Back to Journals » International Journal of Nanomedicine » Volume 18
Advances in the Applications of Extracellular Vesicle for the Treatment of Skin Photoaging: A Comprehensive Review
Received 2 August 2023
Accepted for publication 27 October 2023
Published 7 November 2023 Volume 2023:18 Pages 6411—6423
DOI https://doi.org/10.2147/IJN.S433611
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yan Shen
Chan-Sheng Cai,1 Gui-Juan He,2 Fa-Wei Xu2
1The First Clinical Medical College, Wenzhou Medical University, Wenzhou, Zhejiang, People’s Republic of China; 2Department of Plastic Surgery, Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, Hangzhou, 310016, People’s Republic of China
Correspondence: Fa-Wei Xu, Tel +8615868128520, Email [email protected]
Abstract: Skin photoaging is a complex biological process characterized by the accumulation of oxidative damage and structural changes in the skin, resulting from chronic exposure to ultraviolet (UV) radiation. Despite the growing demand for effective treatments, current therapeutic options for skin photoaging remain limited. However, emerging research has highlighted the potential of extracellular vesicles (EVs), including exosomes, micro-vesicles, apoptotic bodies and liposomes, as promising therapeutic agents in skin rejuvenation. EVs are involved in intercellular communication and can deliver bioactive molecules, including proteins, nucleic acids, and lipids, to recipient cells, thereby influencing various cellular processes. This comprehensive review aims to summarize the current research progress in the application of EVs for the treatment of skin photoaging, including their isolation and characterization methods, roles in skin homeostasis, therapeutic potential and clinical applications for skin photoaging. Additionally, challenges and future directions in EVs-based therapies for skin rejuvenation are discussed.
Keywords: extracellular vesicles, exosomes, liposomes, stem cells, skin photoaging, skin rejuvenation
Graphical Abstract:
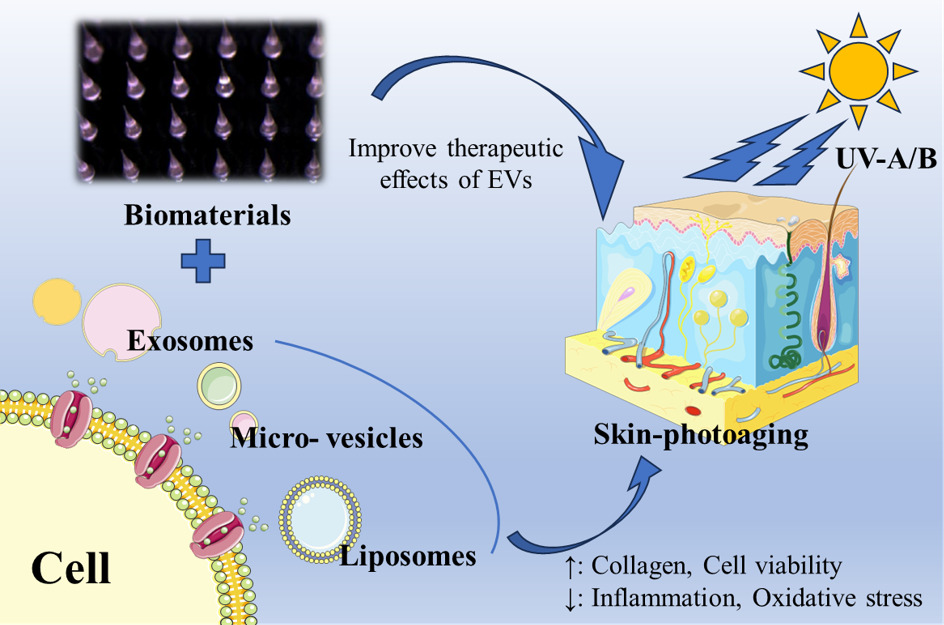
Introduction
Skin, being the largest barrier organ, is susceptible to various injuries caused by genetic factors, lifestyle choices, nutrition, solar radiation, and environmental factors.1–3 Skin aging and diseases impose a significant burden, encompassing mental, social, and financial consequences for individuals, families, and society.4,5 Skin photoaging, a prominent manifestation of cutaneous aging, is primarily induced by chronic exposure to UV radiation.6,7 It is characterized by the appearance of fine lines, wrinkles, pigmentation irregularities, and loss of skin elasticity.8 Photoaged skin exhibits structural alterations, such as the degradation of collagen fibers, the accumulation of abnormal elastic fibers, and the disruption of the epidermal barrier function.9–12 Despite the widespread prevalence and significant socioeconomic impact of skin photoaging, effective treatment modalities are still limited.13 Conventional approaches, such as topical retinoids,14–16 antioxidants,17,18 and laser therapies,19–21 often provide modest and transient results, necessitating the exploration of novel therapeutic strategies.
In recent years, EVs have gained significant attention due to their crucial role in intercellular communication and their potential as therapeutic agents for various diseases, including skin disorders.22,23 EVs comprise a diverse group of membrane-bound vesicles, including exosomes, micro-vesicles, apoptotic bodies and liposomes, which are released by various cell types.24,25 These vesicles, excluding liposomes that are artificially synthesized, encapsulate a cargo of bioactive molecules derived from their parent cells.24 This cargo includes a wide range of bioactive molecules, such as proteins, nucleic acids, lipids, and signaling molecules (Figure 1).26 Through the transfer of these bioactive molecules, EVs play a crucial role in mediating intercellular communication and regulating various physiological and pathological processes, such as proliferation, migration, and differentiation, thus influencing tissue homeostasis and repair.27–29 Through their participation in intercellular communication, EVs play pivotal roles in regulating both physiological and pathological processes.
 |
Figure 1 The structure and bioactive components of EVs were demonstrated and the mechanism of anti-photoaging. |
Among EVs, exosomes, micro-exosomes, apoptotic bodies, and liposomes represent distinct subtypes with unique characteristics and functions.30 Exosomes are natural small extracellular vesicles, derived from the endosomal pathway and secreted by diverse cell types, including keratinocytes, fibroblasts, immune cells, and many kinds of plant cells, are small vesicles ranging from 30 to 150 nm in diameter and carry a cargo of bioactive molecules, including proteins, nucleic acids, lipids, and signaling molecules.31,32 Micro-vesicles, larger in size with a diameter of 100 to 1000 nm, are directly shed from the plasma membrane and transfer a wide range of molecules.33,34 Apoptotic bodies, the largest vesicles ranging from 500 to 5000 nm, are released during programmed cell death and play a role in cellular clearance and immune regulation.35,36 In contrast, liposomes are artificial vesicles composed of lipid bilayers, engineered for drug delivery purposes.37–40 Understanding the distinct characteristics, functions, and origins of these EV subtypes is essential for harnessing their potential in intercellular communication and biomedical applications. Given the ability of EVs to mediate cellular communication and their potential cargo-based therapeutic applications, researchers have begun exploring their role in skin homeostasis and rejuvenation.
This review aims to provide a comprehensive overview of the current research progress in the application of EVs for the treatment of skin photoaging. It encompasses various types of extracellular vesicles, focusing on exosomes and liposomes, their biogenesis, cargo composition, and mechanisms of action. The review also covers methods for isolating and characterizing EVs from different sources, including skin cells and body fluids. Moreover, preclinical and clinical studies investigating the therapeutic potential of extracellular vesicles for skin rejuvenation and anti-aging effects will be examined. Finally, we will discuss the challenges and future directions in the development of EVs-based therapies for the treatment of skin photoaging.
Features of Skin Photoaging
Skin photoaging is a complex biological process characterized by premature aging and damage caused by chronic exposure to ultraviolet (UV) radiation from the sun.41 It is a major concern globally, given its significant impact on the appearance, health, and well-being of individuals. This section provides a comprehensive overview of the mechanisms underlying skin photoaging, including UV-induced molecular and cellular alterations. It explores the clinical manifestations of photoaged skin, such as wrinkles, pigmentation irregularities, and loss of elasticity.
UV radiation induces a cascade of molecular and cellular events that contribute to the accelerated aging of the skin. It triggers the generation of reactive oxygen species (ROS), leading to oxidative stress and DNA damage.42,43 Activation of inflammatory pathways, upregulation of matrix metalloproteinases (MMPs), and disruption of collagen and elastin synthesis further contribute to skin degradation and loss of elasticity.44 Additionally, UV radiation promotes the formation of abnormal pigmentation, such as solar lentigines and uneven skin tone.45
Multiple therapeutic strategies are employed to prevent and treat skin photoaging. Broad-spectrum sunscreens and photoprotective measures, such as avoiding excessive sun exposure and using protective clothing, form the cornerstone of prevention.46 Topical antioxidants, including vitamins C and E, have shown promise in reducing oxidative stress and protecting against UV-induced damage.47 Retinoids, derived from vitamin A, have demonstrated efficacy in improving skin texture, promoting collagen synthesis, and reducing pigmentation.48 Advanced technologies, including laser therapy, intense pulsed light (IPL),49 and photodynamic therapy (PDT),50 offer targeted approaches to address specific manifestations of photoaging. Furthermore, emerging interventions, such as regenerative medicine approaches involving stem cells, growth factors, and tissue engineering, hold potential for skin rejuvenation and repair.51
Extraction, Synthesis and Characterization of EVs
EVs have emerged as key players in intercellular communication and have garnered significant attention in various fields of research.52 To harness their potential in diagnostics, therapeutics, and biomarker discovery, it is crucial to establish reliable methods for the extraction, synthesis, and characterization of EVs. This section provides an overview of the current techniques used for the extraction of EVs from biological samples, the synthesis of liposomes, and the identification and characterization of different types of EVs.
A major challenge in the clinical application of EVs in regenerative medicine is the lack of standardized methods for their isolation.53 The isolation of EVs from complex biological samples is a critical step in their study. Common extraction methods include ultracentrifugation, filtration, precipitation, and size-exclusion chromatography.54 Ultracentrifugation involves sequential centrifugation steps to pellet EVs based on their density.55 Filtration methods employ filters with defined pore sizes to retain EVs while allowing other components to pass through.56 Precipitation methods, such as polyethylene glycol (PEG) precipitation, selectively precipitate EVs from the sample.57 Consequently, combining two or more isolation methods has gained popularity to enhance EV purity. Size-exclusion chromatography separates EVs based on their size using columns with porous beads. To accurately identify and characterize EVs, multiple methods can be employed. Electron microscopy (EM) provides morphological information, allowing visualization of EVs’ membrane-bound structures.57 Techniques such as dynamic light scattering (DLS) and nanoparticle tracking analysis (NTA) measure EVs’ size distribution and concentration.57 Immunological methods, including flow cytometry and Western blotting,58 utilize specific markers for different types of EVs. For example, exosomes commonly express tetraspanins (CD9, CD63, CD81) and heat shock proteins (HSP70, HSP90), while micro-vesicles may express CD40, CD44, and tissue factor (TF).59,60 Apoptotic bodies are characterized by cell surface-exposed phosphatidylserine, cleaved caspase-3, and fragmented DNA, often detected using the TUNEL assay (Table 1).61 Mass spectrometry (MS) enables the identification and quantification of EVs’ molecular components, such as proteins, nucleic acids, and lipids.62
 |
Table 1 The Special Markers of Each Type of EVs |
Liposomes, a type of artificial vesicle, have been widely used for drug delivery and research purposes. Common methods for liposome synthesis include thin-film hydration, reverse-phase evaporation, and extrusion.63,64 Thin-film hydration involves dissolving lipids in an organic solvent, followed by the removal of the solvent and hydration of the lipid film with an aqueous solution. Reverse-phase evaporation combines lipids, an aqueous solution, and an organic solvent, followed by evaporation to form multilamellar vesicles (MLVs).64,65 Extrusion involves passing a lipid suspension through membranes with defined pore sizes to obtain unilamellar vesicles (ULVs).66
The extraction, synthesis, and characterization of EVs are crucial for advancing our understanding of their biology and exploring their potential applications. Various extraction methods facilitate the isolation of EVs from complex samples, while liposome synthesis techniques provide artificial vesicles for drug delivery and research purposes. Identification and characterization methods allow for the accurate classification and assessment of EVs based on their morphological, molecular, and functional features. Further advancements in these areas will contribute to the development of EV-based diagnostics, therapeutics, and biomarker discovery.
Properties of EVs for Skin Wound Therapy
In recent years, EVs have emerged as highly promising vehicles for delivering nucleic-acid-based therapeutics due to their inherent biocompatibility, ability to traverse physiological barriers, and minimal immunogenicity. EVs, including exosomes, are naturally generated by the body’s cells, resulting in reduced inflammatory responses. Some cost-effective and streamlined methodologies in lab aimed to facilitate the future efficient and economical production of exosomes in large quantities.67–70 In the future, with the realization of commercialization and standardized production of EVs, it will become a cost-effective and efficient treatment.
Wound healing is a complex biological process that involves a series of coordinated events aimed at restoring tissue integrity and functionality.71–73 In recent years, EVs have emerged as promising candidates for enhancing wound healing due to their unique characteristics.70,74 The diverse composition of EVs enables them to regulate multiple cellular processes involved in wound healing, including inflammation, angiogenesis, cell proliferation, and extracellular matrix remodeling.68,75 Wang et al, in their study, developed a gelatin methacryloyl (GelMA) hydrogel loaded with VH298-loaded EVs (VH-EVs) derived from epidermal stem cells (ESCs). Their findings demonstrated that VH-EVs exhibited promotive effects on the function of human umbilical vein endothelial cells (HUVECs) in vitro by activating the HIF-1α signaling pathway.76 Moreover, in vivo experiments showed that GelMA hydrogel with sustained release of VH-EVs, possessing high biocompatibility and suitable mechanical properties, effectively promoted diabetic wound healing and angiogenesis in diabetic mice.76 When it comes to the regulation of inflammation in wound healing, a study revealed that apoptotic bodies (Abs) derived from MSCs play a crucial role in promoting cutaneous wound healing by inducing the polarization of macrophages towards the M2 phenotype.77 Furthermore, these functionally converted macrophages enhanced the migration and proliferation abilities of fibroblasts, synergistically facilitating the wound healing process.77 In another study, the researchers compared the functions of EVs derived from mesenchymal stem cells (MSCs) isolated from bone marrow (BMSCs) and adipose tissue (ADSCs) in the context of wound healing. Both types of EVs showed potential benefits for tissue regeneration, but they exhibited distinct biological properties. They found that ADSC-EVs contain molecules that are associated with angiogenesis, while BMSC-EVs were correlated with cellular proliferation.78 And Kevin Las Heras found that EVs derived from hair follicle MSCs (HFMSC-EVs) exhibited the capability to enhance the proliferation and migration of human dermal fibroblasts (HDFs). Furthermore, HFMSC-EVs stimulated angiogenesis in HUVECs as well.79
The utilization of EVs in wound healing holds great potential for improving the outcomes of wound repair and regeneration. Their ability to modulate immune responses, promote angiogenesis, stimulate tissue regeneration, and facilitate ECM remodeling makes them valuable tools in promoting efficient wound healing and reducing scar formation.
Skin Rejuvenation of EVs
Skin aging is another major concern and EVs promote wound healing through physiological regulation of cells and, in particular, stem cell-derived external vesicles, which can also alter cell states to achieve cell anti-aging, thereby delaying skin aging and improving skin aging caused by UV light (Table 2).80,81 Some exosomes of plant cells, organoid or probiotics also contribute to skin rejuvenation, and some researchers combine EVs with biological materials to make EVs have better transdermal delivery effect, and finally achieve better efficacy.
 |
Table 2 Exosomes Derived from Different Types of Cells and Their Effects |
Stem Cell Derived EVs for Treatment of Photoaging
The potential of adipose-derived stem cells (ADSCs) for skin rejuvenation has been well-documented. However, a complete understanding of the effects of ADSC-derived exosomes on photoaged skin still requires further clarification. Jun-Xian Liang et al conducted a study to investigate the anti-aging effects of ADSC-derived exosomes on photoaged skin. They extracted exosomes from cultured ADSCs using ultracentrifugation. The exosomes were then subjected to verification through examination of cell morphology using transmission electron microscopy and identification of specific biomarkers.82 Subsequently, the researchers injected the exosomes at an optimal concentration and treatment time into the photoaged skin of Sprague-Dawley rats that had been exposed to ultraviolet B radiation. The results showed that the injected exosomes significantly reduced epidermal thickness and increased dermal thickness of the photoaged skin after 7 days of treatment.82 Additionally, the proportion of the stratum corneum in the epidermis was reduced. Furthermore, real-time RT-PCR analysis demonstrated an increase in the mRNA expression of type I collagen and a decrease in the expression of type III collagen, MMP-1, and MMP-3. Other researchers in Korea have also demonstrated the remarkable effects of ADSC-derived extracellular vesicles (ADSC-EVs) in mitigating the adverse effects of UVB irradiation on skin. Specifically, they observed that ADSC-EVs effectively suppressed the overexpression of MMP-1, MMP-2, MMP-3, and MMP-9 induced by UVB irradiation, thereby promoting the biosynthesis of collagen.83 Notably, the researchers observed an upregulation of tissue inhibitor of metalloproteinase-1 (TIMP-1) and transforming growth factor beta-1 (TGFβ-1) in HDFs treated with ADSC-EVs after UVB irradiation.83 These factors play pivotal roles in suppressing MMP activity and stimulating extracellular matrix (ECM) synthesis. Furthermore, the study revealed that ADSC-EVs also exhibited the remarkable ability to enhance the migration capacity of HDFs that had been subjected to UVB irradiation.83 Peng Xu conducted a study demonstrating that ADSC-derived extracellular vesicles (ADSC-EVs) effectively reduced skin wrinkles in mice with UVB-induced photoaging.84 This effect was attributed, in part, to the attenuation of Raw 264.7 cell differentiation from M0 to M1 macrophages, resulting in a decrease in intracellular reactive oxygen species (ROS) production. Additionally, ADSC-EVs promoted the expression of antioxidant enzymes and rescued fibroblasts (FBs) from cell cycle arrest.84
Subsequently, Myeongsik Oh made an exciting discovery regarding exosomes derived from the conditioned medium of human induced pluripotent stem cells (iPSCs). These exosomes exhibited similar effects to ADSC-EVs when applied to HDFs cells irradiated with UVB. They stimulated the proliferation and migration of HDFs, leading to increased expression levels of collagen type-I. Notably, the expression levels of senescence-associated β-galactosidase (SA-Gal) and MMP-1/3 were significantly reduced, indicating a potential anti-aging effect.87
Human umbilical cord mesenchymal stem cells (HUCMSCs) have gained recognition as a versatile and efficacious therapeutic option in clinical settings.91 Deng’s research group undertook a study in which they isolated and characterized EVs derived from both HUCMSCs and HDFs.85 Subsequently, they assessed and compared the effects of HUCMSC-derived EVs and HDF-derived EVs on UVB-induced photoaging in dermal fibroblasts. Their findings revealed that pretreatment with HUCMSC-derived EVs or Fb-derived EVs effectively upregulated the expressions of GPX-1 and Col-1, while downregulating the expression of MMP-1. Importantly, both HUCMSC-derived EVs and Fb-derived EVs demonstrated a protective effect on dermal fibroblasts against UVB-induced photoaging, likely attributed to their antioxidant activity.85 And Shi-Jie Liu et al demonstrated that HUCMSC-EVs had same ability to modulate the photo-aging of HaCaT keratinocytes.92
In addition to exosomes derived from human stem cells, exosomes obtained from other types of human cells through specific treatments are also being explored for their potential in photoaging therapy, showing promising results. Shiqi Hu et al created three-dimensional spheroids of human dermal fibroblasts (HDFs), from which they isolated exosomes (referred to as 3D HDF-XOs). These exosomes demonstrated an ability to enhance the expression of procollagen type I, a crucial component of the extracellular matrix, while significantly reducing the expression of MMP-1, an enzyme associated with collagen degradation.86 The effects were primarily attributed to the downregulation of tumor necrosis factor-alpha (TNF-α) and the upregulation of transforming growth factor beta (TGF-β) mediated by the 3D HDF-XOs.86 This research suggests that exosomes derived from 3D spheroids of HDFs can modulate the expression of key molecules involved in skin rejuvenation, providing a potential therapeutic approach for addressing photoaging.
Special Ingredients in EVs Promoting Skin Rejuvenation
EVs contains proteins, nucleic acids and other substances, which are involved in various cellular interactions. Many researchers have explored which EVs components have the therapeutic effect on skin photoaging, and some researchers also have specifically increased the expression of certain components in EVs, thereby improving its therapeutic effect on skin photoaging.
Tingting Yan, a Chinese researcher, conducted a study involving the extraction of exosomes derived from bone marrow mesenchymal stem cells (BMSCs). The research aimed to explore the effects of these exosomes on human dermal fibroblasts (HDFs) exposed to UVB irradiation. Notably, the study revealed that the presence of miR-29b-3p in BMSC-derived exosomes played a pivotal role in reversing the inhibitory effects of UVB irradiation on HDFs.88 Specifically, miR-29b-3p counteracted the hindered migration of HDFs, alleviated oxidative stress, and mitigated the promotion of apoptosis induced by UVB irradiation.88 These findings highlight the potential of BMSC-derived exosomes, particularly through the action of miR-29b-3p, in counteracting the detrimental effects of UVB irradiation on skin cells.
Yu Zhang et al conducted a previous study in which they discovered a non-coding circular RNA, circ_0011129, that acts as an adsorption sponge for miR-6732-5p.89 This interaction was found to inhibit the reduction of type I collagen and prevent the denaturation and accumulation of elastin in a UVA-induced photoaging model using human dermal fibroblast (HDF) cells. To enhance the stability and delivery efficiency of circRNA, they loaded it into small extracellular vesicles (sEVs) derived from human adipose-derived stem cells (hADSCs) through overexpression of circ_0011129 in hADSCs.89 Through miRNA sequencing (miRNA-seq) and GEO data analysis, they identified an enrichment of miRNAs in the 3D-circ-sEVs that were associated with apoptosis, cellular senescence, and inflammation-related cellular activities. They demonstrated that 3D-circ-sEVs can interfere with the process of cellular photoaging and protect cells from UVA radiation damage, as well as in an H2O2-induced oxidative stress model.89
In another study by Wei Gao et al, they confirmed miR-1246 as a crucial therapeutic agent employed by ADSCs to protect against UVB-induced photoaging. They generated miR-1246-overexpressing ADSCs and exosomes (OE-EX) through lentivirus infection. Their findings revealed that OE-EX significantly reduced MMP-1 expression by inhibiting the MAPK/AP-1 signaling pathway.93 Moreover, OE-EX markedly increased procollagen type I secretion by activating the TGF-β/Smad pathway. Additionally, OE-EX exhibited anti-inflammatory effects by preventing UVB-induced degradation of IκB-α and inhibiting NF-κB overexpression.93 Animal experiments demonstrated that OE-EX could reduce UVB-induced wrinkle formation, epidermis thickening, and the reduction of collagen fibers in Kunming mice.93
Special Cells Derived EVs for Treatment of Photoaging
Plant-derived ingredients are frequently incorporated into skincare products due to their anti-aging properties. Notably, certain plant-derived cellular exosomes have been shown to possess regenerative, anti-inflammatory, and low immunogenicity characteristics.94,95 In this context, we will provide a brief overview of how specific exosomes secreted by specialized cells contribute to the rejuvenation of photoaged skin.
Phellinus linteus (PL), a medicinal fungus known for its antitumor and anti-inflammatory properties, has also been investigated for its potential anti-photoaging effects.90 Dr. Han et al conducted a study to explore these effects by fungi isolating exosome-like nanovesicles (FELNVs) from PL. They further evaluated the anti-aging activity of PL-FELNVs through clinical volunteer testing, which demonstrated promising results.90
Their research into the molecular mechanisms of PL-FELNVs revealed that a specific microRNA, miR-CM1, present in the FELNVs, played a crucial role. Through cross-kingdom regulation, miR-CM1 inhibited the expression of Mical2 in HaCaT cells, subsequently promoting the expression of COL1A2. Moreover, it was found that miR-CM1 also suppressed MMP1 expression in skin cells. Consequently, the levels of reactive oxygen species (ROS), malondialdehyde (MDA), and senescence-associated β-galactosidase (SA-β) were decreased, suggesting a decrease in oxidative stress and cellular aging processes. There is another research group that has clinical trials about investigating the effect of Lactobacillus plantarum (LP) EVs on skin aging, their results demonstrated that LP-EVs have a great effect on skin aging by inducing cell proliferation of fibroblasts and regulating ECM related genes (MMP-1 decreased, Type I procollagen, Filaggrin and HAS2 increased).
PL-FELNVs possess several notable advantages, particularly in terms of biocompatibility, cellular uptake, and targeting capabilities. Unlike current synthetic drug delivery systems, such as inorganic and polymeric nanoparticles, which are foreign materials and may carry potential risks of toxicity and immunogenicity, PL-FELNVs are derived from natural and endogenous sources. As a result, they are regarded as highly biocompatible, exhibiting compatibility with various biological functions akin to their parent cells. This characteristic renders PL-FELNVs a safer and more reliable option for drug delivery systems.
Biomaterials Strengthen Anti Photoaging Effects of EVs
Microneedle (MN) is a widely utilized and minimally invasive tool for delivering drugs to the dermal layer. Its efficacy stems from its ability to create small injuries and initiate the wound healing process, which in turn leads to neocollagenesis.96 In pursuit of enhancing the treatment efficacy of skin photoaging, Xiuli Wang’s research team explored the use of roller microneedles for delivering adipose-derived stem cell-derived extracellular vesicles (ADSC-EVs) to the skin.97 Their findings validated that microneedles are an effective means of delivering drugs to the skin, thereby improving treatment outcomes.97
More recently, Van Dat Bui et al developed a dissolving microneedle patch (EV@MN) composed of hyaluronic acid and loaded it with human ADSC-derived EVs (hADSC-EVs) for skin photoaging treatment.98 The EV@MN patch facilitated precise and convenient intradermal delivery, enabling sustained release of EVs in the dermis layer. Consequently, EV@MN significantly enhanced the biological functions of hADSC-EVs on dermal fibroblasts by promoting the synthesis of proteins crucial for the extracellular matrix, such as collagen and elastin, while also boosting fibroblast proliferation.98 Comparatively, other administration methods did not yield the same level of improvement. A kind of natural biomaterial, sponge Haliclona sp. spicules (SHSs), were also used as a carrier of EVs to improve its delivery efficiency, SHSs increased the skin absorption of human umbilical cord-derived MSC-Exos (hucMSC-Exos), and improved anti-photoaging effects in mice.99
Apart from naturally produced EVs derived from stem cells, researchers have begun exploring the use of artificial EVs, where specific substances are encapsulated within liposomes. For instance, a research team from Peking University successfully generated COL1A1 mRNA-containing EVs (COL1A1-EVs) from human dermal fibroblasts using their own cellular nanoporation (CNP) technique as shown in Figure 2A, which enhances the loading efficiency of mRNA in EVs.100 The team also demonstrated that intradermal delivery of the mRNA-loaded EVs via a microneedle array, as shown in Figure 2B, resulted in more prolonged and uniform synthesis and replacement of collagen in the dermis, surpassing the effects of traditional injection of COL1A1-EVs.100 Intradermal delivery of EV-based COL1A1 mRNA holds promise as an effective protein-replacement therapy for treating photoaged skin (Figure 2C).
 |
Figure 2 The EVs introduced by microneedle patch has a good therapeutic effect on photoaging. (A) Scheme of CNP EV production process. (B) Scheme of microneedle patch production process. (C) Skin plaster assessment of dorsal skin after COL1A1-EV treatment. Adapted from You Y, Yang Z, Shi J, et al. Intradermally delivered mRNA-encapsulating extracellular vesicles for collagen-replacement therapy. Nat Biomed Eng. 2023;7:887–900, Springer Nature.100. |
With the rapid development of biomaterials, the application of exosomes in skin rejuvenation is anticipated to make substantial advancements. The clinical utilization of exosomes is poised to become more diverse, with a focus on enhancing the treatment of skin photoaging.
The Future Perspectives
Skin photoaging is a common concern resulting from prolonged exposure to ultraviolet (UV) radiation. Researchers have explored various therapeutic approaches to combat the signs of photoaging and restore skin health. One promising avenue is the use of EVs, which are nanosized vesicles released by cells and carry bioactive molecules. EVs derived from adipose-derived stem cells (ADSCs) and human dermal fibroblasts have shown potential in rejuvenating photoaged skin. These EVs can suppress the overexpression of matrix metalloproteinases (MMPs), enhance collagen synthesis, and promote fibroblast proliferation.
However, the clinical application of EVs faces several challenges. The lack of standardized guidelines leads to variations in EV preparation, purification, quality control, and dosing, causing differences among EVs used in different studies. Establishing unified standards and guidelines is crucial to ensure consistency and quality. While EVs are generally considered safe, long-term safety and optimal dosing require further evaluation. Monitoring and assessing potential risks and side effects associated with EVs are essential. Large-scale production of EVs for clinical use remains challenging. Standardizing production processes and optimizing culture and purification methods are necessary to meet patient demands. Additionally, cost-effectiveness is a factor to consider.
In conclusion, EVs hold promise for skin photoaging treatment, but standardization, safety assessment, large-scale production, and cost considerations are important areas for further research and development. Addressing these challenges will advance the clinical application of EVs and enhance their potential in skin rejuvenation.
Acknowledgment
This work was support by grants from Zhejiang Traditional Chinese Medicine Scientific Research Fund Project (No.2022ZB206).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Lee HJ, Kim M. Skin Barrier Function and the Microbiome. Int J Mol Sci. 2022;23:21. doi:10.3390/ijms232113071
2. Sanford JA, Gallo RL. Functions of the skin microbiota in health and disease. Semin Immunol. 2013;25(5):370–377. doi:10.1016/j.smim.2013.09.005
3. Yousef H, Alhajj M, Sharma S. Anatomy, Skin (Integument), Epidermis. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2023.
4. Rittié L, Fisher GJ. Natural and sun-induced aging of human skin. Cold Spring Harb Perspect Med. 2015;5(1):a015370. doi:10.1101/cshperspect.a015370
5. Gu Y, Han J, Jiang C, et al. Biomarkers, oxidative stress and autophagy in skin aging. Ageing Res Rev. 2020;59:101036. doi:10.1016/j.arr.2020.101036
6. Battie C, Jitsukawa S, Bernerd F, et al. New insights in photoaging, UVA induced damage and skin types. Exp Dermatol. 2014;23(Suppl 1):7–12. doi:10.1111/exd.12388
7. Fisher GJ, Kang S, Varani J, et al. Mechanisms of photoaging and chronological skin aging. Arch Dermatol. 2002;138(11):1462–1470. doi:10.1001/archderm.138.11.1462
8. Wang M, Charareh P, Lei X, Zhong JL. Autophagy: multiple mechanisms to protect skin from ultraviolet radiation-driven photoaging. Oxid Med Cell Longev. 2019;2019:8135985. doi:10.1155/2019/8135985
9. Fitsiou E, Pulido T, Campisi J, et al. Cellular senescence and the senescence-associated secretory phenotype as drivers of skin photoaging. J Invest Dermatol. 2021;141(4s):1119–1126. doi:10.1016/j.jid.2020.09.031
10. Pourang A, Tisack A, Ezekwe N, et al. Effects of visible light on mechanisms of skin photoaging. Photodermatol Photoimmunol Photomed. 2022;38(3):191–196. doi:10.1111/phpp.12736
11. Gromkowska-Kępka KJ, Puścion‐Jakubik A, Markiewicz‐żukowska R, et al. The impact of ultraviolet radiation on skin photoaging - review of in vitro studies. J Cosmet Dermatol. 2021;20(11):3427–3431. doi:10.1111/jocd.14033
12. Poon F, Kang S, Chien AL. Mechanisms and treatments of photoaging. Photodermatol Photoimmunol Photomed. 2015;31(2):65–74. doi:10.1111/phpp.12145
13. Riahi RR, Bush AE, Cohen PR. Topical retinoids: therapeutic mechanisms in the treatment of photodamaged skin. Am J Clin Dermatol. 2016;17(3):265–276. doi:10.1007/s40257-016-0185-5
14. Berry K, Hallock K, Lam C. Photoaging and topical rejuvenation. Facial Plast Surg Clin North Am. 2022;30(3):291–300. doi:10.1016/j.fsc.2022.03.003
15. Hubbard BA, Unger JG, Rohrich RJ. Reversal of skin aging with topical retinoids. Plast Reconstr Surg. 2014;133(4):481e–490e. doi:10.1097/PRS.0000000000000043
16. Milosheska D, Roškar R. Use of retinoids in topical antiaging treatments: a focused review of clinical evidence for conventional and nanoformulations. Adv Ther. 2022;39(12):5351–5375. doi:10.1007/s12325-022-02319-7
17. Petruk G, Del Giudice R, Rigano MM, et al. Antioxidants from plants protect against skin photoaging. Oxid Med Cell Longev. 2018;2018:1454936. doi:10.1155/2018/1454936
18. Cui B, Wang Y, Jin J, et al. Resveratrol Treats UVB-induced photoaging by anti-MMP expression, through anti-inflammatory, antioxidant, and antiapoptotic properties, and treats photoaging by upregulating VEGF-B expression. Oxid Med Cell Longev. 2022;2022:6037303. doi:10.1155/2022/6037303
19. Ziai K, Wright HV. Carbon dioxide laser rejuvenation of the facial skin. Facial Plastic Surgery Clinics of North America. 2022;30(3):331–346. doi:10.1016/j.fsc.2022.03.007
20. Dover JS, Hruza GJ. Laser skin resurfacing. Semin Cutan Med Surg. 1996;15(3):177–188. doi:10.1016/S1085-5629(96)80009-5
21. Saluja R, Gentile RD. Picosecond laser: tattoos and skin rejuvenation. Facial Plast Surg Clin North Am. 2020;28(1):87–100. doi:10.1016/j.fsc.2019.09.008
22. Xiong M, Zhang Q, Hu W, et al. The novel mechanisms and applications of exosomes in dermatology and cutaneous medical aesthetics. Pharmacol Res. 2021;166:105490. doi:10.1016/j.phrs.2021.105490
23. Shao S, Fang H, Li Q, et al. Extracellular vesicles in inflammatory skin disorders: from pathophysiology to treatment. Theranostics. 2020;10(22):9937–9955. doi:10.7150/thno.45488
24. Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373–383. doi:10.1083/jcb.201211138
25. Cui Y, Gao, J, He, Y, et al. Plant extracellular vesicles. Protoplasma. 2020;257(1):3–12. doi:10.1007/s00709-019-01435-6
26. Akers JC, Gonda D, Kim R, et al. Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J Neurooncol. 2013;113(1):1–11. doi:10.1007/s11060-013-1084-8
27. Li SR, Man QW, Gao X, et al. Tissue-derived extracellular vesicles in cancers and non-cancer diseases: present and future. J Extracell Vesicles. 2021;10(14):e12175. doi:10.1002/jev2.12175
28. Jeppesen DK, Zhang Q, Franklin JL, et al. Extracellular vesicles and nanoparticles: emerging complexities. Trends Cell Biol. 2023;33(8):667–681. doi:10.1016/j.tcb.2023.01.002
29. Meng W, He C, Hao Y, et al. Prospects and challenges of extracellular vesicle-based drug delivery system: considering cell source. Drug Deliv. 2020;27(1):585–598. doi:10.1080/10717544.2020.1748758
30. Kang T, Atukorala I, Mathivanan S. Biogenesis of Extracellular Vesicles. Subcell Biochem. 2021;97:19–43. doi:10.1007/978-3-030-67171-6_2
31. Pegtel DM, Gould SJ. Exosomes. Annu Rev Biochem. 2019;88:487–514. doi:10.1146/annurev-biochem-013118-111902
32. Kimiz-Gebologlu I, Oncel SS. Exosomes: large-scale production, isolation, drug loading efficiency, and biodistribution and uptake. J Control Release. 2022;347:533–543. doi:10.1016/j.jconrel.2022.05.027
33. Tong M, Chen Q, James JL, et al. Micro- and nano-vesicles from first trimester human placentae carry flt-1 and levels are increased in severe preeclampsia. Front Endocrinol. 2017;8:174. doi:10.3389/fendo.2017.00174
34. Buccheri C, Festucci F, Potestà M, et al. Micro-vesicles of moringa oleifera seeds in heterozygous rats for DAT gene: effects of oral intake on behavioral profile and hematological parameters. Int J Environ Res Public Health. 2021;18(5). doi:10.3390/ijerph18052322
35. Santavanond JP, Rutter SF, Atkin-Smith GK, et al. Apoptotic bodies: mechanism of formation, isolation and functional relevance. Subcell Biochem. 2021;97:61–88. doi:10.1007/978-3-030-67171-6_4
36. Tang H, Luo H, Zhang Z, et al. Mesenchymal stem cell-derived apoptotic bodies: biological functions and therapeutic potential. Cells. 2022;11(23). doi:10.3390/cells11233879
37. Shah S, Dhawan V, Holm R, et al. Liposomes: advancements and innovation in the manufacturing process. Adv Drug Deliv Rev. 2020;154–155:102–122. doi:10.1016/j.addr.2020.07.002
38. Guimarães D, Cavaco-Paulo A, Nogueira E. Design of liposomes as drug delivery system for therapeutic applications. Int J Pharm. 2021;601:120571. doi:10.1016/j.ijpharm.2021.120571
39. Sapkota R, Dash AK. Liposomes and transferosomes: a breakthrough in topical and transdermal delivery. Ther Deliv. 2021;12(2):145–158. doi:10.4155/tde-2020-0122
40. Liu G, Hou S, Tong P, et al. Liposomes: preparation, characteristics, and application strategies in analytical chemistry. Crit Rev Anal Chem. 2022;52(2):392–412. doi:10.1080/10408347.2020.1805293
41. Ansary TM, Hossain MR, Kamiya K, et al. Inflammatory molecules associated with ultraviolet radiation-mediated skin aging. Int J Mol Sci. 2021;22(8). doi:10.3390/ijms22083974
42. Guan LL, Lim HW, Mohammad TF. Sunscreens and photoaging: a review of current literature. Am J Clin Dermatol. 2021;22(6):819–828. doi:10.1007/s40257-021-00632-5
43. Kim DJ, Iwasaki A, Chien AL, et al. UVB-mediated DNA damage induces matrix metalloproteinases to promote photoaging in an AhR- and SP1-dependent manner. JCI Insight. 2022;7(9). doi:10.1172/jci.insight.156344
44. Schuch AP, Moreno NC, Schuch NJ, et al. Sunlight damage to cellular DNA: focus on oxidatively generated lesions. Free Radic Biol Med. 2017;107:110–124. doi:10.1016/j.freeradbiomed.2017.01.029
45. Black JO. Xeroderma Pigmentosum. Head Neck Pathol. 2016;10(2):139–144. doi:10.1007/s12105-016-0707-8
46. Toutfaire M, Bauwens E, Debacq-Chainiaux F. The impact of cellular senescence in skin ageing: a notion of mosaic and therapeutic strategies. Biochem Pharmacol. 2017;142:1–12. doi:10.1016/j.bcp.2017.04.011
47. Jagdeo J, Kurtti A, Hernandez S, et al. Novel vitamin C and E and green tea polyphenols combination serum improves photoaged facial skin. J Drugs Dermatol. 2021;20(9):996–1003. doi:10.36849/JDD.5818
48. Mukherjee S, Date A, Patravale V, et al. Retinoids in the treatment of skin aging: an overview of clinical efficacy and safety. Clin Interv Aging. 2006;1(4):392–412. doi:10.2147/ciia.2006.1.4.327
49. Sales AFS, Pandolfo IL, de Almeida Cruz M, et al. Intense pulsed light on skin rejuvenation: a systematic review. Arch Dermatol Res. 2022;314(9):823–838. doi:10.1007/s00403-021-02283-2
50. Wang Y, Lu Z, Huang Y, et al. Smart nanostructures for targeted oxygen-producing photodynamic therapy of skin photoaging and potential mechanism. Nanomedicine. 2023;18(3):217–231. doi:10.2217/nnm-2022-0170
51. Wolf DA, Beeson W, Rachel JD, et al. Mesothelial stem cells and stromal vascular fraction for skin rejuvenation. Facial Plast Surg Clin North Am. 2018;26(4):513–532. doi:10.1016/j.fsc.2018.06.011
52. Sun Z, Hou X, Zhang J, et al. Diagnostic and therapeutic roles of extracellular vesicles in aging-related diseases. Oxid Med Cell Longev. 2022;2022:6742792. doi:10.1155/2022/6742792
53. Wu J, Piao Y, Liu Q, et al. Platelet-rich plasma-derived extracellular vesicles: a superior alternative in regenerative medicine? Cell Prolif. 2021;54(12):e13123. doi:10.1111/cpr.13123
54. Sidhom K, Obi PO, Saleem A. A review of exosomal isolation methods: is size exclusion chromatography the best option? Int J Mol Sci. 2020;21(18):6466. doi:10.3390/ijms21186466
55. Momen-Heravi F. Isolation of Extracellular Vesicles by Ultracentrifugation. Methods Mol Biol. 2017;1660:25–32. doi:10.1007/978-1-4939-7253-1_3
56. Lane RE, Korbie D, Trau M, et al. Purification protocols for extracellular vesicles. Methods Mol Biol. 2017;1660:111–130. doi:10.1007/978-1-4939-7253-1_10
57. Tiwari S, Kumar V, Randhawa S, et al. Preparation and characterization of extracellular vesicles. Am J Reprod Immunol. 2021;85(2):e13367. doi:10.1111/aji.13367
58. Nolan JP, Duggan E. Analysis of individual extracellular vesicles by flow cytometry. Methods Mol Biol. 2018;1678:79–92. doi:10.1007/978-1-4939-7346-0_5
59. Kowal J, Arras G, Colombo M, et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci U S A. 2016;113(8):E968–77. doi:10.1073/pnas.1521230113
60. Mathieu M, Névo N, Jouve M, et al. Specificities of exosome versus small ectosome secretion revealed by live intracellular tracking of CD63 and CD9. Nat Commun. 2021;12(1):4389. doi:10.1038/s41467-021-24384-2
61. Doyle LM, Wang MZ. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells. 2019;8(7):727. doi:10.3390/cells8070727
62. Crescitelli R, Lässer C, Lötvall J. Isolation and characterization of extracellular vesicle subpopulations from tissues. Nat Protoc. 2021;16(3):1548–1580. doi:10.1038/s41596-020-00466-1
63. Carita AC, Eloy JO, Chorilli M, et al. Recent advances and perspectives in liposomes for cutaneous drug delivery. Curr Med Chem. 2018;25(5):606–635. doi:10.2174/0929867324666171009120154
64. Fan Y, Marioli M, Zhang K. Analytical characterization of liposomes and other lipid nanoparticles for drug delivery. J Pharm Biomed Anal. 2021;192:113642. doi:10.1016/j.jpba.2020.113642
65. Lee Y, Thompson DH. Stimuli-responsive liposomes for drug delivery. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2017;9(5). doi:10.1002/wnan.1450
66. Rideau E, Wurm FR, Landfester K. Self-assembly of giant unilamellar vesicles by film hydration methodologies. Adv Biosyst. 2019;3(6):e1800324. doi:10.1002/adbi.201800324
67. Cabral J, Ryan AE, Griffin MD, et al. Extracellular vesicles as modulators of wound healing. Adv Drug Deliv Rev. 2018;129:394–406. doi:10.1016/j.addr.2018.01.018
68. Bray ER, Oropallo AR, Grande DA, et al. Extracellular vesicles as therapeutic tools for the treatment of chronic wounds. Pharmaceutics. 2021;13(10). doi:10.3390/pharmaceutics13101543
69. Narauskaitė D, Vydmantaitė G, Rusteikaitė J, et al. Extracellular vesicles in skin wound healing. Pharmaceuticals. 2021;14(8):811. doi:10.3390/ph14080811
70. Hade MD, Suire CN, Mossell J, et al. Extracellular vesicles: emerging frontiers in wound healing. Med Res Rev. 2022;42(6):2102–2125. doi:10.1002/med.21918
71. Francesko A, Petkova P, Tzanov T. Hydrogel dressings for advanced wound management. Curr Med Chem. 2018;25(41):5782–5797. doi:10.2174/0929867324666170920161246
72. Broughton G 2nd, Janis JE, Attinger CE. Wound healing: an overview. Plast Reconstr Surg. 2006;117(7 Suppl):1e-S-32e–S. doi:10.1097/01.prs.0000222562.60260.f9
73. Rodrigues M, Kosaric N, Bonham CA, et al. Wound healing: a cellular perspective. Physiol Rev. 2019;99(1):665–706. doi:10.1152/physrev.00067.2017
74. Lu S, Lu L, Liu Y, et al. Native and engineered extracellular vesicles for wound healing. Front Bioeng Biotechnol. 2022;10:1053217. doi:10.3389/fbioe.2022.1053217
75. Jiang M, Jiang X, Li H, et al. The role of mesenchymal stem cell-derived EVs in diabetic wound healing. Front Immunol. 2023;14:1136098. doi:10.3389/fimmu.2023.1136098
76. Wang Y, Cao Z, Wei Q, et al. VH298-loaded extracellular vesicles released from gelatin methacryloyl hydrogel facilitate diabetic wound healing by HIF-1α-mediated enhancement of angiogenesis. Acta Biomater. 2022;147:342–355. doi:10.1016/j.actbio.2022.05.018
77. Liu J, Qiu X, Lv Y, et al. Apoptotic bodies derived from mesenchymal stem cells promote cutaneous wound healing via regulating the functions of macrophages. Stem Cell Res Ther. 2020;11(1):507. doi:10.1186/s13287-020-02014-w
78. Pomatto M, Gai C, Negro F, et al. Differential therapeutic effect of extracellular vesicles derived by bone marrow and adipose mesenchymal stem cells on wound healing of diabetic ulcers and correlation to their cargoes. Int J Mol Sci. 2021;22(8):3851. doi:10.3390/ijms22083851
79. Las Heras K, Royo F, Garcia-Vallicrosa C, et al. Extracellular vesicles from hair follicle-derived mesenchymal stromal cells: isolation, characterization and therapeutic potential for chronic wound healing. Stem Cell Res Ther. 2022;13(1):147. doi:10.1186/s13287-022-02824-0
80. Wu H, Zhang Z, Zhang Y, et al. Extracellular vesicle: a magic lamp to treat skin aging, refractory wound, and pigmented dermatosis? Front Bioeng Biotechnol. 2022;10:1043320. doi:10.3389/fbioe.2022.1043320
81. Kee LT, Ng CY, Al-Masawa ME, et al. Extracellular vesicles in facial aesthetics: a review. Int J Mol Sci. 2022;23(12):6742. doi:10.3390/ijms23126742
82. Liang JX, Liao X, Li S-H, et al. Antiaging properties of exosomes from adipose-derived mesenchymal stem cells in photoaged rat skin. Biomed Res Int. 2020;2020:6406395. doi:10.1155/2020/6406395
83. Choi JS, Lee Cho W, Choi YJ, et al. Functional recovery in photo-damaged human dermal fibroblasts by human adipose-derived stem cell extracellular vesicles. J Extracell Vesicles. 2019;8(1):1565885. doi:10.1080/20013078.2019.1565885
84. Xu P, Xin Y, Zhang Z, et al. Extracellular vesicles from adipose-derived stem cells ameliorate ultraviolet B-induced skin photoaging by attenuating reactive oxygen species production and inflammation. Stem Cell Res Ther. 2020;11(1):264. doi:10.1186/s13287-020-01777-6
85. Deng M, Yu TZ, Li D, et al. Human umbilical cord mesenchymal stem cell-derived and dermal fibroblast-derived extracellular vesicles protect dermal fibroblasts from ultraviolet radiation-induced photoaging in vitro. Photochem Photobiol Sci. 2020;19(3):406–414. doi:10.1039/c9pp00421a
86. Hu S, Li Z, Cores J, et al. Needle-free injection of exosomes derived from human dermal fibroblast spheroids ameliorates skin photoaging. ACS Nano. 2019;13(10):11273–11282. doi:10.1021/acsnano.9b04384
87. Oh M, Lee J, Kim Y, et al. Exosomes derived from human induced pluripotent stem cells ameliorate the aging of skin fibroblasts. Int J Mol Sci. 2018;19(6):1715. doi:10.3390/ijms19061715
88. Yan T, Huang L, Yan Y, et al. Bone marrow mesenchymal stem cell-derived exosome miR −29b-3p alleviates UV irradiation-induced photoaging in skin fibroblast. Photodermatol Photoimmunol Photomed. 2023;39(3):235–245. doi:10.1111/phpp.12827
89. Zhang Y, Zhang M, Yao A, et al. Circ_0011129 encapsulated by the small extracellular vesicles derived from human stem cells ameliorate skin photoaging. Int J Mol Sci. 2022;23:23. doi:10.3390/ijms232315390
90. Han J, Wu T, Jin J, et al. Exosome-like nanovesicles derived from Phellinus linteus inhibit Mical2 expression through cross-kingdom regulation and inhibit ultraviolet-induced skin aging. J Nanobiotechnology. 2022;20(1):455. doi:10.1186/s12951-022-01657-6
91. Ding DC, Chang Y-H, Shyu W-C, et al. Human umbilical cord mesenchymal stem cells: a new era for stem cell therapy. Cell Transplant. 2015;24(3):339–347. doi:10.3727/096368915X686841
92. Liu SJ, Meng M-Y, Han S, et al. Umbilical cord mesenchymal stem cell-derived exosomes ameliorate HaCaT cell photo-aging. Rejuvenation Res. 2021;24(4):283–293. doi:10.1089/rej.2020.2313
93. Gao W, Yuan L-M, Zhang Y, et al. miR-1246-overexpressing exosomes suppress UVB-induced photoaging via regulation of TGF-β/Smad and attenuation of MAPK/AP-1 pathway. Photochem Photobiol Sci. 2023;22(1):135–146. doi:10.1007/s43630-022-00304-1
94. Dad HA, Gu T-W, Zhu A-Q, et al. Plant exosome-like nanovesicles: emerging therapeutics and drug delivery nanoplatforms. Mol Ther. 2021;29(1):13–31. doi:10.1016/j.ymthe.2020.11.030
95. Jo CS, Myung CH, Yoon YC, et al. The effect of lactobacillus plantarum extracellular vesicles from Korean women in their 20s on skin aging. Curr Issues Mol Biol. 2022;44(2):526–540. doi:10.3390/cimb44020036
96. Huang X, Chang Q, Gao J-H, et al. Sustained release microneedles: materials and applications in facial rejuvenation. Tissue Eng Part B Rev. 2023;29(3):190–202. doi:10.1089/ten.teb.2022.0131
97. Cao Z, Jin S, Wang P, et al. Microneedle based adipose derived stem cells-derived extracellular vesicles therapy ameliorates UV -induced photoaging in SKH −1 mice. J Biomed Mater Res A. 2021;109(10):1849–1857. doi:10.1002/jbm.a.37177
98. Bui VD, Son S, Xavier W, et al. Dissolving microneedles for long-term storage and transdermal delivery of extracellular vesicles. Biomaterials. 2022;287:121644. doi:10.1016/j.biomaterials.2022.121644
99. Zhang K, Yu L, Li FR, et al. Topical application of exosomes derived from human umbilical cord mesenchymal stem cells in combination with sponge spicules for treatment of photoaging. Int J Nanomedicine. 2020;15:2859–2872. doi:10.2147/IJN.S249751
100. You Y, Tian Y, Yang Z, et al. Intradermally delivered mRNA-encapsulating extracellular vesicles for collagen-replacement therapy. Nat Biomed Eng. 2023;7(7):887–900. doi:10.1038/s41551-022-00989-w
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
