Back to Journals » Vascular Health and Risk Management » Volume 13
Aerobic fitness is associated with low cardiovascular disease risk: the impact of lifestyle on early risk factors for atherosclerosis in young healthy Swedish individuals – the Lifestyle, Biomarker, and Atherosclerosis study
Authors Fernström M, Fernberg U , Eliason G, Hurtig-Wennlöf A
Received 28 October 2016
Accepted for publication 10 February 2017
Published 15 March 2017 Volume 2017:13 Pages 91—99
DOI https://doi.org/10.2147/VHRM.S125966
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Konstantinos Tziomalos
Maria Fernström,1,* Ulrika Fernberg,2,* Gabriella Eliason,1 Anita Hurtig-Wennlöf1
1Department of Medical Diagnostics, Medical Faculty, School of Health Sciences, 2Medical Faculty, School of Medical Sciences, Örebro University, Örebro, Sweden
*These authors contributed equally to this work
Background: The progression of cardiovascular disease (CVD) and atherosclerosis is slow and develops over decades. In the cross-sectional Swedish Lifestyle, Biomarker, and Atherosclerosis study, 834 young, self-reported healthy adults aged 18.0–25.9 years have been studied to identify early risk factors for atherosclerosis.
Purpose: The aims of this study were to 1) assess selected cardiometabolic biomarkers, carotid intima–media thickness (cIMT) as a marker of subclinical atherosclerosis, and lifestyle-related indicators (food habits, handgrip strength, and oxygen uptake, VO2 max); 2) analyze the assofciations between cIMT and lifestyle factors; and 3) identify subjects at risk of CVD using a risk score and to compare the characteristics of subjects with and without risk of CVD.
Method: Blood samples were taken in a fasting state, and food habits were reported through a questionnaire. cIMT was measured by ultrasound, and VO2 max was measured by ergometer bike test. The risk score was calculated according to Wildman.
Result: cIMT (mean ± standard deviation) was 0.50±0.06 mm, and VO2 max values were 37.8±8.5 and 42.9±9.9 mL/kg/min, in women and men, respectively. No correlation was found between aerobic fitness expressed as VO2 max (mL/kg/min) and cIMT. Using Wildman’s definition, 12% of the subjects were classified as being at risk of CVD, and 15% had homeostasis model assessment of insulin resistance. A total of 35% of women and 25% of men had lower high-density lipoprotein cholesterol than recommended. Food habits did not differ between those at risk and those not at risk. However, aerobic fitness measured as VO2 max (mL/kg/min) differed; 47% of the subjects at risk had low aerobic fitness compared to 23% of the nonrisk subjects (P<0.001).
Conclusion: High aerobic fitness is associated with low CVD risk in Swedish young adults. The high prevalence of young adults observed with unfavorable levels of high-density lipoprotein cholesterol and homeostasis model assessment of insulin resistance raises concerns about future CVD risk.
Keywords: cIMT, cholesterol, insulin resistance, body fat, diet, aerobic fitness
Background
Cardiovascular disease (CVD) is one of the most common and serious diseases in modern society.1 The atherosclerotic process is complex and involves inflammation of the arterial wall with progressive accumulation of lipids that builds up inside the arteries. The process is slow, narrows the arteries, and increases the risk of CVDs such as peripheral arterial disease, coronary infarction, and stroke. A generally accepted marker of the progression of subclinical atherosclerosis in young adults is carotid intima–media thickness (cIMT).2
There are several early signs and well-known risk factors for CVD, such as high blood pressure, hyperlipidemia, elevated percentage of body fat, and homeostasis model assessment of insulin resistance (IR). On the other hand, aerobic fitness is known to prevent insulin resistance due to its effects on insulin sensitivity.3–5 Aerobic fitness also has effects on the blood lipid profile, on the total body fat, and on the inflammation that develops over time in the wall of the arteries and is a hallmark of atherosclerosis.6
It is well known that the lifestyle factors increasing the risk of CVD include unhealthy dietary habits7 and lack of physical activity.8 In Sweden, the intake of saturated fat has increased since 2004 in both men and women.9 In parallel with the dietary shift toward more lipids and less carbohydrates, body mass index (BMI) has increased during the 21st century.9 Whether increased BMI in a young population is an effect of changes in dietary habits or a consequence of a more sedentary lifestyle with low fitness is not known.
It is vital to establish healthy habits already at a young age to prevent atherosclerosis. As recently reviewed, there are several studies on the impact of lifestyle factors on CVD in patients and the elderly.10 Not so much is known about dietary habits, fitness, and their impact on early risk factors for CVD in young Swedish adults.
The aims of the present study were to 1) assess cardiometabolic biomarkers, cIMT as a marker of subclinical atherosclerosis, and lifestyle-related indicators (food habits, handgrip strength, and oxygen uptake, VO2 max), 2) analyze the associations between cIMT and lifestyle factors, and 3) identify subjects at risk of CVD and compare the characteristics of subjects with and without risk of CVD.
Method
Study design
In the cross-sectional Lifestyle, Biomarker, and Atherosclerosis (LBA) study conducted at Örebro University, Sweden, young, self-reported healthy adults have been examined for early signs of atherosclerosis. The study has been ongoing for four University semesters, from October 2014 to June 2016. The subjects have been tested on two different occasions at 7- to 10-day intervals.
For the first visit, the subjects came to the laboratory in a fasting state. At this visit, lifestyle habits were reported through a questionnaire. Blood pressure and body composition were measured, and blood was drawn. After the first visit, the subjects were offered breakfast. Breakfast and feedback on test results were the only reward for participation in the study. During the second visit, not in a fasting state, cIMT, handgrip strength, and VO2 max were tested.
Study population
Recruitment of the study population was done by advertisement at local schools, at the University, and in a local newspaper. Additionally, the University web platform and social media were used for recruitment. The inclusion criteria were as follows: the subjects should be 18.0–25.9 years old, healthy, ie, not diagnosed with any chronic disease, and nonsmokers.
Uppsala Ethics Committee approved the study design, Dnr: 2014/224. All subjects gave their written consent to participate and were informed that they could terminate their participation at any time.
Definition of lifestyle factors
Food habits and fitness have been used to define lifestyle factors. Food habits were measured by a questionnaire from the Swedish National Food Agency and characterized as unhealthy, normal, or healthy.11 Fitness was measured as oxygen uptake, VO2 max (ie, aerobic fitness), by an ergometer bike test, and muscle strength was measured by a handgrip test. Depending on the results, the subjects were categorized as having low, normal, or high aerobic fitness12 and having low, normal, or high muscular strength.13
Definition of early risk factors for atherosclerosis
There are several criteria for categorizing subjects at risk of atherosclerosis and CVD. We used the definition recommended by Wildman,14 with subjects having two or more of the following characteristics classified as being at risk:
Elevated blood pressure: ≥130/85 mmHg
Elevated triglycerides: ≥1.70 mmol/L
Decreased high-density lipoprotein cholesterol (HDL-C): women <1.30 mmol/L, men <1.04 mmol/L
Elevated glucose: ≥5.6 mmol/L
Insulin resistance: HOMA-IR >2.52
Inflammation: high-sensitive C-reactive protein (hs-CRP) >5.07 mg/L
Questionnaires about lifestyle and food habits
The participants filled in a validated15 computerized questionnaire about their general physical and mental health. Additional questions on family background, heredity of CVD and diabetes, and exercise habits were also answered.
They also filled in the Swedish National Food Agency test “Matvanekollen”. This is a short computerized food habit questionnaire. The result from the test is divided in scores from 1 to 12. The test is available on the Internet for private subjects and scientific studies, and it mirrors the food habits of the general Swedish population compared to the National Food Agency’s recommendations on healthy food habits. The expected distribution of results using this specific questionnaire estimates that 20% of the subjects receive 1–4 points=unhealthy. They are recommended to improve their food habits. Approximately 70% score 5–8 points=normal, indicating healthy food habits with some potential for improvements. The last 10% are characterized as healthy=9–12 points with food habits according to the recommendations. A short feedback based on the result was given to the subjects from the software.11
Blood pressure and body composition
After 15 min seated, resting blood pressure and heart rate were measured using a digital automated device (Dinamap V100; GE Healthcare, Buckinghamshire, UK) with Dura-Cuf (GE Critikon Dura-cuf; GE Medical Systems, Milwaukee, WI, USA). Blood pressure was measured in the left arm in supine position. At least three measurements were conducted with 2 min intervals. When the difference between the two latest systolic pressures was less than 5 mmHg, the measurement was ended. The results for blood pressure and heart rate are reported as an average of the two latest results.
Height was measured with a fixed stadiometer to the nearest 0.5 cm, with the subjects standing without shoes, heels together, back straight, and arms extended alongside the body. Waist circumference was measured around the abdomen between the iliac crest and the lowest rib on exhalation, to the nearest 0.5 cm using a flexible, nonstretchable, measuring tape.16
Body weight and percentage body fat were measured on Tanita, a bioelectrical impedance body composition analyzer (Tanita BC-418 MA; Tanita Europe B.V., Amsterdam, the Netherlands). The procedure was performed with the subjects standing barefoot on the metal surface conductive equipment according to the manufacturer’s guidelines. Adjustments were made with 1 kg for clothes and the standard setting was used. BMI was calculated (kg/m2) and categorized into underweight (<18.50), normal (18.50–24.99), overweight (25.00–29.99), or obese (≥30) according to the WHO classification.17
Carotid intima–media thickness
Ultrasound measurements were performed using a high-resolution B-mode system, (Vivid E9; GE Healthcare, Chicago, IL, USA) with a 12 MHz linear array transducer. The subjects were examined in a supine position with their heads slightly extended and turned approximately 45° to the left. The right common carotid artery was scanned with transverse and longitudinal views, and Doppler flow measurement was made to verify the location of the examination. The cIMT was measured over 10 mm in the longitudinal view in the common carotid artery on the far wall and 10 mm proximal to the carotid bulb with a lateral probe position, according to guidelines.18,19 Simultaneous electrocardiogram (ECG) recording enabled measurements in end diastole. The Vivid E9 semiautomated edge-detection program was used to identify intima–media thickness, and for reproducibility, measurements were performed on at least three different images. An average of three measurements with a difference less than 0.05 mm was reported, so was an average of the maximum values.
Measurement of cardiometabolic biomarkers
Blood samples were collected in the morning following an 8–12 h fasting period. The subjects rested for approximately 20 min prior to blood collection. The area for venipuncture was warmed up with a heating pad and cleaned with antiseptics. The tourniquet was placed approximately 10 cm above the venipuncture site. The venipuncture was performed using a 21-gauge butterfly needle (Vacuette®; Greiner Bio-One International GmbH, Rainbach im Mühlkreis, Austria), and as soon as blood flow was established, the tourniquet was released. After blood collection, all tubes (BD Vacutainer; BD AB, Stockholm, Sweden) were gently inverted several times.
For analyzing cholesterol, HDL-C, low-density lipoprotein cholesterol (LDL-C), triglycerides, and hs-CRP, blood was collected in 2×3 mL lithium-heparin tubes. Plasma was obtained by centrifugation at 2000× g for 8 min at room temperature and later placed at +4°C to await transportation to the accredited clinical chemistry laboratory at Örebro University Hospital.
One 3 mL citric acid-citrate-NaF tube was used to collect blood for glucose analysis. After extended inverting of the tube to prevent hemolysis, it was placed at room temperature or +4°C until transportation.
For insulin analysis, serum was obtained by collecting blood in a 4 mL standard serum tube with clot activating substances. Before centrifugation at 2000x g for 8 min at room temperature, the blood was allowed to clot for at least 30 min.
Total cholesterol, HDL-C, LDL-C, triglycerides, and glucose (mmol/L) were analyzed on an Ortho Clinical DiagnosticsTM (Vitros 5.1TM FS; Clinical Chemistry Instruments, Raritan, NJ, USA). The method for all except LDL-C was dry chemistry (colorimetric method) according to the manufacturer instructions. LDL-C was analyzed on the same instruments but with wet chemistry (antibody reaction) reagents from the instrument manufacturer according to their instructions.
Insulin (mU/L) was analyzed on an Architect i2000SR instrument from Abbott (Abbot Park, IL, USA), with their reagent according to their instructions on antibody-based technologies. hs-CRP was analyzed with the method from Siemens20 (ADVIA 1800 Chemistry System; Upplands Väsby, Sweden).
HOMA-IR was calculated using Matthews’ mathematical equation, insulin (mU/mL)×glucose (mmol/L)/22.5. In the present study, the ratio HOMA-IR was used as a measure of insulin resistance.21
Oxygen uptake (VO2 max)
The subjects performed a submaximal exercise test to calculate VO2 max on a Monark 939E (Monark 939E; Monark Sports & Medical, Vansbro, Sweden). First, the height on the cycle saddle was adjusted to fit the subject and then an ECG device was attached to monitor heart rate (EC Sense; Cardiolex, Solna, Sweden). After questions about cycling capacity, the exercise test started on individually adjusted levels from 50 to 100 W. The cycling continued until a steady-state level was reached, and then the workload was increased by 25–50 W to reach the next steady-state level. After two steady-state levels with a heart rate above 130 on the first level and a second level above 150, the test was ended.22 VO2 max was calculated on a computerized program using the straight-line equation from the heart rates at the two steady states and the expected oxygen consumption per work rate.22 Maximal heart rate was estimated through the formula 220 − age in years.
Subjects were categorized as having low, normal, or high VO2 max according to reference values. Limits for VO2 max categories for women were low (≤31 mL/kg/min), normal (31.1–38.9 mL/kg/min), and high (≥39 mL/kg/min) VO2 max.12 For men, the limits were low (≤38 mL/kg/min), normal (38.1–50.9 mL/kg/min), and high (≥51 mL/kg/min) VO2 max.12
Handgrip strength
Muscle strength was assessed by Dynamometer (Baseline® HiRes™ hydraulic hand dynamometer; Fabrication Enterprices Inc, Irvington, NY, USA). Hand size was measured with a measuring tape, and the dynamometer was adjusted to the right size.23 The subjects sat with their arm at 90° and the dynamometer in the dominant hand. All subjects performed four measurements, one practice and three measurements with 1 min rest in-between. The result was calculated as an average of the measurements.
Subjects were categorized as having low, normal, or high muscular strength according to reference values. The limits for handgrip strength categories for women were low (≤22 kg), normal (22.1–34.9 kg), and high (≥35 kg) muscular strength. For men, the limits for handgrip strength categories were low (≤37 kg), normal (37.1–56.9 kg), and high (≥57 kg) muscular strength.13
Statistical methods
Statistical calculations were performed using IBM SPSS Statistics version 23.0 for Windows (IBM Corp, Armonk, NY, USA). All variables were checked for normality of distribution before the analysis. Differences between the sexes were analyzed by unpaired Student’s t-test. Descriptive data on nonparametric variables (ie, the dietary habits and the Wildman risk score) are presented as median and interquartile range (Q1–Q3). Descriptive data on quantitative variables are presented as mean and standard deviation (SD). Statistical analysis on qualitative variables was performed using Spearman’s correlation (rho) and Chi-2 test and Mann–Whitney U-test. Statistical analysis on quantitative variables was performed using Pearson’s correlation coefficient (r) and two-sample t-test. Level of significance was set at P<0.05 for all analyses.
Results
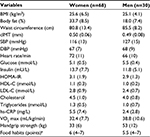
Of the 840 subjects who volunteered to participate, 6 subjects were excluded. The excluded subjects felt and described themselves as healthy, but answers to the questionnaire showed that they were diagnosed and treated for a chronic disease such as diabetes or Crohn’s disease. A total of 834 subjects (577 women and 257 men) were included in the study. In the LBA study, 10% of the participants reported having been born outside Sweden, and 24% of the participants reported to having at least one parent born outside Sweden. There are some missing values due to drop out before visit number 2 or technical difficulties. Results are missing as follows: waist circumference, 1 subject; cIMT 11, subjects; VO2 max, 13 subjects; HDL-C and cholesterol, 5 subjects; LDL-C, 6 subjects; triglycerides, 4 subjects; hs-CRP, 12 subjects; insulin, 19 subjects; glucose, 13 subjects; and HOMA-IR, 25 subjects. The basic characteristics of the subjects divided by sex are shown in Table 1.
Body composition
When divided by sex, BMI was distributed as follows: in women and men, 7.3% and 2.7% were classified as underweight, 77% and 70% had normal BMI (18.50–24.99), 13% and 25% were classified as overweight (25.00–29.99), and 3.5% and 2.3% were categorized as obese (≥30), respectively.
Food habits
In the study population, 24% scored 1–4 points, indicating unhealthy food habits with recommendation for improvement; 67% scored 5–8 points, indicating normal food habits; and 9.1% scored 9–12 points indicating healthy food habits. Compared with the general Swedish population, no statistical difference was found, P=0.65.
Handgrip strength
Handgrip strength showed that 3.3% of the women and 4.3% of the men were classified as having low muscular strength. A total of 49% of the women and 66% of the men had normal muscle strength for their sex and age. The remaining 47% of the women and 30% of the men were characterized as having high muscular strength.
Aerobic fitness measured as oxygen uptake, VO2 max
Low aerobic fitness was present in 26% of the subjects, 40% had normal, and 34% had high aerobic fitness measured as VO2 max (mL/kg/min). The results were divided by sex and distributed as follows: 22% of women had low VO2 max (≤31 mL/kg/min), 37% had normal VO2 max (31.1–38.9 mL/kg/min), and the remaining 41% had high VO2 max (≥39 mL/kg/min). Of men, 36% had low VO2 max (≤38 mL/kg/min), 44% had normal VO2 max (38.1–50.9 mL/kg/min), and 20% had high VO2 max (≥51 mL/kg/min).
cIMT
Average cIMT was 0.50±0.06 mm. In the female population, average cIMT was 0.49±0.06 mm, and in the male population 0.50±0.06 mm. In women, cIMT was correlated with food habits, r=0.09, P<0.05. In men, cIMT was correlated with handgrip, r=0.14, P<0.05. No correlation was found between cIMT and food habits in men, between cIMT and handgrip in women, or between cIMT and VO2 max in both women and men.
Subjects with early risk factors for atherosclerosis according to Wildman
In this young and healthy population of nonsmoking subjects, only four subjects had blood pressure >130/85 mmHg, 27 subjects (3.2%) had triglycerides ≥1.70 mmol/L, and 32 subjects (3.9%) had glucose ≥5.6 mmol/L. High hs-CRP >5.07 mg/L was found in 48 of 822 subjects, indicating that 5.8% of the participating subjects had early signs of general inflammation.
HOMA-IR >2.52 was found in 124 of 809 subjects, and thus 15% were defined as insulin resistant. In women, 201 of 572 subjects had HDL-C <1.30 mmol/L, and in men, 63 of 257 subjects had HDL-C <1.04, showing that in this young population 35% of the women and 25% of the men had HDL-C lower than recommended according to Wildman.14
In the study population, 98 subjects (12%) had two or more risk factors according to Wildman’s definition and are categorized as having a metabolic phenotype vulnerable to CVD, ie, a risk population, designated in the following text as “subjects at risk”. The characteristics of those subjects divided by sex are shown in Table 2. As expected, all variables included in the calculation of the Wildman score showed significant differences between subjects not at risk compared to those classified as being at risk, all P<0.01.
Characteristics of subjects at risk of atherosclerosis
Among the subjects at risk of atherosclerosis according to Wildman, 34% had unhealthy food habits, with recommendation for improvement, compared to 22% in the group of subjects not at risk. Furthermore, 5.1% of the subjects at risk were classified as having low muscle strength when measured by handgrip, as compared to 3.4% in the nonrisk population. Regarding aerobic fitness, 44% of the women and 53% of the men in the risk population had low oxygen uptake (VO2 max in mL/kg/min), compared to 19% of the women and 33% of the men in the nonrisk population. A comparison between subjects at risk and subjects not at risk is presented in Table 3.
In the total studied population, 47% of the subjects with a metabolic abnormal phenotype, ie, persons at risk of CVD, had low aerobic capacity as compared to 23% in the nonrisk group, P<0.001.
When the groups were compared, no significant difference in cIMT was observed between subjects at risk of atherosclerosis and subjects without a metabolic vulnerable phenotype according to Wildman.
Discussion
The main finding in the present study was that high aerobic fitness was associated with low CVD risk. In the study population, 15% were insulin resistant (HOMA-IR), and 35% of the women and 25% of the men had HDL-C lower than recommended. When using Wildman’s definition, 12% of the subjects were classified as being at risk of CVD.
One of the rationales for this study was to evaluate food habits in a young Swedish population, consisting mostly of students at Örebro University. In a previous large study, the Västerbotten Intervention Program in the WHO MONICA project, an increase in BMI had been observed in the Swedish population. The increase started in 2004 and was associated with an increase in the intake of saturated lipids.9 In the present study, 24% of the subjects scored low on the index of food habits, as measured by points from the Swedish National Food Agency test. The low score may be caused by a high intake of saturated lipids but could also depend on lack of fiber, vegetables, or fruit. However, no significant difference in food habits was observed between subjects at risk of atherosclerosis according to Wildman and subjects not at risk. The dietary habits of the study population need to be further elucidated in the future.
In the present study, fitness was measured as VO2 max and handgrip strength. Less than 5% were characterized as having low muscle strength, and >47% of the women and 30% of the men were classified as having high muscle strength compared to age- and sex-specific reference values.13 This was an expected result since data from the questionnaires indicated that strength training is popular in this age group. When handgrip strength was compared between subjects at risk and subjects not at risk of atherosclerosis, we observed a difference between the groups, but only in the female population. Women at risk of atherosclerosis and CVD had significantly lower muscular strength as compared to the women not at risk. One explanation for the lack of difference between the groups in the male population could be the lower number of male subjects in the LBA study.
From a previous large-scale study at the Swedish School of Sport and Health Science, it is known that aerobic fitness is associated with reduced cardiovascular risk in Swedish subjects aged 20–65 years.24 In the LBA study, aerobic fitness measured as maximal oxygen uptake (mL/kg/min) differed between the subjects at risk of atherosclerosis and the nonrisk population. In the subjects with a metabolic abnormal phenotype, ie, at risk, 47% had low aerobic fitness measured as VO2 max compared to 23% in the healthy part of the studied population, P<0.001. The findings suggest that aerobic fitness VO2 max has a beneficial effect on early risk factors for atherosclerosis.
Furthermore, we found that 15% of the study population was already insulin resistant before the age of 26. There were also a large number of persons with dyslipidemia in the form of low HDL-C: 35.1% of the women and 25% of the men had HDL-C lower than recommended. This is in line with the study by Dalleck and Kjelland who studied the prevalence of metabolic syndrome in college students aged 18–24 years in USA. They found that 6.8% of the students suffered from metabolic syndrome. From their results, they concluded that there is a need for primary prevention programs at universities and colleges in USA.25
A study of university students in Brazil found similarly that many young subjects have metabolic syndrome and low HDL-C. They found that 21% of the students were insulin resistant and that as many as 61% had low HDL-C.5 A problem with this type of studies is that there are several definitions of insulin resistance. In the study from Brazil, insulin resistance was defined as HOMA-IR >2.7, while in the present study >2.52 mmol/L was used as the limit. A direct comparison between data is therefore difficult. Despite this, the Brazilian study found even more insulin-resistant subjects than the LBA study. They also found more subjects with low HDL-C as compared to the LBA study. One explanation could be lifestyle differences between Brazil and Sweden. Another explanation could be the age of the subjects: in the study from Brazil, the age of the subjects was 20–30 years. From their results, they concluded that the burden of CVD will be increased in the future.5
To reduce the risk of CVD it is recommended to have HDL-C values above 1.5 mmol/L.26 It is worrying that a young population, more than 30% of the participants in the present study, has HDL-C levels lower than recommended. Nevertheless, according to Wildman’s definition14 used in this study, one biomarker is not enough for a subject to be diagnosed with a metabolically abnormal phenotype. Low HDL-C in combination with high blood pressure, triglycerides, glucose, CRP, or HOMA-IR was however present in 98 of 834 subjects (68 women and 30 men, 12%). A weakness in the present study was that there were more than twice as many female subjects, and this is reflected in the lower number of male subjects at risk. Women showed a greater interest in participation. From a family background perspective, the LBA subjects represent the source population very well. The age group-specific numbers for being born outside Sweden are identical (10%) and having at least one parent born outside Sweden is 25% for Sweden as a whole and 24% for the LBA study population.27
cIMT is a generally accepted marker of subclinical atherosclerosis in young adults. In several studies, it has been found that cIMT progression was associated with obesity and low physical activity.2,28 In the LBA study, physical activity was not presented and no correlation was found between aerobic fitness expressed as VO2 max (mL/kg/min) and cIMT. The reason for the difference between the referred studies and the LBA study is not known. One explanation could be that the majority of the subjects in the LBA study, especially the women, had normal BMI and were well trained. Despite the strong relationship between cIMT and CVD risk reported by previous studies,2,29,30 aerobic fitness appears to have a modest effect on cIMT in young subjects.31,32
In the HELENA study, adolescents 12.5–17.5 years from 10 European centers were studied, and it was concluded that prevention strategies should focus not only on decreasing fatness and increasing cardiorespiratory fitness but also on enhancing muscular strength.33 It has also been shown in the HELENA study that muscular and cardiorespiratory fitness are independently associated.34 Results from the present study support the conclusions from the studies by Dalleck,25 Barbosa,5 and the HELENA study34 that CVD may increase in the future, and that there is need for early prevention aiming to increase both muscle strength and aerobic fitness in young subjects to prevent CVD. If aerobic capacity is regained, it may have a beneficial effect on cardiometabolic health in the future.35
Using Wildman’s definition, 12% of the young healthy Swedish subjects were classified as being at risk of CVD. Food habits did not differ between those at risk and those not at risk. Aerobic fitness measured as VO2 max (mL/kg/min) did however differ: 47% of subjects at risk had low aerobic fitness compared to 23% of the nonrisk subjects, P<0.001. In the present study, no difference in the questionnaire score on food habits was observed compared to the general Swedish population.
Conclusion
High aerobic fitness is associated with low CVD risk in Swedish young adults. The high prevalence of young adults observed with unfavorable levels of HDL-C and HOMA-IR raises concerns about future CVD risk. Our results highlight an urgent need for increased counseling about healthy lifestyle and intervention programs directed toward young adults.
Acknowledgments
We thank all the volunteers who participated in the study. We also thank those who contributed to the collection and processing of data, especially Madelene Johansson, Katya Matusevich, Eva Oskarsson, Natalie Stjernström, and Håkan Wennlöf. The study was supported by grant from AFA insurance.
Disclosure
The authors report no conflicts of interest in this work.
References
Naghavi M, Wang H, Lozano R, et al; GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385(9963):117–171. | ||
Juonala M, Viikari JSA, Kahonen M, et al. Life-time risk factors and progression of carotid atherosclerosis in young adults: the Cardiovascular Risk in Young Finns study. Eur Heart J. 2010;31(14):1745–1751. | ||
Mansikkaniemi K, Juonala M, Taimela S, et al. Cross-sectional associations between physical activity and selected coronary heart disease risk factors in young adults. The Cardiovascular Risk in Young Finns Study. Ann Med. 2012;44(7):733–744. | ||
Gordon B, Chen S, Durstine JL. The effects of exercise training on the traditional lipid profile and beyond. Curr Sports Med Rep. 2014;13(4):253–259. | ||
Barbosa JB, Santos AM, Barbosa MM, et al. Metabolic syndrome, insulin resistance and other cardiovascular risk factors in university students. Cien Saude Colet. 2016;21(4):1123–1136. | ||
Libby P, Lichtman AH, Hansson GK. Immune effector mechanisms implicated in atherosclerosis: from mice to humans. Immunity. 2013;38(6):1092–1104. | ||
Dias JA, Wirfalt E, Drake I, et al. A high quality diet is associated with reduced systemic inflammation in middle-aged individuals. Atherosclerosis. 2015;238(1):38–44. | ||
Palmefors H, DuttaRoy S, Rundqvist B, Borjesson M. The effect of physical activity or exercise on key biomarkers in atherosclerosis – a systematic review. Atherosclerosis. 2014;235(1):150–161. | ||
Johansson I, Nilsson LM, Stegmayr B, Boman K, Hallmans G, Winkvist A. Associations among 25-year trends in diet, cholesterol and BMI from 140,000 observations in men and women in Northern Sweden. Nutr J. 2012;11:40. | ||
Ross R, Blair SN, Arena R, et al; American Heart Association Physical Activity Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Cardiovascular and Stroke Nursing; Council on Functional Genomics and Translational Biology; Stroke Council. Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation. 2016;134(24):E653–E699. | ||
Livsmedelsverket [webpage on the Internet]. Matvanekollen (in Swedish). Available from: https://www.livsmedelsverket.se/matvanor-halsa--miljo/kostrad-och-matvanor/matvanekollen/. Accessed February 6, 2017. | ||
Oja P, Tuxworth B. Eurofit for Adults: Assessment of Health-related Fitness. Strasbourg: Council of Europe. Committee for the Development of Sports; 1995. | ||
Montalcini T, Migliaccio V, Yvelise F, et al. Reference values for handgrip strength in young people of both sexes. Endocrine. 2013;43(2):342–345. | ||
Wildman RP, Muntner P, Reynolds K, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering – prevalence and correlates of 2 phenotypes among the US population (NHANES 1999-2004). Arch Intern Med. 2008;168(15):1617–1624. | ||
Taft C, Karlsson J, Sullivan M. Performance of the Swedish SF-36 version 2.0. Qual Life Res. 2004;13(1):251–256. | ||
Lohamn T, Roche A, Martorell R. Anthropometric Standardization Reference Manual. Champaign, IL: Human Kinetics; 1988. | ||
World Health Organization. Global Database on Body Mass Index. Available from: http://apps.who.int/bmi/index.jsp. Accessed February 6, 2017. | ||
Touboul PJ, Hennerici MG, Meairs S, et al. Mannheim carotid intima-media thickness and plaque consensus (2004–2006–2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc Dis. 2012;34(4):290–296. | ||
Stein JH, Korcarz CE, Hurst RT, et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiography. 2008;21(2):93–111. quiz 189–190. | ||
Siemens [webpage on the Internet]. CardioPhase hsCRP. Available from: https://www.healthcare.siemens.se/point-of-care/poc-cardiac-topics/cardiac-assays/cardiophase-hscrp. Accessed September 17, 2016. | ||
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. | ||
Astrand PO. Quantification of exercise capability and evaluation of physical capacity in man. Prog Cardiovasc Dis. 1976;19(1):51–67. | ||
Ruiz JR, Espana-Romero V, Ortega FB, Sjostrom M, Castillo MJ, Gutierrez A. Hand span influences optimal grip span in male and female teenagers. J Hand Surg Am. 2006;31(8):1367–1372. | ||
Ekblom-Bak E, Hellenius ML, Ekblom O, Engstrom LM, Ekblom B. Fitness and abdominal obesity are independently associated with cardiovascular risk. J Intern Med. 2009;266(6):547–557. | ||
Dalleck LC, Kjelland EM. The prevalence of metabolic syndrome and metabolic syndrome risk factors in college-aged students. Am J Health Promot. 2012;27(1):37–42. | ||
Mayo Clinic [webpage on the Internet]. High cholesterol. Available from: http://www.mayoclinic.org/diseases-conditions/high-blood-cholesterol/diagnosis-treatment/diagnosis/dxc-20181913. Accessed September 14, 2016. | ||
Statistics Sweden [webpage on the Internet]. Population Statistics (31 Dec 2015). Available from: http://www.statistikdatabasen.scb.se. Accessed January 24, 2017. | ||
Ascenso A, Palmeira A, Pedro LM, Martins S, Fonseca H. Physical activity and cardiorespiratory fitness, but not sedentary behavior, are associated with carotid intima-media thickness in obese adolescents. Eur J Pediatr. 2016;175(3):391–398. | ||
Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;115(4):459–467. | ||
Nambi V, Chambless L, Folsom AR, et al. Carotid intima-media thickness and presence or absence of plaque improves prediction of coronary heart disease risk: the ARIC (atherosclerosis risk in communities) study. J Am Coll Cardiol. 2010;55(15):1600–1607. | ||
Thijssen DHJ, Cable NT, Green DJ. Impact of exercise training on arterial wall thickness in humans. Clin Sci (Lond). 2012;122(7):311–322. | ||
Hellsten Y, Nyberg M. Cardiovascular adaptations to exercise training. Compr Physiol. 2015;6(1):1–32. | ||
Jimenez-Pavon D, Ortega FB, Valtuena J, et al. Muscular strength and markers of insulin resistance in European adolescents: the HELENA Study. Eur J Appl Physiol. 2012;112(7):2455–2465. | ||
Artero EG, Ruiz JR, Ortega FB, et al; HELENA Study Group. Muscular and cardiorespiratory fitness are independently associated with metabolic risk in adolescents: the HELENA study. Pediatr Diabetes. 2011;12(8):704–712. | ||
Laitinen TT, Pahkala K, Magnussen CG, et al. Lifetime measures of ideal cardiovascular health and their association with subclinical atherosclerosis: The Cardiovascular Risk in Young Finns Study. Int J Cardiol. 2015;185:186–191. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.