Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Alopecia Areata: Burden of Disease, Approach to Treatment, and Current Unmet Needs
Authors Alhanshali L, Buontempo MG, Lo Sicco KI, Shapiro J
Received 6 January 2023
Accepted for publication 11 March 2023
Published 31 March 2023 Volume 2023:16 Pages 803—820
DOI https://doi.org/10.2147/CCID.S376096
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Lina Alhanshali,1 Michael G Buontempo,2 Kristen I Lo Sicco,3 Jerry Shapiro3
1Department of Dermatology, SUNY Downstate College of Medicine, Brooklyn, NY, USA; 2Department of Dermatology, Hackensack Meridian School of Medicine, Nutley, NJ, USA; 3The Ronald O. Perelman Department of Dermatology, NYU Grossman School of Medicine, New York, NY, USA
Correspondence: Jerry Shapiro, The Ronald O. Perelman Department of Dermatology, New York University Grossman School of Medicine, 240 East 38th Street, 12th Floor, New York, NY, 10016, USA, Email [email protected]
Abstract: Alopecia areata is an autoimmune hair loss disorder with variations in distribution, duration, and severity. The disease is chronic and often follows an unpredictable course, frequently leading to stress and anxiety for those who suffer from it. Throughout the years more knowledge has been gained regarding pathogenesis, diagnostic tools, impact on quality of life, as well as treatment strategies for alopecia areata. However, challenges in treating and alleviating the burden of disease remain. In this article, we discuss updates regarding the pathogenesis and treatment of alopecia areata and highlight unmet needs of the condition, including a review of limitations of current treatments, accessibility to management strategies, and the need for disease awareness and advocacy.
Keywords: alopecia areata, disease burden, unmet needs
Plain Language Summary
Alopecia Areata is a type of alopecia or hair loss disorder. The disease affects men, women, and people of all ages including children. We have learned much about alopecia areata, including more details on how the disease is caused. Many factors contribute to someone having alopecia areata such as genetics, autoimmunity, and environmental factors. There are also new effective treatment options for the disease, for example, Janus Kinase inhibitors, which are small molecules that inhibit the Janus Kinase inflammatory pathway. However, there is still no cure for alopecia areata. As such, we will continue to focus on finding ways of better managing the disease. This includes improving medical therapies, advocating for laws that support patients with alopecia areata, and improving access to resources that help navigate life with alopecia areata.
Introduction
Alopecia areata (AA) is a non-scarring alopecia characterized by an autoimmune attack on the hair follicle. The condition has a lifetime incidence of approximately 2.1%, impacting millions worldwide.1 Hair follicles on the scalp as well as any other parts of the body can be affected, and the condition is categorized based on the pattern and distribution of hair loss. The most common form is patchy AA, which presents as discrete patches of hair loss. Other forms of AA include alopecia totalis (complete loss of scalp hair), alopecia universalis (complete loss of all body hair), alopecia incognita or diffuse AA (diffuse shedding), ophiasis (symmetric band-like alopecia typically involving the occipital region), and sisaipho (involves the top of the scalp, typically sparing the temporal and occipital areas).
The incidence of AA is relatively equal between men and women.2 In AA, the hair follicle is not destroyed, giving the potential for reversibility. The disease can spontaneously resolve and often follows a relapsing-remitting course with and without known triggers. For patchy AA, the spontaneous remission rate is 30–50% in the first 6–12 months of the disease, and complete resolution is seen in up to 66% of patients within 5 years.3 For alopecia totalis, the rate of spontaneous remission is less than 10%.4 Within 20 years, 100% of patients with AA experience disease relapse.5 The typically sudden onset of AA and its unpredictable nature contribute toward the tremendous negative impact on quality of life (QOL).6
The ability of AA to spontaneously resolve can make it challenging to fully assess a therapeutic’s efficacy. In recent years, with more knowledge regarding the pathogenesis of AA, significant advancements have been made to combat the disease.7 However, patients with AA and the clinicians who care for them still face many challenges in longitudinally managing the condition. There is a tremendous unmet need for better therapeutics, improved access to currently available treatments, and public support for people suffering from AA.
Updates on Pathogenesis
The leading mechanism regarding the pathogenesis of AA involves the loss of immune privilege, which has been well studied. More recently, details involving this autoimmune phenomenon leading to disease onset have been further elucidated. Studies regarding the pathogenesis of AA reveal an intricate complexity involving multiple factors including immunology, genetics, the environment, and potentially the microbiome.8
Immunology
Immune privilege (IP) refers to areas of the body where an immune response to antigens is usually suppressed. These areas include the brain, central nervous system, hair follicles, retina, cornea, testicles, and a gravid uterus. In a hair follicle, IP is limited to the hair bulge and bulb and is restricted to the anagen phase only in the bulb.9
In areas exhibiting IP, there are mechanisms to keep immune cells out of the area as well as to downregulate an immune response. The hair follicle’s extracellular matrix is a barrier that prevents immune cells from being transferred in and out of the hair follicle.10 In addition, there is a downregulation of major histocompatibility complex I (MHC-I), which prevents self-antigens from being presented to CD8+ T cells. Major histocompatibility complex II (MHC-II) is also downregulated, preventing antigen-presenting cells from presenting foreign antigens in the region. The epithelium of a healthy hair follicle also secretes immune inhibitors known as “IP guardians” such as interleukin 10 (IL-10), transforming growth factor β- 1 (TGF-β1), and melanocyte-stimulating hormone alpha (αMSH).11 Expression of “IP guardians” by hair follicle epithelium has been demonstrated to be decreased in AA.9
In AA, these processes fail to protect hair follicles from the immune system. In 1993, Paus et al first reported AA as the result of IP loss, an autoimmune phenomenon where T cells attack autoantigens.12 Over the next decade, more details immerged on how this phenomenon occurs. One theory behind the loss of IP in those susceptible to AA is that reactive oxygen species from environmental stress activate proteins such as MHC class I polypeptide-related sequence A, leading to increased expression of MHC class I.9 In what is commonly referred to as a “swarm of bees” on histopathology, inflammatory cells also invade the hair follicle and cause a dysregulation that leads to the clinical presentation of AA.13
The cytokine interferon-gamma (IFN-γ) and inflammasomes play a significant role in the pathogenesis of AA. C3H/HeJ is a mouse model, which shows phenotypic, histologic, and immune similarities to human AA.14 In 2018, Shin et al injected IFN- γ and polyinosinic:polycytidylic acid (a synthetic dsRNA) subcutaneously into C3H/HeJ mice and after 8 weeks, 80% of the mice developed patchy AA.15 One study also showed that IL levels (IL-2, IL-4, and IL-15) were significantly higher in patients with AA and decreased with treatment, which supports their role in pathogenesis.16 However, the change in IL levels did not correlate with the change in Severity of Alopecia Tool (SALT) scores or disease duration.16 Another study also revealed toll-like receptors (TLR) 7 and 9 were elevated in patients with AA and more so in active disease, which further demonstrates the role of the immune system in the pathogenesis of AA.17
While TH1 has long been implicated in AA, TH2 has more recently been reported to contribute to the pathogenesis of AA. A study demonstrated that TH2 markers (IL-13), Chemokine ligand 13 (CCL13), Chemokine ligand 17 (CCL17), Chemokine ligand 22 (CCL22), and Chemokine ligand 26 (CCL 26) were elevated in AA in addition to elevations of natural killer (NK) cell markers (IL-15) and TH1 markers (CCL2, CCL3, and C-X-C motif chemokine ligand 10 (CXCL10)).18 This was further supported by a study showing serum levels of TH1 cytokines (IL-2 and IFN- γ) were elevated along with increased TH2 (IL-17) and regulatory T cells (IL-10) levels.19 IL-23 was not statistically significantly raised, and anti-inflammatory cytokine, IL-4 was decreased in AA patients. IL-2, IL-17, and IL-23 were positively correlated with disease severity as measured by SALT scores.19
In hair follicles impacted by AA, there is expression of MHC class I chain-related A gene (MICA), a ligand that activates natural killer cell receptor NKG2D resulting in increased IFN-y secretion.20 Other ligands of NKG2D including UL-16 binding proteins (ULBPs) are also upregulated in the hair follicle of patients with AA. ULBP3 levels measured through real-time polymerase chain reaction (PCR) have been explored as a potential marker for AA.21 Additionally, in AA hair follicles immune privilege collapse only occurs during the melanogenically active stages of the hair cycle (anagen III–VI).12 AA development involves an immune response to specific melanogenesis and keratinocyte-derived antigens such as trichohyalin, TRP-1, and TRP-2.22
During the COVID-19 pandemic, an increasing number of patients reported a relapse or new onset of AA after infection or vaccination.23,24 AA onset has previously been associated with other viral infections including human immunodeficiency virus (HIV), cytomegalovirus (CMV), dengue virus, as well as the Hepatitis B vaccine.25–27 Immune-related mechanisms have been reported that potentially explain this relationship including virally induced increased interferon production and shared epitopes.26 However, the physiological stress of an infection or vaccination in a genetically predisposed individual may also contribute to the association.28 AA has also been reported after drug reaction with eosinophilia and systemic symptoms (DRESS).29
Genetics
The first clue regarding genetic involvement in AA pathogenesis was through clinical observation that family members of those with AA and identical twins had a higher incidence of the disease.30,31 Mouse models later suggested a polygenic nature to AA.32 Additionally, the association between AA and other autoimmune disorders prompted a selection of specific genes to be analyzed in candidate gene association studies.33 These studies revealed an association with human leukocyte antigen (HLA) alleles. In 2010, the first large genome-wide association study (GWAS) identified 139 single nucleotide polymorphisms that are significantly associated with AA.20 A second large study, a meta-analysis combining data from two GWAS, further revealed two new loci related to AA and data comparisons with other autoimmune diseases.34 More recently, non-single genetic polymorphisms such as short tandem repeats (STR) polymorphisms and copy number variants (CNVs) have been explored and implicated in AA pathogenesis.35–38 Utilizing interviews of index individuals with AA and their relatives, one study estimated a lifetime risk of AA in relatives with the disease as 7.1% in siblings, 7.8% in parents, and 5.7% in offspring.39
Microbiome
The cutaneous and gut microbiome are implicated in many extraintestinal diseases and recently the scalp appears to also be involved. One of the first associations between alopecia and the gut microbiome was through a study in mice, where an antibiotic-induced imbalance of the gut microbiome promoted the development of a generalized alopecia.40 In regard to AA, a case report of two patients with alopecia universalis who underwent a fecal transplant to treat refractory Clostridium difficile developed significant hair regrowth 8 weeks post-transplant.41 A possible reported mechanism for the association between the microbiome and autoimmunity is that intestinal or cutaneous dysbiosis and microbiome disruption results in an imbalance in one’s natural flora and functioning, causing T-cell dysregulation and ultimately triggering of the immune system.42 In a cross-sectional study, patients with AA were shown to have a distinct gut microbiome compared to healthy controls.43 Those with AA had a higher number of certain bacterial species such as Actinobacteria and Candidiate division TM7, and a lower number of Bacteroidetes and Fusobacteria. Bacteroidetes produce butyrate, a short-chain fatty acid essential for differentiating regulatory T cells, which has been demonstrated to be decreased in patients with AA.44 Another study of children with AA and their siblings as controls showed minor differences in gut microbiome composition.45 One species, Ruminococcus bicirculans, was identified to be of decreased abundance in patients with AA and has also been demonstrated to be reduced in other autoimmune disorders.46 More recently, a study found that patients with AA had a more diverse scalp microbiome.47 In the study, the alpha diversity metrics (Shannon index), which measures the diversity of species in a community, was significantly higher in AA patients compared to healthy controls, indicating increased diversity.48 A later study showed a distinct microbiome scalp population in patients with AA.49 Enterobacteriaceae, Streptococcus, Gemella, Porphyromonas, and Granulicatella were relatively more abundant in AA groups, while Staphylococcus and Flavobacterium genera were significantly lower in AA samples than in controls. The significance of these observed changes is not well-defined currently, and it is also unknown whether these changes in the microbiome occur before or after the onset of AA.
Additional studies exploring the relationship between gut, cutaneous, and scalp microbiome and AA are necessary to determine if they have the potential to provide new markers for early disease detection, and possible prevention and treatment strategies.
Treatment
A Brief Review of Prior Treatment Options for Alopecia Areata
Treatment options for AA are highly variable and range from nonmedical interventions (such as abstaining from treatment, microblading, or utilizing hair prosthetics), to medical interventions such as the newer immuno-modulating Janus Kinase (JAK) inhibitors. Smaller lesions of AA, <25% total scalp involvement can have excellent rates of self-resolution. Spontaneous remission rates can vary from 68% in patients with <25% scalp involvement to 8% in cases with >50% scalp involvement.50 In cases where spontaneous resolution is unlikely, the patient is anxious about their disease process, the hair loss is significant, or the hair loss is having a substantial impact on the patient’s lifestyle or quality of life, it is appropriate to pursue treatment.
Treatment options with modest to high treatment success in AA include topical steroids, intralesional steroids, topical immunotherapies, JAK inhibitors, and systemic corticosteroids. Other treatment options with variable success rates that are used less frequently include topical calcineurin inhibitors, topical latanoprost or bimatoprost, cryotherapy (light spray jet), methyl-aminolevulinic acid photodynamic therapy, 308-nm excimer laser, pulsed infrared diode laser (904 nm), and antihistamines. There is limited evidence for the effectiveness of calcineurin inhibitors in treating localized AA, with studies showing a low likelihood of success based on the number of responders in monotherapy.51,52 Topical latanoprost and bimatoprost have shown little to no efficacy in treating AA to cosmetically acceptable results. However, one study demonstrated that 45% of their treatment group reported longer thicker eyelashes with topical latanoprost.53–57 Cryotherapy (light spray jet) has shown limited efficacy in treating AA, and methyl-aminolevulinic acid photodynamic therapy has been found to be unsuccessful in multiple studies.58–60
Additionally, 308-nm excimer laser treatment has been shown to lead to hair regrowth in 41.5–60% of AA patches and is generally safe with few adverse effects. However, it has not been effective in treating alopecia universalis or alopecia totalis and may not be as effective in patients with atopic diathesis. Further research is needed to confirm the efficacy and safety of excimer laser for AA treatment.61–63 In one study, pulsed infrared diode laser treatment (904 nm) showed some response in 94% of patients with AA. LLLT has been shown to be effective in treating androgenetic alopecia (a type of hair loss), but there are limited studies on its use in alopecia areata (AA). Some studies have shown that LLLT can stimulate hair regrowth in AA, but the mechanisms behind this effect are not fully understood. Further research is needed to understand how LLLT works and assess its efficacy in AA treatment.64,65 The antihistamine, fexofenadine, has been found to be a helpful adjuvant therapeutic with contact immunotherapy in the treatment of atopic AA but not non-atopic AA.66,67
Other key factors aside from disease severity that may influence a clinician’s choice of treatment include patient-perceived disease severity, patient distress, and impact on the patient’s social life. While each treatment modality may have variable success within an individual patient’s understanding, involving the patient is key to choosing the correct treatment.
Previously, we developed an algorithm discussing our approach to the management of AA.68 The guideline followed a decision tree outline to help clinicians manage AA in their patients. Below, we have outlined our previous guidelines and added new changes and modifications based on recent literature, changes in the field, drug developments, and clinical expertise (Figure 1). We have also added a discussion below our guidelines to give clinical pearls and expectations for treatment responses. This new guideline aims to help clinicians make treatment choices for their patients with AA and highlight the limitations of the available treatment options.
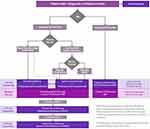 |
Figure 1 An approach to the treatment of Alopecia Areata. |
Decision #1: Age > or < 10
If the patient is under 10, we recommend topical corticosteroids such as clobetasol propionate 0.05% solution and topical 5% minoxidil. If the patient is over 10, a second decision is made depending on the extent of the disease.
Decision #2: Extent of Disease > or < 50%
If the disease process involves less than 50% of the scalp, we suggest using topical corticosteroids with low-dose oral minoxidil (LDOM). LDOM is typically administered at a dose ranging from 0.625 mg (quarter of a 2.5 mg pill) to 5 mg daily with the dose depending on age, gender, weight, and tolerability. An alternative is topical 5% minoxidil solution applied twice daily with or without intralesional (IL) corticosteroids.69,70 If this fails, we then recommend alternating to topical immunotherapy.
If the extent of the disease involves more than 50% of the scalp or the condition is rapidly progressing, we recommend using one of four possible approaches. The first is to use the same treatment mentioned above, topical corticosteroids and LDOM (0.625–5.0 mg/day) or topical 5% minoxidil with or without IL corticosteroids. The second approach would be to use systematic JAK inhibitors with oral minoxidil and with or without intralesional corticosteroids and if this fails switch to topical immunotherapy. The third is topical immunotherapy such as Diphenylcyclopropenone (DPCP), and if DPCP fails attempt to use squaric acid dibutyl ester (SADBE). Finally, at any time if the patient decides to discontinue medical treatment, a scalp prosthesis alone may be utilized.68
Once treatment success is achieved, we recommend decreasing medication dose(es) to the lowest effective dose. In the case where multiple medications are utilized, we recommend first tapering the medication with a higher side effect profile.
Steroids
Steroids, intralesional or topical, are a centerpiece for the treatment of AA and often the first-line therapy in patchy AA.
Intralesional Steroids
IL steroid injections, most commonly intralesional triamcinolone acetonide, colloquially referred to as “IL-TAC are the mainstay of treatment for limited lesions in patchy AA and can serve as an adjunctive treatment in patients with more extensive disease.71 In patients with patchy AA there is often >50% improvement with steroid injections alone after 12 weeks of treatment.72
In our clinical practice, we limit steroid injections to a maximum of 20 mg/month to help mitigate the risk of systemic side effects for patients. Research done by Jarratt et al in 1974 demonstrated no adrenal suppression when utilizing intralesional triamcinolone acetonide at a concentration of (40 mg/mL) at volumes of 0.1 ml or 0.5 ml (equivalent to dosing of 20 mg).73 To help limit side effects, we also typically administer IL-TAC at concentrations of 2.5 mg/cc, 5 mg/cc, or 10 mg/cc once monthly with decreasing frequency as a resolution of the disease process occurs (Table 1). When dosing for injections around the eyebrow, we also recommend utilizing a lower dosing of 2.5 mg/cc. Intralesional triamcinolone acetonide has been associated with the development of Cushing syndrome, especially when dosed multiple times or at high doses in pediatric patients.74 To avoid potential side effects in patients of all ages, clinicians may prefer to utilize lower concentrations of 2.5 mg/cc as it has been shown by Chu et al that doses of Triamcinolone Acetonide beyond 2.5 mg/cc provide no added benefit in the treatment of AA.75 Along the frontal scalp, we suggest using lower concentrations of 2.5 mg/cc to limit the risk of atrophy in a more visible area as well as dyschromia, especially in darker-skinned individuals.68,76
 |
Table 1 Monthly IL-TAC Injection Commonly Used Concentrations and Volumes in the Authors Hair Clinic |
Side effects from steroid dosing within these ranges are typically limited to common skin changes associated with local steroid injection. These side effects can include post-inflammatory hyper/hypopigmentation, striae, atrophy, and telangiectasias. All are reversible except striae which occur after chronic administration of high doses in the same area.77
Topical Steroids
Apart from intralesional steroids, topical steroids often play a role in managing AA. Topical steroids are currently and have long been the mainstay of treatment for AA in patients younger than 10 years or who may be unable to tolerate intralesional steroid injections.78 Topical steroids typically yield a moderate success rate, with topical 0.1% betamethasone valerate foam applied twice daily for 12 weeks achieving 75% hair regrowth in mild-to-moderate AA.79 However, in patients with more severe alopecia such as universalis or totalis, there is typically a lower success rate with topical steroids, such as one study by Tosti et al where only 28.5% (8/28) were deemed, responders when utilizing 2.5 g of clobetasol propionate under occlusion, nightly.80
We recommend topical mometasone 0.1% solution or cream twice daily for children with AA. In our practice, for adults, we commonly use topical clobetasol propionate 0.05% as either a foam, cream, ointment, or solution. Previous split scalp studies have shown the importance of comprehensive application to all affected areas to result in disease resolution, with an occlusive dressing such as a thin plastic film being the preferred application method for topical steroids.80 Consider the curl pattern when choosing the vehicle, understanding that curlier hair is more prone to desiccation and certain vehicles, such as solutions, can be more drying. As such, clobetasol 0.05% solution could be applied daily for patients with straight hair and clobetasol oil for patients with tighter, curlier hair. The side effects from topical steroid treatments are nearly identical to those mentioned above for intralesional steroids.
Topical Immunotherapy (Squaric Acid or Diphenylcyclopropenone)
Topical immunotherapies for the treatment of AA often include diphenylcyclopropenone (DPCP) or squaric acid dibutyl ester (SADBE) which, through a poorly understood mechanism, induce allergic contact dermatitis. This local inflammatory response alters the local immune response by shifting the T-Cell mediated autoimmunity in AA away from targeting the hair follicle.81 DPCP has a success rate of approximately 60% to 70% and is often utilized in more extensive cases spanning more than 50% of the scalp.82 For patients who fail to grow hair after 6–9 months of DPCPm SADBE can be used.83
This treatment modality intentionally induces allergic contact dermatitis, which may be uncomfortable for patients. In addition, the treatment needs to remain on the scalp for 48 hr while it is covered with a wig or protective cap to prevent light exposure; both features of this regimen can be tedious and deterring for patients.68 Finally, patients who opt to wear cranial prostheses might find topical immunotherapy too irritating.
Minoxidil
Minoxidil, most commonly used to treat androgenetic alopecia (AGA), has found its place as a supportive therapy for managing AA. However, minoxidil, in either topical or oral forms, is unlikely to be a reliable monotherapy in patients with severe AA but may serve as an adjuvant therapy in managing AA. In this section, we discuss the evidence regarding the use of minoxidil (in its topical or oral forms) in patients with AA, its side effects, and a guideline summary.
Topical Minoxidil: Low Efficacy Monotherapy in AA
Previous studies utilizing topical Minoxidil in 1% and 3% formulations have failed to demonstrate significant efficacy in patients with severe AA.84,85 While not sufficient at either 1% or 3% concentrations, topical 5% minoxidil has also been investigated. Two placebo-controlled studies previously demonstrated that only 6–14% of patients (3 of 47) vs (6 of 43) who had severe AA treated with 5% topical minoxidil developed a cosmetically acceptable response. The patients enrolled in these studies had hair loss ranging from 25% to 100% hair loss with a mean duration of an alopecic episode of 5.7 years.69,86
While topical minoxidil as monotherapy has not been shown to have high-quality evidence for its use in severe AA at 1%, 3%, or 5% concentrations, its application in limited patchy AA has more established support. An extensive systematic review and meta-analysis by Freire et al found that for both children and adults with limited patchy AA, topical 5% minoxidil has moderate-quality evidence to support its use as an effective and safe monotherapy compared to placebo.87
Potential Areas for Oral Minoxidil to Take Hold
Minoxidil is often utilized as an adjunctive therapy to promote hair growth with concomitant treatments that better target the underlying immune response in AA. While topical minoxidil is readily available over the counter in the United States as a cheap and easy hair treatment when hair loss progresses beyond limited patches, applying topical minoxidil is often cumbersome, especially for patients with more extensive AA, such as alopecia universalis or alopecia totalis. As such, oral minoxidil may be considered more readily. While minoxidil in its oral form is Food and Drug Administration (FDA) approved for the treatment of recalcitrant hypertension, its off-label use in low doses ranging from 0.25 to 5 mg for the treatment of AGA is overall safe and effective.88,89 In adolescents and children over the age of 10, a starting dose of 0.625 mg daily is utilized. Regarding the safety of oral minoxidil in pediatric patients, a retrospective review of 63 patients ages 12 and younger (median age 10 years old) showed no serious side effects.90 Side effects such as hypertrichosis, postural hypotension, and headache were mild and did not result in treatment discontinuation.90 There is a need for more studies assessing the safety and tolerability of oral minoxidil in the pediatric population.
Regarding the possible utilization of oral minoxidil as a monotherapy for AA, one study utilizing oral minoxidil 5 mg twice a day found that 18% of patients with less than 75% hair loss and 9% of the patients with more than 75% hair loss were able to achieve a cosmetic response.91 The benefit from these medications was maintained throughout treatment, with cosmetic response occurring at 34.8 weeks. Of note, strict adherence to a 2-g sodium diet was required for the duration of therapy.91
While the literature does not provide overwhelming support for using oral minoxidil as monotherapy in AA, it should be considered as adjunctive therapy.
Minoxidil Metrics
In both oral and topical forms, minoxidil has been shown to shorten hair follicle telogen phase, increase anagen phase duration, and increase hair diameter and length.92,93 For example, one study by Yin et al showed that LDOM could result in an average addition of 9.4 hairs/cm2 in patients after oral minoxidil alone was added to their treatment regimen, with LDOM ranging from 0.625 to 5 mg a day.93
Minoxidil with JAK Inhibitors
In patients with AA, oral minoxidil may increase global background hair density to promote the masking of alopecic areas of the scalp, thereby decreasing visibility concerns. As oral minoxidil has been shown to increase hair density and diameter in AGA, patients with both AGA and AA may experience more significant benefits from oral minoxidil in overlapping coverage from the medication.
A review by Randolph and Tosti cited multiple studies where both hair density and diameter were found to increase with the initiation of oral minoxidil. In addition, they concluded that oral minoxidil might serve as a safe and effective option for patients with either AA or AGA.94
Recently, the FDA approved the use of the oral JAK inhibitor, baricitinib, for the treatment of AA in patients with SALT scores greater than 50. The BRAVE-AA1 trial and BRAVE-AA2 trials showed that patients with SALT scores ≥50 improved to SALT scores of 20 or less at 36 weeks in 35.9–38.8% of patients on 4 mg of baricitinib, 19.4–22.8% of patients on 2 mg of baricitinib, and 3.3–6.2% of patients on placebo.95
The success of these trials provided enough support for baricitinib to obtain FDA approval for the treatment of AA in patients with SALT scores ≥50 who are over the age of 18 years. In addition, these trials support the use of JAK inhibitors in AA, providing an effective treatment option to patients who may have previously failed to improve with other therapies.96 While JAK inhibitors alone demonstrate a scientific breakthrough for patients with AA, combining JAK inhibitors with oral minoxidil may promote an increased improvement rate in patient SALT scores when used in conjunction with oral JAK inhibitors.97
A recent research letter by Warmbier et al reviewed 12 patients with initial SALT scores of at least 66. These patients took oral minoxidil and tofacitinib with tofacitinib dosed 5–10 mg twice daily and oral minoxidil 2.5 mg two or four times a day. The letter reported that after 3 months, 5 of the 12 patients achieved at least 50% regrowth, further supporting a possible role for minoxidil in combination with JAK inhibitors.98
Interestingly, in 1987 Fiedler-Weiss et al looked at oral minoxidil 5 mg twice a day in patients with severe AA and identified a possible change in cellular infiltrate in response to oral minoxidil.91,99 This study evaluated the effects of topical and oral minoxidil on peripheral blood and tissue lymphocyte populations in patients with alopecia areata. The study found that treatment with topical minoxidil led to a significant increase in peripheral helper-inducer T cell counts in both responders and nonresponders and a significant increase in total T cell counts in responders. No significant differences were seen in the helper/suppressor cell ratios or monocyte counts between groups before or after treatment with topical minoxidil. Tissue lymphocyte populations were not significantly different between responders and nonresponders before treatment. Following treatment with topical minoxidil, responders showed significant decreases in total T cells, helper-inducer T cells, and suppressor-cytotoxic T cells, while nonresponders did not show significant changes. No significant differences were seen in the helper/suppressor cell ratios, activated T cells, or monocyte or Langerhans cell counts between groups or with treatment. Treatment with oral minoxidil was associated with significant suppression of activated T cells and perifollicular Langerhans cells, and a nearly statistically significant decrease in monocyte counts. These results suggest that minoxidil may have different effects on lymphocyte populations depending on the route of administration. These studies found cosmetically acceptable responses in only 18% of patients; on average, the cosmetic response was obtained in 34.8 weeks.99
Minoxidil Side Effects
While LDOM is overall safe, there are several clinical considerations. Clinicians should be cautious when prescribing oral minoxidil to patients who have lower baseline blood pressure, a history of orthostasis, or are taking concomitant antihypertensive medications, as it may precipitate syncopal episodes, especially when approaching a minimum antihypertensive dose of 5 mg per day. Other adverse effects found in a multicenter study of 1404 patients include hypertrichosis, a dose-dependent side effect occurring in approximately 15% of patients, lightheadedness (occurring in 1.7% of patients), fluid retention (occurring in 1.3% of patients), tachycardia (occurring in 0.9%), headache (occurring in 0.4%), periorbital edema (occurring in 0.3% of patients), and insomnia (occurring in 0.2% of patients), leading to drug discontinuation in 29 patients (1.2% of patients). Often these side effects resolve with dose adjustment and rarely is complete medication cessation necessary.88 There is a report of a patient developing pericardial, pleural effusion, and anasarca 3 weeks after LDOM therapy. A recent study assessed biochemical profiles, 24-hr holter monitoring, and 24-hr ambulatory blood pressure monitoring before and 24 weeks after 5 mg oral minoxidil daily in 34 healthy men (aged 21–58). They found no relevant changes in all of the measures.
Minoxidil Guideline Summary
In summary, our previously reported algorithm noted the use of topical 5% minoxidil as a potential add-on for patients with AA. As oral minoxidil gains popularity due to its convenience, cost savings, and efficacy in those with severe disease as well as concomitant AGA, we recommend considering, when reasonable, the use of oral minoxidil (0.25–5 mg daily) in addition to the primary treatment regimen in AA.
Resistant or Extensive Disease (Totalis/Universalis) - JAK Inhibitors
For alopecia that is treatment-resistant or severe such as alopecia universalis or totalis, it may be appropriate to pursue systemic oral JAK inhibitor therapy. We now have an FDA-approved oral JAK inhibitor, baricitinib, and the FDA recently accepted regulatory submissions for ritlecitinib. Oral JAK inhibitor medications, compared to topical, have four times higher odds of achieving a response regardless of the agent.96 Based on initial studies, there appears to be no difference between tofacitinib, ruxolitinib, or baricitinib in their efficacy.96 Depending on the study, hair growth response rates generally range from estimates of 60–75% for tofacitinib, ruxolitinib, and baricitinib, with similar ranges for each at their lower and maximum dosing.100–102
The FDA-approved oral JAK inhibitor, baricitinib, comes in two dosing formulations, 2 mg or 4 mg. As mentioned above, the BRAVE-AA1 trial and BRAVE-AA2 trials showed that patients with SALT scores ≥50 improved to SALT scores of 20 or less at 36 weeks in 35.9–38.8% of patients using 4 mg of baricitinib, 19.4–22.8% of patients using 2 mg of baricitinib, and 3.3–6.2% of patients using placebo.
JAK inhibitors may be effective in treating AA, but their use should be carefully considered due to potential side effects such as malignancy, major adverse cardiovascular events (MACE), leukopenia, neutropenia, anemia, thromboembolic events, reproductive effects, and gastrointestinal effects.103–105 These side effects are more commonly reported in patients with rheumatoid arthritis, but safety data for JAK inhibitors in AA are still limited.96 Therefore, clinicians should be aware of the potential risks associated with using JAK inhibitors, and further research is needed to fully understand their safety and efficacy in the treatment of AA. In one study comparing the effects of baricitinib at different doses in patients with RA, no significant differences between treatment groups were observed in terms of shifts in certain blood cell counts (lymphocytes, neutrophils, and platelets).106
Changes in lymphocyte and neutrophil counts were seen over the long term, with lymphocyte counts initially increasing and then declining to baseline levels, and neutrophil counts initially decreasing within normal levels and then remaining stable. Baricitinib at a dosage of 4 mg was associated with a modest increase in platelet counts that peaked at 2 weeks, returned towards baseline, and remained stable. No association was found between an increase in platelet count and the occurrence of DVT/PE (deep vein thrombosis/pulmonary embolism). Both doses of baricitinib were associated with significant increases in LDL (low-density lipoprotein) and HDL (high-density lipoprotein) levels, but there was no change in the LDL/HDL ratio. Baricitinib treatment was also associated with dose-dependent, largely asymptomatic increases in CPK (creatine phosphokinase) levels. Elevations in ALT (alanine aminotransferase) above the upper limit of normal were more common in the group receiving 4 mg of baricitinib compared to the placebo group. Still, elevations to ≥5 × ULN (upper limit of normal) were not dose-dependent and were more common in the baricitinib group overall. Permanent discontinuations due to hepatobiliary AE (adverse events) were uncommon in this study. Bone marrow suppression may be a dose-related effect of JAK inhibitors. Another study found that patients with RA given baricitinib 4 mg daily had a significant decrease in neutrophil counts.107
However, a review by King et al showed that patients with atopic dermatitis given baricitinib at 2 mg daily had no significant increase in anemia, neutropenia, lymphopenia, or elevated liver enzymes compared to placebo.108
In seven clinical trials, the rate of malignancies (excluding non-melanoma skin cancer) was higher in patients treated with tofacitinib compared to placebo. Specifically, during the first 3 months of treatment, two patients (0.3 cases per 100 patient-years) receiving tofacitinib developed malignancies, compared to none in the placebo group. During the first 12 months of treatment, the rate of malignancies was 0.4 cases per 100 patient-years in the group receiving 5 mg of tofacitinib twice daily and 0.6 cases per 100 patient-years in the group receiving 10 mg of tofacitinib twice daily. The most common types of malignancy observed in these trials were lung and breast cancer, followed by gastric, colorectal, renal cell, prostate cancer, lymphoma, and malignant melanoma.109 It is important to note that a higher rate of lung cancer in patients with rheumatoid arthritis (RA) was observed in current or past smokers treated with the JAK inhibitor compared to those treated with TNF blockers. Additionally, current or past smokers had an increased risk of overall malignancies.110
Regarding reproductive concerns, as per animal studies in rheumatologic literature, JAK inhibitors are contraindicated in pregnancy due to animal teratogenicity at levels 50–100x those given to humans.111 While contraindicated due to teratogenicity concerns during pregnancy, there is no literature that currently suggests a long-term impact on male or female fertility regarding baricitinib or tofacitinib. In RA studies, patients who experienced MACE (defined as myocardial infarction, stroke, or cardiovascular death) were typically ≥50 years of age with ≥1 cardiovascular risk factor treated with another JAK inhibitor, a higher rate of major adverse cardiovascular events was observed when compared with TNF blockers. Two real-world studies investigating the risk of MACE with JAK inhibitors compared to TNF inhibitors in patients with rheumatoid arthritis (RA) have produced conflicting results.112,113 The first study, using the World Health Organization's Global Individual Case Safety Report database, found an increased risk of MACE with JAK inhibitors, particularly tofacitinib, but only in physician-reported cases. Using data from the biologics register RABBIT, the second study did not find an increased risk of MACE with JAK inhibitors in RA patients initiating treatment from 2017 onwards. Studies suggest that the risk of MACE with JAK inhibitors may be limited to patients with known cardiovascular risk. More research is needed to fully understand the potential risks and benefits of these medications in treating RA.
Another review of RA clinical trials found no association was found between baricitinib treatment and the incidence of MACE, ATE, or CHF. Concerning the incidence of DVT/PE, six events occurred in patients treated with 4 mg baricitinib, but no cases of DVT/PE were reported in the placebo group. During a longer-term evaluation, the incidence of DVT/PE was similar between the baricitinib dose groups, with consistent IR values over time.114 Another study was conducted to compare the safety of baricitinib with TNF inhibitor for the treatment of patients with rheumatoid arthritis (RA) in terms of risk of venous thromboembolism (VTE), major adverse cardiovascular events (MACE), and severe infection.115
The study found that, with a mean overall exposure of 9 months, treatment with baricitinib was associated with a significantly increased risk of VTE compared to TNF inhibitor (incidence rate ratio [IRR] = 1.51, 95% confidence interval [CI] 1.10, 2.08). The incidence rate difference (IRD) was 0.26 (95% CI −0.04, 0.57) per 100 patient-years. The risk of MACE was also numerically greater with baricitinib compared to TNF inhibitor, although not statistically significant, during a mean overall exposure of 8 months (IRR = 1.54, 95% CI 0.93, 2.54; IRD = 0.22, 95% CI −0.07, 0.52 per 100 patient-years). Results for serious infection also estimated a numerically greater, non-statistically significant risk with baricitinib compared to TNF inhibitor during a mean overall exposure of 10 months (IRR = 1.36, 95% CI 0.86, 2.13; IRD = 0.57, 95% CI −0.07, 1.21 per 100 patient-GI Perforation (GIP)) is another reported adverse effect from JAK inhibitors with a poorly understood mechanism. One study by Xie et al analyzed real-world data from two large health plan databases in the US to investigate the risk of gastrointestinal perforation (GIP) in patients with rheumatoid arthritis (RA) who were receiving tocilizumab, tofacitinib, or TNF inhibitor. The results showed that the risk of lower GIP was approximately three times higher in tofacitinib users compared to TNF inhibitor users. Although the relative risk was higher for tocilizumab and tofacitinib, the absolute rate differences between treatments were small, with a range of approximately 1 per 1000 patient-years. Glucocorticoid use was also identified as an independent risk factor for GIP. Absolute perforation rates were low, but GIP is a costly and potentially life-threatening adverse event. The mechanisms by which disease-modifying antirheumatic drugs (DMARDs) and biologics might lead to lower gastrointestinal tract perforations are not fully understood but may involve impairments in host defenses.116
In a different study, three cases of gastrointestinal (GI) perforation were reported in patients taking the drug baricitinib to treat RA. These cases included a perforated appendix, a perforated diverticulum, and a proximal intestinal perforation after knee surgery. The patients were all taking background treatment with methotrexate and nonsteroidal anti-inflammatory drugs, and two of them were also taking prednisone.106
Microneedling
Microneedling involves the use of fine needles to puncture the stratum corneum resulting in collagen formation, neovascularization, and the release of growth factors.117,118 While microneedling is most commonly studied for androgenetic alopecia, there have been reports involving its potential use in AA treatment. The use of microneedling was tested on patients with severe or resistant AA and demonstrated hair regrowth.119–121 Randomized controlled trials are necessary to further elucidate the potential benefits of microneedling in AA treatment.
The reported outcomes of the various AA treatments discussed in this section are summarized in Table 2.
 |
Table 2 Alopecia Areata Treatment Outcomes from Select Studies |
Unmet Needs
AA often causes a substantial psychological burden and significantly impacts quality of life. AA is associated with increased depression, anxiety, and impairments in emotions and social functioning.122 A study investigating all-cause and cause-specific mortality in patients with AA found the mortality risk associated with self-harm and psychiatric diseases was significantly higher in AA patients compared to matched controls (HR, 1.21; 95% confidence interval (CI), 1.04–1.41).123 Recently, a comparative study demonstrated that patients with AA have a worse quality of sleep compared to healthy controls, which was associated with anxiety, depression, and a worse QOL.124 Management of AA is a multi-disciplinary effort, and there are unmet needs in various spheres of the disease (Table 3). Efficacious medications, such as JAK inhibitors, are available and increasingly FDA-approved; however, many patients still lack access due to lack of insurance coverage limitations and subsequent cost prohibition. Despite evidence that cranial prostheses (wigs) improve QOL, they remain an underfunded tool in the AA treatment plan.125,126
 |
Table 3 Unmet Needs in Alopecia Areata |
Limitations of Currently Available Medical Therapies
Currently available therapies for AA are limited by their efficacy, side effect profile, cost, and tolerability. In a systematic review and meta-analysis regarding the efficacy and safety of JAK inhibitors, the pooled rate of a good response (defined as 50% improvement in SALT score) was 63%, 28%, and 11% for oral, topical, and sublingual JAK inhibitors, respectively.102 In the same review, the pooled recurrence rate in patients treated with JAK inhibitors was 54%, with drug discontinuation being the most common cause. There is a need to assess the long-term safety and efficacy of JAK inhibitor use.
Another restriction in the use of JAK inhibitors is their cost.127 While the FDA has approved baricitinib for the treatment of severe AA, in our clinical experience many insurances have restrictions regarding medication cost coverage. Despite evidence demonstrating the burden of AA, this autoimmune disease represents a current gap in clinical care. More advocacy is necessary to recognize and substantiate AA as a disease deserving of intervention.
Finally, while JAK inhibitors are overall well tolerated among AA patients in clinical practice, they carry a higher side effect profile than other medications used to treat AA, as discussed in section 3.6. While many of the serious side effects are very rare, they call for specific laboratory testing and monitoring before and during treatment and require contraception until at least 1 week after treatment.128
Serious adverse events such as malignancy and cardiovascular disease may require a prolonged period to develop and as such, clinical trials with longer follow-up periods are needed. Additionally, much of the current data regarding long-term risks are on patients with immune-mediated inflammatory diseases such as RA and inflammatory bowel disease.129 Studies assessing long-term effects of JAK inhibitors in patients with AA specifically are necessary as patients with immune-mediated inflammatory diseases may have an increased baseline risk of severe cardiac events and malignancy compared to patients with AA. Additionally, studies focusing on these long-term severe adverse events are necessary as patients with AA have a higher rate of disease relapse with JAK inhibitor discontinuation and likely require continuation of the lowest effective dose longitudinally.
Access to Wigs and Other Camouflaging Agents
Treatment for AA is limited and may take months before considerable improvement is seen. Additionally, it follows a waxing and waning disease course and relapse may occur multiple times throughout one’s lifetime. As such, wigs as cranial prostheses and camouflaging techniques play an essential role in the management of AA to improve QOL.125,130 Despite research demonstrating the role of wigs as a valuable tool in managing AA, wigs as cranial prostheses are often not fully covered by health insurance. A study analyzing the financial burden of AA demonstrated that spending was highest for camouflaging techniques including wigs and makeup,126 leaving patients of lower socioeconomic backgrounds unable to adequately manage life with AA.
Efforts are underway to address the prohibitive costs of wigs for patients with alopecia. Legislative efforts are an essential way of advocating for the needs of patients suffering from alopecia.131 H. R. 5430 is a bill introduced to the house on September 30, 2021, calling for cranial prostheses to be recognized as durable medical equipment and for coverage under the Medicare program with a letter of medical necessity. The senate companion bill, S.4708 was introduced to the senate on August 2, 2022.132
Psychological Interventions
Support groups can help patients with AA develop coping strategies and learn from others regarding ways of navigating life with AA.133 The National Alopecia Areata Foundation (NAAF) connects patients with AA to support groups throughout the world.134 Support group therapy for AA patients’ family members can also be beneficial in fostering a supportive network.135 Online support networks also exist for patients with AA. However, there is a need for research on whether patients with AA have easy access to supportive non-medical therapy and if online support networks are as beneficial as in-person or virtual support groups. In addition, attending a support group requires free time, transportation (if in-person), webcam, and an internet connection (if virtual), which raises the question on whether there are socioeconomic disparities in access to support groups, a topic that has yet to be studied.
Awareness and Patient Education
There is often a stigma to alopecia stemming from multiple reasons including lack of knowledge and preconceived notions often influenced by the media portrayal of hair loss.136–138 The NAAF promotes education and advocacy for AA as well as the sharing of supportive resources and clinical trials.139 Other groups include the Alopecia Justice League, which aims to raise awareness regarding alopecia and advocate for legislation as well as programs that support patients suffering from the disease.139 The American Academy of Dermatology’s (AAD) Good Skin Knowledge is a youth education program where medical student volunteers teach children in schools about various dermatologic diseases, including alopecia.140
These programs represent recent efforts to increase awareness, knowledge, and advocacy of alopecia, including AA. There is a need for further development and funding for such organizations. Lastly, more efforts are necessary by the community at large to improve acceptance of and inclusion for those with AA. Those with AA who choose not to seek treatment or use camouflaging agents should be free to do so without fear of bullying or social stigma.
Conclusion
Knowledge regarding the pathogenesis and subsequent development of targeted therapies for AA has increased over the last decade. We now have an increased understanding regarding the mechanism of immune privilege collapse and the role of specific inflammatory cytokines. A variety of medical therapies have been developed including JAK Inhibitors and the use of other therapies such as oral minoxidil and microneedling. More research is necessary to explore the interplay between genetics, external or environmental factors, as well as variables leading to a response to therapy. To date, no curative agent exists, and longitudinal studies are necessary to investigate the relationship between cardiovascular disease and malignancy risk in the AA population on long-term JAK inhibitor therapy. Finally, more advocacy, both in legislation and education, is needed to increase access to FDA-approved medications to treat AA as well as cranial prostheses as part of the AA treatment plan.
Abbreviations
AA, Alopecia areata; SALT, Severity of Alopecia Tool; IP, Immune Privilege; MHC-I, major histocompatibility complex I; MHC-II, major histocompatibility complex II; IFn-Y, Interferon-gamma; dsRNA, Double-stranded RNA; TLR-7, Toll-like receptor 7; TLR-9, Toll-like receptor 9; IL-10, Interleukin-10; IL-13, Interleukin-13; IL-15, Interleukin-15; IL-17, Interleukin-17; IL-23, Interleukin-23; CCL2, Chemokine ligand 2; CCL3, Chemokine ligand 3; CCL13, Chemokine ligand 13; CCL17, Chemokine ligand 17; CCL22, Chemokine ligand 22; CCL26, Chemokine ligand 26; CXCL10, C-X-C motif chemokine ligand 10; CXCL10, C-X-C motif chemokine ligand 10; HLA, Human leukocyte antigen; GWAS, genome-wide association study; STR, Short tandem repeat; CNV, Copy number variants; ULBPs, UL-16 binding proteins; JAK, Janus kinase; RA, Rheumatoid arthritis; NAAF, National Alopecia Areata Foundation.
Funding
There is no funding to report.
Disclosure
Lina Alhanshali, Michael G Buontempo, Kristen I Lo Sicco, and Jerry Shapiro contributed equally as co-first and co-senior, respectively. Dr. Shapiro is a consultant for Aclaris Therapeutics, Incyte, and Replicel Life Sciences. Drs. Shapiro and Lo Sicco have been investigators for Regen Lab and are investigators for Pfizer. The authors have no other conflicts of interest to disclose.
References
1. Mirzoyev SA, Schrum AG, Davis MDP, Torgerson RR. Lifetime incidence risk of alopecia areata estimated at 2.1% by Rochester Epidemiology Project, 1990–2009. J Invest Dermatol. 2014;134(4):1141–1142. doi:10.1038/jid.2013.464
2. Harries M, Macbeth AE, Holmes S, et al. The epidemiology of alopecia areata: a population-based cohort study in UK primary care. Br J Dermatol. 2022;186(2):257–265. doi:10.1111/bjd.20628
3. Trüeb RM, Dias MF. Alopecia areata: a comprehensive review of pathogenesis and management. Clin Rev Allergy Immunol. 2018;54(1):68–87. doi:10.1007/s12016-017-8620-9
4. Abbott J, Rapini RP. Totalis Alopecia. In: StatPearls. StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.; 2022.
5. Walker SA, Rothman S. A statistical study and consideration of endocrine influences. J Invest Dermatol. 1950;14(6):403–413. doi:10.1038/jid.1950.52
6. Vélez-Muñiz RDC, Peralta-Pedrero ML, Jurado-Santa Cruz F, Morales-Sánchez MA. Psychological profile and quality of life of patients with alopecia areata. Skin Appendage Disord. 2019;5(5):293–298. doi:10.1159/000497166
7. Zheng C, Tosti A. Alopecia areata: new treatment options including janus kinase inhibitors. Dermatol Clin. 2021;39(3):407–415. doi:10.1016/j.det.2021.03.005
8. Juárez-Rendón KJ, Rivera Sánchez G, Reyes-López M, et al. Alopecia areata. Actualidad y perspectivas. Alopecia areata. Current situation and perspectives. Arch Argent Pediatr. 2017;115(6):e404–e411. doi:10.5546/aap.2017.eng.e404
9. Bertolini M, McElwee K, Gilhar A, Bulfone-Paus S, Paus R. Hair follicle immune privilege and its collapse in alopecia areata. Exp Dermatol. 2020;29(8):703–725. doi:10.1111/exd.14155
10. Paus R, Nickoloff BJ, Ito T. A ‘hairy’ privilege. Trends Immunol. 2005;26(1):32–40. doi:10.1016/j.it.2004.09.014
11. Ito T, Ito N, Bettermann A, Tokura Y, Takigawa M, Paus R. Collapse and restoration of MHC class-I-dependent immune privilege: exploiting the human hair follicle as a model. Am J Pathol. 2004;164(2):623–634. doi:10.1016/s0002-9440(10)63151-3
12. Paus R, Slominski A, Czarnetzki BM. Is alopecia areata an autoimmune-response against melanogenesis-related proteins, exposed by abnormal MHC class I expression in the anagen hair bulb? Yale J Biol Med. 1993;66(6):541–554.
13. Ohyama M. What is behind the ‘swarm of bees’ in alopecia areata. Br J Dermatol. 2018;179(5):1023–1024. doi:10.1111/bjd.17142
14. McElwee KJ, Boggess D, King LE, Sundberg JP. Experimental induction of alopecia areata-like hair loss in C3H/HeJ mice using full-thickness skin grafts. J Invest Dermatol. 1998;111(5):797–803. doi:10.1046/j.1523-1747.1998.00380.x
15. Shin JM, Choi DK, Sohn KC, et al. Induction of alopecia areata in C3H/HeJ mice using polyinosinic-polycytidylic acid (poly[I:C]) and interferon-gamma. Sci Rep. 2018;8(1):12518. doi:10.1038/s41598-018-30997-3
16. Aşkın Ö, Yücesoy SN, Coşkun E, Engin B, Serdaroğlu S. Evaluation of the level of serum Interleukins (IL-2, IL-4, IL-15 andIL-17) and its relationship with disease severity in patients with alopecia areata. An Bras Dermatol. 2021;96(5):551–557. doi:10.1016/j.abd.2021.03.006
17. Kang H, Wu WY, Yu M, Shapiro J, McElwee KJ. Increased expression of TLR7 and TLR9 in alopecia areata. Exp Dermatol. 2020;29(3):254–258. doi:10.1111/exd.14043
18. Song T, Pavel AB, Wen HC, et al. An integrated model of alopecia areata biomarkers highlights both T(H)1 and T(H)2 upregulation. J Allergy Clin Immunol. 2018;142(5):1631–1634.e13. doi:10.1016/j.jaci.2018.06.029
19. Gautam RK, Singh Y, Gupta A, Arora P, Khurana A, Chitkara A. The profile of cytokines (IL-2, IFN-γ, IL-4, IL-10, IL-17A, and IL-23) in active alopecia areata. J Cosmet Dermatol. 2020;19(1):234–240. doi:10.1111/jocd.12970
20. Petukhova L, Duvic M, Hordinsky M, et al. Genome-wide association study in alopecia areata implicates both innate and adaptive immunity. Nature. 2010;466(7302):113–117. doi:10.1038/nature09114
21. Moftah NH, El-Barbary RA, Rashed L, Said M. ULBP3: a marker for alopecia areata incognita. Arch Dermatol Res. 2016;308(6):415–421. doi:10.1007/s00403-016-1652-9
22. Leung MC, Sutton CW, Fenton DA, Tobin DJ. Trichohyalin is a potential major autoantigen in human alopecia areata. J Proteome Res. 2010;9(10):5153–5163. doi:10.1021/pr100422u
23. Capalbo A, Giordano D, Gagliostro N, et al. Alopecia areata in a COVID-19 patient: a case report. Dermatol Ther. 2021;34(2):e14685. doi:10.1111/dth.14685
24. Sgubbi P, Savoia F, Calderoni O, Longo R, Stinchi C, Tabanelli M. Alopecia areata in a patient with SARS-Cov-2 infection. Dermatol Ther. 2020;33(6):e14295. doi:10.1111/dth.14295
25. Kurtz M, Wenner K, Schofield C. Alopecia areata in a human immunodeficiency virus–seropositive young man. Infect Dis Clin Pract. 2014;22(1):57–59. doi:10.1097/IPC.0b013e318287c368
26. Richardson CT, Hayden MS, Gilmore ES, Poligone B. Evaluation of the relationship between alopecia areata and viral antigen exposure. Am J Clin Dermatol. 2018;19(1):119–126. doi:10.1007/s40257-017-0312-y
27. Offidani A, Amerio P, Bernardini ML, Feliciani C, Bossi G. Role of cytomegalovirus replication in alopecia areata pathogenesis. J Cutan Med Surg. 2000;4(2):63–65. doi:10.1177/120347540000400204
28. Rossi A, Magri F, Michelini S, et al. New onset of alopecia areata in a patient with SARS-CoV-2 infection: possible pathogenetic correlations? J Cosmet Dermatol. 2021;20(7):2004–2005. doi:10.1111/jocd.14080
29. Hollingsworth P, Paci K, Evans M, Miedema J, Morrell DS. Alopecia universalis after drug reaction with eosinophilia and systemic symptoms (Dress). Pediatr Dermatol. 2020;37(5):947–949. doi:10.1111/pde.14217
30. Jackow C, Puffer N, Hordinsky M, Nelson J, Tarrand J, Duvic M. Alopecia areata and cytomegalovirus infection in twins: genes versus environment? J Am Acad Dermatol. 1998;38(3):418–425. doi:10.1016/s0190-9622(98)70499-2
31. Shellow WV, Edwards JE, Koo JY. Profile of alopecia areata: a questionnaire analysis of patient and family. Int J Dermatol. 1992;31(3):186–189. doi:10.1111/j.1365-4362.1992.tb03932.x
32. Sundberg JP, Silva KA, Li R, Cox GA, King LE. Adult-onset Alopecia areata is a complex polygenic trait in the C3H/HeJ mouse model. J Invest Dermatol. 2004;123(2):294–297. doi:10.1111/j.0022-202X.2004.23222.x
33. McDonagh AJ, Tazi-Ahnini R. Epidemiology and genetics of alopecia areata. Clin Exp Dermatol. 2002;27(5):405–409. doi:10.1046/j.1365-2230.2002.01077.x
34. Betz RC, Petukhova L, Ripke S, et al. Genome-wide meta-analysis in alopecia areata resolves HLA associations and reveals two new susceptibility loci. Nat Commun. 2015;6:5966. doi:10.1038/ncomms6966
35. Rajabi F, Abdollahimajd F, Jabalameli N, Nassiri Kashani M, Firooz A. The immunogenetics of alopecia areata. Adv Exp Med Biol. 2022;1367:19–59. doi:10.1007/978-3-030-92616-8_2
36. Mingorance Gámez CG, Martínez Chamorro A, Moreno Casares AM, et al. Joint study of the associations of HLA-B and the transmembrane short tandem repeat polymorphism of MICA protein with alopecia areata shows independent associations of both with the disease. Clin Exp Dermatol. 2020;45(6):699–704. doi:10.1111/ced.14208
37. Fischer J, Degenhardt F, Hofmann A, et al. Genomewide analysis of copy number variants in alopecia areata in a Central European cohort reveals association with MCHR2. Exp Dermatol. 2017;26(6):536–541. doi:10.1111/exd.13123
38. Petukhova L, Patel AV, Rigo RK, et al. Integrative analysis of rare copy number variants and gene expression data in alopecia areata implicates an aetiological role for autophagy. Exp Dermatol. 2020;29(3):243–253. doi:10.1111/exd.13986
39. Blaumeiser B, van der Goot I, Fimmers R, et al. Familial aggregation of alopecia areata. J Am Acad Dermatol. 2006;54(4):627–632. doi:10.1016/j.jaad.2005.12.007
40. Hayashi A, Mikami Y, Miyamoto K, et al. Intestinal dysbiosis and biotin deprivation induce alopecia through overgrowth of Lactobacillus murinus in mice. Cell Rep. 2017;20(7):1513–1524. doi:10.1016/j.celrep.2017.07.057
41. Rebello D, Wang E, Yen E, Lio PA, Kelly CR. Hair growth in two alopecia patients after fecal microbiota transplant. ACG Case Rep J. 2017;4:e107. doi:10.14309/crj.2017.107
42. Skogberg G, Jackson S, Åstrand A. Mechanisms of tolerance and potential therapeutic interventions in Alopecia Areata. Pharmacol Ther. 2017;179:102–110. doi:10.1016/j.pharmthera.2017.05.008
43. Lu J, Zhang P, Hu R, et al. Gut microbiota characterization in Chinese patients with alopecia areata. J Dermatol Sci. 2021;102(2):109–115. doi:10.1016/j.jdermsci.2021.04.003
44. Moreno-Arrones OM, Serrano-Villar S, Perez-Brocal V, et al. Analysis of the gut microbiota in alopecia areata: identification of bacterial biomarkers. J Eur Acad Dermatol Venereol. 2020;34(2):400–405. doi:10.1111/jdv.15885
45. Rangu S, Lee JJ, Hu W, Bittinger K, Castelo-Soccio L. Understanding the gut microbiota in pediatric patients with alopecia areata and their siblings: a pilot study. JID Innov. 2021;1(4):100051. doi:10.1016/j.xjidi.2021.100051
46. Bibbò S, Abbondio M, Sau R, et al. Fecal microbiota signatures in celiac disease patients with poly-autoimmunity. Front Cell Infect Microbiol. 2020;10:349. doi:10.3389/fcimb.2020.00349
47. Won EJ, Jang HH, Park H, Kim SJ. A potential predictive role of the scalp microbiome profiling in patients with alopecia areata: Staphylococcus caprae, Corynebacterium, and Cutibacterium species. Microorganisms. 2022;10(5). doi:10.3390/microorganisms10050864
48. Konopiński MK. Shannon diversity index: a call to replace the original Shannon’s formula with unbiased estimator in the population genetics studies. PeerJ. 2020;8:e9391. doi:10.7717/peerj.9391
49. Rinaldi F, Pinto D, Borsani E, Castrezzati S, Amedei A, Rezzani R. The first evidence of bacterial foci in the hair part and dermal papilla of scalp hair follicles: a pilot comparative study in alopecia areata. Int J Mol Sci. 2022;23(19):11956. doi:10.3390/ijms231911956
50. Tosti A, Bellavista S, Iorizzo M. Alopecia areata: a long term follow-up study of 191 patients. J Am Acad Dermatol. 2006;55(3):438–441. doi:10.1016/j.jaad.2006.05.008
51. Gupta AK, Carviel JL, Foley KA, et al. Monotherapy for alopecia areata: a systematic review and network meta-analysis. Skin Appendage Disord. 2019;5(6):331–337. doi:10.1159/000501940
52. Goebel AS, Neubert RH, Wohlrab J. Dermal targeting of tacrolimus using colloidal carrier systems. Int J Pharm. 2011;404(1–2):159–168. doi:10.1016/j.ijpharm.2010.11.029
53. El-Ashmawy AA, El-Maadawy IH, El-Maghraby GM. Efficacy of topical latanoprost versus minoxidil and betamethasone valerate on the treatment of alopecia areata. J Dermatol Treat. 2018;29(1):55–64. doi:10.1080/09546634.2017.1330527
54. Roseborough I, Lee H, Chwalek J, Stamper RL, Price VH. Lack of efficacy of topical latanoprost and bimatoprost ophthalmic solutions in promoting eyelash growth in patients with alopecia areata. J Am Acad Dermatol. 2009;60(4):705–706. doi:10.1016/j.jaad.2008.08.029
55. Faghihi G, Andalib F, Asilian A. The efficacy of latanoprost in the treatment of alopecia areata of eyelashes and eyebrows. Eur J Dermatol. 2009;19(6):586–587. doi:10.1684/ejd.2009.0766
56. Ross EK, Bolduc C, Lui H, Shapiro J. Lack of efficacy of topical latanoprost in the treatment of eyebrow alopecia areata. J Am Acad Dermatol. 2005;53(6):1095–1096. doi:10.1016/j.jaad.2005.06.031
57. Coronel-Pérez IM, Rodríguez-Rey EM, Camacho-Martínez FM. Latanoprost in the treatment of eyelash alopecia in alopecia areata universalis. J Eur Acad Dermatol Venereol. 2010;24(4):481–485. doi:10.1111/j.1468-3083.2009.03543.x
58. Jun M, Lee WS. Therapeutic effect of superficial cryotherapy on alopecia areata: a prospective, split-scalp study in patients with multiple alopecia patches. Ann Dermatol. 2017;29(6):722–727. doi:10.5021/ad.2017.29.6.722
59. Yoo KH, Lee JW, Li K, Kim BJ, Kim MN. Photodynamic therapy with methyl 5-aminolevulinate acid might be ineffective in recalcitrant alopecia totalis regardless of using a microneedle roller to increase skin penetration. Dermatol Surg. 2010;36(5):618–622. doi:10.1111/j.1524-4725.2010.01515.x
60. Lee JW, Yoo KH, Kim BJ, Kim MN. Photodynamic therapy with methyl 5‐aminolevulinate acid combined with microneedle treatment in patients with extensive alopecia areata. Clin Exp Dermatol. 2010;35(5):548–549. doi:10.1111/j.1365-2230.2009.03695.x
61. Al-Mutairi N. 308-nm excimer laser for the treatment of alopecia areata. Dermatol Surg. 2007;33(12):1483–1487. doi:10.1111/j.1524-4725.2007.33320.x
62. Ohtsuki A, Hasegawa T, Komiyama E, Takagi A, Kawasaki J, Ikeda S. 308-nm excimer lamp for the treatment of alopecia areata: clinical trial on 16 cases. Indian J Dermatol. 2013;58(4):326. doi:10.4103/0019-5154.113954
63. Gupta AK, Carviel JL. Meta-analysis of 308-nm excimer laser therapy for alopecia areata. J Dermatol Treat. 2021;32(5):526–529. doi:10.1080/09546634.2019.1687819
64. Waiz M, Saleh AZ, Hayani R, Jubory SO. Use of the pulsed infrared diode laser (904 nm) in the treatment of alopecia areata. J Cosmet Laser Ther. 2006;8(1):27–30. doi:10.1080/14764170600607368
65. Chu H, Kim D-Y. Use of lasers in the treatment of alopecia areata. Med Lasers. 2016;5:71–76. doi:10.25289/ML.2016.5.2.71
66. Lee YB, Lee W-S. Efficacy of antihistamines in combination with topical corticosteroid and superficial cryotherapy for treatment of alopecia areata: a retrospective cohort study. J Am Acad Dermatol. 2021;84(4):1152–1154. doi:10.1016/j.jaad.2020.06.1026
67. Inui S, Nakajima T, Toda N, Itami S. Fexofenadine hydrochloride enhances the efficacy of contact immunotherapy for extensive alopecia areata: retrospective analysis of 121 cases. J Dermatol. 2009;36(6):323–327. doi:10.1111/j.1346-8138.2009.00647.x
68. Strazzulla LC, Wang EHC, Avila L, et al. Alopecia areata: an appraisal of new treatment approaches and overview of current therapies. J Am Acad Dermatol. 2018;78(1):15–24. doi:10.1016/j.jaad.2017.04.1142
69. Fiedler-Weiss VC. Topical minoxidil solution (1% and 5%) in the treatment of alopecia areata. J Am Acad Dermatol. 1987;16(3 Pt 2):745–748. doi:10.1016/s0190-9622(87)80003-8
70. Sharma AN, Michelle L, Juhasz M, Muller Ramos P, Atanaskova Mesinkovska N. Low-dose oral minoxidil as treatment for non-scarring alopecia: a systematic review. Int J Dermatol. 2020;59(8):1013–1019. doi:10.1111/ijd.14933
71. Chang KH, Rojhirunsakool S, Goldberg LJ. Treatment of severe alopecia areata with intralesional steroid injections. J Drugs Dermatol. 2009;8(10):909–912.
72. Tan E, Tay YK, Goh CL, Chin Giam Y. The pattern and profile of alopecia areata in Singapore--a study of 219 Asians. Int J Dermatol. 2002;41(11):748–753. doi:10.1046/j.1365-4362.2002.01357.x
73. Jarratt MT, Spark RF, Arndt KA. The effects of intradermal steroids on the pituitary-adrenal axis and the skin. J Invest Dermatol. 1974;62(4):463–466. doi:10.1111/1523-1747.ep12701707
74. Fredman R, Tenenhaus M. Cushing’s syndrome after intralesional triamcinolone acetonide: a systematic review of the literature and multinational survey. Burns. 2013;39(4):549–557. doi:10.1016/j.burns.2012.09.020
75. Chu TW, AlJasser M, Alharbi A, Abahussein O, McElwee K, Shapiro J. Benefit of different concentrations of intralesional triamcinolone acetonide in alopecia areata: an intrasubject pilot study. J Am Acad Dermatol. 2015;73(2):338–340. doi:10.1016/j.jaad.2015.04.049
76. Atanaskova Mesinkovska N. Emerging unconventional therapies for alopecia areata. J Investig Dermatol Symp Proc. 2018;19(1):S32–s33. doi:10.1016/j.jisp.2017.10.012
77. Firooz A, Tehranchi-Nia Z, Ahmed AR. Benefits and risks of intralesional corticosteroid injection in the treatment of dermatological diseases. Clin Exp Dermatol. 1995;20(5):363–370. doi:10.1111/j.1365-2230.1995.tb01351.x
78. Barton VR, Toussi A, Awasthi S, Kiuru M. Treatment of pediatric alopecia areata: a systematic review. J Am Acad Dermatol. 2022;86(6):1318–1334. doi:10.1016/j.jaad.2021.04.077
79. Mancuso G, Balducci A, Casadio C, et al. Efficacy of betamethasone valerate foam formulation in comparison with betamethasone dipropionate lotion in the treatment of mild-to-moderate alopecia areata: a multicenter, prospective, randomized, controlled, investigator-blinded trial. Int J Dermatol. 2003;42(7):572–575. doi:10.1046/j.1365-4362.2003.01862.x
80. Tosti A, Piraccini BM, Pazzaglia M, Vincenzi C. Clobetasol propionate 0.05% under occlusion in the treatment of alopecia totalis/universalis. J Am Acad Dermatol. 2003;49(1):96–98. doi:10.1067/mjd.2003.423
81. Happle R. Topical immunotherapy in alopecia areata. J Invest Dermatol. 1991;96(5):71s–72s. doi:10.1111/1523-1747.ep12471884
82. Lamb RC, Young D, Holmes S. Retrospective review of diphencyprone in the treatment of alopecia areata. Clin Exp Dermatol. 2016;41(4):352–358. doi:10.1111/ced.12776
83. Ohlmeier MC, Traupe H, Luger TA, Böhm M. Topical immunotherapy with diphenylcyclopropenone of patients with alopecia areata--a large retrospective study on 142 patients with a self-controlled design. J Eur Acad Dermatol Venereol. 2012;26(4):503–507. doi:10.1111/j.1468-3083.2011.04114.x
84. Vestey JP, Savin JA. A trial of 1% minoxidil used topically for severe alopecia areata. Acta Derm Venereol. 1986;66(2):179–180.
85. Price VH. Double-blind, placebo-controlled evaluation of topical minoxidil in extensive alopecia areata. J Am Acad Dermatol. 1987;16(3 Pt 2):730–736. doi:10.1016/s0190-9622(87)70095-4
86. Fiedler-Weiss VC, West DP, Buys CM, Rumsfield JA. Topical minoxidil dose-response effect in alopecia areata. Arch Dermatol. 1986;122(2):180–182. doi:10.1001/archderm.1986.01660140070020
87. Freire PCB, Riera R, Martimbianco ALC, Petri V, Atallah AN. Minoxidil for patchy alopecia areata: systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2019;33(9):1792–1799. doi:10.1111/jdv.15545
88. Vañó-Galván S, Pirmez R, Hermosa-Gelbard A, et al. Safety of low-dose oral minoxidil for hair loss: a multicenter study of 1404 patients. J Am Acad Dermatol. 2021;84(6):1644–1651. doi:10.1016/j.jaad.2021.02.054
89. Pirmez R, Salas-Callo CI. Very-low-dose oral minoxidil in male androgenetic alopecia: a study with quantitative trichoscopic documentation. J Am Acad Dermatol. 2020;82(1):e21–e22. doi:10.1016/j.jaad.2019.08.084
90. de Nicolas-Ruanes B, Moreno-Arrones OM, Saceda-Corralo D, et al. Low-dose oral minoxidil for treatment of androgenetic alopecia and telogen effluvium in a pediatric population: a descriptive study. J Am Acad Dermatol. 2022;87(3):700–702. doi:10.1016/j.jaad.2022.04.030
91. Fiedler-Weiss VC, Rumsfield J, Buys CM, West DP, Wendrow A. Evaluation of oral minoxidil in the treatment of alopecia areata. Arch Dermatol. 1987;123(11):1488–1490. doi:10.1001/archderm.1987.01660350088019
92. Messenger AG, Rundegren J. Minoxidil: mechanisms of action on hair growth. Br J Dermatol. 2004;150(2):186–194. doi:10.1111/j.1365-2133.2004.05785.x
93. Yin L, Svigos K, Gutierrez D, Peterson E, Lo Sicco K, Shapiro J. Low-dose oral minoxidil increases hair density and thickness in androgenetic alopecia: a retrospective analysis of 60 patients. J Eur Acad Dermatol Venereol. 2022;36(3):e200–e202. doi:10.1111/jdv.17731
94. Randolph M, Tosti A. Oral minoxidil treatment for hair loss: a review of efficacy and safety. J Am Acad Dermatol. 2021;84(3):737–746. doi:10.1016/j.jaad.2020.06.1009
95. King B, Ohyama M, Kwon O, et al. Two phase 3 trials of baricitinib for alopecia areata. N Engl J Med. 2022;386(18):1687–1699. doi:10.1056/NEJMoa2110343
96. Phan K, Sebaratnam DF. JAK inhibitors for alopecia areata: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2019;33(5):850–856. doi:10.1111/jdv.15489
97. Dincer D, Tanacan E, Kose Ozkan C. Efficacy of systemic minoxidil and tofacitinib combination in treatment-resistant alopecia universalis. J Cosmet Dermatol. 2021;20(6):1807–1809. doi:10.1111/jocd.13812
98. Wambier CG, Craiglow BG, King BA. Combination tofacitinib and oral minoxidil treatment for severe alopecia areata. J Am Acad Dermatol. 2021;85(3):743–745. doi:10.1016/j.jaad.2019.08.080
99. Fiedler VC, Buys CM. Immunohistochemical characterization of the cellular infiltrate in severe alopecia areata before and after minoxidil treatment. Dermatologica. 1987;175(Suppl 2):29–35. doi:10.1159/000248899
100. Kennedy Crispin M, Ko JM, Craiglow BG, et al. Safety and efficacy of the JAK inhibitor tofacitinib citrate in patients with alopecia areata. JCI Insight. 2016;1(15):e89776. doi:10.1172/jci.insight.89776
101. Mackay-Wiggan J, Jabbari A, Nguyen N, et al. Oral ruxolitinib induces hair regrowth in patients with moderate-to-severe alopecia areata. JCI Insight. 2016;1(15):e89790. doi:10.1172/jci.insight.89790
102. Yan D, Fan H, Chen M, et al. The efficacy and safety of JAK inhibitors for alopecia areata: a systematic review and meta-analysis of prospective studies. Front Pharmacol. 2022;13:950450. doi:10.3389/fphar.2022.950450
103. Serious heart events, cancer, blood clots for certain JAK inhibitors. 2021; Available from: https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death.
104. Wollenhaupt J, Lee EB, Curtis JR, et al. Safety and efficacy of tofacitinib for up to 9.5 years in the treatment of rheumatoid arthritis: final results of a global, open-label, long-term extension study. Arthritis Res Ther. 2019;21(1):89. doi:10.1186/s13075-019-1866-2
105. Winthrop KL, Citera G, Gold D, et al. Age-based (<65 vs ≥65 years) incidence of infections and serious infections with tofacitinib versus biological DMARDs in rheumatoid arthritis clinical trials and the US Corrona RA registry. Ann Rheum Dis. 2021;80(1):134–136. doi:10.1136/annrheumdis-2020-218992
106. Smolen JS, Genovese MC, Takeuchi T, et al. Safety profile of baricitinib in patients with active rheumatoid arthritis with over 2 years median time in treatment. J Rheumatol. 2019;46(1):7–18. doi:10.3899/jrheum.171361
107. Taylor PC, Keystone EC, van der Heijde D, et al. Baricitinib versus placebo or adalimumab in rheumatoid arthritis. N Engl J Med. 2017;376(7):652–662. doi:10.1056/NEJMoa1608345
108. King B, Maari C, Lain E, et al. Extended safety analysis of baricitinib 2 mg in adult patients with atopic dermatitis: an integrated analysis from eight randomized clinical trials. Am J Clin Dermatol. 2021;22(3):395–405. doi:10.1007/s40257-021-00602-x
109. Olumiant (Baricitinib) highlights of prescribing information. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/207924s006lbl.pdf.
110. Curtis J. Malignancies in patients aged ≥ 50 years with RA and ≥ 1 additional cardiovascular risk factor: results from a phase 3b/4 randomized safety study of tofacitinib vs TNF inhibitors. Arthritis Rheumatol. 1940;73(9):54.
111. Harigai M. Growing evidence of the safety of JAK inhibitors in patients with rheumatoid arthritis. Rheumatology. 2019;58(Supplement_1):i34–i42. doi:10.1093/rheumatology/key287
112. Montastruc F, Flumian C, Degboe Y, Constantin A, Ruyssen-Witrand A. OP0268 Comparison of major cardiovascular and thromboembolic events in safety reports between rheumatoid arthritis patients treated with JAK inhibitors versus anti-TNF: results from VigiBase. Ann Rheum Dis. 2022;81(Suppl1):178–179. doi:10.1136/annrheumdis-2022-eular.2686
113. Meissner Y, Albrecht K, Kekow J, et al. OP0135 Risk of cardiovascular events under janus kinase inhibitors in patients with rheumatoid arthritis: observational data from the German rabbit register. Ann Rheum Dis. 2022;81(Suppl1):86–87. doi:10.1136/annrheumdis-2022-eular.779
114. Taylor PC, Weinblatt ME, Burmester GR, et al. Cardiovascular safety during treatment with baricitinib in rheumatoid arthritis. Arthritis Rheumatol. 2019;71(7):1042–1055. doi:10.1002/art.40841
115. Salinas CA, Louder A, Polinski J, et al. Evaluation of VTE, MACE, and serious infections among patients with RA treated with baricitinib compared to TNFi: a multi-database study of patients in routine care using disease registries and claims databases. Rheumatol Ther. 2022;1–23. doi:10.1007/s40744-022-00505-1
116. Xie F, Yun H, Bernatsky S, Curtis JR. Brief report: risk of gastrointestinal perforation among rheumatoid arthritis patients receiving tofacitinib, tocilizumab, or other biologic treatments. Arthritis Rheumatol. 2016;68(11):2612–2617. doi:10.1002/art.39761
117. Fertig RM, Gamret AC, Cervantes J, Tosti A. Microneedling for the treatment of hair loss? J Eur Acad Dermatol Venereol. 2018;32(4):564–569. doi:10.1111/jdv.14722
118. Gowda BHJ, Ahmed MG, Sanjana A. Can microneedles replace hypodermic needles? Resonance. 2022;27(1):63–85. doi:10.1007/s12045-022-1294-5
119. Pei D, Chen L, Yao Y, Zeng L, Zhang G. Microneedling combined with compound betamethasone in treatment of severe alopecia areata: a case report. Front Immunol. 2022;13:939077. doi:10.3389/fimmu.2022.939077
120. Chandrashekar B, Yepuri V, Mysore V. Alopecia areata-successful outcome with microneedling and triamcinolone acetonide. J Cutan Aesthet Surg. 2014;7(1):63–64. doi:10.4103/0974-2077.129989
121. Deepak SH, Shwetha S. Scalp roller therapy in resistant alopecia areata. J Cutan Aesthet Surg. 2014;7(1):61–62. doi:10.4103/0974-2077.129988
122. Mostaghimi A, Napatalung L, Sikirica V, et al. Patient perspectives of the social, emotional and functional impact of alopecia areata: a systematic literature review. Dermatol Ther. 2021;11(3):867–883. doi:10.1007/s13555-021-00512-0
123. Lee S, Lee YB, Kim BJ, Bae S, Lee WS. All-cause and cause-specific mortality risks associated with alopecia areata: a Korean nationwide population-based study. JAMA Dermatol. 2019;155(8):922–928. doi:10.1001/jamadermatol.2019.0629
124. Sánchez-Díaz M, Díaz-Calvillo P, Soto-Moreno A, Molina-Leyva A, Arias-Santiago S. The impact of sleep quality on mood status and quality of life in patients with alopecia areata: a comparative study. Int J Environ Res Public Health. 2022;19(20). doi:10.3390/ijerph192013126
125. Park J, Kim DW, Park SK, Yun SK, Kim HU. Role of hair prostheses (Wigs) in patients with severe alopecia areata. Ann Dermatol. 2018;30(4):505–507. doi:10.5021/ad.2018.30.4.505
126. Li SJ, Mostaghimi A, Tkachenko E, Huang KP. Association of out-of-pocket health care costs and financial burden for patients with alopecia areata. JAMA Dermatol. 2019;155(4):493–494. doi:10.1001/jamadermatol.2018.5218
127. Thompson HJ, Vavra T, Jabbari A. Factors associated with insurance coverage of tofacitinib for alopecia areata: a retrospective review from an academic institution. J Am Acad Dermatol. 2020;83(5):1509–1510. doi:10.1016/j.jaad.2020.06.028
128. Klein B, Treudler R, Simon JC. JAK-inhibitors in dermatology - small molecules, big impact? Overview of the mechanism of action, previous study results and potential adverse effects. J Dtsch Dermatol Ges. 2022;20(1):19–24. doi:10.1111/ddg.14668
129. Maqsood MH, Weber BN, Haberman RH, Lo Sicco KI, Bangalore S, Garshick MS. Cardiovascular and venous thromboembolic risk with janus kinase inhibitors in immune-mediated inflammatory diseases: a systematic review and meta-analysis of randomized trials. ACR Open Rheumatol. 2022;4(10):912–922. doi:10.1002/acr2.11479
130. Inui S, Inoue T, Itami S. Psychosocial impact of wigs or hairpieces on perceived quality of life level in female patients with alopecia areata. J Dermatol. 2013;40(3):225–226. doi:10.1111/1346-8138.12040
131. Klein EJ, Shapiro J, Piraccini B, Cummins D, Krueger LD, Lo Sicco K. Giving a voice: dermatologists’ legislative advocacy for patients with hair loss. Br J Dermatol. 2022;187(4):582–583. doi:10.1111/bjd.21667
132. S.4708-117th Congress (2021-2022): A bill to amend title XVIII of the Social Security Act to provide coverage for wigs as durable medical equipment under the Medicare program, and for other purposes. 2021; Available from: https://www.congress.gov/bill/117th-congress/senate-bill/4708?r=7&s=1.
133. Kalabokes VD. Alopecia areata: support groups and meetings - how can it help your patient? Dermatol Ther. 2011;24(3):302–304. doi:10.1111/j.1529-8019.2011.01418.x
134. The National Alopecia Areata Foundation. 2023; Available from: https://www.naaf.org/.
135. Aschenbeck KA, McFarland SL, Hordinsky MK, Lindgren BR, Farah RS. Importance of group therapeutic support for family members of children with alopecia areata: a cross-sectional survey study. Pediatr Dermatol. 2017;34(4):427–432. doi:10.1111/pde.13176
136. Creadore A, Manjaly P, Li SJ, et al. Evaluation of stigma toward individuals with alopecia. JAMA Dermatol. 2021;157(4):392–398. doi:10.1001/jamadermatol.2020.5732
137. Schielein MC, Tizek L, Ziehfreund S, Sommer R, Biedermann T, Zink A. Stigmatization caused by hair loss - a systematic literature review. J Dtsch Dermatol Ges. 2020;18(12):1357–1368. doi:10.1111/ddg.14234
138. Yeshua-Katz D, Shvarts S, Segal-Engelchin D. Hierarchy of hair loss stigma: media portrayals of cancer, alopecia areata, and cancer in Israeli newspapers. Isr J Health Policy Res. 2019;8(1):68. doi:10.1186/s13584-019-0338-0
139. Alopecia Justice League. 2015; Available from: https://www.alopeciajusticeleague.com/.
140. Good skin knowledge youth education program. Available from: https://www.aad.org/public/public-health/good-skin-knowledge.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
