (Shanghai, China) and are shown in Supplementary Table 1.
RNA Sequencing
RNA sequencing was performed according to a previous study.28 Total RNA was isolated from the brain tissue using TRIzol® Reagent following the manufacturer’s instructions (Thermo Fisher Scientific, CA, USA). Then, RNA quality and quantity were evaluated using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA) and the ND-2000 (Thermo Fisher Scientific, Wilmington, USA). The cDNA library was constructed following the Illu mina TruSeq RNA Sample Prep Kit (Illumina Inc., San Diego, CA) and the Ultra RNA Library Prep Kit for Illumina. Messenger RNA was isolated according to the poly-A selection method by oligo (dT) beads and double-stranded cDNA was synthesized using a SuperScript cDNA synthesis kit (Thermo Fisher Scientific, CA, USA). Quantified by the TBS380, the RNA-seq sequencing library was sequenced in paired-end using the Illumina HiSeq xten/NovaSeq 6000 sequencer (2 × 150 bp read length).
Differential Expression Analysis and Functional Enrichment
After trimming and quality control of the original paired-end readings via SeqPrep (https://github.com/jstjohn/SeqPrep) and Sickle (https://github.com/najoshi/sickle), RSEM (http://deweylab.biostat.wisc.edu/rsem/) was used to quantify on the expression level of genes and transcripts in between two different samples. Differential expression analysis was conducted using DESeq2 with|log2FC| ≥ 2.000 and P value <0.05. Furthermore, to identify functional-enrichment and pathway of candidate genes, the GO and KEGG analysis methods were performed using the online platform of Majorbio Cloud Platform (www.majorbio.com, Shanghai Majorbio Bio-pharm Technology Co., Ltd). Functional enrichment of differentially expressed genes in the GO functional enrichment and KEGG signaling pathway analysis were determined by Goatools (https://github.com/tanghaibao/Goatools) and KOBAS (http://kobas.cbi.pku.edu.cn/home.do). Significance levels for differentially expressed genes (DEGs) enrichment in GO terms and KEGG pathways were calculated using Fisher’s exact test at BH (Benjamini and Hochberg) corrected P-value ≤0.05 comparison with the entire-transcriptome background.
Statistical Analysis
All results were expressed as mean ± S.E.M. (standard error of the mean). The statistical analysis was performed using SPSS 26.0 (IBM, Armonk, NY, USA) and analyzed by one-way ANOVA or Kruskal–Wallis test. All graphs were presented by the software of GraphPad Prism version 9.0.0 (Graph-Pad Software Inc., La Jolla, CA). P<0.05 indicated that the data were significantly different.
Results
Oral Exposure to ZnONPs Caused Neurobehavioral Impairments in Adult Mice
To investigate the effect of ZnONPs treatment on locomotor activity, the open field test was carried out. As displayed in Figure 1, the total distance, distance moved in center and central square duration showed a notable decrease compared to the vehicle group (Figure 1A–D). These results indicated that the main effect of ZnONPs-treatment caused the inhibition of locomotor activity. The rotarod test was carried out to further detect whether ZnONPs affect motor coordination and balance in mice. It was clear that different doses of ZnONPs affected motor coordination and balance in mice as compared with the vehicle group (Figure 1E). The elevated plus maze was employed to test whether oral exposure to ZnONPs induced anxiety-like behavior. Treatment of ZnONPs affected the total distance moved, duration in the open arm, the frequency of mouse entry into the open arm and the number of head dips compared with vehicle control (Figure 2A-E). Our results demonstrated that level of anxiety was drastically increased in a related dose-dependent pattern. Obviously, the damage was significantly worse in the mice treated with high dose ZnONPs. All the above results suggest that oral exposure to ZnONPs for 30 days may potentially lead to neurobehavioral damage in adult mice.
Identification of Differentially Expressed Genes (DEGs) and Pathways Involved in Neurobehavioral Impairments via Oral Exposure to ZnONPs in Adult Mice
Transcriptome analysis was performed to explore the impact of ZnONPs between vehicle group and high-dose group. Due to the circadian rhythm of melatonin, the brain tissues were isolated separately during the day and night from vehicle and ZnONPs-treated mice (n=3 per group) for RNA-Seq assays. The Venn diagram was shown in Figure 3A, the results implied that an overlap of 14,826 genes was identified in mouse brains in the vehicle and high-dose groups during the daytime. A volcano plot was shown that 718 DEGs (|fold change|>2.0, P<0.05) were identified, including 380 upregulated (with top significant containing CD4, Ido1 and Pde10a) and 338 downregulated (with top significant containing Slc6a3, Tph2 and Ucn) in vehicle and ZnONPs-treated mouse brains sampled at daytime (Figure 3B). Gene ontology (GO) enrichment analysis was used further to dissect the primary structure and functions of DEGs. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis was performed to discover biological pathways. GO function enrichment analysis revealed that ZnONPs-treated was involved in biological processes, such as dopamine transport, neuron differentiation and central nervous system neuron differentiation, suggesting the regulation of ZnONPs might occur on the nervous system during the day (Figure 3C). The 278 categories by KEGG pathway enrichment analysis were clustered. The three primary enrichment pathways were neuroactive ligand–receptor interaction, cAMP signaling pathway and calcium signaling pathway. Pathways contained neuromodulation-related and 5-hydroxytryptamine genes, such as Npy, Tph2, Ucn and Ido1 (Figure 3D). To identify the functional categories of DEGs, 718 DEGs were classified into three categories and formed 48 GO terms. Most DEGs were mainly enriched in cellular process and biological regulation in biological processes and involved in binding in molecular functions. Cell part was enriched in the cellular components category (Figure 3E). These results indicate that gene expression regulations and many metabolic and biosynthetic processes dramatically are disturbed by ZnONPs-treated during the day time.
To further investigate whether oral exposure to ZnONPs affects gene expression at night, the brain tissues collected during the night were performed transcriptome analysis. As shown in Figure 4A, the compositional overlap of 15231 core DEGs was confirmed in the vehicle and high-dose groups. Volcano plots showing genes significant 63 up-regulated and 70 down-regulated were identified by RNA-seq analysis in brain tissues sampled at night, in which the widely up-regulated genes consisted of Sh3rf2, Gpr6 and Adora2a; in contrast, down-regulated genes included broadly Camp, Ucn and S100a9 (Figure 4B). GO and KEGG pathway enrichment analysis insighted gavage of ZnONPs suspension enriched inflammatory response, signal transduction and nervous system signaling in brain tissue sampled at night (Figure 4C and D). To further address the biological functions of DEGs after ZnONPs treatment, 133 DEGs were clustered in 44 categories by GO annotations analysis. Notably, cellular component and biological process were involved in cellular process and cell part, but molecular function was enriched in binding (Figure 4E). The 25 common genes of DEGs were reported in brain tissue sampled during the day and night (Figure 4F). These results together reveal that ZnONPs treatment may affect central nervous and immune system via regulation of inflammatory response and neuroactive signal transduction in the day and night time.
Oral Exposure to ZnONPs Affected the mRNA Expression of Melatonin-Limiting Enzyme and Melatonin Receptors in the Intestine of Adult Mice
To investigate whether oral exposure to ZnONPs disturbs the gut-derived melatonin synthetic pathway by influencing speed limiting enzyme Aanat activity, the primary enzyme involved was determined in the mouse intestine. After different doses of ZnONPs treatment, the expression of Aanat was significantly decreased in the jejunum, ileum and colon tissues of day sampling compared to vehicle controls in the day-time. To our surprise, Aanat mRNA content was not significant in the duodenum as compared to vehicle group in the day-time (Figure 5A). Then, to evaluate the state of Aanat expression in mouse intestine during the night, the enzyme activity was determined by qPCR assay. As shown in Figure 5B, the mRNA expression of Aanat in duodenum, jejunum and ileum was significantly reduced, but no significant differences in colon tissue compared to vehicle group at night. These data imply that the key synthetic enzyme expressions of gut-derived melatonin are affected by ZnONPs both day and night. During the day, melatonin receptor Mt1 mRNA expression of duodenum and jejunum showed a significantly decreased level compared to vehicle. However, no significant difference of Mt1 expression was observed in the ileum and colon compared with vehicle group (Figure 5C). Interestingly, the expression of Mt1 mRNA was significantly decreased in the colon, but no significant differences were observed in the duodenum, jejunum and ileum tissues during the night compared to the vehicle group (Figure 5D). After oral exposure to ZnONPs, the similar expression tendency of melatonin receptor Mt2 to Mt1 were observed in duodenum, jejunum, ileum and colon both in the day or night (Figure 5E and F). These results together signify that ZnONPs may influence the expressions of melatonin membrane receptors in adult mice.
Oral Exposure to ZnONPs Affected the mRNA Expression of Npy in the Intestine of Adult Mice
The Npy mRNA expression was detected in this study. During the daytime, the Npy mRNA expression of duodenum and jejunum intestinal segments was downregulated by ZnONPs-treated groups compared with vehicle group, but no significant differences were observed in the ileum and colon tissues compared to the vehicle group (Figure 6A). During the night, the Npy mRNA expression of jejunum and ileum segments had changed after exposure to ZnONPs compared with vehicle control, respectively. However, the Npy mRNA level in duodenum and colon showed no significant differences compared to vehicle group (Figure 6B).
Discussion
ZnONPs are used in many applications due to their special properties, which increase the daily exposure to ZnONPs.29,30 Whereas ZnONPs were thought to have low toxicity in the past, recent studies have reported cytotoxicity, genotoxicity, neurotoxicity and developmental toxicity of ZnONPs in different organs.31 Long-term oral administration via food is one of the main routes of exposure to ZnONPs.32 ZnONPs are known to produce severe neurotoxicity and cause neurobehavioral impairments.33 Intriguingly, melatonin is capable of modulating neuronal inflammatory factors, and oxidative stress, thereby alleviating neurotoxicity.34,35 However, there is limited evidence that melatonin ameliorates the neurotoxicity caused by ZnONPs in mammals. It is unclear whether oral administration of ZnONPs affects melatonin secretion, transport, and circadian rhythms in vivo. Therefore, in this study, adult C57BL/6J male mice were exposed to 0, 6.5, 13 and 26 mg/kg by gavage for 30 days. We found that oral exposure to ZnONPs had potential neuromodulatory effects, including inhibition of movement, impaired motor coordination and balance and anxiety-like behavior. In mammals, the gastrointestinal tract is rich in melatonin, which varies from tissue to tissue and has a distinct daily rhythm.36 We also found that ZnONPs affected gut-derived melatonin in different intestinal segments at day and night. Therefore, we hypothesize that gut-derived melatonin is closely linked to neurobehavioral impairment induced by ZnONPs.
ZnONPs enter the body through the digestive tract, the intestine being the primary target organ. Chen’s study showed that neurobehavioral deficits and specific changes in the neurobehavioral-related genes (Bdnf and Dlg4) were observed in male mice after orally administering of 26 mg/kg of ZnONPs for 30 days.23 Interestingly, our results also showed similar neurobehavioral impairments. Due to their small particle size, ZnONPs can easily cross the blood–brain barrier and enter nerve cells leading to mitochondrial dysfunction, reduced membrane transport capacity and DNA damage.37,38 Our RNA sequencing results showed that oral exposure of mice to ZnONPs, both during the day and at night, led to alterations in immune responses, neuromodulation and some tryptophan metabolism. Examples of upregulated genes were the T-cell cofactor CD4, Ido1 of the tryptophan metabolic pathway and Adora2a of the neurobehavioral G protein-coupled receptor transmission pathway. Significant down-regulation of the neurotransmitter Slc6a3, serotonin synthesis-related enzyme Tph2 and anxiety-related Ucn were observed. Kim et al showed that orally administered to mice with ZnONPs at 750 mg/kg/day for 14 days, they suppressed innate immunity and decreased the CD4+/CD8+ ratio, and implying that the immune status induced by ZnONPs was altered.39
When exposed to environmental stimuli and stress, the adrenocorticotropin-releasing factor CRF and its related neuropeptides, including urocortin 1 (ucn1, ucn2 and ucn3) can bind to corresponding receptors to alter neuronal activity in specific subpopulations of serotonergic neurons and affect stress-related behaviors, such as anxiety-like behaviour.40,41 The GO and KEGG pathways enrichment analysis revealed that oral exposure to ZnONPs at 25 mg/kg body weight of 6-week-old male ICR mice leads to endoplasmic reticulum stress and ROS production. MAPK and NF-JB pathways were activated, inducing an inflammatory response. Furthermore, treatment of BV2 and PC12 cells with ZnONPs elucidated that neuroinflammation was induced through Ca2+-dependent NF-κB, ERK and p38 activation pathways.42,43 In the present study, transcriptome sequencing analysis revealed that the main pathways affected by ZnONPs involve neuroactive ligand–receptor interactions, tryptophan metabolism and inflammatory responses both at day and night. The pathway contained genes such as Npy, Tph2, Ucn and Ido1 that are involved in the synthesis and metabolism of melatonin.44–46 The Npy, a neuropeptide derived from the hypothalamus, plays an essential role in the neuromodulatory.47 Interestingly, the Npy mRNA expression was affected by melatonin administration.48 Therefore, oral exposure of ZnONPs may affect melatonin activity by Npy. Our results showed that the mRNA expression of Npy decreased significantly in both day and night after exposure to ZnONPs, suggesting that oral exposure of ZnONPs possibly disturbs the synthesis and release of gut-derived melatonin via the neuropeptide Y signaling pathway.
Melatonin was synthesized in many mammal tissues and organs, including the ovary, retina, gland, gut, skin and bone marrow.49 Available studies have found that melatonin is also synthesized and secreted in large quantities in the gut, mainly by enterochromaffin cells and melatonin is present in different concentrations in different parts of the gut.50 A study reported that different melatonin levels were detected in the cattle and pigs’ anterior and posterior segments of the gastrointestinal tract.51 Melatonin secretion in different parts of the rat cerebellum, stomach, duodenum and pancreas followed a rhythmic pattern.52 Steful et al, found a specific expression of Aanat in the intestine of rats, suggesting that the intestine is capable of secreting and synthesizing melatonin on its own, further maintaining the stability of melatonin in the intestine. Furthermore, activation of Aanat leads to the release of large amounts of melatonin, and conversely, a decrease in melatonin-related enzymes inhibits melatonin synthesis and secretion.53 Numerous studies have revealed that the highest intestinal melatonin levels are in the duodenum and rectum (mainly in the epithelium).50,54 A similar phenomenon was found in fish, where melatonin was detected from the esophagus to the hindgut and melatonin synthase genes aanat1, aanat2 and hiomt mRNA were rhythmically expressed after removal of the pineal gland in rainbow trout (Oncorhynchus mykiss).55 Our results showed that the expression of melatonin synthase Aanat was highest in the duodenum both during the day and at night, which was consistent with the above findings. Aanat expression was dramatically reduced in the same tissues due to the action of ZnONPs.
Melatonin exerts physiological effects through melatonin membrane receptor-dependent and receptor-independent pathways.56,57 Melatonin membrane receptors are found mainly in the cell membrane and nucleus.58 The wide distribution of melatonin receptors determines the diversity of melatonin functions. In the intestine, melatonin activity is mediated by Mt2 and indirectly affects 5-HT.59 Expression of Mt1 mRNA was detected in the duodenum and colon of rats.60 It has been shown that Mt1 mRNA was located in the epithelium and sub-epithelium of rat intestinal tissues and is expressed in the duodenum, jejunum, ileum and colon.61 Our results showed that transcripts of melatonin receptor Mt1 and Mt2 mRNA were expressed in all intestinal segments but were unevenly distributed. In the presence of ZnONPs, Mt1 mRNA expression was significantly reduced in the duodenum and jejunum during the daytime, and Mt2 mRNA expression showed a significant reduction in all four intestinal segments, especially at a concentration of 26 mg/kg. Melatonin, a neuromodulatory hormone, is cyclic and its receptors may also undergo circadian change.62 Our findings showed a sharp decrease in Mt1 and Mt2 mRNA expression in the duodenum and colon during the night time due to stimulation by ZnONPs, and a pattern of expression might correlate with melatonin receptors distribution. One study evaluated the protective effect of melatonin against ZnONPs toxicity using C6 glial cells. The results showed that melatonin incubation with ZnONPs enhanced the cytotoxic effect and led to cell damage, which might be due to mitochondria-mediated ROS generation and melatonin-induced anti-proliferative effects.63 The stimulation of ZnONPs in this study not only leads to the occurrence of neurological damage in the body but also interferes with the synthesis and physiological effects of melatonin, exacerbating the inflammatory response and producing neurotoxicity in the body. Thus, the interaction between ZnONPs and melatonin leads to cell damage and apoptosis.
There are several limitations exist in the present study. Firstly, due to the rhythmical nature of melatonin secretion and action, we assessed the locomotor activity and anxiety-like behavior in mice by recording only at the daytime conditions but could not reflect whether the same results were obtained at night. Secondly, the doses of ZnONPs used in this study appeared to be lower than those reported in others.32,64,65 Because the doses used herein were calculated from Chinese standards for infant formula additives. Significant differences existed between mice and humans and the daily exposure dose used in the study was not equal to the concentration in the environment. Thirdly, this study did not perform an exogenous melatonin intervention. It is difficult to determine the causal effect of ZnONPs and melatonin on neurobehavioral impairment, which needs to be explored in the future. Of course, our results will also provide a new perspective for the prevention of neurotoxicity from ZnONPs, which means, in the future, we can explore the effects and mechanisms of gut-derived melatonin in ZnONP-induced neurological impairments and find the relevant signaling pathways or target molecules that can regulate melatonin and play a neuroprotective role during exposure to ZnONPs.
Conclusion
In summary, the results of the present study suggest that gut-derived melatonin was affected in neurobehavioral impairments caused by oral exposure to ZnONPs. In addition, melatonin synthesis was reduced and its physiological effects were diminished in different sites of the intestine, as well a phenomenon during the day and night. It may be that ZnONPs interfered with the synthetic pathway and physiological effects of melatonin, making the organism more susceptible to damage caused by ZnONPs. However, the mechanism of ZnONP regulation of gut-derived melatonin needs further exploration. Although this study could not demonstrate the protective effect of gut-derived melatonin against the toxicity of ZnONPs, these findings will provide new insights into the role of gut-derived melatonin in the neurological impairment caused by ZnONPs.
Acknowledgments
This study was supported by NSFC (Grant No. 82001378); the Basic Research and Frontier Exploration Project of Yuzhong District (Grant No. 20180116); Natural Science Foundation of Chongqing (Grant No.cstc2019jcyj-msxmX0294); Science and Technology Research Program of Chongqing Municipal Education Commission (Grant No. KJQN202000444).
Disclosure
The authors report no competing interests related to this study.
References
1. Kim DY, Kadam A, Shinde S, Saratale RG, Patra J, Ghodake G. Recent developments in nanotechnology transforming the agricultural sector: a transition replete with opportunities. J Sci Food Agric. 2018;98(3):849–864. doi:10.1002/jsfa.8749
2. Król A, Pomastowski P, Rafińska K, Railean-Plugaru V, Buszewski B. Zinc oxide nanoparticles: synthesis, antiseptic activity and toxicity mechanism. Adv Colloid Interface Sci. 2017;249:37–52. doi:10.1016/j.cis.2017.07.033
3. Siddiqi KS, Ur Rahman A, Husen A. Properties of zinc oxide nanoparticles and their activity against microbes. Nanoscale Res Lett. 2018;13(1):1–13. doi:10.1186/s11671-018-2532-3
4. Kermani M, Mostafapour A, Sabouri Z, Gheibihayat SM, Darroudi M. The photocatalytic, cytotoxicity, and antibacterial properties of zinc oxide nanoparticles synthesized using Trigonella foenum-graecum L extract. Environ Sci Pollut Res Int. 2022;30(7):19313–19325. doi:10.1007/s11356-022-23518-3
5. Amara S, Ben-Slama I, Mrad I, et al. Acute exposure to zinc oxide nanoparticles does not affect the cognitive capacity and neurotransmitters levels in adult rats. Nanotoxicology. 2014;8(sup1):208–215. doi:10.3109/17435390.2013.879342
6. Shen J, Yang D, Zhou X, et al. Role of autophagy in zinc oxide nanoparticles-induced apoptosis of mouse LEYDIG cells. Int J Mol Sci. 2019;20(16):4042. doi:10.3390/ijms20164042
7. Jeng HA, Swanson J. Toxicity of metal oxide nanoparticles in mammalian cells. J Environ Sci Health A. 2006;41(12):2699–2711. doi:10.1080/10934520600966177
8. Zhang L, Zou L, Jiang X, et al. Stabilization of Nrf2 leading to HO-1 activation protects against zinc oxide nanoparticles-induced endothelial cell death. Nanotoxicology. 2021;15(6):779–797. doi:10.1080/17435390.2021.1919330
9. Alimohammadi S, Hosseini MS, Behbood L. Prenatal exposure to zinc oxide nanoparticles can induce depressive-like behaviors in mice offspring. Int J Pept Res Ther. 2019;25(1):401–409. doi:10.1007/s10989-018-9686-9
10. Jiang Q, Gao Y, Wang C, et al. Nitration of TRPM2 as a molecular switch induces autophagy during brain pericyte injury. Antioxid Redox Signal. 2017;27(16):1297–1316. doi:10.1089/ars.2016.6873
11. Liou S-H, Tsou T-C, Wang S-L, et al. Epidemiological study of health hazards among workers handling engineered nanomaterials. J Nanoparticle Res. 2012;14(8). doi:10.1007/s11051-012-0878-5
12. Chuang H-C, Yang Y-T, Chen H-C, et al. Acute effects of pulmonary exposure to zinc oxide nanoparticles on the brain in vivo. Aerosol Air Qual Res. 2020;20(7):1651–1664.
13. Mousavi SE, Fard MZ, Rezayat SM, Naraki K. Investigation of the effects of zinc oxide nanoparticles on membrane damage of human neuroblastoma cell lineage (SH-SY5Y) and change of tau protein structure by spectroscopic methods. Biointerface Res Appl Chem. 2021;12(5):6032–6045.
14. Emet M, Ozcan H, Ozel L, Yayla M, Halici Z, Hacimuftuoglu A. A review of melatonin, its receptors and drugs. Eurasian J Med. 2016;48(2):135. doi:10.5152/eurasianjmed.2015.0267
15. Tan DX, Hardeland R, Back K, Manchester LC, Alatorre-Jimenez MA, Reiter RJ. On the significance of an alternate pathway of melatonin synthesis via 5-methoxytryptamine: comparisons across species. J Pineal Res. 2016;61(1):27–40. doi:10.1111/jpi.12336
16. Raikhlin N, Kvetnoy I, Tolkachev V. Melatonin may be synthesised in enterochromaffin cells. Nature. 1975;255(5506):344–345. doi:10.1038/255344a0
17. Wang X, Wang Z, Cao J, Dong Y, Chen Y. Melatonin alleviates acute sleep deprivation-induced memory loss in mice by suppressing hippocampal ferroptosis. Front Pharmacol. 2021;12:708645. doi:10.3389/fphar.2021.708645
18. Onaolapo OJ, Onaolapo AY. Melatonin in drug addiction and addiction management: exploring an evolving multidimensional relationship. World J Psychiatry. 2018;8(2):64–74. doi:10.5498/wjp.v8.i2.64
19. Li LB, Fan YG, Wu WX, et al. Novel melatonin-trientine conjugate as potential therapeutic agents for Alzheimer’s disease. Bioorg Chem. 2022;128:106100. doi:10.1016/j.bioorg.2022.106100
20. Song Y, Wang B, Qiu D, et al. Melatonin enhances metallic oxide nanoparticle stress tolerance in rice via inducing tetrapyrrole biosynthesis and amino acid metabolism. Environ Sci. 2021;8(8):2310–2323.
21. Sun L, Song F, Guo J, et al. Nano-ZnO-induced drought tolerance is associated with melatonin synthesis and metabolism in maize. Int J Mol Sci. 2020;21(3):782. doi:10.3390/ijms21030782
22. Jiang X, Tang Q, Zhang J, et al. Autophagy-dependent release of zinc ions is critical for acute lung injury triggered by zinc oxide nanoparticles. Nanotoxicology. 2018;12(9):1068–1091. doi:10.1080/17435390.2018.1513094
23. Chen J, Zhang S, Chen C, et al. Crosstalk of gut microbiota and serum/hippocampus metabolites in neurobehavioral impairments induced by zinc oxide nanoparticles. Nanoscale. 2020;12(41):21429–21439. doi:10.1039/d0nr04563b
24. Pasquereau-Kotula E, Habault J, Kroemer G, Poyet JL, Ma W-L. The anticancer peptide RT53 induces immunogenic cell death. PLoS One. 2018;13(8):e0201220. doi:10.1371/journal.pone.0201220
25. Kraeuter A-K, Guest PC, Sarnyai Z. The open field test for measuring locomotor activity and anxiety-like behavior. In: Pre-Clinical Models. Springer; 2019:99–103.
26. Kraeuter A-K, Guest PC, Sarnyai Z. The elevated plus maze test for measuring anxiety-like behavior in rodents. In: Pre-Clinical Models. Springer; 2019:69–74.
27. Pritchett K, Mulder GB. The rotarod. J Am Assoc Lab Anim Sci. 2003;42(6):49.
28. Jiang X, Li W, Tan M, et al. Identification of miRNAs involved in liver injury induced by chronic exposure to cadmium. Toxicology. 2022;469:153133. doi:10.1016/j.tox.2022.153133
29. Melo A, Amadeu MS, Lancellotti M, Hollanda L, Machado D. The role of nanomaterials in cosmetics: national and international legislative aspects. Química Nova. 2015;38:599–603.
30. Schneider T, Westermann M, Glei M. In vitro uptake and toxicity studies of metal nanoparticles and metal oxide nanoparticles in human HT29 cells. Arch Toxicol. 2017;91(11):3517–3527. doi:10.1007/s00204-017-1976-z
31. Ng CT, Yong LQ, Hande MP, et al. Zinc oxide nanoparticles exhibit cytotoxicity and genotoxicity through oxidative stress responses in human lung fibroblasts and Drosophila melanogaster. Int J Nanomedicine. 2017;12:1621. doi:10.2147/IJN.S124403
32. Yang P, Hong W, Zhou P, Chen B, Xu H. Nano and bulk ZnO trigger diverse Zn-transport-related gene transcription in distinct regions of the small intestine in mice after oral exposure. Biochem Biophys Res Commun. 2017;493(3):1364–1369. doi:10.1016/j.bbrc.2017.09.165
33. Liu H, Yang H, Fang Y, et al. Neurotoxicity and biomarkers of zinc oxide nanoparticles in main functional brain regions and dopaminergic neurons. Sci Total Environ. 2020;705:135809. doi:10.1016/j.scitotenv.2019.135809
34. Lai J, Ji J-M, Luo Y-P, et al. Melatonin ameliorates bupivacaine-induced spinal neurotoxicity in rats by suppressing neuronal NLRP3 inflammasome activation. Neurosci Lett. 2022;772:136472. doi:10.1016/j.neulet.2022.136472
35. Shah SA, Khan M, Jo MH, Jo MG, Amin FU, Kim MO. Melatonin stimulates the SIRT 1/Nrf2 signaling pathway counteracting lipopolysaccharide (LPS)‐induced oxidative stress to rescue postnatal rat brain. CNS Neurosci Ther. 2017;23(1):33–44. doi:10.1111/cns.12588
36. Konturek SJ, Konturek PC, Brzozowska I, et al. Localization and biological activities of melatonin in intact and diseased gastrointestinal tract (GIT). J Physiol Pharmacol. 2007;58(3):381–405.
37. Attia H, Nounou H, Shalaby M. Zinc oxide nanoparticles induced oxidative DNA damage, inflammation and apoptosis in rat’s brain after oral exposure. Toxics. 2018;6(2):29. doi:10.3390/toxics6020029
38. Pan CY, Lin FY, Kao LS, Huang CC, Liu PS, Hsieh H-L. Zinc oxide nanoparticles modulate the gene expression of ZnT1 and ZIP8 to manipulate zinc homeostasis and stress-induced cytotoxicity in human neuroblastoma SH-SY5Y cells. PLoS One. 2020;15(9):e0232729. doi:10.1371/journal.pone.0232729
39. Kim CS, Nguyen HD, Ignacio RM, et al. Immunotoxicity of zinc oxide nanoparticles with different size and electrostatic charge. Int J Nanomedicine. 2014;9(Suppl 2):195–205. doi:10.2147/IJN.S57935
40. Fox JH, Lowry CA. Corticotropin-releasing factor-related peptides, serotonergic systems, and emotional behavior. Front Neurosci. 2013;7:169. doi:10.3389/fnins.2013.00169
41. van der Doelen RHA, Robroch B, Arnoldussen IA, Schulpen M, Homberg JR, Kozicz T. Serotonin and urocortin 1 in the dorsal raphe and edinger-westphal nuclei after early life stress in serotonin transporter knockout rats. Neuroscience. 2017;340:345–358. doi:10.1016/j.neuroscience.2016.10.072
42. Hu H, Guo Q, Fan X, et al. Molecular mechanisms underlying zinc oxide nanoparticle induced insulin resistance in mice. Nanotoxicology. 2020;14(1):59–76. doi:10.1080/17435390.2019.1663288
43. Liang H, Chen A, Lai X, et al. Neuroinflammation is induced by tongue-instilled ZnO nanoparticles via the Ca2+-dependent NF-κB and MAPK pathways. Part Fibre Toxicol. 2018;15(1):1–21. doi:10.1186/s12989-018-0274-0
44. Jenwitheesuk A, Park S, Wongchitrat P, et al. Comparing the effects of melatonin with caloric restriction in the hippocampus of aging mice: involvement of sirtuin1 and the FOXOs pathway. Neurochem Res. 2018;43(1):153–161. doi:10.1007/s11064-017-2369-7
45. Ciani E, Fontaine R, Maugars G, et al. Melatonin receptors in Atlantic salmon stimulate cAMP levels in heterologous cell lines and show season-dependent daily variations in pituitary expression levels. J Pineal Res. 2019;67(3). doi:10.1111/jpi.12590
46. Maher AM, Saleh SR, Elguindy NM, Hashem HM, Yacout GA. Exogenous melatonin restrains neuroinflammation in high fat diet induced diabetic rats through attenuating indoleamine 2,3-dioxygenase 1 expression. Life Sci. 2020;247:117427. doi:10.1016/j.lfs.2020.117427
47. Olcese J. Neuropeptide Y: an endogenous inhibitor of norepinephrine‐stimulated melatonin secretion in the rat pineal gland. J Neurochem. 1991;57(3):943–947. doi:10.1111/j.1471-4159.1991.tb08241.x
48. Conde-Sieira M, Librán-Pérez M, Patiño MAL, Soengas JL, Míguez JM. Melatonin treatment alters glucosensing capacity and mRNA expression levels of peptides related to food intake control in rainbow trout hypothalamus. Gen Comp Endocrinol. 2012;178(1):131–138. doi:10.1016/j.ygcen.2012.04.011
49. Tan DX, Xu B, Zhou X, Reiter RJ. Pineal calcification, melatonin production, aging, associated health consequences and rejuvenation of the pineal gland. Molecules. 2018;23(2):301. doi:10.3390/molecules23020301
50. Pan J, Li F, Wang C, et al. Effects of duodenal 5-hydroxytryptophan perfusion on melatonin synthesis in GI tract of sheep. Molecules. 2021;26(17):5275. doi:10.3390/molecules26175275
51. Bubenik GA, Hacker RR, Brown GM, Bartos L. Melatonin concentrations in the luminal fluid, mucosa, and muscularis of the bovine and porcine gastrointestinal tract. J Pineal Res. 1999;26(1):56–63. doi:10.1111/j.1600-079x.1999.tb00567.x
52. Stebelova K, Anttila K, Manttari S, Saarela S, Zeman M. Immunohistochemical definition of MT2 receptors and melatonin in the gastrointestinal tissues of rat. Acta Histochem. 2010;112(1):26–33. doi:10.1016/j.acthis.2008.03.004
53. Carrillo‐Vico A, Calvo JR, Abreu P, et al. Evidence of melatonin synthesis by human lymphocytes and its physiological significance: possible role as intracrine, autocrine, and/or paracrine substance. FASEB J. 2004;18(3):537–539. doi:10.1096/fj.03-0694fje
54. Bubenik GA. Gastrointestinal melatonin: localization, function, and clinical relevance. Dig Dis Sci. 2002;47(10):2336–2348. doi:10.1023/a:1020107915919
55. Munoz-Perez JL, Lopez-Patino MA, Alvarez-Otero R, Gesto M, Soengas JL, Miguez JM. Characterization of melatonin synthesis in the gastrointestinal tract of rainbow trout (Oncorhynchus mykiss): distribution, relation with serotonin, daily rhythms and photoperiod regulation. J Comp Physiol B. 2016;186(4):471–484. doi:10.1007/s00360-016-0966-4
56. He B, Zhao Y, Xu L, et al. The nuclear melatonin receptor ROR α is a novel endogenous defender against myocardial ischemia/reperfusion injury. J Pineal Res. 2016;60(3):313–326. doi:10.1111/jpi.12312
57. Zhang J, Fang Y, Tang D, et al. Activation of MT1/MT2 to protect testes and leydig cells against cisplatin-induced oxidative stress through the SIRT1/Nrf2 signaling pathway. Cells. 2022;11(10). doi:10.3390/cells11101690
58. Acuna-Castroviejo D, Escames G, Venegas C, et al. Extrapineal melatonin: sources, regulation, and potential functions. Cell Mol Life Sci. 2014;71(16):2997–3025. doi:10.1007/s00018-014-1579-2
59. Esteban-Zubero E, López-Pingarrón L, Alatorre-Jiménez MA, et al. Melatonin’s role as a co-adjuvant treatment in colonic diseases: a review. Life Sci. 2017;170:72–81. doi:10.1016/j.lfs.2016.11.031
60. Huang D, Hu Q, Fang S, Feng J. Dosage effect of zinc glycine chelate on zinc metabolism and gene expression of zinc transporter in intestinal segments on rat. Biol Trace Elem Res. 2016;171(2):363–370. doi:10.1007/s12011-015-0535-9
61. Sotak M, Mrnka L, Pacha J. Heterogeneous expression of melatonin receptor MT1 mRNA in the rat intestine under control and fasting conditions. J Pineal Res. 2006;41(2):183–188. doi:10.1111/j.1600-079X.2006.00355.x
62. Boiko DI, Shkodina AD, Hasan MM, et al. Melatonergic receptors (Mt1/Mt2) as a potential additional target of novel drugs for depression. Neurochem Res. 2022;47(10):2909–2924. doi:10.1007/s11064-022-03646-5
63. Sruthi S, Millot N, Mohanan PV. Zinc oxide nanoparticles mediated cytotoxicity, mitochondrial membrane potential and level of antioxidants in presence of melatonin. Int J Biol Macromol. 2017;103:808–818. doi:10.1016/j.ijbiomac.2017.05.088
64. Baek M, Chung HE, Yu J, et al. Pharmacokinetics, tissue distribution, and excretion of zinc oxide nanoparticles. Int J Nanomedicine. 2012;7:3081–3097. doi:10.2147/Ijn.S32593
65. Yang X, Shao H, Liu W, et al. Endoplasmic reticulum stress and oxidative stress are involved in ZnO nanoparticle-induced hepatotoxicity. Toxicol Lett. 2015;234(1):40–49.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.






