Back to Journals » OncoTargets and Therapy » Volume 9
AEG-1 as a predictor of sensitivity to neoadjuvant chemotherapy in advanced epithelial ovarian cancer
Authors Wang Y, Jin X, Song H, Meng F
Received 17 December 2015
Accepted for publication 2 March 2016
Published 20 April 2016 Volume 2016:9 Pages 2385—2392
DOI https://doi.org/10.2147/OTT.S102648
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Min Li
Yao Wang,1,* Xin Jin,2,* Hongtao Song,3 Fanling Meng4
1Department of Gynecology and Obstetrics, 2Department of Intensive Care Unit, The General Hospital of Daqing Oil Field, Daqing, 3Department of Pathology, 4Department of Gynecology, The Affiliated Tumor Hospital, Harbin Medical University, Harbin, People’s Republic of China
*These authors contributed equally to this work
Objectives: Astrocyte elevated gene-1 (AEG-1) plays a critical role in tumor progression and chemoresistance. The aim of the present study was to investigate the protein expression of AEG-1 in patients with epithelial ovarian cancer (EOC) who underwent debulking surgery after neoadjuvant chemotherapy (NAC).
Materials and methods: The protein expression of AEG-1 was analyzed using immunohistochemistry in 162 patients with EOC. The relationship between AEG-1 expression and chemotherapy resistance was assessed using univariate and multivariate logistic regression analyses with covariate adjustments.
Results: High AEG-1 expression was significantly associated with the International Federation of Gynecology and Obstetrics stage, age, serum cancer antigen-125 concentration, histological grade, the presence of residual tumor after the interval debulking surgery, and lymph node metastasis. Furthermore, AEG-1 expression was significantly higher in NAC-resistant disease than in NAC-sensitive disease (P<0.05). Multivariate analyses indicated that elevated AEG-1 expression predicted poor survival.
Conclusion: Our findings indicate that AEG-1 may be a potential new biomarker for predicting chemoresistance and poor prognoses in patients with EOC.
Keywords: AEG-1, epithelial ovarian cancer, neoadjuvant chemotherapy, interval debulking surgery
Introduction
Epithelial ovarian cancer (EOC) is the most common type of ovarian cancer and the leading cause of mortality among gynecological malignancies. Despite the fact that rapid advances have been made in surgical methods and new chemotherapeutic regimens, the 5-year survival rate for patients with EOC remains <30%.1,2 Currently, the standard therapy regimen for ovarian cancer is cytoreductive surgery combined with platinum-based chemotherapy. In recent years, neoadjuvant chemotherapy (NAC) followed by cytoreduction surgery has been proposed as an alternative approach for treating women with advanced stage EOC with the aim of improving surgical efficiency and the quality of life of patients.3,4 However, resistance to chemotherapeutic agents is a common occurrence that is often attributed to poor response and prognosis. Several studies have investigated the biomarkers that distinguish patients with EOC according to their response to NAC. However, the molecular basis that underlies NAC resistance is largely undefined. Therefore, it is necessary to identify reliable predictive biomarkers for responsiveness to NAC and to address the issue of drug resistance.
Astrocyte elevated gene-1 (AEG-1) was originally cloned as a novel gene that is induced in primary human fetal astrocytes. Recent findings suggest that AEG-1 plays a dominant role in the development and progression of diverse cancers including colorectal cancer,5 pancreatic ductal adenocarcinoma,6 prostate cancer,7 gastric carcinoma,8 rectal cancer,9 hepatocellular carcinoma,10 non-small-cell lung cancer,11 and cervical carcinoma.12 AEG-1 has also been reported to be associated with chemotherapy-associated drug resistance in several cases of cancer.13 In addition, our previous studies have demonstrated that AEG-1 overexpression is associated with a poor prognosis and cisplatin resistance in patients with stage III–IV serous ovarian carcinoma.14 However, this is not the case in patients treated with NAC, and the role of AEG-1 in NAC resistance remains unclear. Therefore, in the present study, we aimed to investigate the expression of AEG-1 in patients with EOC who were treated with debulking surgery after NAC and to define its significance in predicting prognoses.
Materials and methods
Patient population
This retrospective study was approved by the Ethical Committee of the Affiliated Tumor Hospital of Harbin Medical University, Harbin, People’s Republic of China. All patients provided written informed consent. Paraffin-embedded tumor specimens were obtained from 162 patients who had NAC followed by debulking surgery between September 2008 and July 2010 in the Affiliated Tumor Hospital of Harbin Medical University. The initial diagnosis of EOC was made using a core needle biopsy. Core needle biopsy specimens were obtained from all patients before NAC and tissue sections were embedded with paraffin. Tumor stages were assessed based on the staging system of the International Federation of Gynecology and Obstetrics (FIGO). Histological grades were determined based on the World Health Organization classification standards.
The principles of chemotherapy instruct us to follow the National Comprehensive Cancer Network guidelines that were published in 2009.15 Twenty-seven patients had distant metastases, as indicated by surgical and pathological findings. The metastasis locations were as follows: lung (nine cases), pleura (seven cases), brain (five cases), breast (four cases), and bone (two cases). All patients received three cycles of NAC before surgery. The NAC regimen consisted of either cisplatin/carboplatin plus paclitaxel or carboplatin plus docetaxel. The surgeries were performed between 3 and 6 weeks after the last cycle of NAC was administered.
The patients were followed up every 3 months for 2 years and then every 6 months for the next 3 years using clinical and radiological examinations and by monitoring the serum levels of cancer antigen 125 (CA-125). The median follow-up duration was 39 months (range 8–78 months).
Assessment of clinical responses to NAC
Responses to chemotherapy were determined according to the Response Evaluation Criteria in Solid Tumors (version 1.0).16 The size of the primary tumor, as measured by magnetic resonance imaging, was recorded before treatment. The primary tumor was measured across its greatest diameter. The clinical response was classified as complete response, partial response, stable disease, or progressive disease. The first two categories, complete response and partial response, were judged as effective (chemotherapy sensitive). Chemotherapy resistance was defined as stable disease and progressive disease.
Immunohistochemical staining
Biopsy specimens were taken before NAC. We evaluated core biopsy samples from 162 advanced patients with EOC enrolled in this study. AEG-1 expression was detected using immunohistochemistry in paraffin-embedded specimens. Immunohistochemistry staining was performed on paraffin sections using the avidin–biotin immunoperoxidase technique, according to the manufacturer’s instructions. Tissue sections were dewaxed in xylene and rehydrated using standard procedures. After they were washed in phosphate-buffered saline (three times for 5 minutes each), endogenous peroxidase was blocked by treating the tissues with 3% H2O2 for 30 minutes at room temperature. The slides were incubated with a primary antibody against human AEG-1 (dilution 1:50; Abcam, Cambridge, UK) overnight at 4°C. After the slides were washed with phosphate-buffered saline, the sections were incubated with a biotin-labeled secondary antibody (Santa Cruz Biotechnology Inc., Dallas, TX, USA). Finally, the sections were immersed in diaminobenzidine (Dako Denmark A/S, Glostrup, Denmark) and then counterstained with hematoxylin. Negative control slides were stained with rabbit serum instead of primary antibody.
Evaluation procedures
The expression levels of AEG-1 were semiquantitatively classified based on the total combined scores for the percentage of positively stained tumor cells and the staining intensity. The staining was scored according to the number of positive tumor cells as follows: 0, no positive tumor cells; 1, <10% positive tumor cells; 2, 10%–50% positive tumor cells; and 3, >50% positive tumor cells. Staining intensity was scored as follows: score 0 (no staining), 1 (weak staining), 2 (moderate staining), and 3 (strong staining) in at least five different high-power fields. The final score for AEG-1 expression was defined as “low expression” if the product of the positive score and the corresponding intensity score was <3.
The evaluation procedure was performed twice by two independent pathologists who were blinded to the clinicopathological information and the corresponding hematoxylin and eosin slides of the patients.
Statistical analysis
We used χ2 tests or Fisher’s exact tests to assess differences in categorical variables. The relationship between AEG-1 overexpression and chemotherapy resistance was assessed using univariate and multivariate logistic regression with covariate adjustment. Overall survival (OS) and disease-free survival (DFS) were measured using the Kaplan–Meier method with a log-rank test. Finally, a Cox proportional hazards regression was performed in the multivariate analysis of prognostic predictors. All statistical analyses were performed using Statistical Package for the Social Sciences 21.0 software (IBM Corporation, Armonk, NY, USA). P-values were considered statistically significant when <0.05.
Results
Patient characteristics
The demographic characteristics of the 162 patients with EOC are summarized in Table 1. The 162 ovarian cancer cases included 118 (72.8%) cases of serous ovarian carcinoma and 44 (27.2%) cases of nonserous ovarian carcinoma. A total of 135 patients had EOC that was classified as FIGO stage III, and 27 had FIGO stage IV EOC. The tumor size after cytoreductive surgery was smaller than 1 cm in 120 (74.1%) of the patients. The serum CA-125 concentration was >35 U mL−1 in 107 (66.0%) of the patients. Lymph node metastases were found in 45 (27.8%) of the patients.
AEG-1 expression
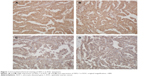
AEG-1 was localized in the cytoplasm of tumor cells (Figure 1). Of the 162 EOC samples that were analyzed, low AEG-1 expression was observed in 54 (33.3%) patients and high AEG-1 expression in 108 (66.7%) patients. AEG-1 expression was significantly associated with the FIGO stage, age, serum CA-125 concentration, histological grade, the presence of residual tumor after the interval debulking surgery, and tumor lymph node metastasis, but not histological type (Table 1).
The effect of AEG-1 overexpression on prognosis in patients with EOC who received NAC
In a univariate Kaplan–Meier analysis, high levels of AEG-1 expression were associated with poor OS or DFS in patients with EOC (P<0.001) (Figure 2A and B; Table 2).
The multivariate Cox regression analysis demonstrated that AEG-1 expression, FIGO stage, and large residual tumor size were independent prognostic factors for both OS and DFS (P<0.001) (Table 3).
Effect of AEG-1 overexpression on NAC resistance in EOC
Among the 162 patients with EOC included in this study, 57 exhibited chemotherapy resistance and 105 exhibited chemotherapy sensitivity. Among the 57 patients with chemotherapy resistance, AEG-1 was overexpressed in 48 (84.2%) patients. Drug resistance to NAC was significantly associated with AEG-1 expression, histological grade, FIGO stage, and residual tumor size (P<0.05) (Table 4).
The results of the multivariate analysis showed that AEG-1 overexpression was independently associated with chemotherapy resistance (odds ratio: 3.325; 95% confidence interval: 1.153–9.593; P=0.026) (Table 5).
Discussion
AEG-1 plays an important role in multiple mechanisms of tumorigenesis, including proliferation, invasion, apoptosis, angiogenesis, and cell survival.17 In addition, AEG-1 regulates tumor cell sensitivity to chemotherapy and radiation therapy.11 Our previous studies demonstrated that AEG-1 was overexpressed in human EOC and that it was significantly associated with clinicopathological features, such as FIGO stage, histological grade, and the presence of residual tumor after interval debulking surgery.18 We also found that AEG-1 functions as a vital regulator through which miR-137 acts to inhibit cancer growth.19 We also revealed that AEG-1 is related to cisplatin resistance in patients with stage III–IV serous ovarian carcinoma.14 These findings indicate that AEG-1 might be an important proto-oncogene during the development of EOC.
NAC is regarded as an efficient treatment for many locally advanced human cancers, including breast and gastrointestinal cancers and bone and soft tissue malignancies.20–22 Currently, NAC is supported by the National Comprehensive Cancer Network guidelines for patients with advanced ovarian cancer. However, the value of AEG-1 as a predictor of chemosensitivity to NAC in human cancer has rarely been addressed. This study was conducted to analyze the expression of AEG-1 in patients with EOC who were treated with NAC and to determine the correlation between AEG-1 expression and chemosensitivity and survival. This is the first report to show that AEG-1 is overexpressed in patients with EOC who received NAC and is positively correlated with FIGO stage, age, serum CA-125 concentration, histological grade, residual tumor size after the primary surgery, tumor lymph node metastasis, and poor prognosis. High AEG-1 expression plays a critical role in EOC progression and may therefore serve as an important biomarker for predicting chemosensitivity to NAC. The protein expression of AEG-1 has also been explored and found to be correlated with clinicopathologic factors and poor prognosis in various cancers such as oral squamous cell carcinoma,23 primary gallbladder carcinoma,24 renal carcinoma,25 tongue carcinoma,26 gastric cancer,27 pancreatic ductal adenocarcinoma,27 and hepatocellular carcinoma.22 Our results are consistent with previous findings regarding the role of AEG-1 in tumor progression in various cancers. All studies performed to date suggest that AEG-1 plays an important biological role in carcinogenesis and tumor progression.
Recently, AEG-1 has been identified as a marker that contributes to the sensitivity of tumor cells to chemotherapy and radiation therapy. The AEG-1 DNA copy number was found to be inversely correlated with sensitivity to chemotherapeutic agents in the first report that examined the role of AEG-1 in chemoresistance.28 Lu et al11 also showed that patients with low AEG-1 expression in non-small-cell lung cancer experienced the best results from both postoperative chemotherapy and radiotherapy. Follow-up studies have revealed that AEG-1 overexpression confers resistance to several chemotherapeutic drugs, including 5-fluorouracil, doxorubicin, paclitaxel, and cisplatin.13 In the present study, we identified an association between AEG-1 and NAC resistance and found that AEG-1 expression is an independent predictor of NAC resistance, which implies that AEG-1 plays a crucial role in chemotherapy resistance.
Some clues that help to explain the mechanisms by which AEG-1 mediates chemotherapy resistance have recently been discovered. These clues include evidence showing the involvement of AEG-1 in the inhibition of apoptosis, protective autophagy, the activation of the transcription factor nuclear factor kappa-light-chain-enhancer of activated B cells, the regulation of translation, the inhibition of stress granule formation, the regulation of gene silencing, and the regulation of the tumor microenvironment.13 Another potential mechanism has also been reported through which AEG-1 might contribute to drug resistance by facilitating the translation of the multidrug resistance gene 1.29 In addition, AEG-1 has a newly identified role as an RNA-binding protein. All of these studies indicate that AEG-1 regulates the expression of a collection of multidrug resistance-related genes. Finally, the effects of AEG-1 on drug resistance in various cancers may also be mediated by microRNAs.30
The fact that AEG-1 plays important roles in drug resistance suggests that AEG-1 is a potential target for anticancer therapies. At the present time, there are some reports showing that therapies can target AEG-1. For example, Hu et al28 showed that inhibiting AEG-1 expression using RNA interference reduced lung metastasis in a xenograft model of breast cancer lung metastasis, and Qian et al showed that AEG-1-based DNA vaccines act as an effective treatment for prostate cancer by inhibiting tumor growth and producing better prognoses in carcinoma-bearing animals.31 These data indicate that AEG-1 is a potential novel agent for therapies to treat EOC.
In conclusion, AEG-1 is overexpressed in patients with EOC, and high AEG-1 expression was a good predictor when estimating responsiveness to NAC and poor prognoses in patients with EOC. Confirmation of this pilot study in larger multicenter studies is needed.
Acknowledgments
We express our thanks to Dr H-T Song for the evaluation procedures.
This work was supported by grants of the National Natural Science Foundation of China (81201613), the Specialized Research Fund for the Doctoral Program of Higher Education (20122307120027), the Postdoctoral Foundation of Heilongjiang Province of China (LBH-Z11067), the scientific research project of Health Department of Heilongjiang Province (663), the Haiyan Foundation of the Affiliated Tumor Hospital of Harbin Medical University/the Foundation of the Affiliated Tumor Hospital of Harbin Medical University (JJZ2011-04) and the Research Fund for the Xiansheng Anti tumor vascular targeted therapy of CSCO (Y-S2015-003). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351:2519–2529. | ||
Jemal A, Tiwari RC, Murray T, et al. Cancer statistics, 2004. CA Cancer J Clin. 2004;54:8–29. | ||
Schwartz PE, Zheng W. Neoadjuvant chemotherapy for advanced ovarian cancer: the role of cytology in pretreatment diagnosis. Gynecol Oncol. 2003;90:644–650. | ||
Vergote I, Trope CG, Amant F, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363:943–953. | ||
Huang S, Wu B, Li D, et al. Knockdown of astrocyte elevated gene-1 inhibits tumor growth and modifies microRNAs expression profiles in human colorectal cancer cells. Biochem Biophys Res Commun. 2014;444:338–345. | ||
Huang Y, Ren GP, Xu C, et al. Expression of astrocyte elevated gene-1 (AEG-1) as a biomarker for aggressive pancreatic ductal adenocarcinoma. BMC Cancer. 2014;14:479. | ||
Bhatnagar A, Wang Y, Mease RC, et al. AEG-1 promoter-mediated imaging of prostate cancer. Cancer Res. 2014;74:5772–5781. | ||
Dong L, Qin S, Li Y, et al. High expression of astrocyte elevated gene-1 is associated with clinical staging, metastasis, and unfavorable prognosis in gastric carcinoma. Tumour Biol. 2015;36:2169–2178. | ||
Wang P, Yin B, Shan L, et al. RNA interference-mediated knockdown of astrocyte elevated gene-1 inhibits growth, induces apoptosis, and increases the chemosensitivity to 5-fluorouracil in renal cancer Caki-1 cells. Mol Cells. 2014;37:857–864. | ||
Srivastava J, Robertson CL, Gredler R, et al. Astrocyte Elevated Gene-1 (AEG-1) contributes to nonthyroidal illness syndrome (NTIS) associated with hepatocellular carcinoma (HCC). J Biol Chem. 2015;290(25):15549–15558. | ||
Lu S, Xu J, Xu X, Hu S, Li B, Li W. The expression of astrocyte elevated gene-1 in human non-small cell lung cancer and its relationship with postoperative chemotherapy and radiotherapy. Histopathology. 2015;67(6):817–826. | ||
Yu JQ, Zhou Q, Zhu H, Zheng FY, Chen ZW. Overexpression of astrocyte elevated gene-1 (AEG-1) in cervical cancer and its correlation with angiogenesis. Asian Pac J Cancer Prev. 2015;16:2277–2281. | ||
Meng X, Thiel KW, Leslie KK. Drug resistance mediated by AEG-1/MTDH/LYRIC. Adv Cancer Res. 2013;120:135–157. | ||
Li C, Li Y, Wang X, et al. Elevated expression of astrocyte elevated gene-1 (AEG-1) is correlated with cisplatin-based chemoresistance and shortened outcome in patients with stages III–IV serous ovarian carcinoma. Histopathology. 2012;60:953–963. | ||
Morgan RJ AR, Armstrong DK, et al. Ovarian cancer including fallopian tube cancer and primary peritoneal cancer. NCCN Clinical Practice Guidelines in Oncology. 2009. Available from: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed April 11, 2016. | ||
Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. | ||
Hu G, Wei Y, Kang Y. The multifaceted role of MTDH/AEG-1 in cancer progression. Clin Cancer Res. 2009;15:5615–5620. | ||
Meng F, Luo C, Ma L, Hu Y, Lou G. Clinical significance of astrocyte elevated gene-1 expression in human epithelial ovarian carcinoma. Int J Gynecol Pathol. 2011;30:145–150. | ||
Guo J, Xia B, Meng F, Lou G. miR-137 suppresses cell growth in ovarian cancer by targeting AEG-1. Biochem Biophys Res Commun. 2013;441:357–363. | ||
King TA, Morrow M. Surgical issues in patients with breast cancer receiving neoadjuvant chemotherapy. Nat Rev Clin Oncol. 2015;12(6):335–343. | ||
Smith JJ, Garcia-Aguilar J. Advances and challenges in treatment of locally advanced rectal cancer. J Clin Oncol. 2015;33(16):1797–1808. | ||
Yaffee P, Osipov A, Tan C, Tuli R, Hendifar A. Review of systemic therapies for locally advanced and metastatic rectal cancer. J Gastrointest Oncol. 2015;6:185–200. | ||
Xia X, Du R, Zhao L, Sun W, Wang X. Expression of AEG-1 and microvessel density correlates with metastasis and prognosis of oral squamous cell carcinoma. Human Pathol. 2014;45:858–865. | ||
Sun W, Fan YZ, Xi H, Lu XS, Ye C, Zhang JT. Astrocyte elevated gene-1 overexpression in human primary gallbladder carcinomas: an unfavorable and independent prognostic factor. Oncol Rep. 2011;26:1133–1142. | ||
Erdem H, Oktay M, Yildirim U, Uzunlar AK, Kayikci MA. Expression of AEG-1 and p53 and their clinicopathological significance in malignant lesions of renal cell carcinomas: a microarray study. Pol J Pathol. 2013;64:28–32. | ||
Ke ZF, He S, Li S, Luo D, Feng C, Zhou W. Expression characteristics of astrocyte elevated gene-1 (AEG-1) in tongue carcinoma and its correlation with poor prognosis. Cancer Epidemiol. 2013;37:179–185. | ||
Li G, Wang Z, Ye J, et al. Uncontrolled inflammation induced by AEG-1 promotes gastric cancer and poor prognosis. Cancer Res. 2014;74:5541–5552. | ||
Hu G, Chong RA, Yang Q, et al. MTDH activation by 8q22 genomic gain promotes chemoresistance and metastasis of poor-prognosis breast cancer. Cancer Cell. 2009;15:9–20. | ||
Yoo BK, Chen D, Su ZZ, et al. Molecular mechanism of chemoresistance by astrocyte elevated gene-1. Cancer Res. 2010;70:3249–3258. | ||
Santhekadur PK, Das SK, Gredler R, et al. Multifunction protein staphylococcal nuclease domain containing 1 (SND1) promotes tumor angiogenesis in human hepatocellular carcinoma through novel pathway that involves nuclear factor kappaB and miR-221. J Biol Chem. 2012;287:13952–13958. | ||
Qian BJ, Yan F, Li N, et al. MTDH/AEG-1-based DNA vaccine suppresses lung metastasis and enhances chemosensitivity to doxorubicin in breast cancer. Cancer Immunol Immunother. 2011;60(6):883–893. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.