Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Aloe Extracts Inhibit Skin Inflammatory Responses by Regulating NF-κB, ERK, and JNK Signaling Pathways in an LPS-Induced RAW264.7 Macrophages Model
Authors Wang F, Liu J, An Q, Wang Y, Yang Y, Huo T , Yang S, Ju R, Quan Q
Received 29 September 2022
Accepted for publication 10 January 2023
Published 28 January 2023 Volume 2023:16 Pages 267—278
DOI https://doi.org/10.2147/CCID.S391741
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Fei Wang,1– 3,* Jitao Liu,1– 3 Quan An,1– 4,* Yiming Wang,1– 3 Yang Yang,1– 3 Tong Huo,1– 3 Simin Yang,5 Ruijun Ju,5 Qianghua Quan1– 4
1Research and Development Department, Yunnan Baiyao Group Health Products Co., Ltd., Kunming, People’s Republic of China; 2East Asia Skin Health Research Center, Beijing, People’s Republic of China; 3Research and Development Department, REAL DermaSci & Biotech Co., Ltd., Beijing, People’s Republic of China; 4Research and Development Department, Yunnan Baiyao Group Shanghai Science & Technology Co., Ltd., Shanghai, People’s Republic of China; 5Beijing Key Laboratory of Enze Biomass Fine Chemicals, Department of Pharmaceutical Engineering, Beijing Institute of Petrochemical Technology, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qianghua Quan, Yunnan Baiyao Group Health Products Co., Ltd, Kunming, People’s Republic of China, Email [email protected]
Introduction: Inflammation generally refers to the body’s defensive response to stimuli, and skin inflammation is still one of the major problems that affect human physical and mental health. While current pharmacological treatments are reported to have cytotoxicity and various side effects, herbal medicines with few side effects and low cytotoxicity are considered as alternative therapeutic approaches.
Methods: In order to investigate anti-inflammatory effects and mechanisms of ALOE, the potential cytotoxicity of A. vera extracts (ALOE) was determined in vitro at first. The production of the pro-inflammatory proteins (ie, IL-6, TNF-α) in lipopolysaccharides (LPS) and ultraviolet A (UVA)-stimulated HaCaT and RAW264.7 cells were then treated with ALOE to test its inhibitory effects using enzyme-linked immunosorbent assay (ELISA). To further explore the anti-inflammatory mechanisms of ALOE, quantitative Polymerase Chain Reaction (qPCR) was used to analyze the mRNA expression of inflammatory genes iNOS, COX-2 and NO production. For NF-κB and MAPK signaling pathways analysis, Western blotting and nuclear fluorescence staining were used to evaluate the expression of key factors.
Results: ALOE did not exhibit obvious cytotoxicity (0– 3 mg/mL) in vitro. ALOE was able to inhibit the expression of pro-inflammatory cytokines IL-6, TNF-α and functioned more prominently in LPS-induced model. ALOE could also suppress the mRNA expression of LPS-induced iNOS and COX-2 and further down-regulate NO level. Furthermore, ALOE reduced the protein expression of P65 in NF-κB signaling pathway and suppressed LPS-induced activation of ERK and JNK, instead of p38 MAPK pathway.
Conclusion: Taken together, these results demonstrated that ALOE is a potential treatment in suppressing LPS-stimulated inflammation reactions targeting NF-κB, JNK and ERK signaling pathways. The anti-inflammatory effects of ALOE indicated that it has the potential to become an effective cosmetic ingredient.
Keywords: Aloe vera extracts, anti-inflammation, LPS, iNOS, COX-2, NO, P65, MAPK, NF-κB
Introduction
Inflammation generally refers to the body’s defensive response to stimuli. Cutaneous inflammation is currently obstructing more than 20% of the population and is induced by several potent cytokines. Topical treatments such as corticosteroids are sometimes not feasible because of the side effects.1 The application of steroids also has the potential to cause disease rebounds in an even more serious situation. Therefore, developing more effective strategies to regulate inflammatory responses with fewer side effects and identifying new therapeutic targets are crucial for improving patient outcomes. Macrophages play a major role in inflammation response and the activation of multiple inflammatory pathways.2 Bacterial lipopolysaccharide (LPS) is a key potent macrophage activator.3 UV radiation, a major environmental hazard, can stimulate the secretion of ROS, induce DNA damage, and result in inflammation development.4–7 LPS and UV stimulation are two factors used to study the mechanism of inflammation widely. Once stimulated with LPS or UV, macrophage activities are initiated via secreting a series of pro-inflammatory cytokines, eg, interleukin-6 (IL-6)8 and tumor necrosis factor-α (TNF-α),9,10 inflammatory mediators, eg, nitric oxide (NO),3 and pro-inflammatory mediators, eg, inducible NO synthase (iNOS), cyclooxygenase-2(COX-2).11–13 The inducible form of nitric oxide synthases is induced in response to cytokines and LPS, and expressed in macrophages. Overexpressed inflammatory cytokines and mediators can recruit more immune cells to infected or injury sites, which links with many autoimmune disorders and inflammatory diseases.11,14,15
Typically, LPS can be recognized by toll-like receptor 4 (TLR4),16 a pattern recognition receptor on the cell membrane.17 TLR418 recruits MyD88 through intracellular toll interleukin 1 receptor domain-containing adaptor protein (TIRAP), and further activates downstream stress-sensitive pathways such as mitogen-activated protein kinase (MAPK) and nuclear transcription factor kappa B (NF-κB).19 The activation of cellular pathways, including MAPK and NF-κB pathways, is an important modulator in the pathophysiological mechanism of skin inflammation. However, the therapeutic options to treat skin inflammation are limited to immunosuppressive agents such as glucocorticosteroids,20 which have been reported to have various side effects.21
Due to the unstable safety of synthetic raw materials used in cosmetics or medicines today, consumers are increasingly favoring those rich in natural ingredients, such as plant extracts. Recently, natural plant extracts rich in polysaccharide are widely explored due to their antitumorigenic, antioxidant, antiviral activities. In the 21st century, the pharmacological effects of medicinal plants have been regarded as a promising future cosmetic or medicine for health-quality improvement. Aloe barbadensis Miller (commonly referred to as A. vera) is a valuable medical plant, as it shows wound healing,22 antimicrobial,23 anti-inflammatory,24 anti-oxidant,25 anti-tumor,26 and anti-viral properties.27 A. vera extracts contain complex active compounds, which are not necessarily the final cosmetic products, but rather can serve as sources of new structures. It is economically beneficial to study natural A. vera extracts currently, because the enhancement of bioactive natural A. vera extracts is a potential strategy in cosmetics development, but reports on A. vera extracts (the proportion of molecular weight ≥ 1000kD accounts for 75.83%) to treat skin inflammation are still inadequate, and its anti-inflammatory activities involved with molecular mechanisms still need to be demonstrated.
To address these limitations, we established LPS- and UVA- induced inflammatory models to test effect of ALOE to regulate relevant inflammatory cytokines (ie, IL-6, TNF-α) in HaCaT and RAW264.7 cells using enzyme-linked immunosorbent assay (ELISA), with cytotoxicity pre-evaluated. We then confirmed effect of ALOE on the production of NO, the expression of iNOS and COX-2 in LPS-stimulated RAW264.7 cells using Quantitative Polymerase Chain Reaction (qPCR). To investigate how ALOE is involved in cellular signaling pathways, we performed Western blotting and fluorescence staining to identify the expression levels of NF-κB P65, and further tested the expression of main factors in MAPK signaling pathway using the same methods. Based on these results, we verified that the proportion of macromolecular content in A. vera extracts has an impact on anti-inflammatory activity.
Materials and Methods
Preparation of ALOE Extracts
The fresh Aloe plant was planted in Yuanjiang County, Yuxi City, Yunnan Province, and purchased from Yunnan Evergreen Biological Corporation legally without any other access. The Aloe plant purchased is a cultivated species grown by Yunnan Evergreen Biological Corporation rather than a wild species. The plant was identified as Aloe barbadensis Miller (A. vera) by (YUKU) Herbarium of Yunnan University, which is affiliated with the School of Life Sciences, Yunnan University. The voucher specimens (both dried extracts and plants) are deposited at Yunnan Baiyao Group Health Products Co., Ltd. The dried Aloe extracts (ALOE) were prepared by firstly removing the outer skin from the fresh Aloe vera leaves to take the gel; the extract was then filtered and concentrated after reflux extraction for 1 h with a 1 round repeat by adding 2 times amount of pure water into the gel. The concentrate was then extracted by mass of 1:1 ethyl acetate, and the aqueous layer was collected after another concentration process to remove ethyl acetate. The aqueous layer was dried under reduced pressure and crushed to obtain Aloe extract.
Reagents
The Escherichia coli (O111:B4) LPS was obtained from Sigma-Aldrich (St. Louis, MO, USA). DMEM medium, L-glutamine, penicillin, streptomycin, and fetal bovine serum (FPS) were purchased from Gibco. All antibodies in Western blot were purchased from Abcam. All other chemicals otherwise indicated were from Servicebio.
Cell Culture
The murine RAW264.7 cell was purchased from American Type Culture Collection. RAW264.7 cells were then cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 1% penicillin/streptomycin and 10% FBS. Cells were incubated at 37°C with 5% CO2. For assays, the RAW264.7 macrophages were plated in a 6-well plate (2 × 106 cells) for 12 h, or in a 96-well plate (1.2 × 105 cells), or a 75 cm2 cell culture flask (2 × 106 cells) before being treated with LPS (final concentration: 10 μg/mL). After cell plate treatment, the cells were treated with different concentrations of ALOE (0.75–3.00 mg/mL) and dexamethasone (DEX, 0.05 mg/mL, positive control drug) for 24 h.
In addition, the inflammatory model based on UVA stimulation was established. RAW264.7 macrophages were plated in a 6-well plate (2 × 106 cells) for 12 h and phosphate buffered saline (PBS) was used to wash away the non-adherent cells; 1mL PBS per well for UV irradiation (UVA, 7 J/cm2) was then added; and finally the remaining cells were treated as follows: DMEM medium alone, LPS, the combination of LPS and ALOE, the combination of LPS and DEX (0.05 mg/mL).
Cell Viability by Sulforhodamine B Assay (SRB)
For SRB assay, cells were treated with increasing concentrations of ALOE (0.15–3.00 mg/mL) and 10 μg/mL of LPS for 24 h. 100 µL 0.4% SRB solution was added to each well, and incubated for 10 min. The unbound SRB was removed by washing with 150 µL 10 mM Tris base (pH = 10.5) five times. Then the OD (570 nm) of solution was measured with microplate reader.
Enzyme-Linked Immunosorbent Assay (ELISA)
After UVA and LPS stimulation treatment, cells were rinsed twice with PBS and lysed in Radio Immunoprecipitation Assay Lysis buffer with 1 mM phenylmethanesulfonyl fluoride. Supernatants of cell lysate were used for cytokine assays. The total protein concentration was measured by BCA protein assay kit according to the manufacturer’s protocol. Expressions of IL-6 and TNF-α were detected by ELISA, according to the manufacturer’s instructions. Absorbance measurements were performed at 450 nm for two cytokines. The background wavelength (OD) was used to deduct from the main OD (450 nm). All measurements were performed in triplicate.
Nitric Oxide (NO) Measurement
The NO production in the cell culture medium was estimated using Griess Reagent kit. The level of nitrite (NO2–) (a stable end-product of NO metabolism) was detected for the accumulation of NO. After incubation, 50 µL cell suspensions were taken from each well to new 96-well plates. Then 50 µL Griess Reagent I and Griess Reagent II were added, respectively. The absorbance was measured at 540 nm. The data from each concentration was measured using five technical replicates. Ibuprofen (1 mM) was used as positive control group.
RNA Isolation and Quantitative Polymerase Chain Reaction (qPCR)
Cells were seeded in 75 cm2 cell culture flask, treated with or without ALOE (3 mg/mL) while stimulating with LPS (10 μg/mL) for 24 h. Total RNA was extracted using Trizol reagent, according to the manufacturer’s instructions. The RNA samples were treated with DNase I to remove genomic DNA and then reverse transcribed using oligo-dT primers. The first-strand cDNA served as a template for the real-time PCR reaction. qPCR was performed using SYBR Green qPCR kit reagent, following the instructions of the manufacturer. The primers for detecting the expression of iNOS, COX-2 and GAPDH were designed using the Primer Premier 5.0 software program, as follows:
iNOS-Forward-5′-AGCAACTACTGCT GGTGGTG-3′,
iNOS-Reverse-5′-TCTTCAGAGTCTGCCCATTG-3′;
COX-2-Forward-5′-CTGGAACATGGACTCACTCAGTTTG −3′,
COX-2-Reverse-5′- AGGCCTTTGCCACTGCTTGT-3′;
GAPDH-Forward-5′-GGCCTTCCGTGTTCCTACC-3′,
GAPDH-Reverse-5′-TGCCTGCTTCACCACCTTC-3′.
GAPDH was used as an internal control. The relative quantity of target mRNA was determined using the comparative threshold (Ct) method by normalizing target mRNA Ct values to those for GAPDH (ΔCt).
Western Blotting
For Western blotting, proteins were separated by 10% sodium dodecyl sulfate gel electrophoresis and electro-transferred to polyvinylidene difluoride membranes. The membrane was blocked with 5% nonfat milk in Tris-buffered saline and Tween 20 buffer for 2 h. Membranes were probed with specific primary antibodies against p65, H3, p-JNK, JNK, p-ERK, ERK, p-p38, p38 and β-actin, respectively, (1:1000 diluted in blocking solution) at 4°C overnight, followed by incubation with appropriate HRP-conjugated secondary antibodies (1:5000 diluted in blocking solution) for 2 h; the intensities of the bands were quantified using a chemiluminescence detection system. Grayscale analysis of bands was performed using Image J.
P65 Nuclear Fluorescence Staining
A confocal microscopic analysis was performed to identify and evaluate the translocation of NF-κB/p65 in RAW264.7 cells. Cells were seeded on cover slides in the 6-well culture plate and incubated overnight. RAW264.7 cells were treated according to the design of experiment, then fixed with 4% paraformaldehyde for 15 min and permeabilized with 0.5% Triton X-100 for 15 min. Next, the fixed cells were incubated with 3% BSA for 30 min. Then, the preparation was incubated with a primary Ab (JAK1 antibody) at 4°C overnight. Afterwards, cells were washed three times with PBS and incubated with the secondary Ab (Cy3 Conjugated Goat Anti-Rabbit IgG) for 50 min. The primary (SELO) and secondary (Goat Anti-Rabbit IgG - H&L (Alexa Fluor® 488)) antibodies were incubated in the same procedure. Cells were then washed three times with PBS and counter-stained with Antifade Reagent with 4′, 6-diamidino-2-phenylindole (DAPI) for 10 min, protected from light. The cellular localization of NF-κB/p65 was detected by fluorescence microscope at the excitation and emission wavelengths of 495 and 517 nm for FITC (Green), 550 and 590 nm for Cy3 (Red), and 358 and 420 nm for DAPI (Blue) nuclear staining, respectively.
Statistical Analysis
All experimental data are presented as mean ± standard deviation (SD) and accompanied by the number of experiments. Statistically significant differences between two groups were determined by Student’s t-test. All analyses were performed using R (v 4.1.0).
Results
ALOE Exhibited No Cytotoxic Effects in a Range of Working Concentrations
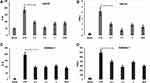
The cytotoxicity of either medicine or cosmetic agent is of chief importance when used. To evaluate whether ALOE has toxicity on human skin, the cell viability of ALOE at various concentrations on the LPS-stimulated HaCaT cells (Figure 1A) and RAW264.7 macrophages (Figure 1B) was assessed. These results showed that ALOE treatment did not exhibit obvious cytotoxicity between 0.30–3.00 mg/mL in both LPS-stimulated HaCaT and RAW264.7 cells, which was within acceptable limits and indicated that ALOE treatment had no significant side effects on HaCaT or RAW264.7 cell viability.
ALOE Suppressed Inflammatory Cytokine Expression Effectively in an LPS-Induced Inflammatory Model
To evaluate the effect of ALOE against inflammatory cytokines, both LPS and UVA stimulation were selected to determine the expression of IL-6 and TNF-α by ELISA in HaCaT cell model (Figure 2). Compared with the blank groups, IL-6 (Figure 2A, p < 0.001) and TNF-α (Figure 2B, p < 0.001) productions were significantly increased in LPS groups.
Similarly, the expressions of IL-6 (Supplementary Figure 1A, p < 0.001) and TNF-α (Supplementary Figure 1B, p < 0.001) were significantly upregulated in the UVA groups in comparison to the blank groups (Supplementary Figure 1A and B). These results indicated that LPS- and UVA-stimulated inflammatory models were both established successfully.
DEX, as a positive control agent, has a great effect on downregulating the expression levels of pro-inflammatory cytokines.28–30 Similar to DEX treated group, the expression levels of IL-6 and TNF-α were reduced significantly under ALOE treatments compared with the LPS or UVA groups. The levels of IL-6 (p < 0.001) and TNF-α (p < 0.01) in LPS groups with ALOE treatments (0.3 mg/mL, 1.5mg/mL, 3mg/mL) reduced nearly 3-fold (Figure 2B, p < 0.001) in comparison to the LPS-only groups, while they reduced nearly 2-fold in comparison to UVA group (Supplementary Figure 1A and B).
In the RAW264.7 cell model, LPS could stimulate the production of IL-6 (Figure 2C, p < 0.001) and TNF-α (Figure 2D, p < 0.001). Compared with LPS group, ALOE and DEX were able to significantly inhibit IL-6 and TNF-α production, and the difference was not statistically significant. Being consistent with the HaCaT cell model, the effect of ALOE on UVA-stimulated RAW264.7 model was also examined (Supplementary Figure 2). It showed that DEX could inhibit the increases of IL-6 (p < 0.01) and TNF-α (p<0.001) caused by UVA significantly, while ALOE had no obvious inhibitory effect on IL-6 and TNF-α caused by UVA (Supplementary Figure 2A and B).
Together, these results indicated that ALOE functions more prominently in inhibiting LPS-induced inflammation than UVA-induced inflammation.
ALOE Inhibited Pro-Inflammatory Mediators
As stated previously, LPS stimulation can produce NO and modulate the expression of inflammatory genes at the mRNA level, directly or indirectly. NO synthesis is greatly amplified by LPS, and NO level is closely correlated with inflammation.31 Therefore, LPS-stimulated NO production in RAW264.7 macrophages were determined in this study. Figure 3A showed that LPS stimulation induced a 5-fold increase in NO concentration compared to the control group, which indicated that LPS markedly stimulates the secretion of NO in RAW264.7 cells. On the other hand, treatment with ALOE lead to NO inhibition in LPS-stimulated RAW264.7 cells. Above all, ALOE is an effective inhibitor for NO release in this cell model.
The iNOS enzyme can continuously promote NO synthesis, and expression of iNOS is up-regulated in LPS-induced macrophages.32,33 COX-2 is a key enzyme during the conversion of arachidonic acid to prostaglandins; it also plays a critical role in the inflammatory process.34 Therefore, iNOS and COX-2 are barely detectable in control condition and highly inducible in acute inflammatory response. Based on that, we then employed qPCR analysis to compare the mRNA expression encoding iNOS and COX-2. The stimulation of LPS significantly increased the expression of iNOS (Figure 3B, p < 0.01) and COX-2 (Figure 3C, p < 0.001). ALOE treatment significantly inhibited the expression of LPS-induced iNOS (p < 0.01) and COX-2 (p < 0.05). To conclude, it was possible that ALOE might suppress the expression of iNOS and COX-2, thereby possessing beneficial effects against cell inflammations.
ALOE Suppressed NF-κB Signaling Pathway by Targeting p65
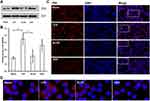
To investigate the anti-inflammation mechanism of ALOE, NF‑κB signaling pathway was explored further. NF‑κB plays pivotal role in controlling cellular responses to cytokines and stresses, and is known to be important for inflammatory inhibition.35,36 To further confirm the regulation of the NF‑κB signaling pathway by ALOE, the expression and translocation of P65 study were performed by Western blotting and P65 nuclear fluorescence staining, respectively. As shown in Figure 4A and B, LPS lead to an increase of P65 protein, while treatment with DEX and ALOE attenuated the expression of nuclear P65 protein levels that were pre-stimulated with LPS (Figure 4B). Figure 4C showed that P65 was present at the highest level in cell plasma of RAW264.7 in the blank group and redder fluorescent P65 protein expression in the LPS stimulation group and co-localization with blue fluorescent nuclei were found by cell staining, indicating that LPS activated P65 protein expression and entered the nucleus. From Figure 4D of enlarged nuclear translocation, following the ALOE treatment, the expression and nuclear translocation of P65 reduced, which was similar to DEX.
ALOE Inhibited MAPK Signaling Pathways by Targeting JNK and ERK
MAPK signaling pathways are known to function as transcriptional factors to regulate pro-inflammatory cytokines in activated macrophages and play critical roles in inflammatory diseases.37,38 Therefore, the effects of ALOE on MAPK signaling pathways were investigated in LPS‑induced model. As shown in Figure 5 and Supplementary Figure 3, following LPS treatments, the expression levels of p38 (Figure 5D) and phosphorylation levels of p38 were inhibited and p-p38/p-38 ratios were decreased. On the other hand, phosphorylation levels of JNK (Figure 5B) and ERK (Figure 5C) were increased; p-JNK/JNK (Supplementary Figure 3A) and p-ERK/ERK (Supplementary Figure 3B) ratios were also increased and decreased when treating ALOE, indicating that ALOE suppressed the LPS-stimulated activation of ERK (Figure 5C) and JNK (Figure 5B), instead of p38 (Figure 5D) MAPK pathway. Protein expression and activation were inhibited by ALOE treatments at different concentrations, and there was no significant difference in inhibition rate among different concentrations.
Discussion
A. vera has been reported to have biological effects including antimicrobial, anti-oxidant, anti-tumor and anti-viral properties, and isolated compounds of A. vera, such as aloe-emodin, aloin, aloesin, emodin, and acemannan, have been pointed out to relieve symptoms related to chronic inflammation.5 However, the anti-inflammatory mechanism of ALOE, a natural plant extract of A. vera, is still not clear. In this research, we confirmed that ALOE exhibits anti-inflammatory activities in vitro via inhibiting the production of pro-inflammatory cytokines (eg, IL-6 and TNF-α), mediators (eg, NO, iNOS and COX-2) and through downregulating NF-κB and MAPK signaling pathways.
Inflammation is inevitable during physiological responses to the injury or infection, and activated macrophages mainly participate in cellular inflammatory responses by releasing pro-inflammatory cytokines.39 Previous research investigated whether LPS and UV can both activate macrophages directly to start inflammatory processes5,11 through induction of iNOS, COX-2 and TNF-α expressions, and these processes are key events in the progression of skin inflammation. However, few investigations studied the differences between anti-inflammatory properties of ALOE in relation to both LPS and UV stimulations. In our study, we found that ALOE can attenuate LPS-induced inflammation through inhibiting pro-inflammatory cytokines IL-6 and TNF-α (Figure 2), which is consistent with previous research.40 TNF-α plays critical roles during inflammation induction that is activated by immune responses from macrophages and other cytokines. Similarly, IL-6 is a kind of pro-inflammatory cytokine that is regulated by an NF-κB signaling pathway and therefore affects immune responses. Treatments of ALOE in LPS-induced models lead to the down-regulation of IL-6 and TNF-α, a reminder of a potential anti-inflammatory property for ALOE. DEX has been found to reduce inflammatory response effectively in the past decade,28 and, surprisingly, we found that ALOE and DEX have similar anti-inflammatory effects in LPS-stimulated macrophages (Figure 2). With advantages shown before, another advantage of ALOE is that it has no cytotoxic effect (Figure 1).
NO is a free radical and acts as a pro-inflammatory mediator in many cell types, including macrophages.41 High levels of NO in macrophages could further result in inflammation and autoimmune disorders.42 Therefore, suppression of NO production may provide a potential strategy for the development of anti-inflammatory products. Many studies have already demonstrated that NO and PGE2 would be up-regulated massively by induction of the pro-inflammatory proteins iNOS and COX-2, respectively.43 In our study, the results showed that NO production (Figure 3A) at the site of inflammation was significantly inhibited by ALOE, and decreased NO level is accompanied by both iNOS (Figure 3B) and COX-2 (Figure 3C) mRNA expressions. Several studies have suggested an interaction between NO and COX-2, which may provide an explanation for why NO production is generated by COX-2.44 Moreover, iNOS can induce massive NO production in inflammatory sites, hence up-regulating COX-2 expression.45 By reasons of the foregoing, we confirmed that ALOE inhibits LPS-induced iNOS and COX-2 gene expressions, and further decreases NO production.
To probe the cellular mechanism of anti-inflammatory properties provided by ALOE, NF-κB signaling pathway is further investigated, because it is essential for modulating several pro-inflammatory molecules (eg, iNOS, IL-6 and TNF-α),46 and is composed of P65 and P50 subunit proteins. In resting cells, NF-κB presents as an inactive form, while in LPS-stimulated cells, activated IκB kinase complexes lead to the phosphorylation and degradation of IκB-α, which further activates NF-κB P65 nuclear translocation.47 In this study, our results showed that ALOE treatment reduced the expression of cellular P65 protein in LPS-induced RAW264.7 cells (Figure 4). These results indicated that ALOE could suppress the activation of NF-κB by inhibiting LPS-induced phosphorylation of P65 protein. Additionally, ALOE exhibited similar property in inhibiting the expression level of NF-κB P65 compared to DEX, which suggests that ALOE may be an alternative treatment with less cytotoxicity when treating LPS-induced inflammation. Taken together, it is demonstrated that ALOE suppressed LPS-induced NF-κB signaling pathway by down-regulating the expression levels of NF-κB P65 in RAW264.7 cells.
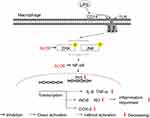
MAPKs, including JNK, ERK and p38, are known to play critical roles in controlling inflammation and immune responses through regulation of pro-inflammatory molecules (eg, iNOS, IL-6 and TNF-α) and NF-κB pathways.37,48 MAPKs family has also been shown to be crucial in LPS-stimulated up-regulation of iNOS and COX-2.49 In the present study, ALOE inhibited the LPS-induced ERK and JNK phosphorylation, instead of p38 phosphorylation in RAW264.7 cells (Figure 5), suggesting that ALOE could have inhibitory effects on ERK and JNK signaling pathways involved in the suppression of pro-inflammatory molecules. Based on our experiments in vitro, ALOE prompted effective anti-inflammatory activity in LPS-induced conditions by down-regulating inflammatory iNOS and COX-2 gene expressions, further decreasing the production of NO, and inhibiting the activation of NF-κB signaling pathway via interfering with JNK and ERK MARK instead of P38 (Figure 6). In the future, in vivo verifications might be performed to provide more confirmed results to guide the development of a new anti-inflammatory agent.
Conclusion
In summary, our study demonstrated that ALOE has an effective anti-inflammatory activity in LPS-stimulated RAW264.7 cells through inhibiting the production of pro-inflammatory molecules (eg, IL-6, TNF-α and NO) and the expression of inflammation-associated genes (eg, iNOS, COX-2), with no marked effects on viability of both HaCaT and RAW264.7 cells (0–3.00 mg/mL). Specifically, ALOE suppresses the activation of MAPK signaling pathways involved, JNK and ERK, and is able to inhibit NF-κB P65 expression, which in turn regulates the production of pro-inflammatory molecules. Taken together, these results provide a better understanding of the anti-inflammatory mechanism of Aloe vera extract in macrophages, in which lies the foundation for the development of novel therapeutic agents in skin inflammation treatment.
Permission to Collect Aloe Extracts
The Aloe plant is a cultivated species grown by Yunnan Evergreen Biological Corporation, rather than wild species, and was purchased from Yunnan Evergreen Biological Corporation legally through mutual communication and with any other access. The Aloe plant purchased is not a rare and endangered wild plant in China, so the acquisition of it is legal and compliant. The plant was identified as Aloe barbadensis Miller (A. vera) by Dr. Wang Huanchong, associate professor from Herbarium of Yunnan University (YUKU), which is affiliated with the School of Life Sciences, Yunnan University. The voucher specimens (both dried extracts and plant) are deposited at Yunnan Baiyao Group Health Products Co., Ltd. The voucher ID is Aloe vera (L.) Burm.f. 0102.
Study complied with relevant institutional, national, and international guidelines and legislation.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article and its Supplementary Information.
Ethics Approval and Consent to Participate
Ethics approval and consent to participate is not applicable in this study, as no clinical test was involved.
Consent for Publication
Not applicable to this section as no human data involved in the study.
Acknowledgments
The authors would like to acknowledge the Beijing Key Laboratory of Enze Biomass Fine Chemicals, Department of Pharmaceutical Engineering, Beijing Institute of Petrochemical Technology.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Yunnan Science and technology project (2018ZF005).
Disclosure
All authors except Simin Yang and Ruijun Ju are affiliated with Yunnan Baiyao Group Health Products Co., Ltd., Kunming, People’s Republic of China, East Asia Skin Health Research Center, Beijing, People’s Republic of China, and Research and Development Department, REAL DermaSci & Biotech Co., Ltd., Beijing, People’s Republic of China. Simin Yang and Ruijin Ju are affiliated with Beijing Key Laboratory of Enze Biomass Fine Chemicals, Department of Pharmaceutical Engineering, Beijing Institute of Petrochemical Technology, Beijing, People’s Republic of China. The authors report no other conflicts of interest in this work.
References
1. Tu H, Zhang D, Barksdale AN, et al. Dexamethasone improves wound healing by decreased inflammation and increased vasculogenesis in mouse skin frostbite model. Wilderness Environ Med. 2020;31(4):407–417. doi:10.1016/j.wem.2020.07.003
2. Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11(11):723–737. doi:10.1038/nri3073
3. Matsuno R, Aramaki Y, Arima H, et al. Contribution of CR3 to nitric oxide production from macrophages stimulated with high-dose of LPS. Biochem Biophys Res Commun. 1998;244(1):115–119. doi:10.1006/bbrc.1998.8231
4. Takasawa R, Nakamura H, Mori T, et al. Differential apoptotic pathways in human keratinocyte HaCaT cells exposed to UVB and UVC. Apoptosis. 2005;10(5):1121–1130. doi:10.1007/s10495-005-0901-8
5. Ao J, Yuan T, Gao L, et al. Organic UV filters exposure induces the production of inflammatory cytokines in human macrophages. Sci Total Environ. 2018;635(SEP.1):926–935. doi:10.1016/j.scitotenv.2018.04.217
6. Ryser S, Schuppli M, Gauthier B, et al. UVB-induced skin inflammation and cutaneous tissue injury is dependent on the MHC class I–like protein, CD1d. J Invest Dermatol. 2014;134(1):192–202. doi:10.1038/jid.2013.300
7. Ichihashi M, Ueda M, Budiyanto A, et al. UV-induced skin damage. Toxicology. 2003;189(1–2):21–39. doi:10.1016/S0300-483X(03)00150-1
8. Dhabhar FS. Acute stress enhances while chronic stress suppresses skin immunity. The role of stress hormones and leukocyte trafficking. Ann N Y Acad Sci. 2006;917:876–893. doi:10.1111/j.1749-6632.2000.tb05454.x
9. Padgett D, Glaser R. How stress influences the immune response. Trends Immunol. 2003;24(8):444–448. doi:10.1016/S1471-4906(03)00173-X
10. Yarilina A, Park-Min K-H, Antoniv T, et al. TNF activates an IRF1-dependent autocrine loop leading to sustained expression of chemokines and STAT1-dependent type I interferon–response genes. Nat Immunol. 2008;9(4):378–387. doi:10.1038/ni1576
11. Bruscia EM, Bonfield TL. Cystic fibrosis lung immunity: the role of the macrophage. J Innate Immun. 2016;8(6):550–563. doi:10.1159/000446825
12. De Cano JL, Mattson PC, Aikawa M. Macrophages in vascular inflammation: origins and functions. Curr Atheroscler Rep. 2016;18(6):34. doi:10.1007/s11883-016-0585-2
13. Han BH, Lee YJ, Yoon JJ, et al. Hwangryunhaedoktang exerts anti-inflammation on LPS-induced NO production by suppressing MAPK and NF-κB activation in RAW264.7 macrophages. J Integr Med. 2017;15(4):326–336. doi:10.1016/S2095-4964(17)60350-9
14. Bon De Son J. The mechanisms of action of disease-modifying antirheumatic drugs: a review with emphasis on macrophage signal transduction and the induction of proinflammatory cytokines. Gen Pharmacol. 1997;29(2):127–150. doi:10.1016/s0306-3623(96)00419-3
15. Ahmad N, Chen LC, Gordon MA, et al. Regulation of cyclooxygenase-2 by nitric oxide in activated hepatic macrophages during acute endotoxemia. J Leukoc Biol. 2002;71(6):1005–1011. doi:10.1189/jlb.71.6.1005
16. Kim SJ, Kim HM. Dynamic lipopolysaccharide transfer cascade to TLR4/MD2 complex via LBP and CD14. BMB Rep. 2017;50(2):55. doi:10.5483/BMBRep.2017.50.2.011
17. Fukata M, Hernandez Y, Conduah D, et al. Innate immune signaling by toll-like receptor-4 (TLR4) shapes the inflammatory microenvironment in colitis-associated tumors. Inflamm Bowel Dis. 2009;15(7):997–1006. doi:10.1002/ibd.20880
18. Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4(7):499–511.
19. Kang JY, Lee J-O. Structural biology of the toll-like receptor family. Annu Rev Biochem. 2011;80(1):917–941. doi:10.1146/annurev-biochem-052909-141507
20. Thomsen SF. Atopic dermatitis: natural history, diagnosis, and treatment. Int Sch Res Notices. 2014;2014:1.
21. Siegfried EC, Jaworski JC, Kaiser JD, et al. Systematic review of published trials: long-term safety of topical corticosteroids and topical calcineurin inhibitors in pediatric patients with atopic dermatitis. BMC Pediatr. 2016;16(1):75. doi:10.1186/s12887-016-0607-9
22. Wahedi HM, Jeong M, Chae JK, et al. Aloesin from Aloe vera accelerates skin wound healing by modulating MAPK/Rho and Smad signaling pathways in vitro and in vivo. Phytomedicine. 2017;28:19. doi:10.1016/j.phymed.2017.02.005
23. Bałan BJ, Niemcewicz M, Kocik J, Jung L, Skopińska-Różewska E, Skopiński P. Oral administration of Aloe vera gel, anti-microbial and anti-inflammatory herbal remedy, stimulates cell-mediated immunity and antibody production in a mouse model. Cent Eur J Immunol. 2014;39(2):125.
24. Yu CS, Yu FS, Chan JK, et al. Aloe-emodin affects the levels of cytokines and functions of leukocytes from Sprague-Dawley rats. In Vivo. 2006;20(4):505–509.
25. Liu F-W, Liu F-C, Wang Y-R, et al. Aloin protects skin fibroblasts from heat stress-induced oxidative stress damage by regulating the oxidative defense system. PLoS One. 2015;10(12):e0143528. doi:10.1371/journal.pone.0143528
26. Esmat AY, Said MM, Khalil SA. Aloin: a natural antitumor anthraquinone glycoside with iron chelating and non-atherogenic activities. Pharm Biol. 2015;53(1):138. doi:10.3109/13880209.2014.912239
27. Li SW, Yang TC, Lai CC. Antiviral activity of aloe-emodin against influenza A virus via galectin-3 up-regulation. Eur J Pharmacol. 2014;738(1):125–132. doi:10.1016/j.ejphar.2014.05.028
28. Fujimoto M, Higuchi H, Honda-Wakasugi Y, et al. Dexmedetomidine inhibits LPS-induced inflammatory responses through peroxisome proliferator-activated receptor gamma (PPARγ) activation following binding to α2 adrenoceptors. Eur J Pharmacol. 2021;892:173733. doi:10.1016/j.ejphar.2020.173733
29. Wang Y, Wang X, Yang Y, et al. Comparison of the in vitro anti-inflammatory effect of cannabidiol to dexamethasone. Clin Cosmet Investig Dermatol. 2022;15:1959–1967. doi:10.2147/CCID.S378798
30. Narumi S, Hamilton TA. Dexamethasone selectively regulates LPS-inducible gene expression in murine peritoneal macrophages. Immunopharmacology. 1990;19(2):93–101. doi:10.1016/0162-3109(90)90044-F
31. Kuo PC, Schroeder RA. The emerging multifaceted roles of nitric oxide. Ann Surg. 1995;221(3):220–235. doi:10.1097/00000658-199503000-00003
32. Luo X, Zhang H, Wei X, et al. Aloin suppresses lipopolysaccharide-induced inflammatory response and apoptosis by inhibiting the activation of NF-κB. Molecules. 2018;23(3):517. doi:10.3390/molecules23030517
33. Vane JR, Mitchell JA, Appleton I, et al. Inducible isoforms of cyclooxygenase and nitric-oxide synthase in inflammation. Proc Natl Acad Sci U S A. 1994;91(6):2046–2050. doi:10.1073/pnas.91.6.2046
34. Cui J, Jia J. Natural COX-2 inhibitors as promising anti-inflammatory agents: an update. Curr Med Chem. 2021;28(18):3622–3646.
35. Shu FL, Malik AB. NF-κB activation as a pathological mechanism of septic shock and inflammation. Am J Physiol Lung Cell Mol Physiol. 2006;290(4):L622. doi:10.1152/ajplung.00477.2005
36. Pahl HL. Activators and target genes of NF-kappaB transcription factors. Oncogene. 1999;18(49):6853–6866. doi:10.1038/sj.onc.1203239
37. Huang GJ, Huang SS, Deng JS. Anti-inflammatory activities of inotilone from phellinus linteus through the inhibition of MMP-9, NF-κB, and MAPK activation in vitro and in vivo. PLoS One. 2012;7(5):e35922.
38. Chen Y, Ji N, Pan S, et al. Roburic acid suppresses NO and IL-6 production via targeting NF-κB and MAPK pathway in RAW264.7 cells. Inflammation. 2017;40(6):1959–1966. doi:10.1007/s10753-017-0636-z
39. Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454(7203):428–435. doi:10.1038/nature07201
40. Li C-Y, Suzuki K, Hung Y-L, et al. Aloe metabolites prevent LPS-induced sepsis and inflammatory response by inhibiting mitogen-activated protein kinase activation. Am J Chin Med. 2017;45(4):1–15. doi:10.1142/S0192415X17500458
41. Zhang C, Li C, Jia X, et al. In vitro and in vivo anti-inflammatory effects of polyphyllin VII through downregulating MAPK and NF-κB pathways. Molecules. 2019;24(5):875.
42. Rui HL, Hotchkiss JH. Potential genotoxicity of chronically elevated nitric oxide: a review. Mutat Res. 1995;339(2):73–89. doi:10.1016/0165-1110(95)90004-7
43. Omote K, Hazama K, Kawamata T, et al. Peripheral nitric oxide in carrageenan-induced inflammation. Brain Res. 2001;912(2):171–175.
44. Salvemini D, Misko TP, Masferrer JL, et al. Nitric oxide activates cyclooxygenase enzymes. Proc Natl Acad Sci U S A. 1993;90(15):7240–7244.
45. Toriyabe M, Omote K, Kawamata T, et al. Contribution of interaction between nitric oxide and cyclooxygenases to the production of prostaglandins in carrageenan-induced inflammation. Anesthesiology. 2004;101(4):983–990. doi:10.1097/00000542-200410000-00025
46. Napetschnig J, Wu H. Molecular basis of NF-κB signaling. Annu Rev Biophys. 2013;42(1):443–468. doi:10.1146/annurev-biophys-083012-130338
47. Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-??B activity. Annu Rev Immunol. 2000;18(1):621–663. doi:10.1146/annurev.immunol.18.1.621
48. Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298(5600):1911–1912.
49. Chen C, Chen YH, Lin WW. Involvement of p38 mitogen-activated protein kinase in lipopolysaccharide-induced iNOS and COX-2 expression in J774 macrophages. Immunology. 1999;97(1):124–129. doi:10.1046/j.1365-2567.1999.00747.x
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.