Back to Journals » Drug Design, Development and Therapy » Volume 16
Aframomum melegueta Seed Extract with Standardized Content of 6-Paradol Reduces Visceral Fat and Enhances Energy Expenditure in Overweight Adults – A Randomized Double-Blind, Placebo-Controlled Clinical Study
Authors Sudeep HV , Aman K, Jestin TV, Shyamprasad K
Received 5 April 2022
Accepted for publication 29 September 2022
Published 28 October 2022 Volume 2022:16 Pages 3777—3791
DOI https://doi.org/10.2147/DDDT.S367350
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Georgios Panos
Heggar Venkataramana Sudeep,1 Khanna Aman,2 Thomas V Jestin,3 Kodimule Shyamprasad1
1Department of Biomedicinal Research (R&D), Vidya Herbs Pvt Ltd, Bangalore, Karnataka, 560 105, India; 2Aman Hospital and Research Center, Vadodara, Gujarat, 390021, India; 3Leads Clinical Research and Bio Services Private Ltd, Bangalore, India
Correspondence: Heggar Venkataramana Sudeep, Research Scientist, R&D Center for Excellence, Vidya Herbs Pvt Ltd, No. 14/A, KIADB, Jigani Industrial Area, Anekal Taluk, Bangalore, Karnataka, 560 105, India, Tel +91 80-42094158, Email [email protected]; [email protected]
Purpose: Aframomum melegueta (grains of paradise) seeds have been demonstrated to possess thermogenic potential. However, it is necessary to validate the functional attributes of A. melegueta seed extract in human subjects.
Methods: In a double-blind, placebo-controlled clinical trial design, we have examined the thermogenic effects of a standardized A. melegueta seed extract (AfperFit). A total of 70 overweight male and female subjects (BMI ≥ 25.0 to ≤ 30.0 kg/m2) aged 20– 50 years were enrolled and administered with either 250 mg of AfperFit or placebo in capsule form twice daily for 12 weeks. The primary efficacy endpoints included energy expenditure (indirect calorimetry), body composition (dual-energy X-ray absorptiometry (DEXA)) and fat distribution (computed tomography (CT scan)), analyzed at baseline and after 12 weeks of treatment. The effect of intervention on the quality of life was examined using SF-12 questionnaire.
Results: Consumption of AfperFit significantly increased the energy expenditure (p< 0.01), visceral fat area (p< 0.001) and visceral to subcutaneous fat ratio (p< 0.01) compared to placebo group. Consequently, there was significant body weight loss and reduction in BMI of subjects in AfperFit group compared to placebo (p< 0.01). The safety evaluation showed that biochemical and hematological parameters were in the normal range. Supplementation of AfperFit was well tolerated during the study and no adverse effects were observed.
Conclusion: Overall, this study validates the health benefits of A. melegueta seed extract as fat burner and recommends its use as a functional ingredient to improve the quality of life and general health.
Keywords: grains of paradise, fat burner, 6-paradol, visceral fat
Introduction
In the modern world, obesity is a global health concern with alarming consequences such as cardiovascular diseases, diabetes, and cancer.1–3 Of the various approaches to manage obesity, stimulation of thermogenic program has gained significant attention.4 Natural thermogenic supplements are publicly available in the market to promote weight loss alongside the diet restrictions and exercise.5,6 These supplements include combination of herbal extracts that can enhance the resting metabolic rate and reduce body fat.7 In this regard, the physiological functions of several herbal constituents and preparations have been demonstrated by researchers. Green tea extract rich with polyphenols has been reported to induce thermogenesis via adiponectin signaling.8 Recently, Caffeine was demonstrated to enhance the physical activity-driven caloric cost in rats.9 Recently Katada et al10 reported that a 2-week consumption of catechins with caffeine markedly increased the energy expenditure in middle-aged human subjects. Use of such thermogenic agents is a promising strategy to mitigate obesity and associated metabolic diseases.
Aframomum melegueta (Fam. Zingiberaceae) is a perennial herb originated from Western parts of Africa.11 A. melegueta is commonly known as grains of paradise or melegueta pepper or guinea grains. The seeds of the plant are used in food and ethnomedicinal preparations. The plant is reported to contain phenolic compounds such as paradols, shogaols and gingerols.12 These bioactive vanilloid compounds possess various biological functions including antioxidant, anti-inflammatory, and anti-proliferative activities.13–15 Further, there are reports on the usefulness of these compounds against metabolic diseases. In a recent study from Hattori et al,16 the synthetic vanilloid compounds were investigated for anti-obesity effects in male mice. A 2-week oral administration of 6-paradol significantly reduced the visceral and subcutaneous fat depots, while the effect was not observed with 6-gingerol and 6-shogaol.
The thermogenic effects of A. melegueta seed extract and its active compound 6-paradol have been studied previously.17,18 Earlier, we found that oral administration of AfperFit, a standardized A. melegueta seed extract, significantly induced beige adipocytes in high-fat diet-induced obese mice via activation of browning genes.19 To further validate the therapeutic claims of AfperFit, we have investigated its weight loss effects in healthy overweight humans.
Materials and Methods
Ethical Approval and Consent
The Institutional Ethics Committee of Aman Hospital and Research Center, Vadodara, India, approved the study protocol and informed consent form (AHRC/IEC/030/2018). This clinical study was registered in Clinical Trials Registry – India (CTRI/2019/03/017956 dated 07/03/2019).
Investigational Product
AfperFit is a proprietary herbal extract from Aframomum melegueta seeds formulated in powder form to contain not less than 2% 6-paradol. The pungent active constituents of AfperFit viz., 6-paradol (Supplementary Figure 1), 6-gingerol and 6-shogaol (Supplementary Figure 2) were analyzed by HPLC (Supplementary File; Characterization of 6-paradol, 6-gingerol and 6-shagaol in AfperFit). Each 250 mg capsule contained 5 mg of 6-paradol. The placebo capsules had similar appearance and color. The investigational product was stored at 15–35 °C protected from light and moisture at the clinical study site.
Subjects
Seventy overweight (BMI ≥25.0 to ≤30.0 kg/m2) male and female subjects aged 20–50 years were enrolled in the trial. Before enrolment, the volunteers were explained the study protocol. All the subjects provided the informed consent. Subject enrolment was initiated on May 13, 2019 and ended on September 27, 2019.
Subjects suffering from chronic health conditions (eg, diabetes, hypertension, chronic renal failure, heart, and liver disease), fasting blood glucose above normal limits, history of chronic metabolic disease, psychiatric illness, drug abuse, smoking, addiction to alcohol, endocrine abnormalities, and history of surgery were excluded from the study.
Study Design
The clinical study to investigate the safety and efficacy of AfperFit was conducted in compliance with ICH-GCP (International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use –Good Clinical Practice) guidelines and Helsinki Declaration Standards. This clinical study adheres to the CONSORT guidelines (Supplementary File).
A randomized, double-blind, placebo-controlled, two arm, single centered clinical study was conducted in healthy overweight male and female human subjects. The study was conducted at Aman Hospital and Research Center, Vadodara, Gujarat, India. A population size of 70 subjects were randomized to two treatment arms: AfperFit and placebo on 1:1 ratio (n = 35 in each group). The subjects received oral doses of 250 mg capsules twice daily (before breakfast and dinner) for 84 days (12 weeks). The study treatment details are provided in Supplementary Table 1. After the baseline/screening visit, two visits were scheduled at the study site during the intervention period at an interval of 6 weeks.
Sample Size
A sample size of 70 subjects (35 per group) was considered sufficient to detect a clinically important difference between groups with 80% power and a 5% level of significance. Considering an estimated potential dropout rate of 12%, the sample size was finalized as 70 (35 per group). The details are provided in the Supplementary File.
Randomization
The interventions were masked and assigned a code for each. The subjects were randomized using block randomization with a block size of 4. The random allocation sequence was generated by the biostatistician and the subjects enrolled by the investigator. The investigator and the subjects were blinded by the interventions.
Study Outcomes
The primary endpoint analyses included determination of energy expenditure, body composition, and fat distribution at baseline and at the end of study (after 12 weeks of treatment).
Energy expenditure was measured by indirect calorimetry (FitMate WM, Cosmed, USA). The subjects were fasted for 8 h and examined early in the morning. The measurement was performed with subjects in supine position for 10–15 min, in a ventilated room void of external noise. The device was calibrated before each measurement.
Body composition analysis at baseline and after 12 weeks of treatment was performed using dual-energy X-ray absorptiometry (DEXA) (Lunar iDXA, GE Healthcare, India).
The fat distribution was examined by computed tomography (CT scan). The CT scan was performed with subjects in the supine position. One slice was collected at fourth and fifth lumbar vertebrae (L4-L5). Before segmentation of body fat, the background noise and unnecessary regions were removed. Body fat was then detected with pixels between −190 and −30 Hounsfield units (HU). Segmentation mask was generated from the binary images to differentiate the visceral and subcutaneous fat. The images were processed using ImageJ software.
The secondary endpoints included safety assessment (serum lipid profile, blood biochemical parameters and complete blood count). The 12-term Short Form Survey (SF-12) questionnaire was used to assess the quality of life.
Statistical Analysis
All the data were analyzed for normality of distribution. The normally distributed data were analyzed by paired sample t-test (within the group) and independent t test (between the groups). Skewed data were analyzed by non-parametric tests: Mann–Whitney test for two independent groups and Wilcoxon test for paired data (within the group). The covariates were analyzed using ranked ANCOVA. The values were presented as mean ± SD. Data were considered statistically significant at p<0.05. In the case of dropouts, the missing subject data for ITT analysis were handled by imputation analysis (last observation carried forward).
Results
A total of 94 human volunteers were screened for inclusion in the trial. Seventy subjects meeting the inclusion criteria were enrolled in the study. Of the 70 subjects randomized to two treatment groups (n = 35 in each group), 60 subjects completed the study. There were 10 dropouts in the study (reason: lost to follow up). The intention-to-treat (ITT) analysis was used to assess the outcome of the study. The participant flow through the study is presented in Figure 1. The demographic characteristics of subjects between the groups were not significantly different (Table 1). The efficacy and safety analysis were performed using ITT population.
 |
Table 1 Demographic Characteristics of Subjects |
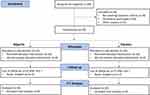 |
Figure 1 Study participant flow diagram in accordance with CONSORT 2010. Adapted from Schulz KF, Altman DG, Moher D, for the CONSORT Group. CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomised Trials. PLoS Med. 2010;7(3): e1000251. Copyright: © 2010 Schulz et al. Creative Commons Attribution License.20 |
Efficacy Analysis
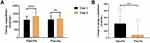
Table 2 shows the effect of AfperFit on energy expenditure in overweight subjects. The energy expenditure was measured using indirect calorimetry at baseline and after 12 weeks of treatment. AfperFit group showed 18.55% increase in the mean energy expenditure from baseline at the end of study (p<0.001). The placebo group showed an insignificant increase of 3.32% in the energy expenditure (Figure 2A). The change in energy expenditure was significantly higher in the AfperFit group as compared to placebo group (p<0.01) (Figure 2B).
 |
Table 2 Effect of AfperFit on Whole-Body Energy Expenditure (Kcal/Day) |
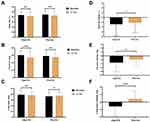
The body composition parameters were determined using DEXA analysis (Table 3) (Figure 3). A significant reduction in body fat (%) was noticed in both the groups (p<0.01). The body fat reduction from baseline to the end of treatment was slightly superior in AfperFit group. However, the data were not significant between the groups. There was a significant decrease in body fat mass observed in both AfperFit (p<0.001) and placebo groups (p<0.01) at the end of study. The mean change in fat mass was higher in AfperFit group than the placebo group.
 |
Table 3 Summary of Changes in Body Composition Parameters of Subjects (DEXA Analysis) |
Interestingly, a decreasing trend was observed in lean mass of AfperFit group from baseline to the end of treatment (p<0.01). On the contrary, placebo group showed an increase in lean mass (p<0.05). The change in lean mass of AfperFit group was significant compared to the placebo (p<0.001).
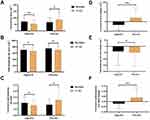
Another aspect of efficacy analysis was to examine the effect of AfperFit on the body fat distribution. Table 4 shows the summary of fat distribution among the subjects. After 12-week supplementation of AfperFit, there was a noticeable reduction (p<0.01) in the visceral fat area, whereas the same was increased to a significant extent in the placebo group (p<0.05). The mean change from baseline to the end of treatment in AfperFit group was significant (p<0.001) as compared to placebo. The subcutaneous fat area was reduced in either groups and the mean change between the groups was not significant. The visceral-to-subcutaneous fat ratio was reduced to a significant extent in the AfperFit group (p<0.05). On the contrary, the ratio was increased from baseline to the end of study in placebo group (p<0.05). The reduction in the visceral-to-subcutaneous fat ratio from baseline was significant in AfperFit compared to placebo group (p<0.01) (Figure 4).
 |
Table 4 Effect of AfperFit on Body Fat Distribution of Subjects |
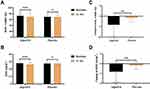
The mean body weight and BMI changes were recorded (Table 5). The DEXA analysis revealed that the AfperFit group showed significant reduction in the body weight and BMI from baseline to the end of treatment (p<0.001). The mean changes in body weight and BMI from baseline were significant in AfperFit group as compared to placebo (p<0.01) (Figure 5).
 |
Table 5 Effect of AfperFit on Body Weight and BMI |
The efficacy parameters were not significantly changed with respect to age and gender as covariates (Table 6).
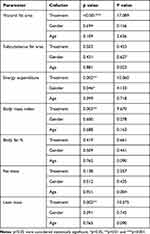 |
Table 6 Results of Rank Analysis of Covariance (ANCOVA) (with Age and Gender as Covariates) of Changes in Efficacy Parameters from Baseline |
The effect of AfperFit administration on general health was determined using quality of life questionnaire (SF-12). The objective assessment included total SF-12 score, physical component score (PCS-12) and mental component score (MCS-12). SF-12 total score in AfperFit group was significantly increased from baseline (29.40 ± 1.58) to the end of study (31.69 ± 2.10) (p<0.001). The change in the total score of AfperFit group (−2.29 ± 1.76) was found to be significant (p<0.001) as compared to placebo (0.20 ± 2.70). Similar trend was observed in MCS-12 score. However, there was no significant change in the PCS-12 score of the treatment groups (Table 7).
 |
Table 7 Summary of Short-Form Health Survey (SF-12) Questionnaire |
To summarise the efficacy measures, a 12-week oral supplementation of 500 mg/day AfperFit could exert thermogenic effect by significantly increasing the energy expenditure and reducing the body fat % and visceral fat. There was an obvious reduction in the body weight and BMI of the subjects in AfperFit group.
Safety Analysis
No serious adverse events were recorded during the study. Liver function and renal function parameters were evaluated at baseline and the end of the study. The parameters were found to be within the normal range throughout the study. No clinically significant differences in the biochemical parameters were observed between the groups at the end of study (Table 8).
 |
Table 8 Safety Analysis – Serum Biochemical Parameters |
Serum lipid parameters were found to be within normal levels throughout the study period. A significant (p<0.05) reduction from baseline in total cholesterol, LDL cholesterol, and LDL: HDL ratio was observed in AfperFit group. No clinically significant difference was found between the AfperFit and placebo groups (Table 9). Table 10 shows the haematological assessment of subjects. In the AfperFit group, there were marginal variations in the parameters except for significant increase in the hemoglobin, mean cell volume (MCV) and mean cell haemoglobin (MCH) and a decrease in platelet count (p<0.05).
 |
Table 9 Safety Analysis – Serum Lipid Profile |
 |
Table 10 Safety Analysis – Haematological Assessment |
Discussion
The present study investigated the weight loss effects of AfperFit, a standardized A. melegueta extract containing not less than 2% 6-paradol, in overweight subjects of either sex. A 12-week ingestion of AfperFit at 500 mg/day significantly increased the energy expenditure compared to the placebo group. Further, the CT analysis of subjects revealed that AfperFit reduced the visceral fat in subjects to a significant extent compared to placebo. On the contrary, the change in subcutaneous fat was insignificant between AfperFit and placebo groups. The preference of AfperFit to the visceral fat is interesting, because visceral adipocytes are associated more with the risk of cardiovascular diseases in humans.21,22 Also, in humans, the browning genes are predominantly expressed in the visceral fat than the subcutaneous fat.23 These findings are agreeing with the previous study from Sugita et al24 who reported that daily consumption of 30 mg A. melegueta extract for 4 weeks soared the energy expenditure and reduced visceral fat accumulation. Further, the present clinical study data are in line with our previous findings that AfperFit mediated browning of visceral fat via increased expression of UCP1, PPARγ and PGC-1α in high fat diet-fed mice.19
In the present study, body composition was measured using the validated DEXA analysis. There was a significant reduction in the body fat % and fat mass of subjects treated with both AfperFit and placebo. However, the changes were more pronounced in the active treatment group.
The lean mass was significantly reduced in the AfperFit-treated subjects. Several other studies on the antiobesity effects of dietary supplements have shown 14–50% reduction in lean body mass.25–27 Based on the observed trend, it can be presumed that AfperFit could not offset the loss of lean mass. A moderate to intense physical activity contributes to retain lean mass in a weight loss program.28 Hence, supplementation of AfperFit with augmented dietary manifestations and exercise could help preserve lean body mass.
Interestingly, none of the efficacy endpoint parameters were influenced by the age and gender of the subjects.
The quality of life was assessed using validated multipurpose SF-12 questionnaire. AfperFit treated subjects showed a significant increase in mental component score and the total score compared to the placebo group. These results clearly suggest that AfperFit has a positive impact on the general health. Furthermore, there was no abnormal change observed in the biochemical and hematological parameters. The observed changes in hemoglobin, MCV, MCH and platelet count of AfperFit group were not significant compared to placebo and can be considered incidental.
AfperFit has a standardized content of 6-paradol along with the presence of other pungent compounds such as 6-gingerol and 6-shogaol. These compounds have been reported to possess different biological activities including antihyperglycemic and lipid lowering effects.29,30 The presence of these compounds could be attributed to the observed thermogenic effects of AfperFit. Iwami et al17 have previously demonstrated the thermogenic potential of A. melegueta seed extract and particularly 6-paradol in rats. The authors reported the mechanism of action as activation of brown adipose tissue (BAT) through the stimulation of sympathetic nerve activity. In another study, 6-paradol was shown to have profound anti-obesity effects in high fat diet-fed mice via activation of brown adipose tissue.18 These reports further support the outcome of the present study.
The limitation of the present study is that we have not measured the impact of AfperFit on substrate oxidation alongside resting energy expenditure. Secondly, the study had considerable drop-outs. The strength of the study is that the primary outcome measures were obtained using instrumental analyses, which guaranteed the data accuracy and reliability.
Conclusion
In conclusion, the present study demonstrated that daily intake of 500 mg of AfperFit for 12 weeks significantly increased the energy expenditure while reducing the body fat and visceral fat. The study outcome strongly supports the weight loss potentials of AfperFit.
Data Sharing Statement
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The authors acknowledge Mr. Sathish Mukashi, Vin Super Foods, Bangalore, India, for assistance in formulation.
Disclosure
The authors Heggar Venkataramana Sudeep and Kodimule Shyamprasad are employed by Vidya Herbs Pvt Ltd., Khanna Aman is employed by Aman Hospital and Research Center, Vadodara, India. Thomas V Jestin is employed by Leads Clinical Research and Bio services Private Ltd., Bangalore, India. The authors report no other conflicts of interest in this work.
References
1. Piché ME, Poirier P, Lemieux I., et al. Overview of epidemiology and contribution of obesity and body fat distribution to cardiovascular disease: an update. Prog Cardiovasc Dis. 2018;61:103–113. doi:10.1016/j.pcad.2018.06.004
2. Chobot A, Górowska-Kowolik K, Sokołowska M, et al. Obesity and diabetes-not only a simple link between two epidemics. Diabetes Metab Res Rev. 2018;34(7):e3042. doi:10.1002/dmrr.3042
3. Fujita K, Hayashi T, Matsushita M, et al. Obesity, inflammation, and prostate Cancer. J Clin Med. 2019;8(2):201. doi:10.3390/jcm8020201
4. Trayhurn P. Origins and early development of the concept that brown adipose tissue thermogenesis is linked to energy balance and obesity. Biochimie. 2017;134:62–70. doi:10.1016/j.biochi.2016.09.007
5. Stohs SJ, Badmaev V. A review of natural stimulant and non-stimulant thermogenic agents. Phytother Res. 2016;30:732–740. doi:10.1002/ptr.5583
6. Ruiz-Moreno C, Del Coso J, Giráldez-Costas V, et al. Effects of p-Synephrine during Exercise: a Brief Narrative Review. Nutrients. 2021;13(1):233. doi:10.3390/nu13010233
7. Clark JE, Welch S. Comparing effectiveness of fat burners and thermogenic supplements to diet and exercise for weight loss and cardiometabolic health: systematic review and meta-analysis. Nutr Health. 2021;27(4):445–459. doi:10.1177/0260106020982362
8. Bolin AP, Sousa-Filho CPB, Marinovic MP, et al. Polyphenol-rich green tea extract induces thermogenesis in mice by a mechanism dependent on adiponectin signaling. J Nutr Biochem. 2020;78:108322. doi:10.1016/j.jnutbio.2019.108322
9. Clark KS, Coleman C, Shelton R, Heemstra LA, Novak CM. Caffeine enhances activity thermogenesis and energy expenditure in rats. Clin Exp Pharmacol Physiol. 2019;46(5):475–482. doi:10.1111/1440-1681.13065
10. Katada S, Yanagimoto A, Matsui Y, et al. Effect of tea catechins with caffeine on energy expenditure in middle-aged men and women: a randomized, double-blind, placebo-controlled, crossover trial. Eur J Nutr. 2020;59:1163–1170. doi:10.1007/s00394-019-01976-9
11. Iwu MM. Handbook of African Medicinal Plants.
12. Fernandez X, Pintaric C, Lizzani-Cuvelier L, et al. Chemical composition of absolute and supercritical carbon dioxide extract of Aframomum melegueta. Flavour Fragr J. 2006;21:162–165. doi:10.1002/ffj.1554
13. Kawamoto Y, Ueno Y, Nakahashi E, et al. Prevention of allergic rhinitis by ginger and the molecular basis of immunosuppression by 6-gingerol through T cell inactivation. J Nutr Biochem. 2016;27:112–122. doi:10.1016/j.jnutbio.2015.08.025
14. Saha A, Blando J, Silver E, et al. 6-Shogaol from dried ginger inhibits growth of prostate cancer cells both in vitro and in vivo through inhibition of STAT3 and NF-κB signaling. Cancer Prev Res. 2014;7:627–638. doi:10.1158/1940-6207.CAPR-13-0420
15. Kim SM, Kim C, Bae H, et al. 6-Shogaol exerts anti-proliferative and pro-apoptotic effects through the modulation of STAT3 and MAPKs signaling pathways. Mol Carcinog. 2015;54:1132–1146. doi:10.1002/mc.22184
16. Hattori H, Mori T, Shibata T, et al. 6-Paradol acts as a potential anti-obesity vanilloid from Grains of Paradise. Mol Nutr Food Res. 2021;65(16):e2100185. doi:10.1002/mnfr.202100185
17. Iwami M, Mahmoud FA, Shiina T, et al. Extract of Grains Of Paradise and its active principle 6-paradol trigger thermogenesis of brown adipose tissue in rats. Auton Neurosci. 2011;161(1–2):63–67. doi:10.1016/j.autneu.2010.11.012
18. Haratake A, Watase D, Setoguchi S, et al. Relationship between the acyl chain length of paradol analogues and their antiobesity activity following oral ingestion. J Agric Food Chem. 2014;62(26):6166–6174. doi:10.1021/jf500873a
19. Sudeep HV, Ramanaiah I, Amritha R, et al. A standardized Aframomum melegueta seed extract regulates browning of white adipose tissue in high-fat diet model mice. Nat Prod Commun. 2021;16:1–10.
20. Schulz KF, Altman DG, Moher D, for the CONSORT Group. CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomised Trials. PLoS Med. 2010;7(3):e1000251. https://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1000251.
21. Pou KM, Massaro JM, Hoffmann U, et al. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study. Circulation. 2007;116(11):1234–1241. doi:10.1161/CIRCULATIONAHA.107.710509
22. Abraham TM, Pedley A, Massaro JM, et al. Association between visceral and subcutaneous adipose depots and incident cardiovascular disease risk factors. Circulation. 2015;132(17):1639–1647. doi:10.1161/CIRCULATIONAHA.114.015000
23. Zuariga MA, Fuster JJ, Gokce N, et al. Humans and mice display opposing patterns of “Browning” gene expression in visceral and subcutaneous white adipose tissue depots. Front Cardiovasc Med. 2017;4:27. doi:10.3389/fcvm.2017.00027
24. Sugita J, Yoneshiro T, Sugishima Y, et al. Daily ingestion of grains of paradise (Aframomum melegueta) extract increases whole-body energy expenditure and decreases visceral fat in humans. J Nutr Sci Vitaminol. 2014;60:22–27. doi:10.3177/jnsv.60.22
25. Grube B, Chong PW, Lau KZ, et al. A natural fiber complex reduces body weight in the overweight and obese: a double-blind, randomized, placebo-controlled study. Obesity. 2013;21:58–64. doi:10.1002/oby.20244
26. Grube B, Chong PW, Alt F, et al. Weight maintenance with litramine (IQP-G-002AS): a 24-week double-blind, randomized, placebo-controlled study. J Obes. 2015;2015:953138. doi:10.1155/2015/953138
27. Chong PW, Beah ZM, Grube B, et al. IQP-GC-101 reduces body weight and body fat mass: a randomized, double-blind, placebo-controlled study. Phytother Res. 2014;28:1520–1526. doi:10.1002/ptr.5158
28. Chaston TB, Dixon JB, O’Brien PE. Changes in fat-free mass during significant weight loss: a systematic review. IJO. 2007;31:743–750.
29. Bouchonville M, Armamento-Villareal R, Shah K, et al. Weight loss, exercise or both and cardiometabolic risk factors in obese older adults: results of a randomized controlled trial. Int J Obes. 2014;38(3):423–431. doi:10.1038/ijo.2013.122
30. Wei CK, Tsai YH, Korinek M, et al. 6-Paradol and 6-Shogaol, the pungent compounds of ginger, promote glucose utilization in adipocytes and myotubes, and 6-paradol reduces blood glucose in high-fat diet-fed mice. Int J Mol Sci. 2017;18:168. doi:10.3390/ijms18010168
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.