Back to Journals » International Journal of Nanomedicine » Volume 18
Advances in the Study of Bioactive Nanoparticles for the Treatment of HCC and Its Postoperative Residual Cancer
Authors Li Y, Zou H, Zheng Z, Liu Z, Hu H, Wu W, Wang T
Received 25 November 2022
Accepted for publication 4 May 2023
Published 22 May 2023 Volume 2023:18 Pages 2721—2735
DOI https://doi.org/10.2147/IJN.S399146
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yan Shen
Yanxu Li,1 Hao Zou,2,* Zekun Zheng,2 Zhuoheng Liu,2,* Huiyuan Hu,2,* Wei Wu,3 Tao Wang4
1Medical College of Yangzhou University, Yangzhou University, Yangzhou City, Jiangsu Province, People’s Republic of China; 2Dalian Medical University, Affiliated Hospital of Yangzhou University, Yangzhou City, Jiangsu Province, People’s Republic of China; 3Affiliated Hospital of Yangzhou University, Yangzhou University, Yangzhou City, Jiangsu Province, People’s Republic of China; 4College of Veterinary Medicine, Yangzhou University, Yangzhou City, Jiangsu Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Wei Wu; Tao Wang, Tel/Fax +86-0514-87991201 ; +86-187 5255 5147, Email [email protected]; [email protected]
Abstract: Primary hepatocellular carcinoma (HCC, hepatocellular carcinoma) is the third leading cause of tumor death in the world and the second leading cause in China. The high recurrence rate at 5 years after surgery also seriously affects the long-term survival of HCC patients. For reasons such as poor liver function, large tumors, or vascular invasion, only relatively limited palliative treatment is available. Therefore, effective diagnostic and therapeutic strategies are needed to improve the complex microenvironment and block the mechanism of tumor development in order to treat the tumor and prevent recurrence. A variety of bioactive nanoparticles have been shown to have therapeutic effects on hepatocellular carcinoma and have the advantages of improving drug solubility, reducing drug side effects, preventing degradation in the blood, increasing drug exposure time, and reducing drug resistance. The development of bioactive nanoparticles is expected to complete the current clinical therapeutic approach. In this review, we discuss the therapeutic advances of different nanoparticles for hepatocellular carcinoma and discuss their potential for postoperative applications with respect to possible mechanisms of hepatocellular carcinoma recurrence. We further discuss the limitations regarding the application of NPs and the safety of NPs.
Keywords: HCC, NPs, treatment
Introduction
Hepatocellular carcinoma (HCC) is the fifth most prevalent cancer in the world.1 The incidence and mortality of HCC continue to rise in many countries, accounting for 90% of HCC in China with a recurrence rate of over 50% 5 years after resection in HCC patients.1–3 Infection with hepatitis B or C virus,4 exposure to carcinogens,5 excessive alcohol consumption,6 obesity,7 and diabetes8 are all important causes of HCC. The aforementioned etiology that leads to HCC encompasses multiple stages, including multiple processes of hepatocyte injury, regeneration, fibrosis, and heterogeneous proliferation.9
Most patients are already in the progressive stage upon diagnosis because they are asymptomatic, which has a greater impact on survival. HCC can currently be diagnosed by a variety of means, such as ultrasound, CT, MRI, and puncture biopsy, but the accuracy and potential for medically induced metastases make it even more difficult to diagnose. HCC can be treated with liver transplantation, surgical resection, and local ablation; however, only 30% of newly diagnosed liver cancer patients are eligible for treatment.10 Despite recent advancements in the systemic treatment of HCC, long-term survival in patients with advanced disease is uncommon.11 Currently, systemic chemotherapy, transhepatic arterial chemoembolization, and radiation therapy comprise the most prevalent adjuvant treatments for HCC. However, the side effects and toxic effects of systemic chemotherapy are substantial and have obvious killing effects on normal cells.12,13 Transhepatic arterial chemoembolization is easily affected by the formation of cancer thrombi or distant metastases in the blood vessels, and radiation therapy is ineffective or discontinued because patients are unable to tolerate it.14 Accordingly, HCC remains one of the most challenging cancers to till date.
With existing therapies, patient survival rates have not improved significantly, and continuous administration of drugs has exacerbated drug resistance in HCC cells. In addition, HCC, as a highly malignant tumor, is relatively less sensitive to chemotherapy. Therefore, there is a great need to utilize nanodrug delivery systems that can not only reduce HCC drug resistance but also increase chemotherapy sensitivity while promoting tumor cell apoptosis. This review will summarize the research progress on the therapeutic effects of different NPs in HCC and discuss the application prospects of this new form of HCC therapy. At the same time, we further summarized the relevant mechanism of HCC postoperative recurrence and further discussed whether NPs that can play a role in HCC treatment can also play a certain role in residual cancer after HCC.
Advantages of Nanoparticles in the Treatment of HCC
In recent years, the development of nanotechnology and biomaterials has led to applications in many areas of cancer treatment, as well as new ideas for the treatment of patients with recurrence of HCC following palliative resection. Nanoparticles (NPs) are solid particles with diameters between 10 and 1000 nm that can encapsulate or adsorb drugs for disease-specific diagnosis or treatment.15 Biologically active NPs are able to carry a wide variety of drugs and protect them from degradation in harsh environments while achieving long-term controlled drug release function via biocompatibility and target recognition properties, thereby significantly reducing the side effects of drugs and demonstrating a very positive role in the treatment of tumors.16–18 In addition, NPs can be modified by specific ligands, such as galactose, mannose, lactose and maltose, which can enhance specificity, lower toxicity, lower immunogenicity, and prolong circulation time. And can aid in the targeting and internalization of specific cell populations, including cancer cells.19 Furthermore, endocytosis allows for intracellular drug staging, and drugs can be released with altered cellular microenvironment acidity and alkalinity, particularly in the acidic environment of cancer cells. In comparison to conventional radiotherapy and chemotherapy, the NPs-mediated hepatic-targeted drug delivery system (HTDDS) enhances the therapeutic efficacy of targeted therapy for HCC.20 Correspondingly, the ability to achieve liver-targeted drug delivery, temporarily store drugs in the liver, and actively identify liver cancer cells is highly promising for the treatment of postoperative residual carcinoma patients.21
Nanoparticles have been widely used as carriers for loading bioactive agents, particularly those with low solubility in water, due to their unique properties.22,23 The advantages of encapsulating drugs in NPs include increased drug solubility, decreased drug side effects, prevention of drug degradation in the blood, increased drug exposure time, and decreased drug resistance.24 Additionally, NPs facilitate the development of protein-based therapeutics. Meanwhile, NPs have a number of benefits, including enhancing protein uptake and cellular responses, activating specific genes and intracellular signaling, and modulating cellular responses to soluble factors.25,26 During their action, NPs can mimic the natural morphology and function of the extracellular matrix (ECM) and deliver and release bioactive substances, including proteins, peptides, and small molecules, to numerous tissues.27–30 During the course of action, not only is drug target recognition achieved but also the zero-level drug release characteristics at the tumor site are controlled, decreasing the frequency of local drug delivery, and the ability to maintain the drug release rate (Figure 1).31,32
 |
Figure 1 Schematic diagram of NPs acting on tumor cells. Reprinted with permission from Figdraw (www.figdraw.com). Abbreviation: NPs, nanoparticles. |
Application of NPs in the Treatment of HCC
NPs and Chemotherapy for HCC
Metal and non-metal NPs can be loaded with chemotherapeutic agents that inhibit tumor cell proliferation and angiogenesis during therapy, such as sorafenib (Sora),33 thereby reducing the dose of chemotherapeutic drugs and achieving relatively satisfactory therapeutic effects. After modification, metal nanoparticles (metal NPs) such as iron, gold, and silver can significantly reduce their biotoxicity and be used to treat HCC. Among them, Fe NPs can be loaded with Sora and i RGD peptide with amino acid sequence CRGDK/RGPD/EC (MIL-101(Fe)@sor + i RGD), which decreases glutathione (GSH) and glutathione peroxidase 4 (GPX-4) levels while providing iron ions to effectively inhibit tumor growth, with good biosafety.34 Fe3O4 has very low biotoxicity and can be doped with poly (ADP-ribose) polymerase 1 (PARP-1) inhibitor (ABT-888) and temozolomide (TMZ) in Fe3O4 / Fe nano-scaffolds, Compared with drug alone, ABT-888/TMZ/NPs can significantly cause DNA damage, cell cycle arrest, PARP-1 fragmentation, Caspase-3 gene activation, and reduce the expression of poor prognostic related genes, so as to achieve the objective of treating tumors.35 Raptinal is a novel anticancer drug that can initiate the apoptotic pathway through the release of cytochrome C and caspase 3, encapsulate mitochondrial function, and significantly induce the expression of apoptotic genes. In addition, Raptinal loaded with Ag NPs significantly reduced bilirubin and AFP levels in the treated group compared with free Raptinal, which indicates a significant reduction in the aggressiveness of the tumor.36 Additionally, platinum has some utility in the treatment of recurrent tumors. Due to the high glucose consumption of HCC cells and the excessive production of reactive oxygen species (ROS), Shoshan et al prepared titanium-coated, non-oxidized platinum nanoparticles. The titanium-coated, non-oxidized platinum nanoparticles not only enable tumor cells to take up Pt NPs more efficiently but also cause DNA damage when the internal Pt NPs are oxidized to oxidized platinum when they come into contact with reactive oxygen species. Thus, the therapeutic effect on HCC cells can be achieved.37
Arsenic trioxide (ATO) was initially discovered and utilized in the treatment of acute promyelocytic leukemia (APL), but the current formulation has applications in the treatment of other types of cancer.38 Huang et al,39 prepared ATO-loaded ZnAs@SiO2 nanoparticles (NPs) to test the efficacy of ATO in the treatment of HCC, and found that the SHP-1/ JAK2/STAT3 signaling pathway was activated, which significantly inhibited the growth and metastasis of hepatocellular carcinoma cells and could be applied to the treatment of HCC. DNA methyltransferases 1 (Dnmt1) protein and PCNA were highly correlated with the prognosis of HCC, and ATO-filled m PEG-PLGA-PLL NPs were able to decrease the expression of Dnmt1 gene and DNA methylesterase gene, induce caspase 3 activation, release free N-terminal structural domain of gasdermin-E (GSDME), and ultimately induce apoptosis in HCC cells.40 Chitosan is a nontoxic, cation-rich, biodegradable carrier that can protect DNA from nuclease degradation, and chitosan nanoparticles have shown high activity against hepatocellular carcinoma cells.41,42 In this regard, triptolide (TP) is highly effective against a variety of cancers, including hepatocellular carcinoma; however, its high toxicity, low solubility in water, and unknown therapeutic targets limit its clinical application.43,44 Nevertheless, galactosylated chitosan TP nanoparticles (GC-TP-NPs) with high drug-carrying capacity can overcome this problem, as they have a sustained release pattern, effective in vitro cellular uptake, and high hepatic tumor accumulation in vivo, in addition to exhibiting lower systemic toxicity and androgenic toxicity, with the same pro-apoptotic and anti-proliferative effects on HCC cells in vitro and in vivo.44 In addition, dextran-based nanocarriers are biocompatible, have low toxicity, and can be used to target and control the release of curcumin to hepatocellular carcinoma cells L929 and HepG2 with the potential to treat the HCC.45 Accordingly, poly (lactic acid)-glycolic acid [PLGA], one of the linear polyesters in polymeric nanoparticles, has been used in the treatment of hepatic carcinoma, since it can be degraded in vivo to lactic acid (LA) and glycolic acid (GA), which is further degraded to carbon dioxide and water and is thus not toxic to the organism. Pan et al,46 encapsulated artemisinin (Artesunate, ART) in GA-modified NPs, and in vitro cytotoxicity experiments demonstrated that the GA-modified NPs had a higher affinity for HCC cells, a higher cellular uptake capacity, a lower cancer cell survival rate, and significant targeting properties, which can be utilized for the targeted treatment of HCC. Bile acids are synthesized in the liver from cholesterol, and in the human enterohepatic circulation, bile acids circulate frequently and efficiently. The concentration of GSH in the cytoplasm of tumor cells is approximately 2–10 mM, which is significantly higher than its concentration in the extracellular matrix (approximately 2–20 μm).47 T Fang et al,48 fabricated redox-sensitive PLGA nano-NPs (TSP/FP) loaded with oridonin (ORI) and GSH, which can apply to the high affinity of the APDTKTQ (Ala-Pro-Asp-Thr-Lys-Thr-Gln) peptide for the receptor of advanced glycation end-products (RAGE) can help cells uptake TSP or FP to release ORI, thus maximizing the therapeutic effect on HCC.
In the chemotherapy of liver cancer, NPs mainly promote the apoptosis of tumor cells by activating Caspase 3, and they can also eliminate tumor cells by regulating gene expression and activating signaling pathways (Figure 2).
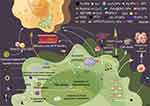 |
Figure 2 Schematic diagram of NPs and chemotherapy for HCC. NPs, nanoparticles. iRGD, Peptide chain of the amino acid sequence CRGDK/RGPD/EC. GSH, glutathione. PARP-1, poly (ADP-ribose) polymerase 1. ABT-888, PARP-1 inhibitor. Reprinted with permission from Figdraw (www.figdraw.com). Abbreviations: TMZ, temozolomide; ROS, reactive oxygen species; ATO, arsenic trioxide; Dnmt, DNA methyltransferases; GSDEM, gasdermin-E; Cyt C, cytochrome C; ORI, oridonin; TP, triptolide; TAP/FP, redox-sensitive PLGA nano-NPs; APDTKTQ, a peptide Ala-Pro-Asp-Thr-Lys-Thr-Gln; RAGE, the receptor of advanced glycation end-products; ART, Artesunate. |
NPs and Photothermal Therapy for HCC
Photothermal therapy (PTT) is a specialized treatment modality in which bioactive materials with high photothermal conversion efficiency are injected into the body, and light energy is converted into heat energy to kill tumor cells using target recognition technology and external light sources.49 Currently, numerous NPs have been validated for use in the photothermal therapy of tumors.
With good biocompatibility and relatively good therapeutic effects, graphene derivatives and their hybrids have garnered a great deal of attention from researchers in recent years and are widely used in cancer nanomedicine; they also have a certain amount of potential in the PTT tumor species. Graphene quantum dots (QDS)-mediated chitosan magnetic nanodelivery system (DOX-Fe3O4@CGA), with conjugated bonds, can adsorb photons and convert them into heat, promote the heating of the surrounding environment and the production of reactive oxygen species (ROS), and realize the targeted photothermal synergistic chemotherapy of HCC.50
Metal NPs play a relatively broad role in PTT treatment, and superparamagnetic iron oxide (SPION) NPs can induce lysosomal membrane permeabilization (LMP) in tumor cells, protect small interfering RNA (siRNA) from degradation by biological systemic nucleotides, improve the heating efficiency of π -π conjugation enhanced magnetic saturation, and induce apoptosis in hepatocellular carcinoma (HCC) cells in a controlled manner, which can be applied for the treatment of HCC.51–53 Adipose-derived mesenchymal cells (AD-MSCs) have the ability to homing and damage the liver. After encapsulation of AD-MSCs with SPIO-coated gold nanoparticles (SPIO @ AuNPs), they can be successfully transfected into AD-MSCs. SPIO @ AuNP-loaded AD-MSCs can thermally ablate surrounding liver cancer tumor cells.54
PTT achieves targeted therapy for HCC patients, thereby improving patient comfort and therapeutic outcomes (Table 1).
 |
Table 1 Photothermal Therapy |
Altered Gene Expression Levels of HCC
Inhibiting the cell cycle, modifying gene expression levels, and regulating signaling pathways can be used to treat HCC. To achieve tumor cell-specific apoptosis and death, Fe3O4 can be combined with FITC-binding cyclic the exposed arginine-glycine-aspartic (RGD) tripeptide to create nanoprobes that target and identify genes regulating integrin αvβ3 and vascular endothelial growth factor receptors (VEGFRs), which are highly expressed in many tumor tissues.55–57 In targeted gene therapy for HCC Fe3O4 NPs are anticipated to be applied to postoperative residual carcinoma in HCC. HCC cell lines and tissues can negatively regulate the expression of miR326 through the PI3K/AKT/c-myc axis, and its down-regulation is positively correlated with the prognosis of HCC. AuNPs carrying miR326 can inhibit the expression of cell cycle factors in vitro and in vivo, leading to tumor cell cycle transition disorders and inhibiting the PI3K/AKT/c-myc axis through a negative feedback loop, further inhibiting the proliferation of HepG2, Hep3B, Huh-7, and other cell lines.58 Enterococci-mediated AuNPs inhibit the proliferation of HepG2 cells via intracellular ROS-mediated apoptosis, decrease the expression of the proliferating cell nuclear antigen (PCNA) gene, and have therapeutic potential for HCC.59
MSN carries ursolic acid (UA) (USMNs-CL) with good anticancer activity and hepatoprotective effects, which exhibited strong proliferation and cell cycle inhibition and apoptosis in HepG2 cells at the G2/M phase, inhibition and cell cycle arrest, blocking tumor cell DNA replication, and significantly causing early and late apoptosis in HepG2 cells, thereby providing a means to improve the bioavailability and prolong the release of anticancer drugs.60 Xue et al,61 discovered that MSNs containing Adriamycin hydrochloride (DOX) and miR-375 significantly increased DOX uptake, and miR-375 reduced p-glycoprotein (P-gp) overexpression, which leads to multidrug resistance (MDR) in tumor cells, by inhibiting the expression of astrocyte elevated gene 1 (AEG-1) and targeting AEG-1-induced apoptosis.
Chitosan has numerous benefits, including biodegradability and low toxicity, and can be used in gene therapy. Accordingly, folic acid-chitosan nanoparticles (FA-CS) loaded with mouse interferon-inducible protein-10 (IP-10) plasmid DNA may achieve antitumor effects by inhibiting HCC cell proliferation and inducing HCC cell apoptosis by modulating immune responses and inhibiting tumor neovascularization, which is a novel HCC therapy.62 In addition, Xue et al,63 discovered that the preparation of galactosylated carboxymethyl chitosan-magnetic iron oxide nanoparticles (Gal-CMCS- Fe3O4 -NPs) enhanced the transfection efficiency of the Ras-related region family 1A (RASSF1A) gene, which is capable of inhibiting hepatocellular carcinoma cells, inhibiting tumor growth, and increasing the sensitivity of hepatocellular. Chitosan NPs can also encapsulate anionic albumin, which can alter redox homeostasis and inhibit NF-κB expression and ALDH1A1 in cancer cells, causing high apoptosis-mediated toxicity with great potential for inhibiting CSCs and treating HCC (Figure 3).64
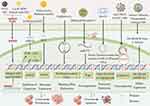 |
Figure 3 Schematic diagram of Altered Gene Expression Levels of HCC. Reprinted with permission from Figdraw (www.figdraw.com). Abbreviations: NPs, nanoparticles; ROS, reactive oxygen species; VEGDF, vascular endothelial growth factor receptors; RGD, the exposed arginine-glycine-aspartic tripeptide; P-gp, p-glycoprotein; FA-CS, folic acid-chitosan nanoparticles; Gal-CMCS- Fe3O4 -NPs, galactosylated carboxymethyl chitosan-magnetic iron oxide nanoparticles; RASSF1A, Ras-related region family 1A. |
Combination Therapy for HCC
Combination therapy refers to the rational combination of drugs or other cellular metabolites with comparable but distinct effects for the treatment of disease. In addition to reducing the toxic side effects of drugs, combination therapy also inhibits the development of drug resistance in tumor cells.
MSNs can target tumors via surface modification and accumulate sufficiently within tumor cells for tumor combination therapy, as well as capture/insert metals for PTT.65,66 Yang et al,67 utilized MSNs loaded with Sora and PTT near-infrared PTT reagent indocyanine green (ICG), which was found to increase the secretion of IFN-G from CD8+ T cells and enhance the number of immune cells in the tumor and spleen, as well as reduce angiogenesis, with potent immune response and recurrence-preventing activity, having a wide application for the treatment of HCC. Meanwhile, Zheng et al,68 prepared MSNs co-loaded with Sora and vascular endothelial growth factor-targeting siRNA (siVEGF) NPs (Sora/ siVEGF@MSNs-LA), which could target the induction of S-cell cycle arrest, enhanced the anti-cancer effect of Sora and siVEGF, and had great potential in the treatment of HCC. In addition, MSN modified by polyamidoamine ligand (PAMAM-APT) co-loaded Sora and pEGFR (SEHPA), which effectively promoted the uptake of Sora by HCC cells while synergistically inhibiting the expression of EGFR and downstream PI3K-Akt pathway, jointly inhibiting angiogenesis and achieving efficient EGFR gene therapy, is a promising dual gene-chemotherapy drug delivery system,69 which has promising applications in the treatment of HCC. Among the shape-controlled magnetic mesoporous silica nanoparticles (M-MSNs), rod-shaped MSNs have stable drug release function and low cytotoxicity, which can assist clinicians in monitoring treatment outcomes by MRI, which might used to suicide gene therapy of HCC.70
The use of N-galactosylated chitosan-5-fluorouracil (GC-FU) for electrostatic condensation with miRNA-122 and the co-delivery of miRNA-122 and the anticancer drug 5-Fu, which improved blood salt stability, effectively induced apoptosis and inhibited the proliferation of HCC cells,71 may have great potential for the future synergistic treatment of HCC.
Multiple NPs contribute to the reduction of tumor cell resistance. Sora inhibits multiple receptor tyrosine kinases and downstream Raf signaling molecules (Raf-1 and B-Raf), but within 6 months, the vast majority of patients develop resistance to sorafenib.72 Using CXCR4-targeted PLGA-PEG NPs to encapsulate sorafenib and mestinon and modifying the surface of PLGA-PEG NPs with the CXCR4 antagonist LFC131 peptide enhanced the delivery and accumulation of anticancer drugs at the tumor site, thereby enhancing the antitumor effect.73 Polyethylene glycol (PEG) and polyethyleneimine (PEI) conjugated ultrasmall nano-graphene oxide (NGO-PEG-PEI) loaded with C6 - ceramide in combination with Sora (NGO-PEG-PEI/Cer) exhibited a synergistic effect, significantly inhibiting tumor growth and improving survival time in vivo, and may also play a role in the destruction of HCC by inactivating MDR and Akt signaling in HCC cells role as a promising potential therapeutic strategy for the treatment of drug-resistant HCC.74
Other Treatment Modalities
In addition to their therapeutic effects in the aforementioned therapeutic modalities, NPs can also be altered to exert therapeutic effects. Mesoporous hollow alumina nanoparticles (MHA) prepared using alumina and grafted with hyaluronic acid (HA) exhibited significantly enhanced targeting effects and significant pro-apoptotic and tumor suppressive effects.75 Moreover, Zhang et al,76 synthesized an amphiphilic polymer containing bile acid (CA) and adsorbed it onto the surface of PLGA NPs. According, they demonstrated that the binding of CA to the bile acid transporter on the cell membrane increased the adhesion of NPs to cells, accelerated the intracellularization of NPs, and inhibited the proliferation of HCC cells. Comparative in vitro cytotoxicity studies of silver-containing reduced graphene oxide (rGO-Ag) nanoparticles revealed that compared with normal liver cells, hepatocellular carcinoma cells (HepG2) cells were more susceptible to the effects of oxidases such as lipid peroxidase, superoxide dismutase, and catalase, and the GSH level decreased and DNA damage was more obvious (Table 2).77
 |
Table 2 Combination Therapy and Other Treatment Modalities |
Discussion
For HCC patients, the advent of NPs has opened up more possibilities for their treatment. However, surgery is still the effective means of patient treatment, and postoperative recurrence represents a major challenge for clinicians. Surgery is challenging, and treatment modalities such as chemotherapy are still used to prevent postoperative HCC recurrence. In the study of postoperative tumor recurrence, it was found that it was mainly related to surgical stimulation and postoperative changes in the tumor microenvironment, and these factors have very positive implications for the prevention and treatment of surgical residual cancer and future HCC recurrence.
Mechanism of Residual Carcinoma After HCC Surgery
Effect of Surgical Stimulation in the Recurrence of HCC Residual Carcinoma
Although surgery offers patients with cancer the opportunity to be cured, it is also a significant factor in the recurrence of residual carcinoma following HCC surgery. Researchers have demonstrated that anesthetic drugs administered during surgical anesthesia may also promote cancer recurrence and metastasis, with intravenous anesthetic isoproterenol and inhaled volatile anesthetics having more profound effects on inflammation, immune cell phenol types, and cancer progression.78 Additionally, local recurrence can rapidly exceed the initial tumor’s volume, despite the fact that surgery can remove solid tumors.79,80 Moreover, studies have demonstrated an increased risk of metastatic growth following the resection of primary tumors.81 The primary reason for this is that surgery causes cancer cells to be shed and enter the circulatory system and upregulates the expression of adhesion molecules in organs.82 In addition, it induces changes in the target tissue as well as changes in the cancer cells themselves, thereby increasing the ability of the cells to migrate and invade. In light of the preceding, the effect of wide and narrow margins on macroscopic HCC has been studied in liver surgery, with 5-year survival rates of 74.9 and 49.1% in the wide margin and narrow margin groups, respectively; since the recurrence of HCC was observed in the narrow margin group, the wide margin procedure was, therefore, deemed advantageous for patient survival.83
Surgery also causes alterations in the physiological functions of the patient, primarily manifesting as a state of stress.84 Herein, the role of natural killer cells (NK cells) in recognizing and killing tumor cells is inhibited and postoperative immune function is impaired, with residual tumor cells gaining the potential to metastasize. In addition, it may also permit the continued growth of cancer cells remaining at the tumor cut edge. Surgical procedures may also induce hypoxia in the liver, thereby activating NF-B and hypoxia-inducing factors (HIFs) and accelerating tumor cell growth in regions where hypoxia and inflammation are prevalent.85 Moreover, when hepatectomy is performed, repeated liver damage occurs, and hepatic stellate cells (HSC) are activated to dedifferentiate into myofibroblast-like cells, which leads to the development of hepatic fibrosis and the occurrence of HCC.86
Effect of Tumor Microenvironment on Residual Carcinoma Development After HCC Surgery
Numerous pro-angiogenic factors, including TGF-1, have been identified in studies involving angiogenic factors that may stimulate the growth of HCSCs and accelerate the proliferation and metastasis of residual carcinoma after HCC surgery.87,88 Accordingly, increased postoperative levels of the inflammatory mediator prostaglandin E2 (PGE2) have been found to mediate the transfer of anti-tumor T helper (TH1) cytokines to TH2 cytokines in tumor cells, thereby promoting the proliferation of regulatory T (Treg) cells, a decrease in the number of activated CD8+ T cells, and the promotion of an immunosuppressive tumor microenvironment.89–91 Surgery not only activates -adrenergic nerve fibers and receptors, thereby accelerating tumor progression, but also reshapes the tumor microenvironment, increases venous and tumor pressures, causes interstitial edema, and promotes tumor-associated neovascularization and neoplastic capillary lymphatics.92–94 This explains why tumors are considered “unhealable wounds”.95
Extracellular matrix (ECM) and carcinoma nodal stratification protein-5 regulate many essential cellular processes in postoperative residual carcinoma of HCC that are closely linked to HCC proliferation and metastasis in tumor tissues.96,97 In addition, it has been discovered that tumor stem cells (HCSCs) persist in cancer cell screenings and may reside in a particular microenvironment that maintains the balance between self-renewal and differentiation of HCSCs by providing the necessary substances.98,99 Accordingly, those present in interstitial microdeposits and micrometastases (mesenchymal or hematogenous), a type of occult tumor that remains in situ following therapeutic resection, are referred to as microresidual disease (MRD).100 Following primary tumor resection, the level of inhibitory factors secreted by cancer cells decreases, and dormant metastases or tumors in the primary lesion begins to rejuvenate, leading to a decrease in systemic anti-angiogenic factors, an increase in angiogenesis, and continued cancer cell growth (Figure 4).101
 |
Figure 4 Mechanisms of the development of postoperative residual carcinoma in HCC. Factors such as surgical anesthesia, altered immune status, surgical stimulation, tumor cell entry, inadequate resection, and altered tumor microenvironment provide survival opportunities for postoperative participating tumors and are important mechanisms for the development of postoperative residual carcinoma in HCC. Reprinted with permission from Figdraw (www.figdraw.com). |
The Potential Application of NPs in the Recurrence of Residual Cancer After HCC Surgery
Recurrent HCC after surgery can be of monoclonal (single-center) origin due to intrahepatic metastasis or of polyclonal (multicenter) origin with ab initio carcinoma, but the determination of the mode of origin of recurrent HCC is not easy.102 Moreover, based on the existing studies, we have little understanding of the mechanisms of HCC recurrence, which is a major obstacle for us to determine its origin and type. In addition, due to the complexity of postoperative recurrence of HCC, we are not able to identify the molecular types of recurrent tumor cells or find the exact targets to target for treatment, which is one of the directions of our future research.
Above, we mentioned that multiple NPs play an important role in the treatment of HCC. In PTT treatment, MSN@Sora-ICG, however, showed strong anti-recurrence activity and has potential in the prevention of recurrence after HCC treatment.67 However, no studies have confirmed that NPs can play a role in the treatment of residual cancer after HCC surgery or in the prevention of recurrence.
In the process of tumor recurrence, the tumor microenvironment plays an important role. It is not difficult to find that many nanoactive carriers can alter the tumor microenvironment, among which IL-6, TNF-α, etc. are prone to increase the secretion of solid tumors and the tumor microenvironment and promote the recurrence of HCC after surgery.103 The cells and molecules in the tumor microenvironment are in a dynamic process of change, reflecting the nature of tumor microenvironment evolution, which culminates in the massive accumulation of inflammatory-related factors such as IL-6, TNF-α, MMP, etc., which accumulate in large quantities in the tumor microenvironment and together promote tumor immune escape, tumor growth, and metastasis.104,105 Therefore, can we use bioactive nanocarriers to improve the tumor microenvironment so as to achieve prevention of tumor recurrence or therapeutic effects after tumor resection?
Traditional herbal medicine (THM) has shown a role in tumor recurrence, and it has been demonstrated that some THMs can play a role in the prevention of recurrence after HCC surgery. For example, cinobufacini (Huachansu), an aqueous extract from Bufo gargari-zans Cantor, the root of Salvia chinensis Benth [Shi-jian-chuan], the gizzard membrane of Gallus gallus domesticus Brisson [Ji-nei-jin], the root of Actinidia valvata Dunn [Mao-ren-shen], and the tuber of Pseudobulbus cremastrae seu Pleiones [Shan-ci-gu], which is anticipated to inhibit tumor growth and prolong the survival of patients.106 Then, can we carry the drug into the nanocarrier to achieve controlled release of the drug to achieve long-term therapeutic effects? In addition, GC-TP-NPs also show a certain tumor inhibitory effect in vitro, and have a certain application potential in the treatment of residual cancer and prevention of recurrence of HCC after surgery.44
At present, electrostatic spinning technology is also widely used in the medical industry, so we can prepare a nanofiber membrane by dispersing bioactive nanoparticles in solvent through electrostatic spinning technology and implant it after surgery, which not only achieves sustainable release of chemotherapy drugs after surgery but also has the effect of treating residual cancer and preventing tumor recurrence after surgery. Our group has successfully prepared nanofiber membranes with such functions. Moreover, the research on NPs for the treatment of HCC is becoming more and more extensive, and some scholars have also found that their NPs themselves can promote apoptosis of HCC cells. For example, TiO2 NPs and ZnO NPs can effectively inhibit hepatocellular carcinoma HepG2 cells through the production of ROS, but the cancer-inhibiting effect is reduced after the combination of the two due to the relative reduction of pores, which also provides a new idea for future research on the treatment of HCC.107
In conclusion, bioactive nanoparticles have great potential in the treatment and prevention of recurrence of residual cancer after HCC surgery.
Limitations of NPs in the Treatment of HCC
Although NPs are becoming more prevalent in clinical research, they still have certain limitations. First, the reproducibility of NPs production is highly variable, and even minute variations in the preparation process can result in new NPs that are distinct from the original NPs. Because new particles in any ensemble have different surface location distributions (eg, small surfaces, vertices, defects, etc.) and the size and shape of new particles can vary widely, it is reasonable to anticipate that the characterization of individual new particles may deviate significantly from that of the original new particles; therefore, to ensure their accuracy, comprehensive characterization of the nanoparticles at each stage and in each batch is required prior to application.108,109 Second, in the construction of HCC models, 3D models are superior to the original 2D models for simulating the key roles of the tumor microenvironment and for understanding the interactions between HCC and NPs; therefore, whenever possible, 3D models are chosen to maximize the restoration of the normal life state of the model.110,111 Moreover, since the target recognition receptors of target organs may mutate, this may result in off-targeting of targeted NPs and produce off-target side effects.112 In addition, NPs are foreign to the patient’s body, have a small diameter, and are likely to be captured by macrophages during vascular infiltration.113 However, if NPs are modified, the clearance of NPs in vivo may pose a new and significant challenge. Lastly, NPs should be used sparingly, rather than in pursuit of therapeutic effects that would lead to the accumulation of NPs in vivo, causing toxic effects and putting the cart before the horse.
Warm therapy is contraindicated in the treatment of mesoendometriosis, which can lead to worsening of the disease.114 In the treatment of patients who are not on anticoagulants and do not have cardiovascular or diabetic morbidity, systemic and topical a-type TXA appears to significantly reduce postoperative bleeding and the need for RBC transfusion after TKA.115 When treating NPs, we should also be concerned about the effects of NPs on other organs; for example, Ni NPs can be toxic to testes.116 Therefore, we cannot ignore the safety of drug administration, and we have to test NPs for the treatment of HCC to ensure that there are no or few adverse effects other than therapeutic effects to maximize the safety of patients’ lives.
Summary and Outlook
HCC is one of the most malignant tumors known, and after diagnosis, most patients with advanced hepatocellular carcinoma have limited clinical options and a poor prognosis.117 The development of a tumor is dependent not only on the tumor cells themselves but also on the “soil” in which they reside, ie, the tumor microenvironment. Therapeutic tools mediated by NPs can modify the tumor microenvironment by targeting one or more cytokines in the tumor microenvironment, thereby significantly inhibiting the biological behavior of HCC cells, such as their proliferation, metastasis, and apoptosis. In addition, NPs can effectively target HCC cells and CSCs, as well as capture CTCs, which are extremely rare in vivo, to facilitate early monitoring of residual carcinoma recurrence after HCC surgery and buy more treatment time for recurrence patients. It is also observed that drug-loaded nanoparticles exert their toxic killing effects on HCC cells by affecting signaling pathways, regulating the cell cycle, and inducing apoptosis, which can be used for the treatment of HCC.
HCC recurrence and postoperative residual cancer are also a major difficulty in the treatment of HCC, which is caused by the recurrence and metastasis of HCC is a multistage, multigene process characterized by dynamic alterations. NPs play a role in the treatment of HCC by regulating gene expression, inhibiting signaling pathways, and the production of pro-apoptotic substances to achieve the effect of cancer suppression, therefore, whether NPs can be placed on the surgical wound by other means to achieve prolonged release of chemotherapeutic drugs and further achieve postoperative treatment of residual cancer and prevent the effect of NPs on the surgical wound can be achieved by other means. This is also one of the directions of our group’s subsequent research.
We also should be noted that the drug-release effect of various NPs may not achieve the desired targeting effect and that there is no assurance that there is no toxic effect on normal tissues or organs. Therefore, more in-depth research on NPs is required to develop a multifunctional and multifaceted cancer treatment modality, which would be a boon for patients with HCC. And simultaneously, With the advancement of technology and extensive research by scholars, it is believed that NPs will play an irreplaceable role in the treatment of residual carcinoma following HCC surgery.
Acknowledgments
This article is supported by the National Natural Science Foundation of China (Project number, 82001964) and Yangzhou University Medical Innovation and transformation Special fund (Project number, AHYZUCXTD202104). Special thanks to the various publishers and Figdraw (www.figdraw.com) for licensing some of the figures in this article. We thank Bullet Edits Limited for the linguistic editing and proofreading of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Lu SD, Li L, Liang XM, et al. Updates and advancements in the management of hepatocellular carcinoma patients after hepatectomy. Expert Rev Gastroenterol Hepatol. 2019;13(11):1077–1088. doi:10.1080/17474124.2019.1684898
2. Valery PC, Laversanne M, Clark PJ, Petrick JL, McGlynn KA, Bray F. Projections of primary liver cancer to 2030 in 30 countries worldwide. Hepatology. 2018;67(2):600–611. doi:10.1002/hep.29498
3. El-Serag HB, Kanwal F. Epidemiology of hepatocellular carcinoma in the United States: where are we? Where do we go? Hepatology. 2014;60(5):1767–1775. doi:10.1002/hep.27222
4. D’Souza S, Lau KC, Coffin CS, Patel TR. Molecular mechanisms of viral hepatitis induced hepatocellular carcinoma. World J Gastroenterol. 2020;26(38):5759–5783. doi:10.3748/wjg.v26.i38.5759
5. Rushing BR, Selim MI. Aflatoxin B1: a review on metabolism, toxicity, occurrence in food, occupational exposure, and detoxification methods. Food Chem Toxicol. 2019;124:81–100. doi:10.1016/j.fct.2018.11.047
6. Huang DQ, Mathurin P, Cortez-Pinto H, Loomba R. Global epidemiology of alcohol-associated cirrhosis and HCC: trends, projections and risk factors. Nat Rev Gastroenterol Hepatol. 2023;20(1):37–49. doi:10.1038/s41575-022-00688-6
7. Marengo A, Rosso C, Bugianesi E. Liver cancer: connections with obesity, fatty liver, and cirrhosis. Annu Rev Med. 2016;67:103–117. doi:10.1146/annurev-med-090514-013832
8. Plaz Torres MC, Jaffe A, Perry R, Marabotto E, Strazzabosco M, Giannini EG. Diabetes medications and risk of HCC. Hepatology. 2022;76(6):1880–1897. doi:10.1002/hep.32439
9. Affo S, Yu LX, Schwabe RF. The role of cancer-associated fibroblasts and fibrosis in liver cancer. Annu Rev Pathol. 2017;12:153–186. doi:10.1146/annurev-pathol-052016-100322
10. Yarchoan M, Agarwal P, Villanueva A, et al. Correction: recent developments and therapeutic strategies against hepatocellular carcinoma. Cancer Res. 2019;79(22):5897. doi:10.1158/0008-5472.CAN-19-2958
11. Llovet JM, Zucman-Rossi J, Pikarsky E, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018. doi:10.1038/nrdp.2016.18
12. Soydal C, Araz M, Nak D, et al. Analysis of prognostic factors in patients receiving transarterial radioembolization for unresectable hepatocellular carcinoma. Nucl Med Commun. 2020;41(1):73–77. doi:10.1097/MNM.0000000000001122
13. Mouli SK, Goff LW. Local arterial therapies in the management of unresectable hepatocellular carcinoma. Curr Treat Options Oncol. 2017;18(11):67. doi:10.1007/s11864-017-0509-6
14. Affonso BB, Galastri FL, da Motta Leal Filho JM, et al. Long-term outcomes of hepatocellular carcinoma that underwent chemoembolization for bridging or downstaging. World J Gastroenterol. 2019;25(37):5687–5701. doi:10.3748/wjg.v25.i37.5687
15. Kishan A, Cosgriff-Hernandez E. Recent advancements in electrospinning design for tissue engineering applications: a review. J Biomed Mater Res A. 2017;105(10):2892–2905. doi:10.1002/jbm.a.36124
16. Babitha S, Rachita L, Karthikeyan K, et al. Electrospun protein nanofibers in healthcare: a review. Int J Pharm. 2017;523(1):52–90. doi:10.1016/j.ijpharm.2017.03.013
17. Kenawy E-R, Bowlin GL, Mansfield K, et al. Release of tetracycline hydrochloride from electrospun poly(ethylene-co-vinylacetate), poly(lactic acid), and a blend. J Control Release. 2002;81(1):57–64. doi:10.1016/S0168-3659(02)00041-X
18. Katti DS, Robinson KW, Ko FK, Laurencin CT. Bioresorbable nanofiber-based systems for wound healing and drug delivery: optimization of fabrication parameters. J Biomed Mater Res B Appl Biomater. 2004;70(2):286–296. doi:10.1002/jbm.b.30041
19. Bian Y, Guo D. Targeted therapy for hepatocellular carcinoma: co-delivery of sorafenib and curcumin using lactosylated pH-responsive nanoparticles. Drug Des Devel Ther. 2020;14:647–659. doi:10.2147/DDDT.S238955
20. Anwanwan D, Singh SK, Singh S, Saikam V, Singh R. Challenges in liver cancer and possible treatment approaches. Biochim Biophys Acta Rev Cancer. 2020;1873(1):188314. doi:10.1016/j.bbcan.2019.188314
21. Zhou F, Teng F, Deng P, Meng N, Song Z, Feng R. Recent progress of nano-drug delivery system for liver cancer treatment. Anticancer Agents Med Chem. 2018;17(14):1884–1897. doi:10.2174/1871520617666170713151149
22. El Fawal G, Hong H, Song X, et al. Polyvinyl alcohol/hydroxyethylcellulose containing ethosomes as a scaffold for transdermal drug delivery applications. Appl Biochem Biotechnol. 2020;191(4):1624–1637. doi:10.1007/s12010-020-03282-1
23. Li B, Yang X. Rutin-loaded cellulose acetate/poly(ethylene oxide) fiber membrane fabricated by electrospinning: a bioactive material. Mater Sci Eng C Mater Biol Appl. 2020;109:110601. doi:10.1016/j.msec.2019.110601
24. Najlah M, Ahmed Z, Iqbal M, et al. Development and characterisation of disulfiram-loaded PLGA nanoparticles for the treatment of non-small cell lung cancer. Eur J Pharm Biopharm. 2017;112:224–233. doi:10.1016/j.ejpb.2016.11.032
25. Zhuang Y, Lin K, Yu H. Advance of nano-composite electrospun fibers in periodontal regeneration. Front Chem. 2019;7:495. doi:10.3389/fchem.2019.00495
26. Campiglio CE, Marcolin C, Draghi L. Electrospun ECM macromolecules as biomimetic scaffold for regenerative medicine: challenges for preserving conformation and bioactivity. AIMS Mater Sci. 2017;4(3):638–669. doi:10.3934/matersci.2017.3.638
27. Qasim SB, Najeeb S, Delaine-Smith RM, Rawlinson A, Ur Rehman I. Potential of electrospun chitosan fibers as a surface layer in functionally graded GTR membrane for periodontal regeneration. Dent Mater. 2017;33(1):71–83. doi:10.1016/j.dental.2016.10.003
28. Eskitoros-Togay ŞM, Bulbul YE, Tort S, Demirtaş Korkmaz F, Acartürk F, Dilsiz N. Fabrication of doxycycline-loaded electrospun PCL/PEO membranes for a potential drug delivery system. Int J Pharm. 2019;565:83–94. doi:10.1016/j.ijpharm.2019.04.073
29. Huang ZM, He CL, Yang A, et al. Encapsulating drugs in biodegradable ultrafine fibers through co-axial electrospinning. J Biomed Mater Res A. 2006;77(1):169–179. doi:10.1002/jbm.a.30564
30. Kim K, Luu YK, Chang C, et al. Incorporation and controlled release of a hydrophilic antibiotic using poly(lactide-co-glycolide)-based electrospun nanofibrous scaffolds. J Control Release. 2004;98(1):47–56. doi:10.1016/j.jconrel.2004.04.009
31. Ulery BD, Kumar D, Ramer-Tait AE, Metzger DW, Wannemuehler MJ, Narasimhan B. Design of a protective single-dose intranasal nanoparticle-based vaccine platform for respiratory infectious diseases. PLoS One. 2011;6(3):e17642. doi:10.1371/journal.pone.0017642
32. Jiang H, Fang D, Hsiao B, Chu B, Chen W. Preparation and characterization of ibuprofen-loaded poly(lactide-co-glycolide)/poly(ethylene glycol)-g-chitosan electrospun membranes. J Biomater Sci Polym Ed. 2004;15(3):279–296. doi:10.1163/156856204322977184
33. Chang YS, Adnane J, Trail PA, et al. Sorafenib (BAY 43-9006) inhibits tumor growth and vascularization and induces tumor apoptosis and hypoxia in RCC xenograft models. Cancer Chemother Pharmacol. 2007;59(5):561–574. doi:10.1007/s00280-006-0393-4
34. Liu X, Zhu X, Qi X, Meng X, Xu K. Co-Administration of iRGD with sorafenib-loaded iron-based metal-organic framework as a targeted ferroptosis agent for liver cancer therapy. Int J Nanomedicine. 2021;16:1037–1050. doi:10.2147/IJN.S292528
35. Munoz-Gamez JA, Lopez Viota J, Barrientos A, et al. Synergistic cytotoxicity of the poly (ADP-ribose) polymerase inhibitor ABT-888 and temozolomide in dual-drug targeted magnetic nanoparticles. Liver Int. 2015;35(4):1430–1441. doi:10.1111/liv.12586
36. Taha H, Elfar N, Haffez H, Hassan ZA. Raptinal silver nanoparticles: new therapeutic advances in hepatocellular carcinoma mouse model. Naunyn Schmiedebergs Arch Pharmacol. 2021;394(2):279–289. doi:10.1007/s00210-020-01973-4
37. Shoshan MS, Vonderach T, Hattendorf B, Wennemers H. Peptide-coated platinum nanoparticles with selective toxicity against liver cancer cells. Angew Chem. 2019;58(15):4901–4905. doi:10.1002/anie.201813149
38. Wang ZY, Chen Z. Acute promyelocytic leukemia: from highly fatal to highly curable. Blood. 2008;111(5):2505–2515. doi:10.1182/blood-2007-07-102798
39. Huang Y, Zhou B, Luo H, et al. ZnAs@SiO2 nanoparticles as a potential anti-tumor drug for targeting stemness and epithelial-mesenchymal transition in hepatocellular carcinoma via SHP-1/JAK2/STAT3 signaling. Theranostics. 2019;9(15):4391–4408. doi:10.7150/thno.32462
40. Hu J, Dong Y, Ding L, et al. Local delivery of arsenic trioxide nanoparticles for hepatocellular carcinoma treatment. Signal Transduct Target Ther. 2019;4:28. doi:10.1038/s41392-019-0062-9
41. Qi L, Xu Z, Chen M. In vitro and in vivo suppression of hepatocellular carcinoma growth by chitosan nanoparticles. Eur J Cancer. 2007;43(1):184–193. doi:10.1016/j.ejca.2006.08.029
42. Jiang H-L, Kim Y-K, Arote R, et al. Mannosylated chitosan-graft-polyethylenimine as a gene carrier for Raw 264.7 cell targeting. Int J Pharm. 2009;375(1):133–139. doi:10.1016/j.ijpharm.2009.03.033
43. Gali-Muhtasib H, Hmadi R, Kareh M, Tohme R, Darwiche N. Cell death mechanisms of plant-derived anticancer drugs: beyond apoptosis. Apoptosis. 2015;20(12):1531–1562. doi:10.1007/s10495-015-1169-2
44. Zhang YQ, Shen Y, Liao MM, et al. Galactosylated chitosan triptolide nanoparticles for overcoming hepatocellular carcinoma: enhanced therapeutic efficacy, low toxicity, and validated network regulatory mechanisms. Nanomedicine. 2019;15(1):86–97. doi:10.1016/j.nano.2018.09.002
45. Anirudhan TS. Binusreejayan. Dextran based nanosized carrier for the controlled and targeted delivery of curcumin to liver cancer cells. Int J Biol Macromol. 2016;88:222–235. doi:10.1016/j.ijbiomac.2016.03.040
46. Pan X, Liu S, Ju L, et al. Preparation, evaluation, and in vitro cytotoxicity studies of artesunate-loaded glycyrrhetinic acid decorated PEG-PLGA nanoparticles. Drug Dev Ind Pharm. 2020;46(11):1889–1897. doi:10.1080/03639045.2020.1825475
47. Zhang Y, Peng L, Chu J, et al. pH and redox dual-responsive copolymer micelles with surface charge reversal for co-delivery of all-trans-retinoic acid and paclitaxel for cancer combination chemotherapy. Int J Nanomedicine. 2018;13:6499–6515. doi:10.2147/IJN.S179046
48. Fang XB, Xu YQ, Chan HF, et al. A Redox-Sensitive and RAGE-targeting nanocarrier for hepatocellular carcinoma therapy. Mol Pharm. 2016;13(11):3613–3625. doi:10.1021/acs.molpharmaceut.6b00116
49. Zhi D, Yang T, O’Hagan J, Zhang S, Donnelly RF. Photothermal therapy. J Control Release. 2020;325:52–71. doi:10.1016/j.jconrel.2020.06.032
50. Chen L, Hong W, Duan S, Li Y, Wang J, Zhu J. Graphene quantum dots mediated magnetic chitosan drug delivery nanosystems for targeting synergistic photothermal-chemotherapy of hepatocellular carcinoma. Cancer Biol Ther. 2022;23(1):281–293. doi:10.1080/15384047.2022.2054249
51. Tian G, Pan R, Zhang B, et al. Liver-targeted combination therapy basing on glycyrrhizic acid-modified DSPE-PEG-PEI nanoparticles for co-delivery of doxorubicin and Bcl-2 siRNA. Front Pharmacol. 2019;10:4. doi:10.3389/fphar.2019.00004
52. Mezghrani O, Tang Y, Ke X, et al. Hepatocellular carcinoma dually-targeted nanoparticles for reduction triggered intracellular delivery of doxorubicin. Int J Pharm. 2015;478(2):553–568. doi:10.1016/j.ijpharm.2014.10.041
53. Kandasamy G, Sudame A, Luthra T, Saini K, Maity D. Functionalized hydrophilic superparamagnetic iron oxide nanoparticles for magnetic fluid hyperthermia application in liver cancer treatment. ACS Omega. 2018;3(4):3991–4005. doi:10.1021/acsomega.8b00207
54. Zhao J, Vykoukal J, Abdelsalam M, et al. Stem cell-mediated delivery of SPIO-loaded gold nanoparticles for the theranosis of liver injury and hepatocellular carcinoma. Nanotechnology. 2014;25(40):405101. doi:10.1088/0957-4484/25/40/405101
55. Shen JM, Li XX, Fan LL, et al. Heterogeneous dimer peptide-conjugated polylysine dendrimer-Fe(3)O(4) composite as a novel nanoscale molecular probe for early diagnosis and therapy in hepatocellular carcinoma. Int J Nanomedicine. 2017;12:1183–1200. doi:10.2147/IJN.S126887
56. Massaguer A, González-Cantó A, Escribano E, et al. Integrin-targeted delivery into cancer cells of a Pt(IV) pro-drug through conjugation to RGD-containing peptides. Dalton Trans. 2015;44(1):202–212. doi:10.1039/C4DT02710H
57. Zheng Y, Ji S, Czerwinski A, Valenzuela F, Pennington M, Liu S. FITC-conjugated cyclic RGD peptides as fluorescent probes for staining integrin αvβ3/αvβ5 in tumor tissues. Bioconjug Chem. 2014;25(11):1925–1941. doi:10.1021/bc500452y
58. Mo Y, He L, Lai Z, et al. Gold nano-particles (AuNPs) carrying miR-326 targets PDK1/AKT/c-myc axis in hepatocellular carcinoma. Artif Cells Nanomed Biotechnol. 2019;47(1):2830–2837. doi:10.1080/21691401.2018.1489266
59. Nandhini JT, Ezhilarasan D, Rajeshkumar S. An ecofriendly synthesized gold nanoparticles induces cytotoxicity via apoptosis in HepG2 cells. Environ Toxicol. 2020;36(1):24–32. doi:10.1002/tox.23007
60. Li T, Chen X, Liu Y, et al. pH-Sensitive mesoporous silica nanoparticles anticancer prodrugs for sustained release of ursolic acid and the enhanced anti-cancer efficacy for hepatocellular carcinoma cancer. Eur J Pharm Sci. 2017;96:456–463. doi:10.1016/j.ejps.2016.10.019
61. Xue H, Yu Z, Liu Y, et al. Delivery of miR-375 and doxorubicin hydrochloride by lipid-coated hollow mesoporous silica nanoparticles to overcome multiple drug resistance in hepatocellular carcinoma. Int J Nanomedicine. 2017;12:5271–5287. doi:10.2147/IJN.S135306
62. Lai C, Yu X, Zhuo H, et al. Anti-tumor immune response of folate-conjugated chitosan nanoparticles containing the IP-10 gene in mice with hepatocellular carcinoma. J Biomed Nanotechnol. 2014;10(12):3576–3589. doi:10.1166/jbn.2014.2051
63. Xue WJ, Feng Y, Wang F, et al. Asialoglycoprotein receptor-magnetic dual targeting nanoparticles for delivery of RASSF1A to hepatocellular carcinoma. Sci Rep. 2016;6:22149. doi:10.1038/srep22149
64. Abu-Serie MM. Evaluation of the selective toxic effect of the charge switchable diethyldithiocarbamate-loaded nanoparticles between hepatic normal and cancerous cells. Sci Rep. 2018;8(1):4617. doi:10.1038/s41598-018-22915-4
65. Cheng YJ, Qin SY, Ma YH, Chen XS, Zhang AQ, Zhang XZ. Super-pH-sensitive mesoporous silica nanoparticle-based drug delivery system for effective combination cancer therapy. ACS Biomater Sci. 2019;5(4):1878–1886. doi:10.1021/acsbiomaterials.9b00099
66. Wang J, Zhang Y, Liu L, et al. Combined chemo/photothermal therapy based on mesoporous silica-Au core-shell nanoparticles for hepatocellular carcinoma treatment. Drug Dev Ind Pharm. 2019;45(9):1487–1495. doi:10.1080/03639045.2019.1629688
67. Yang H, Liu HS, Hou W, et al. An NIR-responsive mesoporous silica nanosystem for synergetic photothermal-immunoenhancement therapy of hepatocellular carcinoma. J Mater Chem B. 2020;8(2):251–259. doi:10.1039/C9TB01891C
68. Zheng G, Zhao R, Xu A, Shen Z, Chen X, Shao J. Co-delivery of sorafenib and siVEGF based on mesoporous silica nanoparticles for ASGPR mediated targeted HCC therapy. Eur J Pharm Sci. 2018;111:492–502. doi:10.1016/j.ejps.2017.10.036
69. Zhang BC, Luo BY, Zou JJ, et al. Co-delivery of Sorafenib and CRISPR/Cas9 based on targeted core-shell hollow mesoporous organosilica nanoparticles for synergistic HCC therapy. ACS Appl Mater Interfaces. 2020;12(51):57362–57372. doi:10.1021/acsami.0c17660
70. Wang Z, Chang Z, Lu M, et al. Shape-controlled magnetic mesoporous silica nanoparticles for magnetically-mediated suicide gene therapy of hepatocellular carcinoma. Biomaterials. 2018;154:147–157. doi:10.1016/j.biomaterials.2017.10.047
71. Ning Q, Liu YF, Ye PJ, et al. Delivery of Liver-Specific miRNA-122 using a targeted macromolecular prodrug toward synergistic therapy for hepatocellular carcinoma. ACS Appl Mater Interfaces. 2019;11(11):10578–10588. doi:10.1021/acsami.9b00634
72. Aoki M, Fujishita T. Oncogenic Roles of the PI3K/AKT/mTOR Axis. Curr Top Microbiol Immunol. 2017;407:153–189. doi:10.1007/82_2017_6
73. Zheng N, Liu W, Li B, et al. Co-delivery of sorafenib and metapristone encapsulated by CXCR4-targeted PLGA-PEG nanoparticles overcomes hepatocellular carcinoma resistance to sorafenib. J Exp Clin Cancer Res. 2019;38(1):232. doi:10.1186/s13046-019-1216-x
74. Wang SB, Ma YY, Chen XY, Zhao YY, Mou XZ. Ceramide-graphene oxide nanoparticles enhance cytotoxicity and decrease HCC xenograft development: a novel approach for targeted cancer therapy. Front Pharmacol. 2019;10:69. doi:10.3389/fphar.2019.00069
75. Gao Y, Hu L, Liu Y, Xu X, Wu C. Targeted delivery of paclitaxel in liver cancer using hyaluronic acid functionalized mesoporous hollow alumina nanoparticles. Biomed Res Int. 2019;2019:2928507. doi:10.1155/2019/2928507
76. Zhang J, Yu C, Jiang G. Synthesis of cholic-acid-carrying polymer and in-vitro evaluation of hepatoma-targeting nanoparticles decorated with the polymer. J Biomater Sci Polym Ed. 2016;27(9):865–879. doi:10.1080/09205063.2016.1168764
77. Ali D, Alarifi S, Alkahtani S, Almeer RS. Silver-doped graphene oxide nanocomposite triggers cytotoxicity and apoptosis in human hepatic normal and carcinoma cells. Int J Nanomedicine. 2018;13:5685–5699. doi:10.2147/IJN.S165448
78. Shapiro J, Jersky J, Katzav S, Feldman M, Segal S. Anesthetic drugs accelerate the progression of postoperative metastases of mouse tumors. J Clin Invest. 1981;68(3):678–685. doi:10.1172/JCI110303
79. Lange PH, Hekmat K, Bosl G, Kennedy BJ, Fraley EE. Accelerated growth of testicular cancer after cytoreductive surgery. Cancer. 1980;45(6):1498–1506. doi:10.1002/1097-0142(19800315)45:6<1498::AID-CNCR2820450633>3.0.CO;2-7
80. deVere White R, Deitch AD, Hong WK, Olsson CA. The influence of cytoreductive surgery on the response to chemotherapy of a rat renal cancer. Urol Res. 1985;13(1):35–38. doi:10.1007/BF00571754
81. O’Reilly MS, Boehm T, Shing Y, et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88(2):277–285. doi:10.1016/S0092-8674(00)81848-6
82. Tohme S, Simmons RL, Tsung A. Surgery for Cancer: a Trigger for Metastases. Cancer Res. 2017;77(7):1548–1552. doi:10.1158/0008-5472.CAN-16-1536
83. Shi M, Guo RP, Lin XJ, et al. Partial hepatectomy with wide versus narrow resection margin for solitary hepatocellular carcinoma: a prospective randomized trial. Ann Surg. 2007;245(1):36–43. doi:10.1097/01.sla.0000231758.07868.71
84. Ceelen W, Pattyn P, Mareel M. Surgery, wound healing, and metastasis: recent insights and clinical implications. Crit Rev Oncol Hematol. 2014;89(1):16–26. doi:10.1016/j.critrevonc.2013.07.008
85. Govaert KM, Emmink BL, Nijkamp MW, et al. Hypoxia after liver surgery imposes an aggressive cancer stem cell phenotype on residual tumor cells. Ann Surg. 2014;259(4):750–759. doi:10.1097/SLA.0b013e318295c160
86. Wang XM, Yu DM, McCaughan GW, Gorrell MD. Fibroblast activation protein increases apoptosis, cell adhesion, and migration by the LX-2 human stellate cell line. Hepatology. 2005;42(4):935–945. doi:10.1002/hep.20853
87. Nakamura T, Sakai K, Nakamura T, Matsumoto K. Hepatocyte growth factor twenty years on: much more than a growth factor. J Gastroenterol Hepatol. 2011;26(Suppl 1):188–202. doi:10.1111/j.1440-1746.2010.06549.x
88. Deng H, Wang HF, Gao YB, Jin XL, Xiao JC. Hepatic progenitor cell represents a transitioning cell population between liver epithelium and stroma. Med Hypotheses. 2011;76(6):809–812. doi:10.1016/j.mehy.2011.02.024
89. Zhao H, Feng Y, Wang Y, Yang B, Xing Z. Comparison of different loading dose of celecoxib on postoperative anti-inflammation and analgesia in patients undergoing endoscopic nasal surgery-200 mg is equivalent to 400 mg. Pain Med. 2011;12(8):1267–1275. doi:10.1111/j.1526-4637.2011.01196.x
90. Ruan D, So SP. Prostaglandin E2 produced by inducible COX-2 and mPGES-1 promoting cancer cell proliferation in vitro and in vivo. Life Sci. 2014;116(1):43–50. doi:10.1016/j.lfs.2014.07.042
91. Wang D, Dubois RN. Eicosanoids and cancer. Nat Rev Cancer. 2010;10(3):181–193. doi:10.1038/nrc2809
92. Swartz MA, Lund AW. Lymphatic and interstitial flow in the tumour microenvironment: linking mechanobiology with immunity. Nat Rev Cancer. 2012;12(3):210–219. doi:10.1038/nrc3186
93. Le CP, Nowell CJ, Kim-Fuchs C, et al. Chronic stress in mice remodels lymph vasculature to promote tumour cell dissemination. Nat Commun. 2016;7:10634. doi:10.1038/ncomms10634
94. Sloan EK, Priceman SJ, Cox BF, et al. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2010;70(18):7042–7052. doi:10.1158/0008-5472.CAN-10-0522
95. Abramovitch R, Marikovsky M, Meir G, Neeman M. Stimulation of tumour growth by wound-derived growth factors. Br J Cancer. 1999;79(9–10):1392–1398. doi:10.1038/sj.bjc.6690223
96. Sánchez A, Alvarez AM, Pagan R, et al. Fibronectin regulates morphology, cell organization and gene expression of rat fetal hepatocytes in primary culture. J Hepatol. 2000;32(2):242–250. doi:10.1016/S0168-8278(00)80069-0
97. Kim SH, Turnbull J, Guimond S. Extracellular matrix and cell signalling: the dynamic cooperation of integrin, proteoglycan and growth factor receptor. J Endocrinol. 2011;209(2):139–151. doi:10.1530/JOE-10-0377
98. Li L, Xie T. Stem cell niche: structure and function. Annu Rev Cell Dev Biol. 2005;21:605–631. doi:10.1146/annurev.cellbio.21.012704.131525
99. Fan ST, Yang ZF, Ho DW, Ng MN, Yu WC, Wong J. Prediction of posthepatectomy recurrence of hepatocellular carcinoma by circulating cancer stem cells: a prospective study. Ann Surg. 2011;254(4):569–576. doi:10.1097/SLA.0b013e3182300a1d
100. Coffey JC, Wang JH, Smith MJ, Bouchier-Hayes D, Cotter TG, Redmond HP. Excisional surgery for cancer cure: therapy at a cost. Lancet Oncol. 2003;4(12):760–768. doi:10.1016/S1470-2045(03)01282-8
101. Demicheli R, Retsky MW, Hrushesky WJ, Baum M, Gukas ID. The effects of surgery on tumor growth: a century of investigations. Ann Oncol. 2008;19(11):1821–1828. doi:10.1093/annonc/mdn386
102. Wang B, Xia CY, Lau WY, et al. Determination of clonal origin of recurrent hepatocellular carcinoma for personalized therapy and outcomes evaluation: a new strategy for hepatic surgery. J Am Coll Surg. 2013;217(6):1054–1062. doi:10.1016/j.jamcollsurg.2013.07.402
103. Wu HH, Huang CC, Chang CP, Lin MT, Niu KC, Tian YF. Heat shock protein 70 (HSP70) reduces hepatic inflammatory and oxidative damage in a rat model of liver ischemia/reperfusion injury with hyperbaric oxygen preconditioning. Med Sci Monit. 2018;24:8096–8104. doi:10.12659/MSM.911641
104. Schumacher N, Rose-John S. ADAM17 Activity and IL-6 Trans-signaling in inflammation and cancer. Cancers. 2019;11:11. doi:10.3390/cancers11111736
105. Sun X, Hu F, Hou Z, et al. SIX4 activates Akt and promotes tumor angiogenesis. Exp Cell Res. 2019;383(1):111495. doi:10.1016/j.yexcr.2019.111495
106. Zhai XF, Liu XL, Shen F, Fan J, Ling CQ. Traditional herbal medicine prevents postoperative recurrence of small hepatocellular carcinoma: a randomized controlled study. Cancer. 2018;124(10):2161–2168. doi:10.1002/cncr.30915
107. Khalid AD, Ur-Rehman N, Tariq GH, et al. Functional bioinspired nanocomposites for anticancer activity with generation of reactive oxygen species. Chemosphere. 2023;310:136885. doi:10.1016/j.chemosphere.2022.136885
108. Long BA, Lau CY, Rodriguez DJ, Tang SA, Anderson SL. Sublimation kinetics for individual graphite and graphene nanoparticles (NPs): NP-to-NP variations and evolving structure-kinetics and structure-emissivity relationships. J Am Chem Soc. 2020;142(33):14090–14101. doi:10.1021/jacs.0c01720
109. Tran S, DeGiovanni PJ, Piel B, Rai P. Cancer nanomedicine: a review of recent success in drug delivery. Clin Transl Med. 2017;6(1):44. doi:10.1186/s40169-017-0175-0
110. Fong ELS, Toh TB, Lin QXX, et al. Generation of matched patient-derived xenograft in vitro-in vivo models using 3D macroporous hydrogels for the study of liver cancer. Biomaterials. 2018;159:229–240. doi:10.1016/j.biomaterials.2017.12.026
111. Kolesky DB, Truby RL, Gladman AS, Busbee TA, Homan KA, Lewis JA. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv Mater. 2014;26(19):3124–3130. doi:10.1002/adma.201305506
112. Ruan S, Zhou Y, Jiang X, Gao H. Rethinking CRITID procedure of brain targeting drug delivery: circulation, blood brain barrier recognition, intracellular transport, diseased cell targeting, internalization, and drug release. Adv Sci. 2021;8(9):2004025. doi:10.1002/advs.202004025
113. Baboci L, Capolla S, Di Cintio F, et al. The dual role of the liver in nanomedicine as an actor in the elimination of nanostructures or a therapeutic target. J Oncol. 2020;2020:4638192. doi:10.1155/2020/4638192
114. Habek D, Cerovac A, Kamerić L, Nevačinović E, Šerak A. Balneogynaecology in the 21st century: increasingly recommended primary and complementary treatment of chronic gynaecological diseases. Med Glas (Zenica). 2021;18(1):1–6. doi:10.17392/1263-21
115. De Falco L, Troiano E, Cesar M, et al. Intra-operative local plus systemic tranexamic acid significantly decreases post-operative bleeding and the need for allogeneic blood transfusion in total knee arthroplasty. Med Glas (Zenica). 2021;18(1):267–272. doi:10.17392/1327-21
116. Iftikhar M, Noureen A, Jabeen F, et al. Bioinspired engineered nickel nanoparticles with multifunctional attributes for reproductive toxicity. Chemosphere. 2023;311(Pt 1):136927. doi:10.1016/j.chemosphere.2022.136927
117. Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144(8):1941–1953. doi:10.1002/ijc.31937
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
