Back to Journals » Clinical Interventions in Aging » Volume 18
Age-Related Effect of Uric Acid on Contrast-Induced Acute Kidney Injury of Patients Undergoing Coronary Angiography
Authors Lu J, He Y, Yang Y, Zhong X, Chen S, Wu B, Pan Y, Wang Y, Xiu J, Kang Y, Liu J, Liu Y, Chen S, Chen K, Chen L
Received 29 April 2023
Accepted for publication 8 November 2023
Published 7 December 2023 Volume 2023:18 Pages 2053—2061
DOI https://doi.org/10.2147/CIA.S419370
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Maddalena Illario
Jin Lu,1,* Yibo He,2,3,* Yanfang Yang,1,* Xuejing Zhong,1 Shaowen Chen,1 Bo Wu,1 Yuxiong Pan,1 Yizhang Wang,1 Jiaming Xiu,1 Yu Kang,3,4 Jin Liu,2,3 Yong Liu,2,3 Shiqun Chen,5 Kaihong Chen,1 Liling Chen1
1Department of Cardiology, Longyan First Affiliated Hospital of Fujian Medical University, Longyan, People’s Republic of China; 2Department of Cardiology, Guangdong Cardiovascular Institute, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, 510080, People’s Republic of China; 3Department of Cardiology, Guangdong Provincial Key Laboratory of Coronary Heart Disease Prevention, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Southern Medical University, Guangzhou, 510080, People’s Republic of China; 4Department of Cardiology, Shantou University Medical College, Shantou, 515041, People’s Republic of China; 5Global Health Research Center, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Science, Southern Medical University, Guangzhou, 510100, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Liling Chen; Shiqun Chen, Tel +86 5973082105 ; +86 02083827812-10528, Fax +86 5972100500 ; +86 2083851483, Email [email protected]; [email protected]
Background: The association between uric acid (UA) and contrast-induced acute kidney injury (CI-AKI) following coronary angiography (CAG) has been established. However, whether the association would vary with age remained undetermined.
Methods: We performed the retrospective analysis based on the Cardio-renal Improvement II study, (ClinicalTrials.gov NCT05050877), which enrolled consecutive patients undergoing coronary angiography in 5 teaching hospitals in China from 2007 to 2020. The primary outcome was CI-AKI defined as the rise of serum creatinine (SCr) ≥ 0.5 mg/dL or 25% compared with the baseline value within 48 hours following CAG. The effect of age on the association between uric acid and CI-AKI was assessed by the logistic regression model.
Results: A total of 36,550 patients (mean age 63.08± 5.6-year-old, 41.7% men) were included in the study. After adjusting for the confounders, the risk of CI-AKI between each quartile of uric acid was insignificant in the young group. In patients of the middle group, lower UA was associated with a lower risk of CI-AKI while higher UA was associated with a higher risk (Q1 OR: 0.853, 95% CI: 0.734– 0.993; Q4 OR: 1.797, 95% CI: 1.547– 2.09). In patients of the elder group, lower and higher UA were both associated with a higher risk of CI-AKI (Q1 OR: 1.247, 95% CI: 1.003– 1.553; Q4 OR: 1.688, 95% CI: 1.344– 2.124). The restricted cubic spline indicated a non-linear association between UA and CI-AKI in middle and elder age groups but a linear association in the young age group.
Conclusion: The association between uric acid and CI-AKI vary in patients of different age. Patients with elder age should maintain a middle level of uric acid while patients with middle age should consider a lower level of uric acid to reduce the risk of CI-AKI. The level of UA was an insignificant risk factor for CI-AKI in young patients.
Keywords: uric acid, coronary angiography, CI-AKI, age-related
Introduction
Contrast-induced acute kidney injury (CI-AKI) is one of the most common complications of coronary angiography (CAG), which is usually associated with adverse clinical outcomes.1,2 There is no generally proven measure to treat CI-AKI effectively and the best strategy for the guideline recommendation is prophylaxis for prevention.3 Therefore, the identification of high-risk patients with specific characteristics is essential for the purposes of preventive treatment before the procedure.
A broad spectrum of risk factors of CI-AKI has been reported previously, including impaired renal function, hypotension, congestive heart failure, age, anemia, diabetes, contrast medium volume and so on.4 As an increasing number of populations, especially young people, are undergoing CAG procedures, whether these risk factors affect the development of CI-AKI in patients irrespective of age has seldom been investigated. Serum uric acid (SUA), a degradation metabolite of purines, was also demonstrated to be associated with the development of CI-AKI as well.5,6 However, the level of uric acid is an age-related factor that their filtration and affection on kidney function may vary in patients of different ages.7 Limited research has yet been conducted to examine the potential impact of age on the relationship between uric acid level and CI-AKI.
In this study, we aim to evaluate the association between uric acid level and the risk of CI-AKI at different ages in patients undergoing coronary angiography, to further improve the risk assessment before the procedure and minimize the occurrence of complications.
Method
Study Population
145,256 consecutive patients undergoing CAG were included in the study based on the Cardiorenal Improvement II (CIN-II) study, which was a multi-center cohort study with patients enrolled from 5 teaching hospitals (Cardiorenal Improvement II, ClinicalTrials.gov NCT05050877). The exclusion criteria were as follows: the following: patients without laboratory results of serum uric acid, preoperative and postoperative serum creatinine (SCr) (n=107,282); patients with an estimated Glomerular Filtration Rate (eGFR) < 15 mL/min/1.73 m2 or requiring dialysis (n=1,424). Eventually, 36,550 patients were included (Figure 1). All traceable personal identifiers were removed from the analytic dataset to protect patients’ privacy. The study protocol and informed consent exemption were approved by the ethics committees of Guangdong Provincial People’s Hospital and the participant centers. The study was performed according to the declaration of Helsinki.
 |
Figure 1 Patient flow diagram. |
Data Collection
Data were extracted from the electronic clinical management system (ECMS) of each participant hospital. We had access to all primary and secondary care records. The baseline information included demographic characteristics, coexisting conditions, laboratory examinations and medications. Uric acid was measured at admission. When numerous results of preoperative and postoperative results of SCr existed, the first examination at admission and the highest value within 48h following coronary catheterization were adopted.
Endpoint and Clinical Definition
The primary endpoint was CI-AKI, which was defined as a rise in SCr of ≥0.5 mg/dL or a 25% increase from the baseline value within 48 hours after contrast media administration in the procedure of coronary catheterization.8 The level of UA was quartered into four groups in each age group, respectively (young age group: <50 years, middle age group: 50–70 years, old age group: >70 years). The eGFR was calculated by the Modification of Diet in Renal Disease (MDRD) formula.9 Chronic kidney disease (CKD) was defined according to the kidney disease: Improving Global Outcomes Organization (KDIGO) clinical practice guideline, in which eGFR < 60 mL/min/1.73 m2 was determined as CKD.10 Anemia was defined as a hematocrit ≤39% (male) or ≤36% (female) according to the World Health Organization criteria. Diabetes mellitus (DM) was defined using ICD-10 codes or hypoglycemic medication use. Acute myocardial infarction (AMI), hypertension, coronary artery disease (CAD), atrial fibrillation (AF) and stroke were defined using ICD-10 codes.
Statistical Analysis
The distribution of each variable was evaluated by the Kolmogorov–Smirnov test. Normally distributed variables are reported as mean ± SDs, whereas variables with skewed distribution are reported as median (interquartile interval). Categorical data are reported as frequencies and percentages. Differences of groups in baseline characteristics were compared through the use of chi-square tests for categorical variables and Student’s t-test and One-way ANOVA for continuous variables. Multinomial multiple logistic regression analyses were performed to examine between the UA and CI-AKI adjusted for age, gender,intra-aortic balloon pump (IABP), congestive heart failure (CHF), anemia, DM, pre-SCr, contrast medium volume, AMI and PCI, which were based on the classic Mehran CIN risk score4 and relevant literature.11,12 Furthermore, restricted cubic spline (RCS) was used to investigate the linear tendency of the association between UA value and the risk of CI-AKI. In this method, we selected four SUA values as knots based on UA percentiles, tested the linear and non-linear associations between knots using a cubic function, and presented the smoothly integrated graph. Presented tests were 2-tailed for all, and a p-value<0.05 was considered statistically significant. All statistical analyses were performed using R (ver. 4.0.3).
Result
Baseline Characteristics
The mean age of the study population was 63.08 (±5.6) years, and 71.3% were men. The proportion of male patients was higher in the younger age group, who were more likely to have higher low-density lipoprotein cholesterol (LDL-C) levels. Elderly patients were characterized by a higher level of SCr, more likely to be complicated with DM, CKD, hypertension, CHF and CAD. Both the young and the old age group had higher levels of UA. Detailed data between patients in different age groups is shown in Table 1.
 |
Table 1 Baseline Characteristics of the Patients Undergoing Coronary Angiography in Different Age Groups |
Compared with patients with contrast-associated acute kidney injury (CA-AKI), patients in the CA-AKI group were more likely to be female. Patients with CA-AKI had a higher proportion of CHF, anemia, and higher levels of UA and IABP usage. In contrast, the pre-operative SCr of patients with CA-AKI seemed to be lower than those without CA-AKI. Detailed data between patients in CA-AKI and non-CA-AKI groups is shown in Table 2.
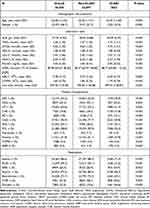 |
Table 2 Baseline Characteristics of the Patients Undergoing Coronary Angiography with or without CI-AKI |
Association of Uric Acid and CI-AKI in Different Age Groups
Multivariate logistic regression showed that in patients of the young-age group, both higher and lower uric acid levels did not correlate with a higher risk of CI-AKI compared with the quartile 2 level of uric acid (OR for Q1: 0.927, 95% CI: 0.648–1.329; Q3: 1.249, 95% CI: 0.875–1.787; Q4: 1.243, 95% CI: 0.841–1.833). In patients in the middle-age group, the lower level of uric acid was associated with a lower risk of CI-AKI while the higher level of uric acid was associated with a higher risk of CI-AKI (OR for Q1: 0.853, 95% CI: 0.935–1.273; Q3: 1.091, 95% CI: 0.935–1.273; Q4: 1.797.95% CI: 1.547–2.09). However, in patients of the old-age group, both higher or lower levels of uric acid were associated with high risks of CI-AKI (OR for Q1: 1.247, 95% CI: 1.003–1.553; Q3: 1.34, 95% CI: 1.074–1.675; Q4: 1.688, 95% CI: 1.00–2.88) (Figure 2).
To further investigate the association between the continuous change of uric acid and risk of CI-AKI, restrict cubic spline showed that the risk of CI-AKI linearly increased as the uric acid level increased but without significance for the most part in the young-age group (P for non-linear=0.483). The risk of CI-AKI remained lower at the lower level of uric acid but sharply increased as the uric acid increased at the higher level in the middle-age group (P for non-linear=0.002). The RCS of the old-age group was a “U” shape which demonstrated both higher or lower levels of uric acid significantly increased the risk of CI-AKI (P for non-linear<0.001) (Figure 3).
 |
Figure 3 Uric acid associated risk of CI-AKI in different age groups. |
Discussion
In this study, our results showed that the association between uric acid level and CI-AKI varied with age. Uric acid did not significantly correlate with the risk of CI-AKI in younger patients. Both higher and lower uric acid levels increased the risk of CI-AKI in elderly patients. For middle-aged patients, only the high level of uric acid was correlated with a higher risk of CI-AKI, while the lower level of uric acid was associated with a lower risk of CI-AKI.
Previous studies have also revealed that increased uric acid levels played a role in the progression of CI-AKI. Wei Guo et al found higher level of uric acid is an independent predictor associated with a risk of CI-AKI in patients undergoing PCI.13 Murat Saritemur et al demonstrated uric acid level is a simple independent early predictor of CI-AKI in patients who underwent primary PCI.14 Although uric acid and renal function affect each other, clinical studies showed that hyperuricemia was significantly associated with the risk of contrast-induced acute kidney injury after adjusting preoperative renal function levels in patients undergoing percutaneous coronary interventions.14,15 Therefore, our study adjusted preoperative creatinine to reduce this aspect of the factor. The previous studies mainly focused on the impact of uric acid on the CI-AKI without considering the differences by age. While Maurice C. Gephardt found uric acid values as related to age.7 Eghlim Nemati et al showed that older patients have relatively higher levels of uric acid than younger.16 Herein, our study has shown that the effect of uric acid on CI-AKI may be age-related. A middle level of uric acid in old-aged patients and a lower level of uric acid in the middle age patients might be more beneficial for reducing the risk of CI-AKI following coronary catheterization.
Previous studies have also shown that excess uric acid is associated with the susceptibility of renal impairment on exposure to contrast medium,17,18 The underlying mechanism of this relationship may be attributed to, the increased oxidative stress and crystals-related inflammation of high-level uric acid, leading to tubular injury, endothelial dysfunction and caused kidney injury following CAG.19–22 However, young patients have been reported as not susceptible to oxidative stress induced than the elderly,23 which may explain the insignificant association between uric acid and CI-AKI. Similarly, Victoria Zigmont has also reported that serum Vitamin D and glioma risk was weak positive associated with younger adults due to the risk alleles in telomerase-related genes being more common among older patients.24 And Victoria Zigmont et al reported that central obesity was identified as an independent factor of renal function impairment for all groups except males under 45 years of age.25 On the other hand, studies have reported that low uric acid levels may reflect inadequate protection against oxidant-mediated stress in elder patients;26–28 Moreover, low uric acid levels in elder patients may reflect malnutrition,27,28 which may lead to immunodeficiency and impaired renal function susceptibility. These may be the potential mechanisms leading to an increased risk of CI-AKI in elderly people with low levels of uric acid in our study. Nonetheless, further studies are needed to illustrate how age differences would affect the association of risk factors, such as uric acid, on CI-AKI.
At what target level of uric acid should be controlled to reduce kidney dysfunction in patients undergoing CAG remained inconsistent. Hyperuricemia, defined as a serum uric acid level of >7 mg/dL (417 mol/L) in males and >6 mg/dL (357 mol/L) in females, was related to the risk of CI-AKI in patients with normal renal function, according to Yong Liu et al.14 Sunil Pillai proposed that those with serum uric acid levels more than 4.05 mg/dL were more likely to develop postoperative AKI.29 According to the result presented in our study, the influence of uric acid on CI-AKI should take age into account, and it is not feasible to establish a universal standard for patients at any age. Given the disparities in renal function and the differences in the ability of uric acid and contrast agent excretion between age groups,30 we suggested that the uric acid management strategies for patients in different age groups should not be identical. In general, a stricter strategy may be considered to control uric acid for middle-aged patients and a modest strategy may be better for old patients to keep uric acid at a median level. As for young patients, intentional treatment of hyperuricemia may not help prevent CI-AKI following CAG.
In patients aged ≥50 years, the mean glycemia was higher than among those aged < 50 years. TC was also higher among the patients aged ≥ 50 years compared to those aged < 50 years.31 Hua-Fen Chen et al showed that very high relative hazards of coronary complications were observed in younger and female diabetic patients in Taiwan.32 Previous studies have shown age, estimated glomerular filtration rate and ejection fraction score can predict contrast-induced acute kidney injury in patients with diabetes and chronic kidney disease.33 Based on the above studies, age plays a great role in the control of blood lipids, blood glucose and uric acid, and different thresholds for human metabolic indicators are formulated according to different ages.
Previous studies have also revealed that increased uric acid levels played a role in the progression of CI-AKI; However, they seldom reported the effect of age and did not pay attention to the part of the young and middle-aged patients.13,34 Our study suggests that for patients before undergoing CAG, not only the level of preoperative renal function should be a concern, but also the risk of CI-AKI caused by age-related uric acid and blood glucose may be further considered, so as to make individualized treatment plans.
Therefore, we may suggest that age differences need to be considered for the risk of contrast nephropathy associated with relevant metabolic indicators besides uric acid.
Limitation
Firstly, due to the nature of the observational study, only associations can be drawn without the causal relationship being inferred. The effect of uric acid-lowering medication on the correlation between age and uric acid-associated risk for CI-AKI needs further prospective studies to validate. Secondly, although some data were missing for the retrospective reason, a fully adjusted analysis was performed in this study, and the sample size was large enough to give adequate power for correlation.
Conclusion
In conclusion, the association between uric acid and CI-AKI in patients undergoing CAG was affected by age. Preoperative assessment of uric acid levels should be taken into account in the context of age. Further research is warranted to determine the optimal target of uric acid for preventing CI-AKI in different age groups.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was sponsored by Longyan City Science and Technology Plan Project (Grant number: 2021LYF17040), Guangdong Provincial science and technology project (2020B1111170011, DFJH2020026, DFJH201919); Guangdong Provincial science and technology project (KJ022021049); Guangdong Provincial Key Laboratory of Coronary Heart Disease Prevention (No. Y0120220151). The funders had no role in the study design, data collection, and analysis, decision to publish, or preparation of the manuscript; the work was not funded by any industry sponsors.
Disclosure
The authors report no conflicts of interest in this work.
References
1. James MT, Samuel SM, Manning MA., et al. Contrast-induced acute kidney injury and risk of adverse clinical outcomes after coronary angiography: a systematic review and meta-analysis. Circ Cardiovasc Interv. 2013;6(1):37–43. doi:10.1161/circinterventions.112.974493
2. Abu Jawdeh BG, Kanso AA, Schelling JR. Evidence-based approach for prevention of radiocontrast-induced nephropathy. J Hosp Med. 2009;4(8):500–506. doi:10.1002/jhm.477
3. Ozkok S, Ozkok A. Contrast-induced acute kidney injury: a review of practical points. World J Nephrol. 2017;6(3):86–99. doi:10.5527/wjn.v6.i3.86
4. Mehran R, Aymong ED, Nikolsky E, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;44(7):1393–1399. doi:10.1016/j.jacc.2004.06.068
5. Mendi MA, Afsar B, Oksuz F, et al. Uric acid is a useful tool to predict contrast-induced nephropathy. Angiology. 2017;68(7):627–632. doi:10.1177/0003319716639187
6. Barbieri L, Verdoia M, Schaffer A, et al. Uric acid levels and the risk of contrast induced nephropathy in patients undergoing coronary angiography or PCI. Nutr Metab Cardiovasc Dis. 2015;25(2):181–186. doi:10.1016/j.numecd.2014.08.008
7. Gephardt MC, Hanlon TJ, Matson CF. BLOOD URIC ACID VALUES AS RELATED TO SEX AND AGE. JAMA. 1964;189:1028–1029. doi:10.1001/jama.1964.03070130048019
8. Goldenberg I, Matetzky S. Nephropathy induced by contrast media: pathogenesis, risk factors and preventive strategies. Cmaj. 2005;172(11):1461–1471. doi:10.1503/cmaj.1040847
9. Aguiar-Souto P, Ferrante G, Del Furia F, Barlis P, Khurana R, Di Mario C. Frequency and predictors of contrast-induced nephropathy after angioplasty for chronic total occlusions. Int J Cardiol. 2010;139(1):68–74. doi:10.1016/j.ijcard.2008.10.006
10. Inker LA, Astor BC, Fox CH, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis. 2014;63(5):713–735. doi:10.1053/j.ajkd.2014.01.416
11. Yin W, Zhou G, Zhou L, et al. Validation of pre-operative risk scores of contrast-induced acute kidney injury in a Chinese cohort. BMC Nephrol. 2020;21(1):45. doi:10.1186/s12882-020-1700-8
12. Lei L, He Y, Guo Z, et al. A simple nomogram to predict contrast-induced acute kidney injury in patients with congestive heart failure undergoing coronary angiography. Cardiol Res Pract. 2021;2021:9614953. doi:10.1155/2021/9614953
13. Guo W, Liu Y, Chen JY, et al. Hyperuricemia is an independent predictor of contrast-induced acute kidney injury and mortality in patients undergoing percutaneous coronary intervention. Angiology. 2015;66(8):721–726. doi:10.1177/0003319714568516
14. Liu Y, Tan N, Chen J, et al. The relationship between hyperuricemia and the risk of contrast-induced acute kidney injury after percutaneous coronary intervention in patients with relatively normal serum creatinine. Clinics. 2013;68(1):19–25. doi:10.6061/clinics/2013(01)oa04
15. Toprak O, Cirit M, Esi E, Postaci N, Yesil M, Bayata S. Hyperuricemia as a risk factor for contrast-induced nephropathy in patients with chronic kidney disease. Catheter Cardiovasc Interv. 2006;67(2):227–235. doi:10.1002/ccd.20598
16. Nemati E, Khosravi A, Einollahi B, Meshkati M, Taghipour M, Abbaszadeh S. The relationship between dialysis adequacy and serum uric acid in dialysis patients; a cross-sectional multi-center study in Iranian hemodialysis centers. J Renal Inj Prev. 2017;6(2):142–147. doi:10.15171/jrip.2017.28
17. Kanbay M, Solak Y, Afsar B, et al. Serum uric acid and risk for acute kidney injury following contrast. Angiology. 2017;68(2):132–144. doi:10.1177/0003319716644395
18. Guo W, Song F, Chen S, et al. The relationship between hyperuricemia and contrast-induced acute kidney injury undergoing primary percutaneous coronary intervention: secondary analysis protocol for the ATTEMPT RESCIND-1 study. Trials. 2020;21(1):567. doi:10.1186/s13063-020-04505-w
19. Schepers MS, van Ballegooijen ES, Bangma CH, Verkoelen CF. Crystals cause acute necrotic cell death in renal proximal tubule cells, but not in collecting tubule cells. Kidney Int. 2005;68(4):1543–1553. doi:10.1111/j.1523-1755.2005.00566.x
20. Lee TS, Lu TM, Chen CH, Guo BC, Hsu CP. Hyperuricemia induces endothelial dysfunction and accelerates atherosclerosis by disturbing the asymmetric dimethylarginine/dimethylarginine dimethylaminotransferase 2 pathway. Redox Biol. 2021;46:102108. doi:10.1016/j.redox.2021.102108
21. Kanbay M, Huddam B, Azak A, et al. A randomized study of allopurinol on endothelial function and estimated glomular filtration rate in asymptomatic hyperuricemic subjects with normal renal function. Clin J Am Soc Nephrol. 2011;6(8):1887–1894. doi:10.2215/cjn.11451210
22. Kanbay M, Siriopol D, Nistor I, et al. Effects of allopurinol on endothelial dysfunction: a meta-analysis. Am J Nephrol. 2014;39(4):348–356. doi:10.1159/000360609
23. Higashi Y, Sasaki S, Nakagawa K, et al. Tetrahydrobiopterin improves aging-related impairment of endothelium-dependent vasodilation through increase in nitric oxide production. Atherosclerosis. 2006;186(2):390–395. doi:10.1016/j.atherosclerosis.2005.07.025
24. Zigmont V, Garrett A, Peng J, et al. Association between prediagnostic serum 25-hydroxyvitamin D concentration and glioma. Nutr Cancer. 2015;67(7):1120–1130. doi:10.1080/01635581.2015.1073757
25. Tsao YC, Chen JY, Yeh WC, Li WC. Gender- and age-specific associations between visceral obesity and renal function impairment. Obes Facts. 2019;12(1):67–77. doi:10.1159/000496626
26. Nieto FJ, Iribarren C, Gross MD, Comstock GW, Cutler RG. Uric acid and serum antioxidant capacity: a reaction to atherosclerosis? Atherosclerosis. 2000;148(1):131–139. doi:10.1016/s0021-9150(99)00214-2
27. Suliman ME, Johnson RJ, García-López E, et al. J-shaped mortality relationship for uric acid in CKD. Am J Kidney Dis. 2006;48(5):761–771. doi:10.1053/j.ajkd.2006.08.019
28. Hsu SP, Pai MF, Peng YS, Chiang CK, Ho TI, Hung KY. Serum uric acid levels show a ‘J-shaped’ association with all-cause mortality in haemodialysis patients. Nephrol Dial Transplant. 2004;19(2):457–462. doi:10.1093/ndt/gfg563
29. Pillai S, Kriplani A, Chawla A, et al. Acute Kidney Injury Post-Percutaneous Nephrolithotomy (PNL): prospective outcomes from a university teaching hospital. J Clin Med. 2021;10(7). doi:10.3390/jcm10071373
30. Yoshida T, Fujimori T, Nabeshima Y. Mediation of unusually high concentrations of 1,25-dihydroxyvitamin D in homozygous klotho mutant mice by increased expression of renal 1alpha-hydroxylase gene. Endocrinology. 2002;143(2):683–689. doi:10.1210/endo.143.2.8657
31. Devroey D, De Swaef N, Coigniez P, Vandevoorde J, Kartounian J, Betz W. Correlations between lipid levels and age, gender, glycemia, obesity, diabetes, and smoking. Endocr Res. 2004;30(1):83–93. doi:10.1081/erc-120029887
32. Chen HF, Li CY. Effect-modifications by age and sex on the risks of coronary artery disease and revascularization procedures in relation to diabetes. Diabetes Res Clin Pract. 2007;75(1):88–95. doi:10.1016/j.diabres.2006.05.020
33. Li J, Li Y, Wang X, et al. Age, estimated glomerular filtration rate and ejection fraction score predicts contrast-induced acute kidney injury in patients with diabetes and chronic kidney disease: insight from the TRACK-D study. Chin Med J. 2014;127(12):2332–2336.
34. Tang H, Chen H, Li Z, et al. Association between uric acid level and contrast-induced acute kidney injury in patients with type 2 diabetes mellitus after coronary angiography: a retrospective cohort study. BMC Nephrol. 2022;23(1):399. doi:10.1186/s12882-022-03030-z
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

