Back to Journals » International Journal of Nanomedicine » Volume 18
Advances in Immunomodulatory Mechanisms of Mesenchymal Stem Cells-Derived Exosome on Immune Cells in Scar Formation
Authors Zhao W , Zhang H, Liu R, Cui R
Received 11 April 2023
Accepted for publication 19 June 2023
Published 3 July 2023 Volume 2023:18 Pages 3643—3662
DOI https://doi.org/10.2147/IJN.S412717
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yan Shen
Wen Zhao,1,2,* Huimin Zhang,1,2,* Rui Liu,1,2 Rongtao Cui1– 3
1Department of Burn and Plastic Surgery, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, People’s Republic of China; 2Medical Science and Technology Innovation Center, Shandong First Medical University & Shandong Academy of Medical Sciences, Jinan, People’s Republic of China; 3Department of Burn and Plastic Surgery, Shandong Provincial Hospital, Cheeloo College of Medicine, Shandong University, Jinan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Rongtao Cui, Department of Burn and Plastic Surgery, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, People’s Republic of China, Tel +86 18653170822, Email [email protected]
Abstract: Pathological scars are the result of over-repair and excessive tissue proliferation of the skin injury. It may cause serious dysfunction, resulting in psychological and physiological burdens on the patients. Currently, mesenchymal stem cells-derived exosomes (MSC-Exo) displayed a promising therapeutic effect on wound repair and scar attenuation. But the regulatory mechanisms are opinions vary. In view of inflammation has long been proven as the initial factor of wound healing and scarring, and the unique immunomodulation mechanism of MSC-Exo, the utilization of MSC-Exo may be promising therapeutic for pathological scars. However, different immune cells function differently during wound repair and scar formation. The immunoregulatory mechanism of MSC-Exo would differ among different immune cells and molecules. Herein, this review gave a comprehensive summary of MSC-Exo immunomodulating different immune cells in wound healing and scar formation to provide basic theoretical references and therapeutic exploration of inflammatory wound healing and pathological scars.
Keywords: exosomes, inflammation, immune cells, immunoregulation, scars
Background
Pathological scars, including hypertrophic scar (HS) and keloids, are abnormal scars at the trauma or surgical sites of the skin and are characterized by the over-proliferation of fibroblasts, abundant production of extracellular matrix (ECM) and excessive deposition of collagen, which normally cause burdens for the patients both psychologically and physiologically.1–3 It’s always a troublesome problem for clinical doctors due to its unclear pathogenesis and shortage of effective therapies.4,5 Therefore, exploring the potential pathogenesis and developing possible therapeutics for pathological scars are urgently needed.
The process of wound healing experiences 4 stages: hemostasis, inflammation, proliferation and remodeling, which we can see that the inflammation is at the beginning of scar formation.6 Although the exact pathogenesis of pathological scars remains unclear, the inflammation was proven to largely influence the formation and development of HS and keloids.7,8 An excessive response of inflammation is deemed to contribute to scar formation and fibrosis.9 Clinical works also showed that infected wounds always need a longer time to heal and will easily develop into more serious scars in most cases than uninfected wounds.10 However, there are lots of immune cells and molecules that participate in this process, and the specific mechanism of immunomodulation is not fully understood. For example, the polarity of macrophages plays an important role in the formation of pathological scars, especially M2 macrophages could promote the formation and development of HS and keloids.11 In addition, mast cells, T lymphocytes, neutrophils, Langerhans cells, and natural killer T (NKT) cells are also essential in the immunoregulatory process of scar formation.12–16 Subsequently, plenty of immune agents were explored for the treatment of HS and keloids, such as triamcinolone acetonide, tacrolimus, imiquimod, fingolimod, et al17–20 Hydrogel biomaterials with inherent antimicrobial properties offer an attractive and viable solution to wound bacterial infections, which might address this issue and also attenuate scar formation.21 Thus, further studying the mechanism of immunomodulation during scar formation would provide theoretical references for other researchers and develop more effective immune therapeutics for pathological scars.
Exosomes are one kind of nano-sized extracellular vesicle (EVs) with a diameter of 30–150nm derived from various cells, and serve as mediators for cell-to-cell communication.22 Two other subpopulations of EVs are microvesicles and apoptotic bodies. Exosomes derived from adipose-derived mesenchymal stem cells (adMSC), umbilical cord blood-derived mesenchyme stem cells (uMSC), bone marrow-derived mesenchymal stem cells (bmMSC), induced pluripotent stem cells-derived mesenchymal stem cells (iPSCs-MSC), epidermal mesenchymal stem cells (eMSC) have advantages like small size, low immunogenicity, and do not require for extra procedures for culture expansion or distribution, which were widely observed to help promote tissue repair and wound healing by controlling various inflammatory, proliferative and remodeling processes in vivo.23–28 Especially, mesenchymal stem cells-derived exosomes (MSC-Exo) have attracted much attention for their immunomodulatory and regenerative functions in the treatment of HS and keloids by promoting wound healing and tissue repair.29,30 As cell-free therapeutics, MSC-Exo mainly modulates the recipient cells through their cargo, including proteins, lipids and nucleic acids, but our understanding of how exosomes activate recipient cells and the cargo is responsible for their subsequent functional impact is limited.23,31 Our previous study demonstrated that the miRNA-138-5p loaded in MSC-Exo could attenuate pathological scars by targeting silent information regulator 1 (SIRT1) and further inhibit the proliferation, migration and fibrotic protein expression of human scar fibroblasts.32 Recent studies suggest that exosomes play an important role as immune modulators.33 The research in MSC-Exo -mediated immunoregulatory mechanisms in wound healing and scar formation achieved great progress due to the importance of inflammation in the formation of HS and keloids.34–36 Yet, the clinical application of MSC-Exo still faces major challenges such as production and isolation methods, and ideal cell sources.34
Here, we provide an overview of how MSC-Exo promotes wound healing and tissue repair processes to attenuate the formation of pathological scars by regulating the inflammation via affecting different immune cells, and discuss current assessment challenges and fundamental insights leading to future clinically relevant exosome therapy directions.
The Application of MSC-Exo in Scar Attenuation
Biological Characteristics of Exosomes
Exosomes are nanoscale vesicles secreted by cells through exocytosis, which are present in almost all body fluids.37 Extracellular vesicles are nanoscale vesicle particles that are actively released by cells. According to their origin, size and biological characteristics, they can be divided into three categories: exosomes (30–150 nm), microbubbles (100–1000 nm) and apoptotic bodies (500–2000 nm).38 Exosomes are mainly derived from multi-vesicles formed by intussusception of lysosomal granules in cells, and the outer membrane of the multi-vesicles fuses with the cell membrane and is released into the extracellular matrix. The exosome which contains protein, mRNA, microRNA and other biological information is just 30–150 nm in diameter, and its surface is rich in cholesterol, sphingomyelin, ceramide and other lipid substances. Exocrine bodies are secreted by almost all cells and are widely distributed in body fluids such as blood, urine, saliva, cerebrospinal fluid, pleural effusion, and ascites.39,40 Mesenchymal stem cells (MSCs), one of the main sources of exosomes, have high differentiation potentials so that are widely concerned by the scientific community.41
The precise mechanism of exosomes generated from MSCs is still unknown at this time, but it mainly plays its role in the following aspects:42 (1) Paracrine effect: It reduces cell apoptosis, encourages cell proliferation, and aids in the regeneration of injured cells by secreting a range of growth hormones and cytokines. (2) Immune regulation: It can inhibit the body’s excessive immune response and induce immune tolerance by interacting with immune cells, and inhibit the response of autoreactive effector T cells by promoting the generation and expansion of regulatory T cells. (3) Adhesion and migration: under the signal stimulation of tissue damage or inflammatory reaction, MSCs can migrate and home to the damaged tissue site, exerting the corresponding tissue repair ability.
Contents in Exosomes
Exosomes from various origins have been demonstrated to contain evolutionarily conservative protein components such as cytoskeleton protein, phospholipase D2, heat shock protein (HSP), signal transduction protein, CD55, and CD59, according to numerous research.43
Heat shock proteins like HSP70 and HSP90, membrane transporters and fusion proteins like GTPases, annexins, and flotillin, and members of the four transmembrane protein superfamily called tetraspanins such as CD9, CD63, CD81 and CD82 are the proteins that are most frequently found in exosomes. Exosomes from various sources also have unique protein molecules on their surfaces, like T cell receptors, granzymes, and perforin, which are employed to stimulate the body’s immune system.44 The exosomes secreted by neurons contain glutamate receptors.45 Integrin CD41a was found on the exocrine membrane derived from platelets; The exosomes secreted by antigen-presenting cells usually express costimulatory factors, such as CD54 and CD48.46 The exosomes secreted by intestinal epithelial cells contain various metabolic enzymes; Dendritic cells secrete exosomes that contain almost all antigen-presenting molecules and can induce or amplify acquired immunity.47
A list of the lipids was found in exosomes, including ceramides (Cer), sphingomyelins (SM), gangliosides GM3 from the class of sphingolipids, phosphatidylserines (PS), phosphatidylethanolamines (PE), phosphatidylcholines (PC), and lysophosphatidylcholine, phosphatidylinositols.48 Exosome lipid content is tightly correlated with the cell type from which they are produced. Exosomes of cholesterol, sphingomyelin, saturated phosphatidylcholine, and phosphatidylethanolamine have larger lipid contents than plasma membranes.49
The nucleic acid in the exosome is an important symbol to distinguish from vesicles. The nucleic acid contained in the exocrine body mainly includes DNA, mRNA and miRNA.50 MiRNA participates in the post-transcriptional regulation of mRNA and is related to disease occurrence and immune regulation. In order to degrade or prevent the expression of target mRNA, miRNA, an endogenous single-stranded non-coding miRNA with a length of 19 to 23 nt, binds to the 3’ non-coding region of mRNA.51 It is reported that exosome miRNA is crucial for intercellular communication. MiR-99a-5p, miR-128, miR-124-3p, miR-22-3p, and miR-99b-5p are the five most prevalent miRNAs.52 Due to the protection of exosomes, the inclusion of miRNAs can be resistant to RNA-dependent enzyme degradation and can be transported remotely in body fluids. Different types of cells from different diseases can secrete exosomes containing miRNAs. When the expression of miRNAs in cells changes, the corresponding miRNAs in exosomes will show the same trend.
Function of Exosome
Exosomes as Biomarkers
As the exosomal membranes are homologous to the cell membranes of the source cells, so using exosomes as a biomarker is easy to detect and less damaging. Since urine samples are the easiest to get, the majority of recent studies on biomarkers have concentrated on pee-derived exosomes. Prostate cancer patients had considerably higher urinary exosome expression of ERG, PCA3, PSMA, CK19, and EpCAM compared to healthy males. Additionally discovered to have diagnostic significance in primary prostate cancer are CK19 and EpCAM.53 A prevalent kind of cancer of the head and neck, oral cavity, pharynx, and larynx mucosal epithelium gives rise to head and neck squamous cell carcinoma (HNSCC). To participate in metabolic reprogramming and microenvironmental remodeling, HNSCC cells produce exosomes, which interact and communicate with recipient cells. This results in metabolic alterations. There is a study revealed that exosomes may be a new therapeutic target for HNSCC cure.54 In the field of lung cancer, research about exosomes is also popular. An earlier study’s findings revealed that non-small cell lung cancer patients had considerably lower amounts of the exosomes tRF-Lys-CTT-049, tRF-Leu-TAA-005, tRF-Asn-GTT-010, tRF-Trp-CCA-057, and tRF-Ala-AGC-036 indicating that these five exosomal tRFs could be promising non-small cell lung cancer diagnostic biomarkers.55
Exosomes as Drug Carriers
An ideal drug carrier should be able to bypass the host immune system, be precisely absorbed by target cells, have a sufficient cycle half-life, be non-toxic, and have the capacity to load a range of different medications. Therefore, as a natural liposome, exosomes are considered to have more advantages than synthetic liposomes widely used at present.55 It has been reported that therapeutic proteins like catalase or brain-derived neurotrophic factor, as well as low molecular chemotherapeutic drugs like PTX and doxorubicin (DOX), are incorporated into the exosomes through electroporation, saponin penetration, extrusion, freeze-thaw cycles, or sonication.56 Gomes et al developed a hybrid nanocarrier of tumor-derived exosomes based on the fusible characteristics of liposomes and exosomes. For the treatment of breast cancer, the exosomes were combined with long-circulation, pH-sensitive liposomes carrying DOX (ExoSpHL-DOX).57 In kanchanapally’s study, MSCs-Exo were exposed to cancer cells by loading with honokiol with the sonication method. And the results showed that these particles had better cytotoxic effects than the free honokiol.58
Exosomes Promote Tissue Repair
As a promising therapeutic strategy, exosome has attracted extensive attention for its application in tissue repair. Based on a systematic review of relevant studies in recent years, exosome plays a positive role after the damage of tissues, such as bone, cartilage, skin, spinal cord and tendons, which means it could widely promote tissue repair.59–63 Studies have shown that exosome participates in various processes of skin tissue repair. It can promote wound healing and skin tissue regeneration by promoting the proliferation and migration of skin cells, promoting angiogenesis, and regulating the immune response.24,64 Interestingly, exosome plays a dynamic role in skin wound healing that it can promote collagen synthesis to accelerate wound repair in the early stage of wound healing, but inhibit collagen synthesis to restrict the scar formation in the later stage.24,30,65 Therefore, exosomes can inhibit the proliferation of scars to a great extent by accelerating wound healing.
The Immune Function of Exosomes
It has been known that exosomes are key substances in intercellular communication and innate immunity is an important part of the host’s defense mechanism. According to recent findings, exosomes could control immunity in the tumor microenvironment. Through exosomes, CD8+T cells can kill the stromal cells that support mesenchymal tumors and prevent tumor cell invasion and metastasis. CD8+T cell-derived exosomes can increase the expression of matrix metalloproteinase-9 in tumor cells, thereby promoting tumor progression. In addition, exosomes derived from CD8+T cells reduce the effect of anti-tumor response by inhibiting the proliferation of CD8+T cells mediated by dendritic cells.58 As innate immune cells, macrophages are divided into M1 macrophages (M1) with proinflammatory phenotype and M2 macrophages with anti-inflammatory phenotype. M1-generated exosomes inhibit tumor growth by polarizing M2 to M1, whereas tumor-derived exosomes enhance M2 polarization. PGE2 and IL1RA were detected in MP and exosomes, which have been proven to be important mediators of MSCs’ anti-inflammatory effect in vitro and inflammatory arthritis. Cosenza showed that MSCs-derived MP and exosomes play a similar immunosuppressive function by reducing the proliferation of T and B lymphocytes and inducing Treg cell population.66 The exosome is one of the paracrine components and the main contributor to the efficacy of stem cells. Chamberlain found that the exposure of CD14 macrophages to the exosomes derived from MSC leads to a decrease of M1/M2 macrophage ratio. They may improve ligament healing and reduce scar formation.67 ADSC-Exo have a profound potential for scar attenuation. By delivering miRNA-125a and miRNA-31 to vascular endothelial cells, they can contribute to the immune response of wound inflammation and stimulate angiogenesis.24 In another study, MSCs secrete secretions containing miR-432-5p to inhibit target gene TRAM2, prevent ECM deposition, and promote stromal regeneration.68
The applications of MSC-Exo in scar formation can be summarized in one picture, which was shown in Figure 1.
Clinical Application of MSC-Exo in Pathological Scars and Existing Difficulties
MSC-Exo can be a promising option as an innovative cell-free approach to support wound healing and skin regeneration as well as scar attenuation. It conveys functional cargos (such as growth factor, cytokine, miRNA, etc.) from MSCs to target cells, thereby affecting the recipient skin cells’ biological events, such as migration, proliferation, and also secretion of ECM components (mainly collagen).30 Moreover, MSC-Exo could also mediate crosstalk between skin cells and immune cells in cutaneous wound healing, which further accelerates wound healing and alleviates pathological scar formation.69
Although lots of studies showed that MSC-Exo plays a positive role in wound healing and tissue regeneration as well as pathological scars attenuation, most of them were just in vitro and animal experiments. Thus, the results of them still need to be verified in clinical trials. But changes from basic to clinical research remain huge challenges.
First, due to technical limitations, it is difficult to obtain high purity and yield MSC-Exo, so further development of technology is needed to improve isolation efficiency and productivity for clinical applications. Second, it is unclear whether the positive effects of MSC-Exo in treatment are directly related to its contents (lipid, protein and nucleic acid). Therefore, the therapeutic efficacy, safety and effective dosage of its contents need to be further studied while using MSC-Exo as a therapeutic agent. Third, non-specific targeted therapy of MSC-Exo may lead to unforeseen side effects. MSC-Exo has been proven to target a variety of cell types, but it is not clear how they choose the priority receptors. Especially in the complex environment of the human body. Therefore, improving the targeting of MSC-Exo could improve its safety and efficiency. Fourth, the use of MSC-Exo as a drug delivery vector is also one of the research hotspots, but drug delivery efficiency is another challenge that needs to be overcome. Therefore, future research needs to develop efficient technologies, which can not only load enough doses of the drug into MSC-Exo but also maintain stable biological activity and physical integrity of MSC-Exo.
Immune Cells in Scar Formation
Macrophages
Overview of macrophages
Macrophage is a kind of immune cell with multiple differentiation sources, which can be self-renewed or supplemented by monocytes. The two primary categories of macrophages are classic activated macrophages (M1, which is also called pro-inflammatory macrophages) and alternative activated macrophages (M2, which is also called anti-inflammatory macrophages).70 In addition, M2 macrophages come in four different subtypes: M2a, M2b, M2c, and M2d.70 Polarization of macrophages is the phenotypic change of macrophages between different types, which is controlled by a number of signal molecules and pathways.71 However, the molecular and cytological mechanisms underlying the complicated process of macrophage polarization are not entirely understood.72 The polarization of macrophages serves several roles and is strongly associated with the onset and progression of illnesses, depending on the local microenvironment.71,73
Macrophages in Scar Formation
Numerous macrophage infiltration is a remarkable feature of the early stage of wound healing. In the early and middle stages of skin wound healing, dermal macrophage depletion has a beneficial effect on cutaneous scarring, which is characterized by decreased vessel density, decreased fibroblast and myofibroblast proliferation, and reduced post-healing fibrosis and scar formation.74 In macrophage phenotypes, M2 macrophages are mainly involved in tissue proliferation and scar formation.75 It has been determined that HS tissue contains substantially more M2 macrophages than does typical scar tissue.76
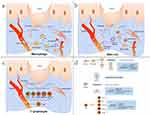
Like other immune cells, macrophages play a role in the formation of scar mainly by secreting pro-fibrosis and pro-proliferation cytokines. Macrophages are the primary source of cytokines like transforming growth factor (TGF)-1, fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), insulin-like growth factor (IGF)-1, etc., which encourage fibroblast proliferation, migration, and differentiation, promote collagen synthesis and wound contraction, encourage ECM collagen deposition or angiogenesis, and play an important role in scarring (Figure 2a–d).76–78 In addition, macrophages can also produce matrix metalloproteinases (MMPs) and play an important role in the remodeling stage of wound healing.79 Therefore, reducing the recruitment of macrophages and the release of cytokines or inhibiting the polarization of M2 macrophages may play a role in the prevention and treatment of scarring.74,80
The Notch signal pathway is an important pathway known to participate in inflammatory reactions and fibrosis processes. The expression of Notch is an important condition to promote fibrosis of the liver, kidney, skin and other organs, while inhibiting the Notch pathway of macrophages can effectively improve the fibrosis process.81–83 The drug emodin can inhibit the inflammatory reaction and collagen deposition by down-regulating the Notch and TGF-β of macrophages.84,85 The expression of the Notch can further inhibit the recruitment and polarization of macrophages in the injured part of the body to achieve the purpose of treating HS.84 Therefore, many studies have focused on the control of Notch signal pathway through drug targeting to regulate the polarization of macrophages. For example, astragalus polysaccharide can activate Notch signal pathway to promote the polarization of M1 macrophages, but capsaicin can inhibit Notch signal pathway to induce the polarization of M2 macrophages.86,87
At present, most of the research is focused on the distribution and quantity of macrophages. In view of high plasticity and diversity of macrophages, the further study of the cellular and molecular biological mechanisms of phenotypic transformation is likely to explore new therapeutic targets in the future in reducing excessive injury or proliferation, improving chronic wound healing and scar formation.
Mast Cell
Overview of Mast Cell
Mast cell (MC) was primordially regarded as the main cell involved in IgE-mediated rapid hypersensitivity.88 Activated MC plays an important role in the process of host resistance to foreign pathogens at early stage of infection by releasing a variety of special particles (such as histamine, 5-hydroxytryptamine, heparin, trypsin, chymotrypsin, etc.) and recruiting other inflammatory cells, including neutrophils, monocytes, macrophages, etc.89–91 Moreover, MC is also involved in adaptive immunity directly or indirectly by secreting a variety of immune molecules. In addition, MC is also an important antigen-presenting cell, which could recognize foreign antigens and present them to T lymphocytes and B lymphocytes. With the development of research, it is found that MC is also closely related to wound healing and scar formation.91,92
Mast Cell in Scar Formation
The role of MC involved in scar formation could be sketched in three aspects: firstly, regulating anticoagulation and coagulation processes in the early stage of wound healing; secondly, triggering a stronger inflammatory reaction by interacting with other immune cells; thirdly, promoting fibroblast proliferation and collagen production.12,93,94
MC migrates to the injury site under the action of stimulation signals, releasing TNF- α to enhance the expression of FXIIIa in dermal dendritic cells.95 Additionally, MC-derived trypsin and chymase inhibit the platelet-induced aggregation of fibrin by decomposing the α chain and β chain of fibrinogen.95 Meanwhile, MC can create heparin, tissue-type plasminogen activator (t-PA), urokinase-type plasminogen activator (u-PA), and plasminogen activator inhibitor (PAI)-1 to control the coagulation and anticoagulation processes (Figure 2b–d).93,96
The MC in the skin connective tissue is highly expressed in chymotrypsin and trypsin, which are mainly trypsin in the inflammatory process and chymotrypsin in the chronic fibrosis process.97 The combination of MC-derived trypsin and protease-activated receptor-2 on the surface of endothelial cells also leads to vasodilation, fluid extravasation, aggravation of local inflammatory reaction, and induction of more inflammatory cells such as monocytes and neutrophils to migrate to the injured site, leading to stronger inflammatory reaction.94,98
Furthermore, MC-derived trypsin could promote the migration and proliferation of keratinocytes and fibroblasts by degrading the basement membranes’ components, such as type IV collagen and laminin. It could also promote fibroblasts to transform into myofibroblasts, synthesize ECM and Alpha-smooth smooth actin (α- SMA), and induce collagen deposition, leading to pathological scars and scar contracture. MC-derived IL-4 acts as the second signal, which could amplify the effect of low FGF and PDGF concentrations and further promote the proliferation of fibroblasts.91 Cell-cell gap junction formed between fibroblasts and MC, the structural basis for the role of MC, enhanced the contact of cells, thus promoting the growth, proliferation, migration, and differentiation of fibroblasts.99,100 Therefore, eliminating the gap connection between them may play a role in preventing scarring or reducing the degree of fibrosis.
Hence, based on the role of MC in the process of wound healing, inhibiting the activation of MC or targeting the cytokines secreted by MC can reduce the inflammatory reaction and scar formation, which may be a potential method to improve wound healing. In recent years, researchers have had a profound understanding of the role of MC in wound healing, but the research is limited to animal experiments, and the results are still different from human experiments. Therefore, the mechanism of MC in human scarring needs further clinical research.
T Lymphocyte
T lymphocytes are derived from bone marrow pluripotent hematopoietic stem cells or lymphoid cells differentiated from them. A complete T lymphocyte system is necessary to ensure normal wound healing, which plays a major regulatory role in wound healing.101 Over-proliferation or the continuous existence of T lymphocytes in the injury site may be one of the reasons for excessive scarring.101,102 However, there are many subtypes of T lymphocytes, and different T lymphocytes play different roles in different microenvironments during wound healing and scar formation.101,103 According to the differentiation of functions and cell surface receptors, T lymphocytes were divided into helper T lymphocytes, cytotoxic T lymphocytes, regulatory T lymphocytes and natural killer T lymphocytes (Figure 2c and d).104,105
Helper T Lymphocytes in Scar Formation
In the process of wound healing, a large number of DAMPs-related chemokines in the injury site induce Th1 cells and Th2 cells to reach there.103 IFN-γ and IL-12 are mostly secreted by Th1 cells. IFN-γ enhances the gene expression of collagenase, and promotes collagen decomposition and remodeling.103 Additionally, cytokines and NOS can be activated by Th1 cells. NOS can enhance the function of collagenase, promote ECM degradation and inhibit fibrosis. Whereas, many other cytokines, including IL-4, IL-5, IL-6, IL-10, IL-13, etc., are secreted by Th2 cells. It was found that IL-4 and IL-13 are the main cytokines leading to skin fibrosis.106 Overexpression of IL-4 and IL-13 will activate and prolong the fibrosis process of skin tissue, leading to the formation of pathological scars. IL-4 and IL-13 can also induce the expression and secretion of the periosteal protein, which further induces TGF-β through RhoA/ROCK pathway.107 In turn, TGF-β further promotes the production and secretion of the periosteal protein. Such a positive feedback loop promotes the formation of scarring. Tredget et al confirmed that the serum of patients with HS included considerably higher levels of the cytokines produced by Th2 cells, IL-4, IL-6, and IL-10.108 On the contrary, IFN-γ and IL-12 produced by Th1 cells were reduced, indicating that Th2 and its cytokines play a significant role in the development of HS after injury.
Regulatory T Lymphocytes in Scar Formation
Skin is rich in Treg cells, which play a role in maintaining skin inflammation and immune homeostasis.109 Treg cells suppress the immune system by blocking other functional T cells and preserving immunological tolerance in the peripheral body, and then directly affecting collagen deposition, inhibiting wound repair and healing.110 Murao et al found that the college synthesis of fibroblasts decreased when co-cultured with T lymphocytes rich in Treg cells compared with lymphocytes lack of Treg cells. However, It was also shown that co-culture of fibroblasts with pure Treg cells can promote the expression and synthesis of collagen without other T lymphocytes.111,112 These two seemingly paradoxical results suggested that Treg could directly or indirectly regulate the role of fibroblasts in the process of collagen deposition and subsequent scar formation through their interaction with other T lymphocytes.113
Natural Killer T Lymphocytes in Scar Formation
Natural Killer T lymphocytes (NKT cells) are natural immune lymphocytes, which simultaneously express TCR and NK cell receptors, and play an important role in infection, autoimmune and tumor.114 It was found that NKT cells infiltration mainly occurred in the first three days of wound repair, and was activated by CD1d (a member of the CD1 family, with a structure similar to MHCI molecule, expressed on the surface of some antigen-presenting cells, mainly presenting glycolipid antigen) related antigen.115 The combination of CD1d-related glycolipid antigen and NKT cell TCR can up-regulate the expression of its surface molecule CD69.116 After intravenous injection of anti-CD1d antibody, the activation of NKT cells is inhibited, and its surface CXCR2 continues to circulate, resulting in the high expression of CXCR2, which promotes NKT cells to migrate to the injury site at the early stage of inflammation, just like neutrophils.117 The activated NKT cells can inhibit the production of some chemokines that act on the chemotaxis of neutrophils, monocytes and macrophages, and negatively regulate the early inflammatory reactions and fibroproliferative signals.117 Therefore, the lack of NTK cells or the use of anti-CD1d antibodies to inhibit the activation of NKT cells can increase the chemokines and TGF-β of the local inflammation. Therefore, the research on NKT cells and their related molecules is likely to be a new therapeutic target for poor wound healing or scarring in the future.117,118 In the view of NKT cells also express NK cell receptors, whether NK cells participate in the process of wound repair and even scar formation requires to be further study.
Natural Killer Cell
Overview of Natural Killer Cell
Natural Killer (NK) cells are the lymphocytes of the innate immune system, which were considered to be an important part of the first defense line of the human body. Human NK cells, or CD3-CD56+, make up about 10% of peripheral blood lymphocytes and are distinguished by CD3 negative and CD56 positive expression. NK cells have two main phenotypes, including CD56bright and CD56dim. CD56bright NK cells can produce higher levels of human immune regulatory cytokines, including IFN- γ, TNF- β, GM-CSF, IL-10 and IL-13, etc., showing that CD56+NK cells have unique natural immune regulation.119,120
NK Cell in Scar Formation
The abnormal infiltration of NKT cells is the characteristic of delayed wound healing, which hinders wound repair and induces over-scarring. Knocking out NK cells with T cell function in vitro during the wound repair and healing process of the mice resulted in significantly accelerated wound healing and less scarring, which confirmed that the continuous inflammatory reaction caused by NK cells can promote the formation of scar.117,118 NK cells may also participate in the process of wound repair and even scar formation in consideration of NKT cells also express NK cell receptors. In this regard, our research team has confirmed that NK cells play a key role in wound repair and immune regulation.120 However, the study of its immune regulatory mechanism on scar formation has not yet been reported home and abroad.
Other Immune Cells
Neutrophils
Neutrophils are the first immune cells to reach the injury site. It begins to apoptosis only after 24 hours of local survival. They could not only kill the pathogenic microorganisms, but also secrete cytokines and growth factors, such as IL-1, IL-17, VEGF, TNF- α, to induce other immune cells to reach the injury site and promote the proliferation of fibroblasts, keratinocytes and endothelial cells.121,122 Therefore, neutrophils may be one of the first cellular signals to activate local cells.
The experimental results of knockout of mouse CXCR2 (the key regulator of neutrophil chemotaxis) gene showed poor wound healing in mice, characterized by reduced migration and proliferation of keratinocytes and fibroblasts, and reduced degree of vascular regeneration and skin regeneration, which suggested the importance of neutrophils to promote wound repair.123 However, some studies have confirmed that neutrophils play a negative regulatory role in the process of wound healing.124 Mice with neutrophil deficiency show accelerated wound healing and epithelial regeneration.125 This suggests that neutrophils play a complex role in the process of wound healing. Therefore, the interaction between neutrophils and other immune cells may be the reason for the complex role in wound healing and scar formation, which needs to be further studied in the future.
Langerhans Cell
Langerhans cells move to the site of the lesion in order to help with damage repair, which are found in the granular layer of the epidermis. Niessen et al showed that the quantity of Langerhans cells in HS tissue was much larger than that in normal skin tissue at the 3rd and 12th months following the operation, which suggested that Langerhans cells were strongly related to the later formation of HS in the remodeling stage of wound healing.15 Continuous interaction between epidermal Langerhans cells and keratinocytes in the dermis inhibits the release of IL-1 α and promotes IL-4, which may be related to less degradation of collagen and promote the formation of HS.126
Immunomodulation Mechanisms of MSC-Exo in Scar Formation
MSC-Exo Immunomodulates the Activities of Macrophages
MSC-Exo Regulates Macrophage Polarity
Macrophages are in charge of controlling the stages of inflammation, proliferation, and remodeling due to their extremely dynamic flexibility.77 In response to the release of cytokines after tissue injury, the recruited macrophage populations reprogram and experience significant phenotypic and functional alterations.127 Consequently, as potentially crucial targets, macrophages emit a variety of inflammatory cytokines and regulate tissue repair processes raising the possibility that they are important targets in the formation of HS and keloids.128–130 It has been shown that different macrophage activation states play specific and important roles in the various stages of wound healing and scar formation.84,131–133
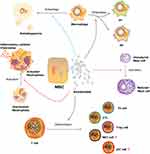
Recent studies have identified the regulatory effects of MSC-Exo on macrophage polarity, suggesting that MSC-Exo may affect the process of wound healing and scarring through immunomodulating macrophage polarity (Figure 3).134–136 Previous research demonstrated that uMSC-Exo promoted angiogenesis and collagen synthesis while triggering M2 macrophage polarization to aid the repair of diabetic wounds.137 However, if the period of this process is prolonged and M2 macrophages continue existing, fibroblast over-proliferation, excessive ECM production and collagen deposition will occur, which eventually causes the formation and progress of HS and keloids. Therefore, MSC-Exo could control this process by regulating the polarity of macrophages, and further inhibiting scar formation.
MSC-Exo Stimulates Macrophage Autophagy
Macrophage autophagy regulates the process of cell proliferation, migration, and neuronal signal transduction, which protects against inflammation- and fibrosis-associated diseases.138,139 Macrophage autophagy reduces inflammation by restricting the release of pro-inflammatory cytokines like IL-1β and inhibiting the activation of the NLRP3-inflammasome via the NF-B-p62-mitophagy pathway.140 Additionally, autophagy controls macrophage polarization via activating the NLRP3 gene and the ROS/ERK signaling pathway.141
Exosomes from human exfoliated deciduous teeth-derived stem cells (SHED-Exo) were previously shown to have potential clinical uses as cell-free therapies for wound healing.142 Their research showed that miR-1246 in SHED-Exo improved autophagy by controlling macrophage activity via the AKT, ERK1/2, and STAT3 signaling pathways. Lipopolysaccharide (LPS)-induced wounds in a mouse model were used to study inflammatory wound healing. The results demonstrated that SHED-Exo enhances wound healing with less itching in an LPS-induced wound model via promoting macrophage autophagy. Therefore, these results suggest that manipulation of macrophage autophagy by stem cell-derived exosomes, which regulates the macrophage phenotype, may offer clinical options for improved wound healing with reduced pruritus and pathological scar amelioration (Figure 3).
MSC-Exo Inhibits Mast Cell Activation
It has been hypothesized that mast cell activation contributes to each stage of the wound healing and scarring processes.12,91,143 Mast cells, which are inflammatory cells that are present in the body, can activate in response to a variety of clinical and environmental factors. Due to their capacity to create a variety of mediators, they facilitate the recruitment of inflammatory cells after skin damage, aid in the prevention of infection, and collaborate with fibroblasts to expedite the creation of scars.94,144,145 Activated mast cells undergo degranulation and the cell numbers increase in response to skin injury during scarring.146 Therefore, blocking mast cell activation would lessen the development of cutaneous scars.92
Recently, Cho et al investigated whether exosomes from tonsil-derived mesenchymal stem cells (tMSC-Exo) have the capacity to control mast cell activation in response to TLR7 stimulation.147 The result of their study showed that TLR7 agonist-treated HMC-1 cells (a cell line of human mast cells) that contained microRNAs that target inflammatory cytokines greatly decreased the expression of inflammatory cytokines. In addition, tMSC-Exo prevented the development of cutaneous mast cells and CD14-positive cells in TLR7 agonist-treated mice. Another previous study demonstrated that exosomes from iPSCs-MSC could inhibit mast cell activation via the HIF-1 signaling pathway.148 Both of these studies suggest that MSC-Exo has a negative effect on mast cell activation under inflammatory conditions (Figure 3).
MSC-Exo Regulates T-Cell Differentiation
The role of T cells in wound healing and scar formation is complex due to the variety of cell subsets. For example, IL-10-producing CD4+ T lymphocyte subsets, specifically Th1, regulate inflammatory cell cytokine expression to selectively attenuate dermal wound fibrosis.149 While the NKG2A/CD94 complex (an inhibitory marker of the T cells) was specifically upregulated, it was demonstrated that the downregulation of cytotoxic CD8+ T cells is a keloid signature in peripheral blood and keloid lesions.150 This may explain the significant decrease in CTLs within the scar tissue boundary. In addition, increased infiltration of CD45RO+ memory T cells in keloid scars indicated that a disrupted T-cell response may contribute to the progression of keloids.151 Therefore, the balance of T-cell differentiation is crucial for scar alleviation.
A recent study examined how Th17 and regulatory T (Treg) cells differentiate from naïve CD4+ T cells in releasing inflammatory factors, Th17, and hypertrophic scar formation to reveal the role of ADCS-Exo.152 The result of their study showed that naïve CD4+ T cells treated with ADCS-Exo in vitro can produce significantly less IL-6, IL-17A, TNF-α RORϒt, and more IL-10 and Foxp3 on mRNA and protein levels. Moreover, mice treated with ADSC-Exo showed reduced collagen deposition, reduced levels of IL-17A, TNF-α, and RORϒt, and elevated levels of IL-10 and Foxp3 production. Furthermore, uMSC-Exo appeared to restore the balance between Th17 and Treg cells, which was accompanied by decreased IL-17 and increased TGF-β and IL-10 levels, indicating that uMSC-Exo serves as a crucial regulator of the balance between Th1/Th17 and Treg cells during immune and inflammatory responses.153,154 Except for Helper T lymphocytes and Treg cells, MSC-Exo could also participate in the differentiation of CTLs. There was a study showed that exosomal PD-L1 suppressed CD8+ T cell numbers in the spleen and peripheral lymph nodes and inhibited cytokine production of CD8+ T cells, indicating that exosome exerts immune inhibitory effects and promotes tissue repair by suppressing T cells differentiate into CD8+ T cells.155 As is known that NKT cells and γδΤ cells are also involved in wound healing and scar formation. But the existing evidence of MSC-Exo regulating the differentiation of NKT cells and γδΤ cells is limited. Whether MSC-Exo could also work on it still need further research (Figure 3).
MSC-Exo Inhibits Neutrophils Activity
Neutrophils are the first immune cells to reach the injury site to defend against pathogens and recruit other immune cells by releasing various of inflammatory cytokines and chemokines. With the antibacterial property of neutrophils, host damage was also caused by neutrophils resulting from the activity of proteases secreted by these cells. Numerous studies demonstrating a link between elevated neutrophil-derived proteases and persistent non-healing wounds and even severe scarring underline the clinical implications of this issue.156 Another study demonstrated that neutrophils might control the movement of the preexisting matrix to incorporate fibroblasts that are active or mature into scars that will stay a long time.14 As such, they could function as a subsequent response to the initial scar-formation process.
The study on the immunomodulatory effects of MSC-Exo on LPS-induced systemic inflammation demonstrates that MSC-Exo reduced neutrophil-to-lymphocyte ratio (NLR) and levels of inflammatory cytokines like IL-6, IL-1, and TNF-α after exosome injection in the mouse model, suggesting that treating mice with MSC-Exo can mitigate the damaging effects of neutrophil-related inflammation brought on by sepsis by reducing inflammatory factors and tissue damage.157 Neutrophil extracellular trap (NET) is the main property of neutrophil activation in response to inflammatory stimuli and the release of extracellular chromatin structures, which was identified plays a promoting role in the differentiation and function of fibroblasts, and therefore resulting in scar formation.158,159 But the C5b-9 assembly-induced production of NETs and IL-17 on neutrophils was suppressed by MSC-Exo, suggesting that MSC-Exo could improve immune dysregulation and break the feed-forward loop between complement and neutrophils to prevent the spread and perpetuation of inflammation.160 Hence, the eMSC-Exo-inhibited NET formation potentially attenuates inflammation conditions and scarring by reducing neutrophil-related hyperinflammation.
Discussion
HS and keloids are pathological cutaneous scars, which are partially induced by chronic dermal inflammation and result from overwhelming fibroblast production of extracellular matrix.8 Their etiology is unclear to a great extent, so they lack effective precautions and treatments. In this study, we reviewed the immunomodulation of MSC-Exo on different immune cells in wound healing and pathological scarring.
Inflammation has long been proven that plays a concrete role in scar formation.7 Chemokines, such as C-C chemokine ligand 2 (CCL2), C-C chemokine ligand 4 (CCL4), C-C chemokine ligand 5 (CCL5), C-X3-C Motif Chemokine Ligand 1 (CX3CL1), C-X-C Motif Chemokine Ligand 10 (CXCL10) and C-X-C Motif Chemokine Ligand 12 (CXCL12), at the injury site recruit a variety of immune cells to participate in wound healing and tissue repair and thus result in HS.103,161 Additionally, excessive expression of fibrotic cytokines, such as transforming growth factor (TGF) β1, fibroblast growth factor (FGF), epithelial growth factor (EGF), vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF) et al, from immune cells at the injury site is the main reason for the overwhelming tissue repair-caused HS and keloids formation.7,76
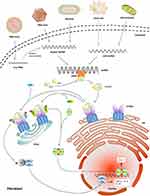
cGAS-STING axis, including the synthase for the second messenger cyclic GMP-AMP (cGAS) and the cyclic GMP-AMP receptor stimulator of interferon genes (STING), is a famous inflammation-related pathway and also a research hotspot in inflammatory diseases, cancer immunotherapy, liver diseases, etc.162–164 cGAS-STING causes inflammation by identifying pathogenic DNA through igniting an innate immune response that results in a potent type I interferon response or a prodigious release of inflammatory cytokines (IL-1, IL-6, TNF-α, etc.) against microbial infections.162 The DNA sensor cGAS can detect endogenous DNA as well as microbial DNA (derived from bacteria or viruses), including extranuclear chromatin produced by genotoxic stress and DNA released from mitochondria, which makes cGAS-STING an important axis in autoimmunity, sterile inflammatory responses, and cellular senescence.165,166 Previous studies showed that the cGAS-STING pathway is also an important signal for fibrotic diseases, such as liver, renal, lung or cardiac fibrosis.167–170 However, there is not any research putting it together with skin wound healing or scarring. And given the predominant role of cGAS-STING in inflammation, it may be involved in wound healing and scarring (Figure 4).
mesenchymal stem cells produce cytokines in a paracrine or autocrine way to control immune response and tissue regeneration. MSC-Exo, on the other hand, has anti-inflammatory and immunomodulatory qualities that may be useful in inflammatory illnesses and diseases such skin damage and cutaneous scarring.171 Oxidative damage is a critical cause of chronic nonhealing wounds. Through the control of oxidative stress, a Chinese research team looked into the likely mechanism by which adMSC-Exo enhances diabetic wound healing. Reactive oxygen species (ROS) levels in keratinocytes, fibroblasts, and endothelial cells were found to be reduced by adMSC-Exo, which also protected these cells from the damaging effects of hypoxia and oxidative stress by activating the exosomal HSP90/LRP1/AKT signaling pathway. One of our previous studies discovered that the cross-talk between human mesenchymal stromal/stem cells and NK cells improved the immune response in both healthy and immunocompromised patients during the process of post-trauma repair.120 We also confirmed that this process was driven by MSC-derived CCL2 acting on NK-expressed C-C chemokine receptor 2 (CCR2), indicating that therapeutic application of MSCs or their soluble factors, which was normally secreted by exosomes, might thus improve the NK function after severe injury. However, the mechanism of NK cells as well as its downstream molecules and pathways in pathological scars requires deeper research.
Emerging evidence has shown that MSC-Exo exerts a critical immunomodulatory function in tissue regeneration and scar attenuation.172–174 Our recently published study also confirmed that MSC-Exo containing miR-138-5p alleviates pathological scars by downregulating the expression of SIRT1 and inhibiting the biological behaviors of fibroblasts.32 Additionally, the result of our study also demonstrated that SIRT1 could enhance the development of pathological scars by promoting the growth of fibroblasts, But the exact downstream mechanism is yet unclear.32 Furthermore, SIRT1 was proven to participate in innate and adaptive immunes via regulating the maturation of dendritic cells and T-cell differentiation.175–178 Taken all together, MSC-Exo may, both directly and indirectly, regulate the immune response during pathological scarring, which still requires further study.
However, the production, isolation, drug loading efficiency, biodistribution, uptake of exosomes and their optimal use remain challenges.179 Meanwhile, the storage stability, low yield, low purity, and weak targeting of exosomes limit its clinical application.180 Especially, exosome production is relatively low as a therapy method. Thus, how to improve its yield has become a research hotspot in recent years. With deeper insights into this field, several ways, such as mechanical loading, 3D cultivation conditions and designer exosomes, have been reported to boost its secretion.181,182 Recently, designer exosomes have been developed to overcome the limitations of exosomes for the targeted delivery of drugs or functional molecules for the healing of damaged tissue.182 And exosomes produced from 3D cultures of MSCs by tangential flow filtration also showed higher yield and improved activity.181 Whereas there are still more advanced strategies for the large-scale production of exosomes will be needed for further research and clinical applications. With the development of technologies in the separation, purification, specificity identification, analysis and detection of exosomes, our understanding of the basic biology of exosomes and their application in medical therapy technology will be greatly promoted.
In a word, the MSC-Exo application is a promising therapeutic for both wound healing acceleration and scar attenuation. Particularly, its immunomodulatory effects on immune cells achieve great progress in improving inflammation conditions and diseases, such as wound healing, cutaneous scarring, organ fibrosis and some other inflammation-related diseases. We are devoted to exploring the immunoregulatory mechanism of NK cells during scar formation. The further of our work will reveal the pathogenesis of pathological scars at a deeper level.
Acknowledgments
This study was financially supported by the National Natural Science Foundation of China (81901968), the Provincial Natural Science Foundation of Shandong province (ZR2019BH051) and the Postdoctoral Science Foundation of China (2018M642667). Special thanks to Dr. Nan Liu for pre-reviewing our manuscript.
Funding
This study was financially supported by the National Natural Science Foundation of China (81901968), the Provincial Natural Science Foundation of Shandong province (ZR2019BH051) and the Postdoctoral Science Foundation of China (2018M642667).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Griffin MF, Borrelli MR, Garcia JT, et al. JUN promotes hypertrophic skin scarring via CD36 in preclinical in vitro and in vivo models. Sci Transl Med. 2021;13(609):eabb3312. doi:10.1126/scitranslmed.abb3312
2. Direder M, Weiss T, Copic D, et al. Schwann cells contribute to keloid formation. Matrix Biol. 2022;108:55–76. doi:10.1016/j.matbio.2022.03.001
3. Berman B, Maderal A, Raphael B. Keloids and hypertrophic scars: pathophysiology, classification, and treatment. Dermatol Surg. 2017;43(Suppl 1):S3–S18. doi:10.1097/DSS.0000000000000819
4. Leszczynski R, da Silva CA, Pinto ACPN, Kuczynski U, da Silva EM. Laser therapy for treating hypertrophic and keloid scars. Cochrane Database Syst Rev. 2022;9(9):CD011642. doi:10.1002/14651858.CD011642.pub2
5. Coentro JQ, Pugliese E, Hanley G, Raghunath M, Zeugolis DI. Current and upcoming therapies to modulate skin scarring and fibrosis. Adv Drug Deliv Rev. 2019;146:37–59. doi:10.1016/j.addr.2018.08.009
6. Xiaojie W, Banda J, Qi H, et al. Scarless wound healing: current insights from the perspectives of TGF-β, KGF-1, and KGF-2. Cytokine Growth Factor Rev. 2022;66:26–37. doi:10.1016/j.cytogfr.2022.03.001
7. Wang Z-C, Zhao W-Y, Cao Y, et al. The roles of inflammation in keloid and hypertrophic scars. Front Immunol. 2020;11:603187. doi:10.3389/fimmu.2020.603187
8. Ogawa R. Keloid and hypertrophic scars are the result of chronic inflammation in the reticular dermis. Int J Mol Sci. 2017;18(3):606. doi:10.3390/ijms18030606
9. Wilgus TA. Inflammation as an orchestrator of cutaneous scar formation: a review of the literature. Plast Aesthet Res. 2020;7:54. doi:10.20517/2347-9264.2020.150
10. Leng T, Wang Y, Cheng W, Wang W, Qu X, Lei B. Bioactive anti-inflammatory antibacterial metformin-contained hydrogel dressing accelerating wound healing. Biomater Adv. 2022;135:212737. doi:10.1016/j.bioadv.2022.212737
11. Xu X, Gu S, Huang X, et al. The role of macrophages in the formation of hypertrophic scars and keloids. Burns Trauma. 2020;8:tkaa006. doi:10.1093/burnst/tkaa006
12. Ud-Din S, Wilgus TA, Bayat A. Mast cells in skin scarring: a review of animal and human research. Front Immunol. 2020;11:552205. doi:10.3389/fimmu.2020.552205
13. Chen Y, Jin Q, Fu X, Qiao J, Niu F. Connection between T regulatory cell enrichment and collagen deposition in keloid. Exp Cell Res. 2019;383(2):111549. doi:10.1016/j.yexcr.2019.111549
14. Fischer A, Wannemacher J, Christ S, et al. Neutrophils direct preexisting matrix to initiate repair in damaged tissues. Nat Immunol. 2022;23(4):518–531. doi:10.1038/s41590-022-01166-6
15. Niessen FB, Schalkwijk J, Vos H, Timens W. Hypertrophic scar formation is associated with an increased number of epidermal Langerhans cells. J Pathol. 2004;202(1):121–129. doi:10.1002/path.1502
16. Tanno H, Kawakami K, Kanno E, et al. Invariant NKT cells promote skin wound healing by preventing a prolonged neutrophilic inflammatory response. Wound Repair Regen. 2017;25(5):805–815. doi:10.1111/wrr.12588
17. Moravej H, Forghanian A, Dadkhahfar S, Mozafari N. Intralesional bleomycin versus intralesional triamcinolone in the treatment of keloids and hypertrophic scars. Dermatol Ther. 2022;35(9):e15730. doi:10.1111/dth.15730
18. Menezes MCS, Buzelin M, Nunes CB, Alberti LR. Tacrolimus action pathways in an ointment base for hypertrophic scar prevention in a rabbit ear model. An Bras Dermatol. 2021;96(4):429–435. doi:10.1016/j.abd.2020.08.019
19. Klotz T, Munn Z, Aromataris EC, Greenwood JE. Imiquimod to prevent keloid recurrence postexcision: a systematic review and meta-analysis. Wound Repair Regen. 2020;28(1):145–156. doi:10.1111/wrr.12766
20. Aoki M, Kondo A, Matsunaga N, et al. The immunosuppressant fingolimod (FTY720) for the treatment of mechanical force-induced abnormal scars. J Immunol Res. 2020;2020:7057195. doi:10.1155/2020/7057195
21. Cheng S, Wang H, Pan X, et al. Dendritic hydrogels with robust inherent antibacterial properties for promoting bacteria-infected wound healing. ACS Appl Mater Interfaces. 2022;14(9):11144–11155. doi:10.1021/acsami.1c25014
22. Huang J, Zhang J, Xiong J, et al. Stem cell-derived nanovesicles: a novel cell-free therapy for wound healing. Stem Cells Int. 2021;2021:1285087. doi:10.1155/2021/1285087
23. Roefs MT, Sluijter JPG, Vader P. Extracellular vesicle-associated proteins in tissue repair. Trends Cell Biol. 2020;30(12):990–1013. doi:10.1016/j.tcb.2020.09.009
24. An Y, Lin S, Tan X, et al. Exosomes from adipose-derived stem cells and application to skin wound healing. Cell Prolif. 2021;54(3):e12993. doi:10.1111/cpr.12993
25. Hu Y, Rao S-S, Wang Z-X, et al. Exosomes from human umbilical cord blood accelerate cutaneous wound healing through miR-21-3p-mediated promotion of angiogenesis and fibroblast function. Theranostics. 2018;8(1):169–184. doi:10.7150/thno.21234
26. Wu D, Kang L, Tian J, et al. Exosomes derived from bone mesenchymal stem cells with the stimulation of FeO nanoparticles and static magnetic field enhance wound healing through upregulated miR-21-5p. Int J Nanomedicine. 2020;15:7979–7993. doi:10.2147/IJN.S275650
27. Kim S, Lee SK, Kim H, Kim TM. Exosomes secreted from induced pluripotent stem cell-derived mesenchymal stem cells accelerate skin cell proliferation. Int J Mol Sci. 2018;19(10):3119. doi:10.3390/ijms19103119
28. Duan M, Zhang Y, Zhang H, Meng Y, Qian M, Zhang G. Epidermal stem cell-derived exosomes promote skin regeneration by downregulating transforming growth factor-β1 in wound healing. Stem Cell Res Ther. 2020;11(1):452. doi:10.1186/s13287-020-01971-6
29. Ha DH, Kim HK, Lee J, et al. Mesenchymal stem/stromal cell-derived exosomes for immunomodulatory therapeutics and skin regeneration. Cells. 2020;9(5):1157. doi:10.3390/cells9051157
30. Bian D, Wu Y, Song G, Azizi R, Zamani A. The application of mesenchymal stromal cells (MSCs) and their derivative exosome in skin wound healing: a comprehensive review. Stem Cell Res Ther. 2022;13(1):24. doi:10.1186/s13287-021-02697-9
31. Vu NB, Nguyen HT, Palumbo R, Pellicano R, Fagoonee S, Pham PV. Stem cell-derived exosomes for wound healing: current status and promising directions. Minerva Med. 2021;112(3):384–400. doi:10.23736/S0026-4806.20.07205-5
32. Zhao W, Zhang R, Zang C, et al. Exosome derived from mesenchymal stem cells alleviates pathological scars by inhibiting the proliferation, migration and protein expression of fibroblasts via delivering miR-138-5p to target SIRT1. Int J Nanomedicine. 2022;17:4023–4038. doi:10.2147/IJN.S377317
33. Silva AM, Teixeira JH, Almeida MI, Gonçalves RM, Barbosa MA, Santos SG. Extracellular vesicles: immunomodulatory messengers in the context of tissue repair/regeneration. Eur J Pharm Sci. 2017;98:86–95. doi:10.1016/j.ejps.2016.09.017
34. Manchon E, Hirt N, Bouaziz J-D, Jabrane-Ferrat N, Al-Daccak R. Stem cells-derived extracellular vesicles: potential therapeutics for wound healing in chronic inflammatory skin diseases. Int J Mol Sci. 2021;22(6):3130. doi:10.3390/ijms22063130
35. Li Y, Zhang J, Shi J, et al. Exosomes derived from human adipose mesenchymal stem cells attenuate hypertrophic scar fibrosis by miR-192-5p/IL-17RA/Smad axis. Stem Cell Res Ther. 2021;12(1):221. doi:10.1186/s13287-021-02290-0
36. Z-Y W, Zhang H-J, Zhou Z-H, et al. The effect of inhibiting exosomes derived from adipose-derived stem cells via the TGF-β1/Smad pathway on the fibrosis of keloid fibroblasts. Gland Surg. 2021;10(3):1046–1056. doi:10.21037/gs-21-4
37. Zhang H, Wang L, Li C, et al. Exosome-induced regulation in inflammatory bowel disease. Front Immunol. 2019;10:1464. doi:10.3389/fimmu.2019.01464
38. Tenchov R, Sasso JM, Wang X, Liaw WS, Chen CA, Zhou QA. Exosomes─nature’s lipid nanoparticles, a rising star in drug delivery and diagnostics. ACS Nano. 2022;16(11):17802–17846. doi:10.1021/acsnano.2c08774
39. Pathan M, Fonseka P, Chitti SV, et al. Vesiclepedia 2019: a compendium of RNA, proteins, lipids and metabolites in extracellular vesicles. Nucleic Acids Res. 2019;47(D1):D516–D519. doi:10.1093/nar/gky1029
40. Doyle LM, Wang MZ. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells. 2019;8(7):727. doi:10.3390/cells8070727
41. Zhang L, Xiang J, Zhang F, Liu L, Hu C. MSCs can be a double-edged sword in tumorigenesis. Front Oncol. 2022;12:1047907. doi:10.3389/fonc.2022.1047907
42. Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21(1):9–17. doi:10.1038/s41556-018-0250-9
43. Mathivanan S, Fahner CJ, Reid GE, Simpson RJ. ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic Acids Res. 2012;40(Database issue):D1241–D1244. doi:10.1093/nar/gkr828
44. Lettau M, Janssen O. Intra- and extracellular effector vesicles from human T and NK cells: same-same, but different? Front Immunol. 2021;12:804895. doi:10.3389/fimmu.2021.804895
45. Wu B, Feng J, Guo J, et al. ADSCs-derived exosomes ameliorate hepatic fibrosis by suppressing stellate cell activation and remodeling hepatocellular glutamine synthetase-mediated glutamine and ammonia homeostasis. Stem Cell Res Ther. 2022;13(1):494. doi:10.1186/s13287-022-03049-x
46. Moura SL, Martín CG, Martí M, Pividori MI. Multiplex detection and characterization of breast cancer exosomes by magneto-actuated immunoassay. Talanta. 2020;211:120657. doi:10.1016/j.talanta.2019.120657
47. Zhang X, Gong W, Duan C, Cai H, Shen Y, Cao J. Echinococcus granulosus protoscoleces-derived exosome-like vesicles and Egr-miR-277a-3p promote dendritic cell maturation and differentiation. Cells. 2022;11(20):3220. doi:10.3390/cells11203220
48. Egea-Jimenez AL, Zimmermann P. Lipids in Exosome Biology. Handb Exp Pharmacol. 2020;259:309–336. doi:10.1007/164_2019_220
49. Hullin-Matsuda F, Colosetti P, Rabia M, Luquain-Costaz C, Delton I. Exosomal lipids from membrane organization to biomarkers: focus on an endolysosomal-specific lipid. Biochimie. 2022;203:77–92. doi:10.1016/j.biochi.2022.09.016
50. Perpetuo L, Ferreira R, Thongboonkerd V, Guedes S, Amado F, Vitorino R. Urinary exosomes: diagnostic impact with a bioinformatic approach. Adv Clin Chem. 2022;111:69–99. doi:10.1016/bs.acc.2022.07.002
51. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478):eaau6977. doi:10.1126/science.aau6977
52. Huang X, Yuan T, Tschannen M, et al. Characterization of human plasma-derived exosomal RNAs by deep sequencing. BMC Genomics. 2013;14:319. doi:10.1186/1471-2164-14-319
53. Gan J, Zeng X, Wang X, et al. Effective diagnosis of prostate cancer based on mRNAs from urinary exosomes. Front Med. 2022;9:736110. doi:10.3389/fmed.2022.736110
54. Li T, Li J, Wang H, et al. Exosomes: potential biomarkers and functions in head and neck squamous cell carcinoma. Front Mol Biosci. 2022;9:881794. doi:10.3389/fmolb.2022.881794
55. Zheng B, Song X, Wang L, et al. Plasma exosomal tRNA-derived fragments as diagnostic biomarkers in non-small cell lung cancer. Front Oncol. 2022;12:1037523. doi:10.3389/fonc.2022.1037523
56. Kim MS, Haney MJ, Zhao Y, et al. Engineering macrophage-derived exosomes for targeted paclitaxel delivery to pulmonary metastases: in vitro and in vivo evaluations. Nanomedicine. 2018;14(1):195–204. doi:10.1016/j.nano.2017.09.011
57. Gomes ER, Carvalho AT, Barbosa TC, et al. Fusion of tumor-derived exosomes with long-circulating and pH-sensitive liposomes loaded with doxorubicin for the treatment of breast cancer. AAPS Pharm Sci Tech. 2022;23(7):255. doi:10.1208/s12249-022-02349-y
58. Kanchanapally R, Khan MA, Deshmukh SK, et al. Exosomal formulation escalates cellular uptake of honokiol leading to the enhancement of its antitumor efficacy. ACS Omega. 2020;5(36):23299–23307. doi:10.1021/acsomega.0c03136
59. Zhang Y, Xie Y, Hao Z, et al. Umbilical mesenchymal stem cell-derived exosome-encapsulated hydrogels accelerate bone repair by enhancing angiogenesis. ACS Appl Mater Interfaces. 2021;13(16):18472–18487. doi:10.1021/acsami.0c22671
60. Thomas BL, Eldridge SE, Nosrati B, et al. WNT3A-loaded exosomes enable cartilage repair. J Extracell Vesicles. 2021;10(7):e12088. doi:10.1002/jev2.12088
61. Safari B, Aghazadeh M, Davaran S, Roshangar L. Exosome-loaded hydrogels: a new cell-free therapeutic approach for skin regeneration. Eur J Pharm Biopharm. 2022;171:50–59. doi:10.1016/j.ejpb.2021.11.002
62. Fan L, Liu C, Chen X, et al. Exosomes-loaded electroconductive hydrogel synergistically promotes tissue repair after spinal cord injury via immunoregulation and enhancement of myelinated axon growth. Adv Sci. 2022;9(13):e2105586. doi:10.1002/advs.202105586
63. Yu H, Cheng J, Shi W, et al. Bone marrow mesenchymal stem cell-derived exosomes promote tendon regeneration by facilitating the proliferation and migration of endogenous tendon stem/progenitor cells. Acta Biomater. 2020;106:328–341. doi:10.1016/j.actbio.2020.01.051
64. Qian L, Pi L, Fang BR, Meng XX. Adipose mesenchymal stem cell-derived exosomes accelerate skin wound healing via the lncRNA H19/miR-19b/SOX9 axis. Lab Invest. 2021;101(9):1254–1266. doi:10.1038/s41374-021-00611-8
65. Zhao B, Zhang X, Zhang Y, et al. Human exosomes accelerate cutaneous wound healing by promoting collagen synthesis in a diabetic mouse model. Stem Cells Dev. 2021;30(18):922–933. doi:10.1089/scd.2021.0100
66. Cosenza S, Toupet K, Maumus M, et al. Mesenchymal stem cells-derived exosomes are more immunosuppressive than microparticles in inflammatory arthritis. Theranostics. 2018;8(5):1399–1410. doi:10.7150/thno.21072
67. Chamberlain CS, Kink JA, Wildenauer LA, et al. Exosome-educated macrophages and exosomes differentially improve ligament healing. Stem Cells. 2021;39(1):55–61. doi:10.1002/stem.3291
68. Tang Q, Lu B, He J, et al. Exosomes-loaded thermosensitive hydrogels for corneal epithelium and stroma regeneration. Biomaterials. 2022;280:121320. doi:10.1016/j.biomaterials.2021.121320
69. Zhou X, Brown BA, Siegel AP, et al. Exosome-mediated crosstalk between keratinocytes and macrophages in cutaneous wound healing. ACS Nano. 2020;14(10):12732–12748. doi:10.1021/acsnano.0c03064
70. Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8(12):958–969. doi:10.1038/nri2448
71. Atri C, Guerfali FZ, Laouini D. Role of human macrophage polarization in inflammation during infectious diseases. Int J Mol Sci. 2018;19(6):1801. doi:10.3390/ijms19061801
72. He L, Jhong JH, Chen Q, et al. Global characterization of macrophage polarization mechanisms and identification of M2-type polarization inhibitors. Cell Rep. 2021;37(5):109955. doi:10.1016/j.celrep.2021.109955
73. Shapouri-Moghaddam A, Mohammadian S, Vazini H, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233(9):6425–6440. doi:10.1002/jcp.26429
74. Sim SL, Kumari S, Kaur S, Khosrotehrani K. Macrophages in skin wounds: functions and therapeutic potential. Biomolecules. 2022;12(11):1659. doi:10.3390/biom12111659
75. Hesketh M, Sahin KB, West ZE, Murray RZ. Macrophage phenotypes regulate scar formation and chronic wound healing. Int J Mol Sci. 2017;18(7):1545. doi:10.3390/ijms18071545
76. Williams H, Suda S, Dervish S, Yap YT, Holland AJA, Medbury HJ. Monocyte M1/M2 profile is altered in paediatric burn patients with hypertrophic scarring. Wound Repair Regen. 2021;29(6):996–1005. doi:10.1111/wrr.12960
77. Chen L, Wang J, Li S, et al. The clinical dynamic changes of macrophage phenotype and function in different stages of human wound healing and hypertrophic scar formation. Int Wound J. 2019;16(2):360–369. doi:10.1111/iwj.13041
78. Witherel CE, Sao K, Brisson BK, et al. Regulation of extracellular matrix assembly and structure by hybrid M1/M2 macrophages. Biomaterials. 2021;269:120667. doi:10.1016/j.biomaterials.2021.120667
79. Kloc M, Ghobrial RM, Wosik J, Lewicka A, Lewicki S, Kubiak JZ. Macrophage functions in wound healing. J Tissue Eng Regen Med. 2019;13(1):99–109. doi:10.1002/term.2772
80. Strudwick XL, Adams DH, Pyne NT, Samuel MS, Murray RZ, Cowin AJ. Systemic delivery of anti-integrin alphal antibodies reduces early macrophage recruitment, inflammation, and scar formation in murine burn wounds. Adv Wound Care. 2020;9(12):637–648. doi:10.1089/wound.2019.1035
81. Condorelli AG, El Hachem M, Zambruno G, Nystrom A, Candi E, Castiglia D. Notch-ing up knowledge on molecular mechanisms of skin fibrosis: focus on the multifaceted Notch signalling pathway. J Biomed Sci. 2021;28(1):36. doi:10.1186/s12929-021-00732-8
82. Mancarella S, Gigante I, Serino G, et al. Crenigacestat blocking notch pathway reduces liver fibrosis in the surrounding ecosystem of intrahepatic CCA viaTGF-β inhibition. J Exp Clin Cancer Res. 2022;41(1):331. doi:10.1186/s13046-022-02536-6
83. Yu C, Xiong C, Tang J, et al. Histone demethylase JMJD3 protects against renal fibrosis by suppressing TGFβ and Notch signaling and preserving PTEN expression. Theranostics. 2021;11(6):2706–2721. doi:10.7150/thno.48679
84. Xia Z, Wang J, Yang S, et al. Emodin alleviates hypertrophic scar formation by suppressing macrophage polarization and inhibiting the Notch and TGF-β pathways in macrophages. Braz J Med Biol Res. 2021;54(8):e11184. doi:10.1590/1414-431X2021e11184
85. He T, Bai X, Jing J, et al. Notch signal deficiency alleviates hypertrophic scar formation after wound healing through the inhibition of inflammation. Arch Biochem Biophys. 2020;682:108286. doi:10.1016/j.abb.2020.108286
86. Wei W, Li ZP, Bian ZX, Han QB. Astragalus polysaccharide RAP induces macrophage phenotype polarization to M1 via the notch signaling pathway. Molecules. 2019;24(10):2016. doi:10.3390/molecules24102016
87. Sheng J, Zhang B, Chen Y, Yu F. Capsaicin attenuates liver fibrosis by targeting Notch signaling to inhibit TNF-α secretion from M1 macrophages. Immunopharmacol Immunotoxicol. 2020;42(6):556–563. doi:10.1080/08923973.2020.1811308
88. Kanagaratham C, El Ansari YS, Lewis OL, Oettgen HC. IgE and IgG antibodies as regulators of mast cell and basophil functions in food allergy. Front Immunol. 2020;11:603050. doi:10.3389/fimmu.2020.603050
89. Mendoza RP, Anderson CC, Fudge DH, Roede JR, Brown JM. Metabolic consequences of IgE- and Non-IgE-mediated mast cell degranulation. J Immunol. 2021;207(11):2637–2648. doi:10.4049/jimmunol.2001278
90. Kim HS, Kawakami Y, Kasakura K, Kawakami T. Recent advances in mast cell activation and regulation. F1000Res. 2020;9:F1000. doi:10.12688/f1000research.22037.1
91. Komi DEA, Khomtchouk K, Santa Maria PL. A review of the contribution of mast cells in wound healing: involved molecular and cellular mechanisms. Clin Rev Allergy Immunol. 2020;58(3):298–312. doi:10.1007/s12016-019-08729-w
92. Chen L, Schrementi ME, Ranzer MJ, Wilgus TA, DiPietro LA. Blockade of mast cell activation reduces cutaneous scar formation. PLoS One. 2014;9(1):e85226. doi:10.1371/journal.pone.0085226
93. Marshall JS, Portales-Cervantes L, Leong E. Mast cell responses to viruses and pathogen products. Int J Mol Sci. 2019;20(17):4241. doi:10.3390/ijms20174241
94. Bacci S. Fine regulation during wound healing by mast cells, a physiological role not yet clarified. Int J Mol Sci. 2022;23(3):1820. doi:10.3390/ijms23031820
95. Lipitsä T, Siiskonen H, Naukkarinen A, Harvima IT. Mast cell chymase degrades fibrinogen and fibrin. Br J Dermatol. 2019;181(2):296–303. doi:10.1111/bjd.17534
96. Maccarana M, Jia J, Li H, Zhang X, Vlodavsky I, Li JP. Implications of heparanase on heparin synthesis and metabolism in mast cells. Int J Mol Sci. 2022;23(9):4821. doi:10.3390/ijms23094821
97. Badertscher K, Brönnimann M, Karlen S, Braathen LR, Yawalkar N. Mast cell chymase is increased in chronic atopic dermatitis but not in psoriasis. Arch Dermatol Res. 2005;296(10):503–506. doi:10.1007/s00403-005-0542-3
98. West PW, Bulfone-Paus S. Mast cell tissue heterogeneity and specificity of immune cell recruitment. Front Immunol. 2022;13:932090. doi:10.3389/fimmu.2022.932090
99. Pincha N, Hajam EY, Badarinath K, et al. PAI1 mediates fibroblast-mast cell interactions in skin fibrosis. J Clin Invest. 2018;128(5):1807–1819. doi:10.1172/JCI99088
100. Kishimoto T, Ishida W, Nakajima I, et al. Promotion of conjunctival fibroblast-mediated collagen gel contraction by mast cells through up-regulation of matrix metalloproteinase release and activation. Exp Eye Res. 2022;218:108980. doi:10.1016/j.exer.2022.108980
101. Zhang M, Zhang S. T cells in fibrosis and fibrotic diseases. Front Immunol. 2020;11:1142. doi:10.3389/fimmu.2020.01142
102. Jin W, Zheng Y, Zhu P. T cell abnormalities in systemic sclerosis. Autoimmun Rev. 2022;21(11):103185. doi:10.1016/j.autrev.2022.103185
103. Chen B, Li H, Xia W. The role of Th1/Th2 cell chemokine expression in hypertrophic scar. Int Wound J. 2020;17(1):197–205. doi:10.1111/iwj.13257
104. Piris MA, Rodriguez-Pinilla SM, Santonja C, et al. Update on peripheral T-cell lymphomas with T-helper phenotype: are there too many subtypes? Semin Diagn Pathol. 2020;37(1):24–31. doi:10.1053/j.semdp.2019.12.005
105. Andreatta M, Corria-Osorio J, Müller S, Cubas R, Coukos G, Carmona SJ. Interpretation of T cell states from single-cell transcriptomics data using reference atlases. Nat Commun. 2021;12(1):2965. doi:10.1038/s41467-021-23324-4
106. Nguyen JK, Austin E, Huang A, Mamalis A, Jagdeo J. The IL-4/IL-13 axis in skin fibrosis and scarring: mechanistic concepts and therapeutic targets. Arch Dermatol Res. 2020;312(2):81–92. doi:10.1007/s00403-019-01972-3
107. Maeda D, Kubo T, Kiya K, et al. Periostin is induced by IL-4/IL-13 in dermal fibroblasts and promotes RhoA/ROCK pathway-mediated TGF-β1 secretion in abnormal scar formation. J Plast Surg Hand Surg. 2019;53(5):288–294. doi:10.1080/2000656X.2019.1612752
108. Tredget EE, Yang L, Delehanty M, Shankowsky H, Scott PG. Polarized Th2 cytokine production in patients with hypertrophic scar following thermal injury. J Interferon Cytokine Res. 2006;26(3):179–189. doi:10.1089/jir.2006.26.179
109. Ali N, Rosenblum MD. Regulatory T cells in skin. Immunology. 2017;152(3):372–381. doi:10.1111/imm.12791
110. Boothby IC, Cohen JN, Rosenblum MD. Regulatory T cells in skin injury: at the crossroads of tolerance and tissue repair. Sci Immunol. 2020;5(47):eaaz9631. doi:10.1126/sciimmunol.aaz9631
111. Murao N, Seino K, Hayashi T, et al. Treg-enriched CD4+ T cells attenuate collagen synthesis in keloid fibroblasts. Exp Dermatol. 2014;23(4):266–271. doi:10.1111/exd.12368
112. Li J, Tan J, Martino MM, Lui KO. Regulatory T-cells: potential regulator of tissue repair and regeneration. Front Immunol. 2018;9:585. doi:10.3389/fimmu.2018.00585
113. Lei H, Schmidt-Bleek K, Dienelt A, Reinke P, Volk HD. Regulatory T cell-mediated anti-inflammatory effects promote successful tissue repair in both indirect and direct manners. Front Pharmacol. 2015;6:184. doi:10.3389/fphar.2015.00184
114. Schneider DF, Glenn CH, Faunce DE. Innate lymphocyte subsets and their immunoregulatory roles in burn injury and sepsis. J Burn Care Res. 2007;28(3):365–379. doi:10.1097/bcr.0b013e318053d40b
115. Bendelac A, Lantz O, Quimby M, Yewdell J, Bennink JR, Brutkiewicz R. CD1 recognition by mouse NK1+ T lymphocytes. Science. 1995;268(5212):863–865.
116. Moretta A. CD69-mediated pathway of lymphocyte activation: anti-CD69 monoclonal antibodies trigger the cytolytic activity of different lymphoid effector cells with the exception of cytolytic T lymphocytes expressing T cell receptor alpha/beta. J Exp Med. 1991;174(6):1393–1398.
117. Schneider DF, Palmer JL, Tulley JM, Kovacs EJ, Gamelli RL, Faunce DE. Prevention of NKT cell activation accelerates cutaneous wound closure and alters local inflammatory signals. J Surg Res. 2011;171(1):361–373. doi:10.1016/j.jss.2010.03.030
118. Schneider DF, Palmer JL, Tulley JM, et al. A novel role for NKT cells in cutaneous wound repair. J Surg Res. 2011;168(2):325–333. doi:10.1016/j.jss.2009.09.030
119. Cooper MA, Fehniger TA, Turner SC, et al. Human natural killer cells: a unique innate immunoregulatory role for the CD56 (bright) subset. Blood. 2001;97(10):3146–3151. doi:10.1182/blood.v97.10.3146
120. Cui R, Rekasi H, Hepner-Schefczyk M, et al. Human mesenchymal stromal/stem cells acquire immunostimulatory capacity upon cross-talk with natural killer cells and might improve the NK cell function of immunocompromised patients. Stem Cell Res Ther. 2016;7(1):88. doi:10.1186/s13287-016-0353-9
121. Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83(3):835–870. doi:10.1152/physrev.2003.83.3.835
122. Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13(3):159–175. doi:10.1038/nri3399
123. Devalaraja RM, Nanney LB, Du J, et al. Delayed wound healing in CXCR2 knockout mice. J Invest Dermatol. 2000;115(2):234–244. doi:10.1046/j.1523-1747.2000.00034.x
124. Brubaker AL, Schneider DF, Kovacs EJ. Neutrophils and natural killer T cells as negative regulators of wound healing. Expert Rev Dermatol. 2011;6(1):5–8. doi:10.1586/edm.10.66
125. Dovi JV, He LK, DiPietro LA. Accelerated wound closure in neutrophil-depleted mice. J Leukoc Biol. 2003;73(4):448–455. doi:10.1189/jlb.0802406
126. Niessen FB, Andriessen MP, Schalkwijk J, Visser L, Timens W. Keratinocyte-derived growth factors play a role in the formation of hypertrophic scars. J Pathol. 2001;194(2):207–216. doi:10.1002/path.853
127. Gan J, Liu C, Li H, et al. Accelerated wound healing in diabetes by reprogramming the macrophages with particle-induced clustering of the mannose receptors. Biomaterials. 2019;219:119340. doi:10.1016/j.biomaterials.2019.119340
128. Shook BA, Wasko RR, Rivera-Gonzalez GC, et al. Myofibroblast proliferation and heterogeneity are supported by macrophages during skin repair. Science. 2018;362(6417):eaar2971. doi:10.1126/science.aar2971
129. Kurachi I, Kurita E, Takushima A, Suga H. Human CD206+ macrophages show antifibrotic effects on human fibroblasts through an IL-6-dependent mechanism in vitro. Plast Reconstr Surg. 2021;147(2):231e–239e. doi:10.1097/PRS.0000000000007563
130. Hao Y, Dong X, Zhang M, Liu H, Zhu L, Wang Y. Effects of hyperbaric oxygen therapy on the expression levels of the inflammatory factors interleukin-12p40, macrophage inflammatory protein-1β, platelet-derived growth factor-BB, and interleukin-1 receptor antagonist in keloids. Medicine. 2020;99(16):e19857. doi:10.1097/MD.0000000000019857
131. Kim S, Kim Y, Hyun Y-S, Choi H, Kim S-Y, Kim T-G. Exosomes from human cord blood plasma accelerate cutaneous wound healing by promoting fibroblast function, angiogenesis, and M2 macrophage differentiation. Biomater Sci. 2021;9(8):3028–3039. doi:10.1039/d0bm01801e
132. Li T, Chen W, Zhang Q, Deng C. Human-specific gene CHRFAM7A mediates M2 macrophage polarization via the Notch pathway to ameliorate hypertrophic scar formation. Biomed Pharmacother. 2020;131:110611. doi:10.1016/j.biopha.2020.110611
133. Zhou B, Gao Z, Liu W, Wu X, Wang W. Important role of mechanical microenvironment on macrophage dysfunction during keloid pathogenesis. Exp Dermatol. 2022;31(3):375–380. doi:10.1111/exd.14473
134. Shi R, Jin Y, Zhao S, Yuan H, Shi J, Zhao H. Hypoxic ADSC-derived exosomes enhance wound healing in diabetic mice via delivery of circ-Snhg11 and induction of M2-like macrophage polarization. Biomed Pharmacother. 2022;153:113463. doi:10.1016/j.biopha.2022.113463
135. He X, Dong Z, Cao Y, et al. MSC-derived exosome promotes M2 polarization and enhances cutaneous wound healing. Stem Cells Int. 2019;2019:7132708. doi:10.1155/2019/7132708
136. Luo Z, Lin J, Sun Y, Wang C, Chen J. Bone marrow stromal cell-derived exosomes promote muscle healing following contusion through macrophage polarization. Stem Cells Dev. 2021;30(3):135–148. doi:10.1089/scd.2020.0167
137. Teng L, Maqsood M, Zhu M, et al. Exosomes derived from human umbilical cord mesenchymal stem cells accelerate diabetic wound healing via promoting M2 macrophage polarization, angiogenesis, and collagen deposition. Int J Mol Sci. 2022;23(18):10421. doi:10.3390/ijms231810421
138. Wen J-H, Li D-Y, Liang S, Yang C, Tang J-X, Liu H-F. Macrophage autophagy in macrophage polarization, chronic inflammation and organ fibrosis. Front Immunol. 2022;13:946832. doi:10.3389/fimmu.2022.946832
139. Lodder J, Denaës T, Chobert M-N, et al. Macrophage autophagy protects against liver fibrosis in mice. Autophagy. 2015;11(8):1280–1292. doi:10.1080/15548627.2015.1058473
140. Zhong Z, Umemura A, Sanchez-Lopez E, et al. NF-κB restricts inflammasome activation via elimination of damaged mitochondria. Cell. 2016;164(5):896–910. doi:10.1016/j.cell.2015.12.057
141. Liu T, Wang L, Liang P, et al. USP19 suppresses inflammation and promotes M2-like macrophage polarization by manipulating NLRP3 function via autophagy. Cell Mol Immunol. 2021;18(10):2431–2442. doi:10.1038/s41423-020-00567-7
142. Xie Y, Yu L, Cheng Z, et al. SHED-derived exosomes promote LPS-induced wound healing with less itching by stimulating macrophage autophagy. J Nanobiotechnology. 2022;20(1):239. doi:10.1186/s12951-022-01446-1
143. Wilgus TA, Ud-Din S, Bayat A. A review of the evidence for and against a role for mast cells in cutaneous scarring and fibrosis. Int J Mol Sci. 2020;21(24):9673. doi:10.3390/ijms21249673
144. Mukai K, Tsai M, Saito H, Galli SJ. Mast cells as sources of cytokines, chemokines, and growth factors. Immunol Rev. 2018;282(1):121–150. doi:10.1111/imr.12634
145. Zimmermann C, Troeltzsch D, Giménez-Rivera VA, et al. Mast cells are critical for controlling the bacterial burden and the healing of infected wounds. Proc Natl Acad Sci U S A. 2019;116(41):20500–20504. doi:10.1073/pnas.1908816116
146. Wilgus TA, Wulff BC. The importance of mast cells in dermal scarring. Adv Wound Care. 2014;3(4):356–365. doi:10.1089/wound.2013.0457
147. Cho K-A, Cha J-E, Kim J, Kim Y-H, Ryu K-H, Woo S-Y. Mesenchymal stem cell-derived exosomes attenuate TLR7-mediated mast cell activation. Tissue Eng Regen Med. 2022;19(1):117–129. doi:10.1007/s13770-021-00395-4
148. Gao R, Ye T, Zhu Z, et al. Small extracellular vesicles from iPSC-derived mesenchymal stem cells ameliorate tendinopathy pain by inhibiting mast cell activation. Nanomedicine. 2022;17(8):513–529. doi:10.2217/nnm-2022-0036
149. Short WD, Wang X, Li H, et al. Interleukin-10 producing T lymphocytes attenuate dermal scarring. Ann Surg. 2021;274(4):627–636. doi:10.1097/SLA.0000000000004984
150. Xu H, Zhu Z, Hu J, et al. Downregulated cytotoxic CD8 T-cell identifies with the NKG2A-soluble HLA-E axis as a predictive biomarker and potential therapeutic target in keloids. Cell Mol Immunol. 2022;19(4):527–539. doi:10.1038/s41423-021-00834-1
151. Chen Z, Zhou L, Won T, Gao Z, Wu X, Lu L. Characterization of CD45RO memory T lymphocytes in keloid disease. Br J Dermatol. 2018;178(4):940–950. doi:10.1111/bjd.16173
152. Wang H, Li Y, Yue Z, et al. Adipose-derived stem cell exosomes inhibit hypertrophic scaring formation by regulating Th17/Treg cell balance. Biomed Res Int. 2022;2022:9899135. doi:10.1155/2022/9899135
153. Xu K, Ma D, Zhang G, et al. Human umbilical cord mesenchymal stem cell-derived small extracellular vesicles ameliorate collagen-induced arthritis via immunomodulatory T lymphocytes. Mol Immunol. 2021;135:36–44. doi:10.1016/j.molimm.2021.04.001
154. Fu Y, Li J, Zhang Z, et al. Umbilical cord mesenchymal stem cell-derived exosomes alleviate collagen-induced arthritis by balancing the population of Th17 and regulatory T cells. FEBS Lett. 2022;596(20):2668–2677. doi:10.1002/1873-3468.14460
155. Su D, Tsai HI, Xu Z, et al. Exosomal PD-L1 functions as an immunosuppressant to promote wound healing. J Extracell Vesicles. 2019;9(1):1709262. doi:10.1080/20013078.2019.1709262
156. Wilgus TA, Roy S, McDaniel JC. Neutrophils and wound repair: positive actions and negative reactions. Adv Wound Care. 2013;2(7):379–388. doi:10.1089/wound.2012.0383
157. Eshghi F, Tahmasebi S, Alimohammadi M, et al. Study of immunomodulatory effects of mesenchymal stem cell-derived exosomes in a mouse model of LPS induced systemic inflammation. Life Sci. 2022;310:120938. doi:10.1016/j.lfs.2022.120938
158. Chrysanthopoulou A, Mitroulis I, Apostolidou E, et al. Neutrophil extracellular traps promote differentiation and function of fibroblasts. J Pathol. 2014;233(3):294–307. doi:10.1002/path.4359
159. Jin Z, Sun J, Song Z, et al. Neutrophil extracellular traps promote scar formation in post-epidural fibrosis. NPJ Regen Med. 2020;5(1):19. doi:10.1038/s41536-020-00103-1
160. Loh JT, Zhang B, Teo JKH, et al. Mechanism for the attenuation of neutrophil and complement hyperactivity by MSC exosomes. Cytotherapy. 2022;24(7):711–719. doi:10.1016/j.jcyt.2021.12.003
161. Rees PA, Greaves NS, Baguneid M, Bayat A. Chemokines in wound healing and as potential therapeutic targets for reducing cutaneous scarring. Adv Wound Care. 2015;4(11):687–703. doi:10.1089/wound.2014.0568
162. Decout A, Katz JD, Venkatraman S, Ablasser A. The cGAS-STING pathway as a therapeutic target in inflammatory diseases. Nat Rev Immunol. 2021;21(9):548–569. doi:10.1038/s41577-021-00524-z
163. Jiang M, Chen P, Wang L, et al. cGAS-STING, an important pathway in cancer immunotherapy. J Hematol Oncol. 2020;13(1):81. doi:10.1186/s13045-020-00916-z
164. Chen R, Du J, Zhu H, Ling Q. The role of cGAS-STING signalling in liver diseases. JHEP Rep. 2021;3(5):100324. doi:10.1016/j.jhepr.2021.100324
165. Hopfner KP, Hornung V. Molecular mechanisms and cellular functions of cGAS-STING signalling. Nat Rev Mol Cell Biol. 2020;21(9):501–521. doi:10.1038/s41580-020-0244-x
166. Chen C, Xu P. Cellular functions of cGAS-STING signaling. Trends Cell Biol. 2022. doi:10.1016/j.tcb.2022.11.001
167. Xie X, Wu X, Zhao D, et al. Fluvoxamine alleviates bleomycin-induced lung fibrosis via regulating the cGAS-STING pathway. Pharmacol Res. 2023;187:106577. doi:10.1016/j.phrs.2022.106577
168. Chung KW, Dhillon P, Huang S, et al. Mitochondrial damage and activation of the STING pathway lead to renal inflammation and fibrosis. Cell Metab. 2019;30(4):784–799.e785. doi:10.1016/j.cmet.2019.08.003
169. Shen R, Yang K, Cheng X, et al. Accumulation of polystyrene microplastics induces liver fibrosis by activating cGAS/STING pathway. Environ Pollut. 2022;300:118986. doi:10.1016/j.envpol.2022.118986
170. Hu S, Gao Y, Gao R, et al. The selective STING inhibitor H-151 preserves myocardial function and ameliorates cardiac fibrosis in murine myocardial infarction. Int Immunopharmacol. 2022;107:108658. doi:10.1016/j.intimp.2022.108658
171. Arabpour M, Saghazadeh A, Rezaei N. Anti-inflammatory and M2 macrophage polarization-promoting effect of mesenchymal stem cell-derived exosomes. Int Immunopharmacol. 2021;97:107823. doi:10.1016/j.intimp.2021.107823
172. Toh WS, Zhang B, Lai RC, Lim SK. Immune regulatory targets of mesenchymal stromal cell exosomes/small extracellular vesicles in tissue regeneration. Cytotherapy. 2018;20(12):1419–1426. doi:10.1016/j.jcyt.2018.09.008
173. Zhu Z, Zhang Y, Wu L, Hua K, Ding J. Regeneration-related functional cargoes in mesenchymal stem cell-derived small extracellular vesicles. Stem Cells Dev. 2020;29(1):15–24. doi:10.1089/scd.2019.0131
174. Guillamat-Prats R. The role of MSC in wound healing, scarring and regeneration. Cells. 2021;10(7):1729. doi:10.3390/cells10071729
175. Yu Q, Dong L, Li Y, Liu G. SIRT1 and HIF1α signaling in metabolism and immune responses. Cancer Lett. 2018;418:20–26. doi:10.1016/j.canlet.2017.12.035
176. Elesela S, Morris SB, Narayanan S, Kumar S, Lombard DB, Lukacs NW. Sirtuin 1 regulates mitochondrial function and immune homeostasis in respiratory syncytial virus infected dendritic cells. PLoS Pathog. 2020;16(2):e1008319. doi:10.1371/journal.ppat.1008319
177. Limagne E, Thibaudin M, Euvrard R, et al. Sirtuin-1 activation controls tumor growth by impeding Th17 differentiation via STAT3 deacetylation. Cell Rep. 2017;19(4):746–759. doi:10.1016/j.celrep.2017.04.004
178. Dong L, Bi Y, Jia A, et al. Crucial role of histone deacetylase SIRT1 in myeloid-derived suppressor cell-mediated reprogramming of CD4 T-cell differentiation. Cell Mol Immunol. 2020;17(7):785–787. doi:10.1038/s41423-020-0419-6
179. Kimiz-Gebologlu I, Oncel SS. Exosomes: large-scale production, isolation, drug loading efficiency, and biodistribution and uptake. J Control Release. 2022;347:533–543. doi:10.1016/j.jconrel.2022.05.027
180. Zhang Y, Bi J, Huang J, Tang Y, Du S, Li P. Exosome: a review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int J Nanomedicine. 2020;15:6917–6934. doi:10.2147/ijn.S264498
181. Haraszti RA, Miller R, Stoppato M, et al. Exosomes produced from 3D cultures of MSCs by tangential flow filtration show higher yield and improved activity. Mol Ther. 2018;26(12):2838–2847. doi:10.1016/j.ymthe.2018.09.015
182. Jafari D, Shajari S, Jafari R, et al. Designer exosomes: a new platform for biotechnology therapeutics. BioDrugs. 2020;34(5):567–586. doi:10.1007/s40259-020-00434-x
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.