Back to Journals » International Journal of Nanomedicine » Volume 18
Advances in Formulations of Microneedle System for Rheumatoid Arthritis Treatment
Authors Guo P, Huang C, Yang Q, Zhong G, Zhang J, Qiu M, Zeng R, Gou K, Zhang C, Qu Y
Received 8 September 2023
Accepted for publication 5 December 2023
Published 18 December 2023 Volume 2023:18 Pages 7759—7784
DOI https://doi.org/10.2147/IJN.S435251
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor R.D.K. Misra
Peng Guo,1,* Chi Huang,2,* Qin Yang,1 Guofeng Zhong,1 Junbo Zhang,1 Mengyu Qiu,1 Rui Zeng,3 Kaijun Gou,3 Chen Zhang,1 Yan Qu1
1State Key Laboratory of Southwestern Chinese Medicine Resources, School of Pharmacy, Chengdu University of Traditional Chinese Medicine, Chengdu, 611137, People’s Republic of China; 2Department of Pharmacy, Jiang’an Hospital of Traditional Chinese Medicine, Yibin, 644200, People’s Republic of China; 3Institute of Tibetan Plateau, Southwest Minzu University, Chengdu, 610225, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yan Qu; Chen Zhang, State Key Laboratory of Southwestern Chinese Medicine Resources, Chengdu University of Traditional Chinese Medicine, Chengdu, 610072, People’s Republic of China, Email [email protected]; [email protected]
Abstract: Rheumatoid arthritis (RA) is a systemic autoimmune disease characterized by chronic joint inflammation, eventually leading to severe disability and premature death. At present, the treatment of RA is mainly to reduce inflammation, swelling, and pain. Commonly used drugs are non-steroidal anti-inflammatory drugs (NSAIDs), glucocorticoids, and disease-modifying anti-rheumatic drugs (DMARDs). These drugs lack specificity and require long-term, high-dose administration, which can cause serious adverse effects. In addition, the oral, intravenous, and intra-articular injections will reduce patient compliance, resulting in high cost and low bioavailability. Due to these limitations, microneedles (MNs) have emerged as a new strategy to efficiently localize the drugs in inflamed joints for the treatment of RA. MNs can overcome the cuticle barrier of the skin without stimulating nerves and blood vessels. Which can increase patient compliance, improve bioavailability, and avoid systemic circulation. This review summarizes and evaluates the application of MNs in RA, especially dissolving MNs (DMNs). We encourage the use of MNs to treat RA, by describing the general properties of MNs, materials, preparation technology, drug release mechanism, and advantages. Furthermore, we discussed the biological safety, development prospects, and future challenges of MNs, hoping to provide a new strategy for the treatment of RA.
Keywords: rheumatoid arthritis, dissolving microneedles, transdermal delivery system, degradability, biosafety
Corrigendum for this paper has been published.
Graphical Abstract:

Introduction
Rheumatoid arthritis (RA), the most common type of autoimmune arthritis, is accompanied by chronic inflammation and joint swelling, stiffness, and erosion.1 Severe RA can lead to dysfunction, organ failure, infection, and even death.2 At present, the pathogenesis of RA is still in the exploratory stage. Related studies showed that RA occurrence might be connected to autoimmune, genetic, infection, smoking, and other factors.3 Various inflammatory mediators, such as tumor necrosis factor-α (TNF-α), C-reactive protein, CD40L, interleukin (IL)-18, IL-20, monocyte chemoattractant protein-1, nuclear factor-κB receptor activator ligand fractal protein, matrix metalloproteinase-9, and adhesion molecules, play an important role in the development of the disease.4–6 Although the cause of RA is complicated, several classes of drugs are commonly used in clinical practice, to control the development of the disease and reduce joint damage such as nonsteroidal anti-inflammatory drugs (NSAIDs), disease-modifying antirheumatic drugs (DMARDs), immunosuppressive drugs, biological agents, small interfering RNA (siRNA), and natural small-molecule compounds.7,8 Although the above-mentioned drugs have some therapeutic effects, they still have some disadvantages including the need for high doses, poor patient compliance, less drug accumulation in joints, and extraarticular adverse reactions. In addition, most drugs through intravenous/intra-articular injection have lower bioavailability and faster systemic clearance.9–11 Nano drug delivery system, as a new drug delivery technology, can increase the solubility of drugs, change the distribution of drugs in the body, improve the targeting of drugs, and has become a research hotspot for the treatment of RA.12–14 However, as the drug delivery method is mainly injected into the joint cavity, there are still many shortcomings in patient compliance. To overcome these drawbacks, researchers are trying to create new drug delivery strategies.
An interesting physical penetration enhancement technique is the use of microneedle (MN) arrays, composed of small micron-sized needles. They do not touch nerve endings, so there is no pain.15 MNs can efficiently deliver compounds with different molecular weights to the skin, such as NSAIDs,16 biological agents,17 peptides,18 and even cells.19 Compared with the traditional delivery system, MNs have many unique advantages. First, unlike oral or intravenous injection, drugs must pass through the systemic blood circulation to reach the joint cavity. The MNs can bypass the first pass effect and can directly locate the drug release at the joint, thus avoiding the waste of the drug and improving the curative effect. Secondly, the MNs avoid the diffusion of drugs to healthy tissues outside the joints as much as possible, which greatly reduces the toxic and side effects of drugs. Thirdly, compared with intra-articular injection, MNs can use lower doses to obtain a better curative effect. It can continuously and stably deliver drugs to the intra-articular cavity to produce the sustained-release effect and avoid the high drug concentration caused by local injection. Fourthly, the MNs may have drug-responsive release functions depending on the intra-articular environment, such as ROS and acid-responsive properties,20 which is conducive to the targeted treatment of RA.21 In addition, the convenience and painless characteristics of MNs administration have high patient compliance. In conclusion, these advantages make it possible for MNs to be used in the clinical treatment of RA.
Although MNs have good advantages and prospects in RA treatment, they are not summarized in detail. (Figure 1) showed different MNs applied in RA. We described the general properties of MNs, materials, preparation technology, drug release mechanism, and advantages. Then we discuss the great contribution of different types of MNs in the treatment of RA. Furthermore, we discussed the biological safety, development prospects, and future challenges of MNs, hoping to provide new thoughts for further investigations of effective RA therapy.
 |
Figure 1 Application of different types of microneedles in RA. |
Fabrication Techniques for MNs
With the continuous development of industrialization, the preparation technology of MNs is also constantly updated. The traditional preparation methods include Microelectromechanical Systems (MEMS),22 Laser cutting,23 Laser Ablation,24 Micro-molding Method,25 Atomization spraying method,26 and Droplet-Born Air Blowing method.27 The most widely used method is Micro-molding. In short, the male mold is prepared by MEMS, and then the male mold is put into the dry PDMS mold to prepare the female mold. The prepared drug solution is poured by centrifugal or vacuum method to prepare the MNs matrix, and the MNs are dried, solidified, and peeled. The prepared PDMS mold can be reused. However, the above methods require the support of expensive equipment and professional and technical personnel, which takes a long time and cumbersome steps, which is not conducive to expanding scale production. Therefore, a simpler and more economical preparation technique is the 3D printing method, also known as Additive Manufacturing. Through computer-aided design and adding materials layer by layer, a fine-designed MNs array can be created. For example, Barnum et al.28 Prepared and characterized 3D printing hydrogel MNs (Figure 2A and B). The results showed that MNs had good mechanical properties and were an outstanding carrier for transdermal drug delivery. The earliest 3D printing technology is Stereolithography (SLA), which uses UV light and digital micromirrors to cure liquid polymers to form MNs through photochemical processes. It can produce MNs with high quality and fine spatial resolution, but it has the disadvantages of slow printing speed, high price and limited printing materials. Another 3D printing technology is digital light processing (DLP). Its preparation process is similar to SLA, which can form photopolymers by layer curing. It has shorter construction time than SLA, less oxygen inhibition, better surface quality, but limited mechanical properties. Fused Deposition Modeling (FDM) is also a 3D printing technology. It has the characteristics of low cost, high speed and simplicity, but the mechanical properties of the prepared MNs is weak, the surface is rough, and the high temperature in the extrusion process also limits its application. Another common 3D printing technique is Two/multi-photon polymerization (TPP/MPP), which has high spatial resolution, but is too slow to build, and the limited materials used will limit its use in industrial MNs production. In conclusion, 3D printing technology has the ability to quickly and accurately customize MNs with complex shapes. Its emergence is conducive to the development of biomedicine and material science, and provides the possibility for rapid and large-scale production of MNs. Table 1 summarizes the different preparation materials and technologies and their advantages and disadvantages.
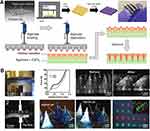 |
Figure 2 (A) Schematic illustration of the fabrication process using 3D printing followed by cleaning with NaOH to remove support material, and actual demonstration of semiflexible MNs arrays. (B) Concept drawing of loading mechanism for filling MNAs. 1. Setup employed for the mechanical testing of MNAs 2. Pig skin insertion testing configuration. Reprinted from Eur J Pharm Biopharm, 82(2), Gomaa YA, Garland MJ, McInnes F, El-Khordagui LK, Wilson C, Donnelly RF. Laser-engineered dissolving microneedles for active transdermal delivery of nadroparin calcium. 299–307, copyright 2012, with permission with Elsevier.23 |
 |
Table 1 Advantages and Disadvantages of Different Microneedles Preparation Technologies and Materials |
Classification, Characteristics, and Drug Release Mechanisms of MNs in RA
MNs consist of small micron-sized needles ranging in length from 50 to 900 µm.44 It has the combined advantages of noninvasive (transdermal delivery) and invasive (injection administration) drug delivery.45 MNs can penetrate the SC (10–40 µm thick) into the epidermis/dermis layer.46–48 It also produces short-term microchannels to help drug penetration, greatly increasing the amount of transdermal drug absorption without stimulating nerves and damaging blood vessels to achieve a painless effect.49,50 It can be classified according to different factors, such as the material prepared by the MNs and the application type of the MNs (diagnosis, treatment, transdermal). According to the morphological classification of the MNs, they can be divided into solid MNs, coated MNs, dissolving MNs, and hollow MNs.51 However, according to previous studies, classification according to morphology and materials is the most common. The different types of MNs and their drug-release behavior are shown in (Figure 3A–E).
Types of MNs
Solid MNs
The concept of solid MNs for drug delivery was first proposed in the 1970s, but it was not until the 1990s that advances in microfabrication technology made it easier to prepare solid MNs.52 Solid MNs are usually made of silicon, glass, ceramic, metal, and nondegradable polymer by wet or dry etching, laser cutting, electropolishing, metal plating, and so on.53 The drug release of solid MNs follows the principles of “poke and patch” or “patch and poke”.54 Simply put, solid MNs are inserted into the skin to create a channel for the drug to penetrate the skin to facilitate drug absorption.54 Then MNs are removed, and the drug is applied to the skin.55 Akmal et al.56 The oscillating solid MNs device Dermapen was applied before and after 5% w/w imiquimod cream. The results showed that the “patch and poke” strategy could greatly enhance the penetration of 5% w/w imiquimod cream, generate an intradermal reservoir within 24h, and enable the continuous intradermal delivery of drugs to deeper tumor lesions. It has been proven that the use of solid MNs to pretreat the skin to produce microscale pores can greatly improve the penetration efficiency of drugs, but solid MNs are prone to breakage during use and transportation, leaving biological residues that cause the risk of infection.57,58 The biocompatibility and safety of the materials used in solid MNs, such as metal and silicon, need to be further investigated.59 Penetration efficiency is affected by the size of pores or densities produced by solid MNs in the skin, and the lipophilicity, particle size, and molecular weight of the drug.60,61
Coated MNs
The preparation materials and methods of coated MNs are similar to solid MNs, and the drug release of coated MNs is carried out by “coating and poking”. The drug solution or dispersion layer is evenly coated on the MNs. After MNs are inserted into the skin, the drug is quickly dissolved and released, and then MNs are removed.62 Pearton et al63 have proven that coated MNs have satisfactory stability by uniformly coating the plasma DNA on steel MNs, and the coated plasmid DNA has good dissolution and permeability from MNs in situ. The expressed gene product can be detected in the local live human epidermis at the MN puncture site. Methods such as dipping or spraying enable the drug to be coated on MNs, and some surfactants can also be used to increase the wettability of the MN surface.64 Coated MNs need to be coated, but some drugs are unsuitable for coating technology, so coated MNs have some limitations in delivering drugs. In addition, Coated MNs require additional complex preparation techniques, and the drug loading is difficult to control, which limits its application.65
Dissolving MNs
Most rapid DMNs have high toughness so that they will not be brittle and fractured when inserted into the skin to avoid harmful sharp waste.11,35,36,66 The preparation process of DMNs is simple and the production conditions are mild, which can ensure the stability of the drug during the preparation process (Figure 4A). Rapid DMNs are highly biocompatible and will not cause serious side effects after insertion into the skin. Also, some molded materials are biodegradable or have the characteristic of rapid solubility (Figure 4B and C). The drugs were prepared into different dosage forms according to their characteristics, such as liposomes,67 nanoparticles, micelles,68 etc., and loaded into DMNs. After the DMNs are inserted into the skin, the drug will be released with the dissolution of the DMNs matrix, so no medical waste will be generated. The dissolution behavior of rapid DMNs is shown in (Figure 4D), enabling the drugs loaded in the rapid solubility MNs to be slowly released as MNs dissolve in the skin, acting as a slow drug release.69 At present, there are many reports that DMNs can be used to treat RA and achieve good therapeutic effects. As a result, rapid DMNs have received increasing attention and are considered the most promising MNs.70 However, rapid DMNs also have some defects. One of the biggest problems is that the mechanical strength of rapid DMNs is not enough. When inserted into the skin, it may be offset by skin elasticity, resulting in needle tip damage and cannot reach the ideal depth of drug release.71 Lin et al72 added hydroxypropyl-β-cyclodextrin (HP-β-CD) to the needle material, sodium hyaluronate (HA). The hydrogen interaction between HP-β-CD and HA restricted the mobility of the molecular chains and subsequently increased the elastic modulus of the complex materials. The mechanical strength of polymer MNs is improved so they can be successfully inserted into the dense and hard scar tissue. In addition, DMNs are easy to break and absorb water during transportation, which will affect its ability to insert into the skin.
Hollow MNs
The drug release mode of hollow MNs is mainly through “poke and flow”. There is a cavity inside hollow MNs for drug solution or drug dispersion to enter. After MNs are inserted into the skin, the drug solution is driven by pressure to flow from the cavity above hollow MNs into the cavity and then into the skin, similar to subcutaneous (s.c) injection.73 Therefore, devices that control flow and velocity can be used to maintain a constant flow rate to control the drugs entering the skin and drug release time, which is beneficial to clinical safety. The main advantage of hollow MNs over the previous three MNs is that they deliver the drug solution directly to the skin, without the need for additional drying, and can be applied to heat-labile drugs, such as a vaccine, siRNA, Biological agents, etc.74 Ma et al17 developed hollow MNs that mimic the activities of mosquitoes based on the bionic principle, and the results showed good skin penetration. Vivek et al75 used 3D printing technology to prepare hollow MNs, and successfully delivered rifampicin, an antibiotic with gastrointestinal side effects and severe liver toxicity, into isolated pig skin. The bioavailability study of rodents showed that rifampicin could effectively penetrate through hollow MNs. However, the preparation of hollow MNs is more complicated than other types of MNs due to their cavity structure.39 The hollow structure of hollow MNs may result in the risk of fracture if MNs are not mechanically strong enough to be inserted into the skin. The length of hollow MNs used for tissue sampling or bio-signal monitoring is at least 1500 μm, enabling them to reach effective skin depth.76 However, the high aspect ratio of MNs means that MNs are more difficult to prepare and more prone to fracture. Therefore, expensive manufacturing equipment and hollow MNs inserted into dense skin may cause obstruction, which will limit its clinical application.
Hydrogel MNs
The hydrogel formation of MNs was first reported in 2012. Hydrogel MNs act as a needle to penetrate the SC and open the skin channel. After entering the skin, hydrogel MNs act as a rate-controlled membrane to slowly release the drug,77 and hydrogel MNs can be completely peeled off from the skin. Compared to solid MNs, hydrogel MNs will not break and leave residues, causing the risk of biological infection, which has better biocompatibility.78 Hydrogel MNs can not only deliver drugs but also absorb tissue permeate to help wound healing due to their strong water absorption effect, a feature that other types of MNs do not have. At present, hydrogel MNs have applications in antibody/drug delivery, skin diseases, and wound healing. For example, oral metformin, a large side-effect of oral administration, was used to study the transdermal drug delivery of MNs.79 The results showed that MNs are used to enhance the permeability and bioavailability of drugs and reduce the side effects of oral administration. However, hydrogel MNs also have some limitations. Hydrogel MNs slowly dissolve in the body after insertion into the skin, making the absorption of drugs in the plasma slower than oral administration, and the release of hydrogel MNs in vivo is a sudden release followed by an increase in swelling followed by a slow release. The sudden release of hydrogel MNs may result in the risk of poisoning due to high blood concentration.80 Because of long-term swelling and release of hydrogel MNs in the body, hydrogel MNs used should be sterile and have a high degree of biocompatibility. Otherwise, there is a risk of tissue damage and infection.78
Characteristics of MNs
From preparation to applications, MNs need to be comprehensively evaluated by a series of tests. Through the data continuously optimize the preparation of prescriptions, to screen out suitable materials for different drugs to ensure their safety and effectiveness. To promote the transformation of MNs from experimental research to clinical application products, the common characterization methods are as follows:
Morphological Characterization of MNs
Through the optical imaging system, the shape of the MNs, the dissolution after penetrating the skin, and the penetration depth of the skin can be characterized. The shape, height, spacing, aspect ratio, and an array of MNs are characterized by electron microscopy. A scanning electron microscope can observe the three-dimensional structure of the MNs.81 In addition, Rhodamine B, coumarin-6, and other fluorescent chromogenic agents were loaded into the MNs as probes.82 Scan the direction of the Z-axis under the laser scanning confocal microscopy, and reconstruct the three-dimensional form structure through the software. We can analyze the fluorescent strength and depth of the drug penetrating through the MNs. The above instrument is simple to operate. It can be used to evaluate whether the MNs are formed or sharp.
Mechanical Property Test of MNs
As a new transdermal drug delivery system, MNs need to overcome the stratum corneum (SC) barrier to play an effective role. Therefore, it must have sufficient mechanical properties. 0.098 N/needle is the minimum force for the MNs to penetrate the SC.83 The traditional method is to test the breaking force of the axial needle. The tip of the MNs is installed upward on the probe of the texture analyzer, and it is lowered to the flat metal block at a constant speed.84 The force-displacement curve is obtained, and the inflection of the curve is the MNs breaking force. However, this method also has some limitations. For example, the tips of the MNs cannot all break at the same time, and the fracture force obtained is not accurate enough. In addition, a universal testing machine, texture analyzer, dynamometer, force-displacement machine, etc. can also characterize the mechanical properties of the MNs.85 Du et al66 used a nanoindenter to measure the amount of the MNs Yang’s modulus. By using a spherical probe with a diameter of 56 μm, it was measured at a constant speed of 10μm/s. The results showed that Young’s modulus of the MNs was as high as 60.66 mpa. The nanoindenter can control more accurate displacement and capture more fine changes in the MNs tips, but this method is long and expensive. Therefore, it is suggested to comprehensively evaluate the force of the MNs by combining a variety of mechanical strength test methods.
Evaluation of Puncture Performance of MNs
The puncture performance evaluation of the MNs is the key to a successful application. At present, there are many methods to evaluate the puncture performance of the MNs. First, we can simply and quickly judge whether the MNs are successfully inserted by dyeing, Trans-epidermal water loss (TEWL), and resistance method.86 At present, the most commonly used dyes are water-soluble dyes such as trypan blue, methylene trypan blue, and rhodamine B. Pearton63 et al used methylene blue solution to dye and observe the isolated human skin after MNs puncture. It was found that with the increase of MNs force, the whole effect was more obvious. TEWL is a non-invasive, highly sensitive, and real-time method to understand skin water loss. When the MNs are inserted into the skin, they will destroy the SC barrier, and the microchannels will increase the TEWL value. When the channel is closed, the TEWL will return to the baseline value. Wang et al.87 The MNs prepared from Panax notoginseng polysaccharide were inserted into the skin of rats, and the TEWL values increased continuously for 10 minutes, which indicated that the MNs of Panax notoginseng polysaccharide had good skin permeability. However, the TEWL value will be affected by many factors, such as temperature, humidity, skin conditions, and so on. The resistance method is to measure the changes in the electrical impedance of the SC before and after MNs application. The above methods can only indirectly evaluate the puncture ability of the MNs, but cannot detect the puncture depth of the MNs. Histological sections, confocal images, and Optical coherence tomography (OCT) can detect the puncture depth of the MNs. Histological sections are a complex method to prove the depth of the MNs puncture.88 The skin applied by the MNs is paraffin-embedded and dyed to observe the channel depth after the MNs penetration. However, because of the tension and pressure on the skin during the process of fixing and slicing, the original shape of the MNs penetrating the skin is changed. The stimulation of the dye will cause the skin to shrink and make the pores smaller. The fluorescent dye was loaded into the MNs and the penetration depth of fluorescence was observed under a CLSM. For example, Du et al.82 Inserted the MNs loaded with rhodamine 6G into the isolated pig skin for 3 min, and observed under the CLSM, it was found that the penetration depth was as high as 170 μm. This is a non-invasive method that can be used in vitro/vivo, but it can also lead to errors due to the gradual diffusion of fluorescent dyes. The OCT is thought to be a lateral imaging technique that provides the SC, the active epidermis, and the upper dermis. It has a visual depth of up to 2000 μm and provides non-invasive, real-time depth observations. Therefore, different methods can be selected to evaluate the puncture performance of the MNs.
Skin Permeability of MNs
The skin permeability of the MNs was determined by Franz transdermal diffusion method and high-performance liquid chromatography.81 The MNs were inserted into the isolated animal skin, and then the skin was placed in the receiving chamber. The receiving liquid after ultrasonic treatment is added to the receiving pool, and there is no air bubble in the middle. Place the above device in a constant temperature water bath at 37 °C ± 0.5 °C and stir it. Collect samples at a fixed time point to characterize the permeability of the MNs by analyzing the content of the drugs diffused to the receiving chamber. This method has the advantages of simple operation, low cost, and easy repetition. Different skin models can be used to meet the requirements of different MNs. However, the in vitro skin tissue does not contain the subcutaneous layer and cannot truly simulate the inner environment of the skin, and the composition of the receiving liquid is also different from the human body fluid. The optical resolution-optical sound microscope can be used to dynamically characterize the MNs, including insertion depth, and drug release curve, which is a more accurate method.
Drug Release Mechanisms of Microneedle in RA
Different types of MNs have different drug release mechanisms. Table 2 summarizes the advantages and disadvantages of different MNs. The choice of MNs release mechanism depends on drug characteristics and disease types.
 |
Table 2 Advantages and Disadvantages of Different Microneedle Drug Release Mechanisms |
Table 2 Advantages and disadvantages of different microneedle drug release mechanisms
Advantages of MNs in RA
Minimally Invasive and Pain
Patients with RA suffer from joint pain for a long time, and traditional drug delivery methods, such as injection, bring another kind of stimulation to the patient and increase the pain of the patient.93 MNs are a new form of transdermal delivery that delivers drugs to the joints of patients, and because MNs are usually no larger than 800 μm in size, they do not cause pain.94 In addition, the tiny channel created by the MNs will gradually recover after the MNs are removed. The ability of microorganisms to enter the body through the hole formed by the needle tip is low, the risk of bleeding is low, and the risk of infection for patients is reduced. The emergence of MNs avoids people’s dependence on traditional needles and reduces the risk of occupational exposure for medical staff. For example, for RA patients who are afraid of long metal needles, the appearance of MNs will not make them feel fear and improve their compliance.95
Maintain Drug Activity
MNs are an effective carrier for RA, whether the drug can exist stably and maintain effective drug activity is a question worthy of consideration. Previously, there were articles about MNs loaded with vaccines or insulin and other macromolecules.26,96,97 The original activities of vaccine and insulin in MNs were not destroyed, showing similar immune regulation response and blood sugar lowering as subcutaneous injections. Cao et al84 loaded the cryopreserved biological agent etanercept into HA-MNs and cross-linked under ultraviolet light. The secondary structure of etanercept was not destroyed by circular dichroism analysis. The results showed that the etanercept could maintain its original structure and biological activity in the MNs without cold storage. MNs can solve the limitations of traditional injection drug delivery and reduce the cost of refrigerated storage and cold chain transportation. In conclusion, MNs present a great potential for future application of transdermal delivery of RA.
Convenience
As a transdermal patch, MNs are simple to use and easy to operate patient self-administration via MNs is possible or assist others to administer drugs, such as the elderly and children, without the personal operation of professional doctors or nurses. The self-administration of MNs avoids the anxiety and fear of patients, which helps alleviate autoimmune diseases. Patients with RA can self-administer MNs at the time recommended by their doctor, and if any discomfort occurs, the MNs can be removed immediately to interrupt the administration, avoiding a build-up of drugs that can cause side effects. The time for patients to self-administer medication can be more flexible than the time for receiving treatment from doctors and nurses in the hospital, which saves patients’ waiting time and reduces the medical burden from another perspective.98
In addition to the convenience of use, MNs can also reduce administration times, which has also brought convenience to the treatment of patients with RA. MNs is not simply a substitute for subcutaneous injection but has a certain sustained-release function, which can release drugs for the RA in a long-term and stable manner. For example, Du et al95 cross-linked HA with methacrylate to obtain Me-HA. Compared with MNs prepared with ordinary HA, the prepared Me-HA MN can release 56% of the drug within 10 min and continuously release the drug until 480min. These results indicate that MNs have good sustained release performance and can reduce the number of drug administration and toxicity. In addition to the sustained-release function of the material, MNs can also be loaded with slow-release drugs, such as the preparation of anti-inflammatory drugs into liposomes, MNs can effectively accumulate at the site of administration. After the MNs are removed, the drug can be released stably for a long time, reducing the frequency of administration to a certain extent. As a chronic inflammatory disease, RA requires multiple long-term administrations to achieve the purpose of controlling the symptoms of the disease. Therefore, there is an urgent need to develop a sustained-release preparation that can reduce the number of administrations to increase patient compliance. MNs are a promising drug carrier for the treatment of RA because it can achieve sustained drug release.
MN Therapy in RA
RA is an autoimmune disease with chronic polyarticular disease as the main clinical manifestation. In the past, the treatment of RA was mainly based on oral or intra-articular injection of drugs. However, oral drug administration has the disadvantages of low bioavailability and high side effects, which limits the clinical application of oral drug administration; in addition, joint cavity injection also brings pain and the risk of infection, which increases the psychological pressure of patients. As a new transdermal drug delivery technology, MNs can make the drug reach the joint cavity directly through the joint skin, and make the drug gather in the lesion with relatively high concentration, which overcomes the systemic side effects of traditional drug delivery and improves the bioavailability of the drug. In addition, MNs delivery has the advantages of being minimally invasive and non-invasive to skin tissue, which improves patient compliance. Therefore, to address the clinical problems of RA, such as symmetry and multiplicity, the dosage form of MNs can be improved to solve the problems of multiplicity and reflect the value of MNs drug delivery. Table 3 A summary of MNs towards RA therapy.
 |
Table 3 A Summary of MNs Towards RA Therapy |
Solid and Coated MNs Therapy in RA
Solid MNs generally do not directly carry drugs, and release drugs by “poke and patch”. It has strong enough mechanical properties to penetrate the SC to produce microchannels. The application of Solid MNs in RA mainly improves the transdermal absorption of insoluble drugs and bioavailability by enhancing skin penetration.
Liposome, an amphiphilic structure with a phospholipid bilayer, can improve solubility and reduce the toxicity of drugs. The surface of the liposome can also be targeted after modification.117 For example, Jia et al118 developed a new dexamethasone-loaded liposome (Dex-Lip) to treat adjuvant-induced RA in rats. Compared with free dexamethasone, the Dex-Lip can specifically target inflammatory tissue, and the aggregation at the inflammatory site can be up to 72 h, which can effectively reduce the level of inflammatory factors in serum and improve the hyperglycemia caused by free drugs. Intravenous liposomes are effective, but there are inevitable side effects due to systemic circulation. The combination of liposomes and MNs can avoid the influence of conventional drug delivery and enhance the therapeutic effect of the liposome. Chen et al100 prepared a triptolide (TP)-loaded liposome hydrogel patch (TP-LHP). TPL is a diterpenoid trioxide isolated from Tripterygium wilfordii Hook F.119 It has good anti-inflammatory activity. TP-LHP has a stable and long-term release and can avoid first-pass elimination and adverse reactions in the digestive tract. The plasma concentration of TP-LHP mediated by MNs was higher than oral administration, and the maintenance time was as high as 30 h. Pharmacodynamic experiments in rats with collagen-induced arthritis showed that MNs could reduce joint swelling and serum IL-1β and IL-6 levels by regulating the balance of Th1 and Th2 pathways. In conclusion, TP-LHP combined with MNs technology can provide a safe and effective method of administration for RA treatment.
Guo et al99 used total alkaloids isolated from Aconitum sinomontanum to prepare nanostructured lipid carriers (AAS-NLCs). Fluorescence imaging confirmed that MNs could provide a channel through the SC, making AAS-NLCs penetrate deeper. In vivo studies have shown that the combination of MNs and NLC can improve the bioavailability of AAS. The in vitro percutaneous penetration of AAS-NLCs mediated by MNs was higher than other drug delivery methods. Also, AAS-NLC-MNs can eliminate foot swelling, relieve inflammation and pain, and regulate immune function in adjuvant arthritis rats. After administration of AAS-NLC-MNs, rabbit skin showed no irritation, and the arrhythmia symptoms of rats were improved. This work showed that the AAS-NLC-MN transdermal delivery method could provide more effective and safer drug delivery and improve the therapeutic effect of AAS sustained release.
Paeoniflorin is the main active component of total glucosides of paeony (TGP). Cui et al101 prepared paeoniflorin with anti-inflammatory, anti-ulcer, analgesic, and sedative properties into ethosomes and used MNs to assist the penetration of paeoniflorin through the skin. The best auxiliary condition is when the length of MNs is 500 μm, the pressure is 3 N, and the action time is 3 min. Studies have shown that the transdermal amount of TGP solution alone is 24.42 ± 8.35 µg/cm², whereas the transdermal amount of MN-assisted TGP solution is 548.11 ± 10.49 µg/cm². Therefore, MNs can enhance the percutaneous penetration of paeoniflorin. The amount of TGP-E is 54.97 ± 4.72 µg/cm², whereas the transdermal amount of MN-assisted TGP-E is 307.17 ± 26.36 µg/cm². This work showed us that both liposomes and MNs can assist the percutaneous penetration of TGP, but the MNs-mediated penetration is more dramatic.
SiRNA drugs have excellent targeting and specificity and have achieved good efficacy in the application of RA. However, researchers found a lack of suitable drug carriers in the process of drug development. The emergence of MNs has given researchers hope. For example, Rosalind et al116 used coated MNs to deliver siRNA by coating siRNA on MNs so that MNs could be loaded with up to 40 μg siRNA. The in vitro skin penetration test showed that the coating dissolved rapidly and only left residual fluorescence on the surface of the needle. Recovery and quantification of siRNA from MNs after insertion into mouse model skin showed that 50% to 85% of the coated siRNA was deposited in mouse paws and had good bioavailability. The drug solution can be repeatedly coated on coated MNs to improve the loading of MNs. This makes it possible for MNs to deliver enough siRNA to treat RA.
In conclusion, solid MNs and coated MNs are increasingly used in RA. Researchers hope that through the use of MNs, the penetration of anti-inflammatory drugs can be enhanced so that the drugs can reach the synovial tissue as soon as possible, reduce inflammation, and alleviate pain.
Hydrogel-Forming MN Therapy in RA
Methotrexate (MTX) is an immunosuppressant, which is currently the first-line drug for the treatment of RA.120,121 The recommended dose of MTX is 7.5–25 mg per week.122,123 The commonly used method of administration is an oral and subcutaneous injection (SC). Although oral MTX can achieve a good curative effect, gastrointestinal side effects such as nausea and vomiting are unacceptable SC will bring pain to patients and lead to high blood concentration.120,124,125 The emergence of MNs provides a new route of drug delivery. At first, people used the micro-channels generated by the solid MNs to help the penetration of MTX.126 However, in the application process, it was found that the steps of this method were complicated to control the dose. The microchannels produced by solid MNs are easy to close, resulting in the reduction of drug penetration. Hydrogel-forming MNs are composed of crosslinked polymers. After being inserted into the skin, the hydrogel-forming MNs can absorb the interstitial fluid of the skin and swell, forming a continuous channel between the attached drug reservoir and the skin microcirculation. Therefore, a large number of drugs can be continuously delivered through the drug reservoir. Moreover, it is easy to remove from the skin and does not produce sharp waste. Therefore, people began to try to deliver MTX with Hydrogel-forming MN.
Tekko et al114 designed a hydrogel MN array prepared by heat cross-linking of PVA/PVP blend and calcium combined with a patch-like reservoir equipped with MTX. The results showed that the Cmax of the patch group was lower than the oral group, but the AUC0-48 of the transdermal group was 1.2 times higher than the oral group. The maximum concentration (Cmax = 57.4 ± 20.0 nM) was reached in the oral group in the first hour and then decreased gradually, whereas the blood concentration in the integrated patch group increased gradually and reached a peak at 24 h (Cmax = 35.1 ± 5.1 nM). This showed that MTX administered via the patch could maintain a steady-state blood drug concentration for a long time without reaching a high peak concentration, which reduces the toxicity caused by drug aggregation. Because of MTX cytotoxicity, in vitro insertion experiments showed that at 0.5 h after removing the MN patch, a hole formed in the skin can be observed, and the hole completely disappeared after 2 h. Slight erythema was observed only 0.5 h after insertion and then disappeared without adverse skin irritation or local cytotoxicity. In conclusion, this work provides a promising minimally invasive transdermal drug delivery system that can continuously deliver MTX within 24 h. It avoids systemic adverse reactions and can better control the dose, which provides a new strategy for the treatment of RA.
Compared with traditional RA drugs, aptamers have better targeting and therapeutic effects, while being relatively safe and low immunogenic. The abnormality of DEK protein is related to the occurrence of RA. Blocking DEK by intra-articular injection of aptamer DTA has a good anti-inflammatory effect. Cao et al115 developed a methoxy-modified DTA and loaded it into hydrogel microneedles (hMN) based on HA (Figure 5A). DTA was delivered by hMN, which avoids the pain caused by intra-articular injection. hMN by dehydration reduces the exposure time of DTA at room temperature, avoids waste, and inactivates DTA. In vivo, the anti-inflammatory effect showed that hMN could achieve similar or better efficacy than intravenous injection (Figure 5B and C).
The above research shows that Hydrogel-forming MN can be used as a good drug carrier for the treatment of RA. When Hydrogel-forming MN is inserted into the skin, the highly hydrophilic needle will absorb the liquid fluid of the skin and swell. The defect of limited drug loading of MNs was improved by adding a drug reservoir. After the drug is released, Hydrogel-forming MN can be completely removed from the skin without leaving any residues, which solves the concerns of biological safety. In addition, Hydrogel-forming MN does not need to dry for a long time during the preparation process, which is conducive to transporting drugs that are unstable in heat, such as protein, and DNA. In conclusion, Hydrogel-forming MN is expected to become a new way to treat RA instead of intravenous administration.
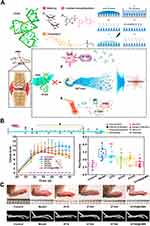 |
Figure 5 (A) Schematic illustration of the present study. (B) In vivo study of aptamers in CIA mice. (C) Mouse paw images at the end of treatments and MicroCT scanning images of mouse paws after the listed treatments on day 50. (*p<0.05, **p<0.01, ***p<0.001, and ns means no significant difference). Reprinted in part with permission from Cao J, Su J, An M, et al. Novel DEK-targeting aptamer delivered by a hydrogel microneedle attenuates collagen-induced arthritis. Mol Pharm. 2021;18(1):305–316.115 Copyright 2020 American Chemical Society. |
Dissolving MN Therapy in RA
NSAIDs
Non-steroidal anti-inflammatory drugs (NSAIDs) are widely used for RA treatment. It can reduce pain and inflammation and has clear targets and activity. Although these drugs have low toxicity, frequent oral administration will bring serious gastrointestinal side effects, such as a gastric ulcer. Therefore, it is necessary to find a new administration method for NSAIDs. Amodwala et al102 prepared meloxicam (NSAIDs with good molecular weight, therapeutic dose, and biological half-life) into rapid DMN patches. The results showed that MNs could dissolve rapidly and release nearly 100% of the drug within 60 min at a PVA/PVP ratio of 9:1. Compared to the transdermal delivery of drug solution alone in their study, the transdermal flux of MN-mediated meloxicam increased by 1.60 μg/cm2/h, the permeability increased by 2.58 times, and the deposition rate was 63.37%. It has the same anti-inflammatory activity as the existing approved oral preparations. In another experiment, Chen et al103 prepared meloxicam into tip-dissolving (TD)MNs. The results showed that the rapid and soluble MNs could achieve explosive drug release, releasing 91.72% of meloxicam in 30 min, delivering the drug to the skin effectively (79.18%), and having a good bioavailability (122.3%). Compared to traditional oral administration, it showed considerable anti-inflammatory and analgesic activities.x
Another example of combination therapy is Lu et al,127 who developed a dissolving MNs delivery system with photothermal properties of polydopamine to co-deliver the NSAID loxoprofen and the Janus kinase inhibitor tofacitinib, which assisted in co-delivering loxoprofen and tofacitinib directly into the articular cavity through a combination of MNs delivery and photothermal therapy (Figure 6A). In addition, MNs significantly facilitate drug penetration and retention in the skin and can promote drug retention in the joint cavity (Figures 6B and C). Compared with direct injection of loxoprofen and tofacitinib into the joint cavity, MNs delivery of the drug more significantly reduced pathological manifestations such as joint swelling, muscle atrophy, and cartilage destruction in rats with RA (Figure 6D).
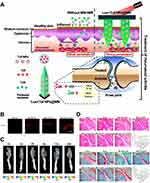 |
Figure 6 (A) Schematic Illustration of the Co-Delivery of Loxoprofen and Tofacitinib by Photothermal MNs for RA Treatment. (B) Visualization of RhB NPs’ permeation in the skin from different formations with CLSM in vivo. (C) In vivo images of RhB retention in the articular with the time up to 24 h after MNs administration with light irradiation. (D) Histological features of knee joint stained with HE and safranin O-fast green. (magnification 100×; scale bar: 1000 µm). (*p<0.05). Reprinted in part with permission from Lu Y, Xiao T, Lai R, et al. Co-delivery of loxoprofen and tofacitinib by photothermal microneedles for rheumatoid arthritis treatment. Pharmaceutics. 2023;15(5):1500.127 Copyright 2023 MDPI. |
DMARDs
Although the current pathogenesis of RA is not clear, studies have shown that oxidative stress plays an important role in the pathogenesis of RA.128,129 Antioxidants can delay the disease process of RA.130 Wu et al.96 Encapsulated MTX and reactive oxygen species (ROS) scavengers (polydopamine/manganese dioxide, termed PDA@MnO2) in DMNs to treat RA (Figure 7A). PDA@MNO2 can quickly reach joint inflammatory parts through the channel generated by DMNs to remove ROS and inhibit inflammation reactions. (Figure 7B) showed that the DMNs had good needle shape and sharp tips. (Figure 7C) showed that the penetration depth of the DMNs can reach 200μm, which is conducive to drug penetration. In addition, the MTX-loaded DMNs group also showed better anti-inflammatory effects than the oral MTX Group (Figure 7D). This work provides a valuable method of DMNs-assisted transdermal drug delivery, and the combination of chemotherapy and antioxidation opens up a new idea for the treatment of RA.
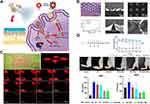 |
Figure 7 (A) Schematic Illustration of the Synthesis of a High-Performance Nanozyme of PDA@MnO2 and the Fabricated Microneedles Successfully Delivered Drugs with Nanocomposites, As Shown by Results in Paw Swelling, MRI Image, and the Level of Cytokines. (B) Characteristics of MNs (C) Skin Penetration in vitro and in vivo. (D) Therapeutic Effects of MNs. (**p<0.01,***p<0.001). Reprinted in part with permission from Wu C, Cheng J, Li W, Yang L, Dong H, Zhang X. Programmable polymeric microneedles for combined chemotherapy and antioxidative treatment of rheumatoid arthritis. ACS Appl Mater Interfaces. 2021;13(46):55559–55568.96 Copyright 2020 American Chemical Society. |
TNF-α is a kind of inflammatory factor secreted by activated monocyte macrophages. Excessive expression of TNF-α plays an important role in the inflammation response of RA, such as promoting synovial hyperplasia and the expression of other inflammatory factors. Etanercept (EN) is a widely used TNF-α inhibitor that can effectively improve the symptoms of patients with RA and delay the progression of RA. However, as a biological agent, the EN administration pathway is limited to subcutaneous (s.c) injection, which will bring pain and increase the risk of infection. Therefore, people began to consider loading EN into DMNs for transdermal administration. Cao et al104 used HA as a DMN material to deliver EN transdermal to the body. HA MNs crosslinked by ultraviolet light have high biocompatibility, bioequivalence, and good mechanical strength. Experiments on mouse skin insertion have shown that MNs can be inserted at 200 µm and have a good drug delivery depth. In vivo experiments in adjuvant-induced arthritis mice showed that, within 10 days, mice were treated with EN via s.c. injection and EN in MNs. The paw swelling rate was reduced from 1.70 to 1.48 and 1.68 to 1.44, respectively. The results of this study showed that DMNs can maintain the good activity of macromolecular drugs and pass them to the joint cavity. The systemic side effects caused by the lack of good targeting ability of biomacromolecules can be avoided by transdermal delivery, which brings new hope for the treatment of RA.
Polypeptides
Yao et al105 prepared DMNs loaded with a neurotoxin (NT: a protein drug) through a two-step centrifugation method. The upper part of each needle is loaded with 15.4 ± 0.5 μg drug, and the prepared DMNs have good mechanical strength and biocompatibility as well as low cytotoxicity. The depth of the skin inserted into the rat can reach 70 μm, and the cumulative penetration of the drug can reach 95.8% within 4 h, but the NT solution cannot penetrate the skin. Pharmacodynamic studies showed that DMNs-NT reduced the production of TNF-α and IL-1β, and inhibited the swelling of joints in RA rats. In conclusion, this work proves that DMNs have great potential to deliver NT, which can provide a more comfortable treatment for RA.
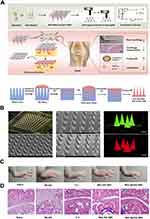 |
Figure 8 (A) Scheme of microneedle-mediated delivery of melittin for RA treatment. (B) Scheme of the micro-molding method for fabrication of microneedles. (C) Morphological characterization of MNs. (D) Representative images of paws from different groups. And H&E staining of mice paws. Scale bar: 400 μm. Reprinted from J Control Release, 336, Du G, He P, Zhao J, et al. Polymeric microneedle-mediated transdermal delivery of melittin for rheumatoid arthritis treatment. 537–548, copyright 2021, with permission from Elsevier.18 |
Du et al18 fabricated melittin-loaded HA-MNs (Figure 8A), MNs consist of 10×10 needles with a needle length of 700 μm (Figure 8B). After modifying HA with cross-linkable groups, the fabricated MNs with sustained release properties could further improve the therapeutic potency. In vivo and in vitro studies have proved that the MNs have a longer drug release than the injection, and the sustained release of melittin shows the potential to further improve the efficacy and reduce the frequency of administration (Figure 8C and D). Cytokine and T cell analysis in the paws and lymphatic organs indicated that the application of MNs suppressed the levels of pro-inflammation cytokines including IL-17 and TNF-α, and increased the percentage of regulatory CD4 T cells. This research lays the foundation for non-invasive and efficient treatment strategies for RA and other autoimmune diseases.
Natural Small-Molecule Compounds
To reduce the side effects of chemical therapy for RA, more and more attention has been paid to natural small-molecule compounds, and more and more small-molecule compounds have been used in RA treatment. Natural medicines can play a good therapeutic role in the advanced and remission phases of RA and have a synergistic effect of multiple targets and multiple pathways to reduce the side effects caused by chemicals.
Hu et al112 designed PEGylated star-shaped PLGA, which hybridized with calcium carbonate from nanoparticles [6s-NPs (CaCO3)]. The nanoparticles improve the low drug-loading and permeability of PLGA and have the characteristics of immune stealth and acid-responsive properties. The bis benzylisoquinoline alkaloid tetrandrine (Tet) isolated from Sinomenium acutum (Thumb) Rehd et wils has been widely used in the clinical treatment of RA. However, Tet has some disadvantages such as poor solubility and difficulty to be absorbed by oral administration. Therefore, Wu et al. Loaded Tet into [6s-NPs (CaCO3)], and the drug loading capacity increased by 3.26 times compared with single-chain PLGA nanoparticles. To improve the permeability of nanoparticles, nanoparticles were loaded into DMNs prepared from peach gum polysaccharides. Compared with the traditional HA DMNs, the DMNs prepared from peach gum polysaccharides have better stability and higher mechanical strength. This integrated transdermal delivery system enhanced the absorption of Tet by synovium, and stronger downregulation of VEGF, JAK2/p-JAK2, and STAT3/p-STAT3 pathways was observed in adjuvant-induced arthritis rats. In conclusion, this work provides a new strategy for the targeted painless treatment of RA.
Sinomenine (SIN), an anti-inflammatory alkaloid derived from the plant, is already available in China in tablets and injections for the treatment of RA. Shu et al109 prepared sinomenine hydrochloride-loaded MNs (SH-MN) by casting method with PVP and chondroitin sulfate (CS). In vitro permeation studies showed that the cumulative permeability and penetration rate of SH-MN were 5.31 and 5.06 times higher than that of SH-gel (g). Pharmacological studies showed that SH-MN could dissolve completely in 4 h, and the drug diffuses through the channel to a depth of 200 μm. The area under the curve entering the skin and blood is 1.43 and 1.63 times higher than SH-gel (g), respectively. These studies suggested that MNs are a good alternative method to overcome the defects of SH administration.
Clinical Trials of MNs in RA Treatment
MNs, as a novel technique for transdermal drug delivery systems, have been developed and innovated in recent years in the hope of breaking through the limitations that exist in conventional RA treatments. However, there are not a large number of clinical trials devoted to the study, therefore, only a very small amount of literature is available as evidence. A clinical trial on the status of adalimumab MNs in healthy volunteers was conducted by the Dutch Centre for Human Analogues Research (ID NCT 03607903). Adalimumab, a highly effective treatment for a variety of autoimmune disorders, has drawbacks such as poor patient adherence as it is mainly administered via subcutaneous injection. MNs, with features such as being minimally invasive, have emerged as a potential alternative to injectable drug delivery. This trial performed MNs administration and subcutaneous injection administration in healthy volunteers and assessed MNs administration by comparing the pain, acceptability, and local tolerance to assess the safety of MNs administration. This study also included a comparison of adalimumab used for the treatment of RA in the two groups, ie, one group intervened with MNs as the intradermal route, and the other group intervened with the subcutaneous route of administration of the same drug. Saline was used as a control for both types of drug delivery. To demonstrate the effectiveness of MN against a single dose of 40 mg SC invasive drug delivery for the treatment of RA.131
Safety Evaluation
In the past few decades, MNs transdermal delivery systems have emerged as a promising drug delivery strategy. In the treatment of many diseases (such as cancer, psoriasis, and diabetes), it has achieved good therapeutic effects. MNs are a good substitute for oral and intravenous administration. Therefore, it is necessary to evaluate the safety of MNs for drug delivery so that MNs can be used in clinical practice. Matrix materials for MNs should have good biocompatibility, biodegradability, and safety, which are very important for the clinical application of MNs. Polyvinyl alcohol, polylactic acid, and sodium carboxymethylcellulose are frequently utilized MNs matrix materials that all have strong biocompatibility and are frequently employed for drug delivery in medical facilities. Sugars, including hyaluronic acid, chitosan, and dextran, among many others, are the major materials that are used to produce dissolving MNs.132 Hyaluronic acid has been extensively studied in drug delivery applications133 and is recognized as biocompatible and safe. In addition, MNs also have an excellent safety performance for skin tissues. Studies have demonstrated19 that by inserting hyaluronic acid MNs patches into the dorsal skin of mice by pressure and removing them, it can be found that no significant skin damage was observed at the site where the MNs were applied, and the MNs could be clearly observed after application, but became invisible within 10 min and disappeared completely after 30 min. H&E staining results showed that There was no obvious inflammatory cell infiltration or pathophysiological reaction in the skin tissue. Shengbo Li et al134 verified the cytotoxicity of the MNs in HACAT and EC by using CCK-8 and Calcium xanthophyll-AM/PI. The experiments demonstrated that cell viability was maintained at more than 95% in all the MNs groups, which indicated that MNs have good biocompatibility. In addition, the haemolysis experiments showed that the MNs also had superior haemocompatibility. In the in vivo compatibility evaluation, the major organs of the mice in the administered MNs group were collected and evaluated by H&E staining, and there were no obvious inflammatory lesions or tissue damage. These showed that the MNs materials have safety and excellent biocompatibility. Chen B Z et al135 prepared different types of poly(lactic acid) (PLA) MNs using the mold method, applied MNs of different specifications and sizes to different parts of 18 participants, and investigated the safety of MNs in the human body through local skin irritation and pain sensation. The study showed that the different sizes of MNs caused less pain than 30-gauge hypodermic needles, the levels of pain sensation and skin irritation showed a strong dependence on the size of the MNs, and the different sizes of MNs were well tolerated and safe when applied to different skin sites in humans.
Conclusions and Future Perspectives
As a chronic inflammatory disease, RA is difficult to cure. At present, most clinical drugs are mainly for controlling symptoms, reducing pain, and protecting cartilage. The commonly used way of administration is oral and joint injection, and there are many inevitable defects. While studying new RA drugs, it is of great significance to develop new ways of administration to improve drug efficacy, reduce toxic and side effects, and improve patient compliance. Nowadays, the emergence of transdermal drug delivery systems, such as MNs (dual advantages with non-invasive and invasive ways), can avoid the first elimination obstacles, showing unique advantages in the treatment of RA. First, MNs can painlessly deliver drugs to the joints, reducing the waste and use of drugs, and avoiding the side effects of drugs to a certain extent. The second MNs can play a slow release role and improve the defect that RA, as a chronic inflammatory disease, needs to be frequently administered for a long time. Third, the MNs can carry several drugs with different effects at the same time, which can play the role of cartilage repair while anti-inflammatory and analgesic. MNs can maintain good drug-targeted release. In addition to the drugs, MNs can also deliver micelles or nano-carriers containing drugs, thereby increasing drug load or having a responsive release function. This combined administration has great significance for the treatment of RA.
Although MNs have good advantages, there are still some problems that have attracted attention. (1) At present, most experiments are carried out on rats, and there are still great differences between human skin and animal skin. (2) The mechanical strength and anti-fracture of MNs need to be improved. (3) The drug release rate of MNs was mainly in vitro, but the drug release rate of MNs in humans needs to be further verified. (4) RA is a systemic joint disease, the size of different joints and the age of patients will also affect the parameters of MNs, such as size, shape, and mechanism. Therefore, it is necessary to establish a standard guide to better guide RA applications to the clinic. (5) As a transdermal preparation, MNs should be sterile after insertion into the skin to avoid infection. (6) At present, there are many kinds of materials for preparing MNs tips, such as HA, PVA, and some natural polysaccharides, such as BSP, and Panax notoginseng polysaccharides. At present, there is no clear provision for MNs materials. Researchers should look for safer and better biocompatible materials. Despite these challenges, MNs have great potential in the treatment of RA. As a new transdermal drug delivery agent, MNs have been used to treat many diseases, such as cancer, diabetes, skin diseases, etc. In the context of scientific and technological progress, the technology of MNs is also in progress. New types of MNs, such as frozen MNs, can better maintain the activity of biological agents and also deliver live cells to the skin. New preparation technologies, such as 3D printing technology, can design the size and type of MNs more freely and flexibly, to better fit the skin, enhance the convenience of drug delivery, and improve the accuracy of drug delivery. The combination of cross-research and inspiration from the disciplines of physical chemistry, biomedicine, and material science is conducive to obtaining lower-cost, safer, and better-performance MNs for the treatment of RA.
Abbreviations
CMC-Na, carboxymethylcellulose sodium; CS, chondroitin sulfate; Dex-Lip, dexamethasone-loaded liposome; DIC, diclofenac; DLP, digital light processing; DMARDs, disease-modifying anti-rheumatic drugs; DMNs, Dissolving MNs; EN, Etanercept; FDM, Fused Deposition Modeling; HA, hyaluronate; hMN, hydrogel microneedles; HP-β-CD, hydroxypropyl-β-cyclodextrin; MEMS, Microelectromechanical Systems; MN, microneedle; MNs, microneedles; MTX, Methotrexate; NSAIDs, non-steroidal anti-inflammatory drugs; NT, neurotoxin; OCT, Optic coherence tomography; PCL, polycaprolactone; PsA, psoriatic arthritis; PVP, polyvinyl pyrrolidone; RA, Rheumatoid arthritis; ROS, reactive oxygen species; SC, stratum corneum; SH-MN, sinomenine hydrochloride-loaded MNs; SIN, Sinomenine; siRNA, small interfering RNA; SLA, Stereolithograph; TAC, tacrolimus; TD, tip-dissolving; Tet, tetrandrine; TEWL, Trans-epidermal water loss; TGP, total glucosides of paeony; TP, triptolide; TP-LHP, triptolide-loaded liposome hydrogel patch; TPP/MPP, two/multi-photon polymerization.
Acknowledgments
The authors acknowledge Prof. Yan Qu and Dr. Chen Zhang for their fruitful discussions and suggestions during the preparation of this manuscript. This work is supported by the Natural Science Foundation of Sichuan Province (No. 2022NSFSC0373), (No.2023YFS0348), the China Postdoctoral Science Foundation (2021M690488), the Central Guidance on Local Science and Technology Development Fund of Sichuan (No.2022ZYD0099).
Author Contributions
All authors have made significant contributions to the reported work, whether in conceptualization, study design, execution, data acquisition, analysis, and interpretation, or all of these areas; participated in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed to the journal in which the article is to be submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. McInnes IB, Schett G. Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet. 2017;389(10086):2328–2337. doi:10.1016/s0140-6736(17)31472-1
2. Wang Q, Qin X, Fang J, Sun X. Nanomedicines for the treatment of rheumatoid arthritis: state of art and potential therapeutic strategies. Acta Pharmaceutica Sinica B. 2021;11(5):1158–1174. doi:10.1016/j.apsb.2021.03.013
3. Catrina AI, Svensson CI, Malmstrom V, Schett G, Klareskog L. Mechanisms leading from systemic autoimmunity to joint-specific disease in rheumatoid arthritis. Nat Rev Rheumatol. 2017;13(2):79–86. doi:10.1038/nrrheum.2016.200
4. Thakur S, Riyaz B, Patil A, Kaur A, Kapoor B, Mishra V. Novel drug delivery systems for NSAIDs in management of rheumatoid arthritis: an overview. Biomed Pharmacother. 2018;106:1011–1023. doi:10.1016/j.biopha.2018.07.027
5. Krishnamurthy A, Joshua V, Haj Hensvold A, et al. Identification of a novel chemokine-dependent molecular mechanism underlying rheumatoid arthritis-associated autoantibody-mediated bone loss. Ann Rheum Dis. 2016;75(4):721–729. doi:10.1136/annrheumdis-2015-208093
6. Patakas A, R-r J, Weir W, et al. Abatacept inhibition of T cell priming in mice by induction of a unique transcriptional profile that reduces their ability to activate antigen-presenting cells. Arthritis Rheumatol. 2016;68(3):627–638. doi:10.1002/art.39470
7. Mueller AL, Payandeh Z, Mohammadkhani N, et al. Recent advances in understanding the pathogenesis of rheumatoid arthritis: new treatment strategies. Cells. 2021;10(11):3017. doi:10.3390/cells10113017
8. Nooreen R, Nene S, Jain H, et al. Polymer nanotherapeutics: a versatile platform for effective rheumatoid arthritis therapy. J Control Release. 2022;348:397–419. doi:10.1016/j.jconrel.2022.05.054
9. Shreya AB, Raut SY, Managuli RS, Udupa N, Mutalik S. Active targeting of drugs and bioactive molecules via oral administration by ligand-conjugated lipidic nanocarriers: recent advances. AAPS PharmSciTech. 2018;20(1):15. doi:10.1208/s12249-018-1262-2
10. Anil U, Markus DH, Hurley ET, et al. The efficacy of intra-articular injections in the treatment of knee osteoarthritis: a network meta-analysis of randomized controlled trials. Knee. 2021;32:173–182. doi:10.1016/j.knee.2021.08.008
11. McNicol ED, Ferguson MC, Schumann R. Single-dose intravenous diclofenac for acute postoperative pain in adults. Cochrane Database Syst Rev. 2018;8:CD012498. doi:10.1002/14651858.CD012498.pub2
12. Chen J, Zeng S, Xue Q, et al. Photoacoustic image-guided biomimetic nanoparticles targeting rheumatoid arthritis. Proc Natl Acad Sci U S A. 2022;119(43):e2213373119. doi:10.1073/pnas.2213373119
13. Chen J, Qi J, Chen C, et al. Tocilizumab-conjugated polymer nanoparticles for NIR-II photoacoustic-imaging-guided therapy of rheumatoid arthritis. Adv Mater. 2020;32(37):e2003399. doi:10.1002/adma.202003399
14. Chen J, Tan J, Li J, et al. Genetically engineered biomimetic nanoparticles for targeted delivery of mRNA to treat rheumatoid arthritis. Small Methods. 2023;7(11):e2300678. doi:10.1002/smtd.202300678
15. Zoudani EL, Soltani M. A new computational method of modeling and evaluation of dissolving microneedle for drug delivery applications: extension to theoretical modeling of a novel design of microneedle (array in array) for efficient drug delivery. Eur J Pharm Sci. 2020;150:105339. doi:10.1016/j.ejps.2020.105339
16. Pireddu R, Schlich M, Marceddu S, et al. Nanosuspensions and microneedles roller as a combined approach to enhance diclofenac topical bioavailability. Pharmaceutics. 2020;12(12):1140. doi:10.3390/pharmaceutics12121140
17. Jacobse J, Ten Voorde W, Tandon A, et al. Comprehensive evaluation of microneedle-based intradermal Adalimumab delivery vs. subcutaneous administration: results of a randomized controlled clinical trial. Br J Clin Pharmacol. 2021;87(8):3162–3176. doi:10.1111/bcp.14729
18. Du G, He P, Zhao J, et al. Polymeric microneedle-mediated transdermal delivery of melittin for rheumatoid arthritis treatment. J Control Release. 2021;336:537–548. doi:10.1016/j.jconrel.2021.07.005
19. Chang H, Chew SWT, Zheng M, et al. Cryomicroneedles for transdermal cell delivery. Nat Biomed Eng. 2021;5(9):1008–1018. doi:10.1038/s41551-021-00720-1
20. Zhang C, Li J, Xiao M, et al. Oral colon-targeted mucoadhesive micelles with enzyme-responsive controlled release of curcumin for ulcerative colitis therapy. Chin. Chem. Lett. 2022. doi:10.1016/j.cclet.2022.03.110
21. Cahill EM, O’Cearbhaill ED, Cahill EM, O’Cearbhaill ED. Toward biofunctional microneedles for stimulus responsive drug delivery. Bioconjug Chem. 2015;26(7):1289–1296. doi:10.1021/acs.bioconjchem.5b00211
22. Moffatt K, Wang Y, Raj Singh TR, Donnelly RF. Microneedles for enhanced transdermal and intraocular drug delivery. Curr Opin Pharmacol. 2017;36:14–21. doi:10.1016/j.coph.2017.07.007
23. Gomaa YA, Garland MJ, McInnes F, El-Khordagui LK, Wilson C, Donnelly RF. Laser-engineered dissolving microneedles for active transdermal delivery of nadroparin calcium. Eur J Pharm Biopharm. 2012;82(2):299–307. doi:10.1016/j.ejpb.2012.07.008
24. Tucak A, Sirbubalo M, Hindija L, et al. Microneedles: characteristics, materials, production methods and commercial development. Micromachines. 2020;11(11):961. doi:10.3390/mi11110961
25. Evens T, Malek O, Castagne S, Seveno D, Van Bael A. A novel method for producing solid polymer microneedles using laser ablated moulds in an injection moulding process. Manuf Lett. 2020;24:29–32. doi:10.1016/j.mfglet.2020.03.009
26. McGrath MG, Vucen S, Vrdoljak A, et al. Production of dissolvable microneedles using an atomised spray process: effect of microneedle composition on skin penetration. Eur J Pharm Biopharm. 2014;86(2):200–211. doi:10.1016/j.ejpb.2013.04.023
27. Park JH, Allen MG, Prausnitz MR. Biodegradable polymer microneedles: fabrication, mechanics and transdermal drug delivery. J Control Release. 2005;104(1):51–66. doi:10.1016/j.jconrel.2005.02.002
28. Barnum L, Quint J, Derakhshandeh H, et al. 3D-printed hydrogel-filled microneedle arrays. Adv Healthc Mater. 2021;10(13):e2001922. doi:10.1002/adhm.202001922
29. Held J, Gaspar J, Ruther P, et al. Design of experiment characterization of microneedle fabrication processes based on dry silicon etching. J Micromech Microeng. 2010;20(2):025024. doi:10.1088/0960-1317/20/2/025024
30. Izumi H, Aoyagi S. Novel fabrication method for long silicon microneedles with three-dimensional sharp tips and complicated shank shapes by isotropic dry etching. IEEJ Trans ElectrElectron Eng. 2007;2(3):328–334. doi:10.1002/tee.20147
31. Wang R, Wang W, Li Z. An improved manufacturing approach for discrete silicon microneedle arrays with tunable height-pitch ratio. Sensors. 2016;16(10). doi:10.3390/s16101628
32. Donnelly RF, Majithiya R, Singh TR, et al. Design, optimization and characterisation of polymeric microneedle arrays prepared by a novel laser-based micromoulding technique. Pharm Res. 2011;28(1):41–57. doi:10.1007/s11095-010-0169-8
33. Dardano P, Calio A, Di Palma V, Bevilacqua MF, Di Matteo A, De Stefano L. A photolithographic approach to polymeric microneedles array fabrication. Materials. 2015;8(12):8661–8673. doi:10.3390/ma8125484
34. Equbal A, Sood AK. Electroless plating of copper on different shaped ABS parts: a comparison. Int J Adv Mater Manuf Charact. 2014;4(1):32–41. doi:10.11127/ijammc.2014.03.05
35. Bystrova S, Luttge R. Micromolding for ceramic microneedle arrays. Microelectron Eng. 2011;88(8):1681–1684. doi:10.1016/j.mee.2010.12.067
36. Ye R, Yang J, Li Y, et al. Fabrication of tip-hollow and tip-dissolvable microneedle arrays for transdermal drug delivery. ACS Biomater Sci Eng. 2020;6(4):2487–2494. doi:10.1021/acsbiomaterials.0c00120
37. Hsu W-L, Huang C-Y, Hsu Y-P, et al. On-skin glucose-biosensing and on-demand insulin-zinc hexamers delivery using microneedles for syringe-free diabetes management. Chem Eng J. 2020;398:125536. doi:10.1016/j.cej.2020.125536
38. Zhou P, Zhao S, Huang C, Qu Y, Zhang C. Bletilla striata polysaccharide microneedle for effective transdermal administration of model protein antigen. Int J Biol Macromol. 2022;205:511–519. doi:10.1016/j.ijbiomac.2022.02.116
39. He Y, Chen Z, Nie X, et al. Recent advances in polysaccharides from edible and medicinal Polygonati rhizoma: from bench to market. Int J Biol Macromol. 2022;195:102–116. doi:10.1016/j.ijbiomac.2021.12.010
40. Zhang C, Wang X, Xiao M, et al. Nano-in-micro alginate/chitosan hydrogel via electrospray technology for orally curcumin delivery to effectively alleviate ulcerative colitis. Mater Des. 2022;221:110894. doi:10.1016/j.matdes.2022.110894
41. Chang H, Zheng M, Yu X, et al. A swellable microneedle patch to rapidly extract skin interstitial fluid for timely metabolic analysis. Adv Mater. 2017;29(37). doi:10.1002/adma.201702243
42. Lim H, Ha S, Bae M, Yoon SH. A highly robust approach to fabricate the mass-customizable mold of sharp-tipped biodegradable polymer microneedles for drug delivery. Int J Pharm. 2021;600:120475. doi:10.1016/j.ijpharm.2021.120475
43. Yadav PR, Munni MN, Campbell L, et al. Translation of polymeric microneedles for treatment of human diseases: recent trends, progress, and challenges. Pharmaceutics. 2021;13(8):1132. doi:10.3390/pharmaceutics13081132
44. Wang M, Hu L, Xu C. Recent advances in the design of polymeric microneedles for transdermal drug delivery and biosensing. Lab Chip. 2017;17(8):1373–1387. doi:10.1039/c7lc00016b
45. Waghule T, Singhvi G, Dubey SK, et al. Microneedles: a smart approach and increasing potential for transdermal drug delivery system. Biomed Pharmacother. 2019;109:1249–1258. doi:10.1016/j.biopha.2018.10.078
46. Bhatnagar S, Dave K, Venuganti VVK. Microneedles in the clinic. J Control Release. 2017;260:164–182. doi:10.1016/j.jconrel.2017.05.029
47. Ma G, Wu C. Microneedle, bio-microneedle and bio-inspired microneedle: a review. J Control Release. 2017;251:11–23. doi:10.1016/j.jconrel.2017.02.011
48. van der Maaden K, Jiskoot W, Bouwstra J. Microneedle technologies for (trans)dermal drug and vaccine delivery. J Control Release. 2012;161(2):645–655. doi:10.1016/j.jconrel.2012.01.042
49. Sivamani RK, Stoeber B, Liepmann D, Maibach HI. Microneedle penetration and injection past the stratum corneum in humans. J Dermatol Treat. 2009;20(3):156–159. doi:10.1080/09546630802512679
50. Wu Y, Qiu Y, Zhang S, Qin G, Gao Y. Microneedle-based drug delivery: studies on delivery parameters and biocompatibility. Biomed Microdevices. 2008;10(5):601–610. doi:10.1007/s10544-008-9171-x
51. Ruan S, Zhang Y, Feng N. Microneedle-mediated transdermal nanodelivery systems: a review. Biomater Sci. 2021;9(24):8065–8089. doi:10.1039/d1bm01249e
52. Ilic T, Savic S, Batinic B, et al. Combined use of biocompatible nanoemulsions and solid microneedles to improve transport of a model NSAID across the skin: in vitro and in vivo studies. Eur J Pharm Sci. 2018;125:110–119. doi:10.1016/j.ejps.2018.09.023
53. Shu W, Heimark H, Bertollo N, Tobin DJ, O’Cearbhaill ED, Annaidh AN. Insights into the mechanics of solid conical microneedle array insertion into skin using the finite element method. Acta Biomater. 2021;135:403–413. doi:10.1016/j.actbio.2021.08.045
54. Kolli CS, Banga AK. Characterization of solid maltose microneedles and their use for transdermal delivery. Pharm Res. 2008;25(1):104–113. doi:10.1007/s11095-007-9350-0
55. Wei-Ze L, Mei-Rong H, Jian-Ping Z, et al. Super-short solid silicon microneedles for transdermal drug delivery applications. Int J Pharm. 2010;389(1–2):122–129. doi:10.1016/j.ijpharm.2010.01.024
56. Sabri A, Ogilvie J, McKenna J, Segal J, Scurr D, Marlow M. Intradermal Delivery of an Immunomodulator for basal cell carcinoma; expanding the mechanistic insight into solid microneedle-enhanced delivery of hydrophobic molecules. Mol Pharm. 2020;17(8):2925–2937. doi:10.1021/acs.molpharmaceut.0c00347
57. Zhang L, Guo R, Wang S, Yang X, Ling G, Zhang P. Fabrication, evaluation and applications of dissolving microneedles. Int J Pharm. 2021;604:120749. doi:10.1016/j.ijpharm.2021.120749
58. Pukfukdee P, Banlunara W, Rutwaree T, et al. Solid composite material for delivering viable cells into skin tissues via detachable dissolvable microneedles. ACS Appl. Bio Mater. 2020;3(7):4581–4589. doi:10.1021/acsabm.0c00498
59. Witting M, Obst K, Pietzsch M, Friess W, Hedtrich S. Feasibility study for intraepidermal delivery of proteins using a solid microneedle array. Int J Pharm. 2015;486(1–2):52–58. doi:10.1016/j.ijpharm.2015.03.046
60. Abiandu I, Ita K. Transdermal delivery of potassium chloride with solid microneedles. J Drug Delivery Sci Technol. 2019;53:101216. doi:10.1016/j.jddst.2019.101216
61. Makvandi P, Kirkby M, Hutton ARJ, et al. Engineering microneedle patches for improved penetration: analysis, skin models and factors affecting needle insertion. Nanomicro Lett. 2021;13(1):93. doi:10.1007/s40820-021-00611-9
62. Gill HS, Prausnitz MR. Coated microneedles for transdermal delivery. J Control Release. 2007;117(2):227–237. doi:10.1016/j.jconrel.2006.10.017
63. Pearton M, Saller V, Coulman SA, et al. Microneedle delivery of plasmid DNA to living human skin: formulation coating, skin insertion and gene expression. J Control Release. 2012;160(3):561–569. doi:10.1016/j.jconrel.2012.04.005
64. Vinayakumar KB, Hegde GM, Nayak MM, Dinesh NS, Rajanna K. Fabrication and characterization of gold coated hollow silicon microneedle array for drug delivery. Microelectron Eng. 2014;128:12–18. doi:10.1016/j.mee.2014.05.039
65. Koutsonanos DG, Del Pilar Martin M, Zarnitsyn VG, et al. Transdermal influenza immunization with vaccine-coated microneedle arrays. PLoS One. 2009;4(3):e4773. doi:10.1371/journal.pone.0004773
66. Ranganayakulu SV, Kucheludu A, Veera Bhadraiah B, Ramesh Kumar B. Defect location and sizing by ultrasonic phased array on aero grade material aluminum He-15. Int J Adv Mater Manuf Charact. 2014;4(1):47–50. doi:10.11127/ijammc.2014.03.07
67. Zhou P, Chen C, Yue X, et al. Strategy for osteoarthritis therapy: improved the delivery of triptolide using liposome-loaded dissolving microneedle arrays. Int J Pharm. 2021;609:121211. doi:10.1016/j.ijpharm.2021.121211
68. Jing Q, Ruan H, Li J, et al. Keratinocyte membrane-mediated nanodelivery system with dissolving microneedles for targeted therapy of skin diseases. Biomaterials. 2021;278:121142. doi:10.1016/j.biomaterials.2021.121142
69. Ita K. Dissolving microneedles for transdermal drug delivery: advances and challenges. Biomed Pharmacother. 2017;93:1116–1127. doi:10.1016/j.biopha.2017.07.019
70. Vora LK, Donnelly RF, Larraneta E, Gonzalez-Vazquez P, Thakur RRS, Vavia PR. Novel bilayer dissolving microneedle arrays with concentrated PLGA nano-microparticles for targeted intradermal delivery: proof of concept. J Control Release. 2017;265:93–101. doi:10.1016/j.jconrel.2017.10.005
71. Li Z, He Y, Deng L, Zhang ZR, Lin Y. A fast-dissolving microneedle array loaded with chitosan nanoparticles to evoke systemic immune responses in mice. J Mater Chem B. 2020;8(2):216–225. doi:10.1039/c9tb02061f
72. Lin S, Quan G, Hou A, et al. Strategy for hypertrophic scar therapy: improved delivery of triamcinolone acetonide using mechanically robust tip-concentrated dissolving microneedle array. J Control Release. 2019;306:69–82. doi:10.1016/j.jconrel.2019.05.038
73. Szeto B, Aksit A, Valentini C, et al. Novel 3D-printed hollow microneedles facilitate safe, reliable, and informative sampling of perilymph from Guinea pigs. Hear Res. 2021;400:108141. doi:10.1016/j.heares.2020.108141
74. Ingrole RSJ, Azizoglu E, Dul M, Birchall JC, Gill HS, Prausnitz MR. Trends of microneedle technology in the scientific literature, patents, clinical trials and internet activity. Biomaterials. 2021;267:120491. doi:10.1016/j.biomaterials.2020.120491
75. Yadav V, Sharma PK, Murty US, et al. 3D printed hollow microneedles array using stereolithography for efficient transdermal delivery of rifampicin. Int J Pharm. 2021;605:120815. doi:10.1016/j.ijpharm.2021.120815
76. Kato N, Kawashima T, Shibata T, Mineta T, Makino E. Micromachining of a newly designed AFM probe integrated with hollow microneedle for cellular function analysis. Microelectron Eng. 2010;87(5–8):1185–1189. doi:10.1016/j.mee.2009.12.025
77. Angkawinitwong U, Courtenay AJ, Rodgers AM, et al. A novel transdermal protein delivery strategy via electrohydrodynamic coating of PLGA Microparticles onto microneedles. ACS Appl Mater Interfaces. 2020;12(11):12478–12488. doi:10.1021/acsami.9b22425
78. Kim YC, Park JH, Prausnitz MR. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev. 2012;64(14):1547–1568. doi:10.1016/j.addr.2012.04.005
79. Migdadi EM, Courtenay AJ, Tekko IA, et al. Hydrogel-forming microneedles enhance transdermal delivery of metformin hydrochloride. J Control Release. 2018;285:142–151. doi:10.1016/j.jconrel.2018.07.009
80. Turner JG, White LR, Estrela P, Leese HS. Hydrogel-forming microneedles: current advancements and future trends. Macromol Biosci. 2021;21(2):e2000307. doi:10.1002/mabi.202000307
81. Mansoor I, Lai J, Ranamukhaarachchi S, et al. A microneedle-based method for the characterization of diffusion in skin tissue using doxorubicin as a model drug. Biomed Microdevices. 2015;17(3):9967. doi:10.1007/s10544-015-9967-4
82. Du H, Liu P, Zhu J, et al. Hyaluronic acid-based dissolving microneedle patch loaded with methotrexate for improved treatment of psoriasis. ACS Appl Mater Interfaces. 2019;11(46):43588–43598. doi:10.1021/acsami.9b15668
83. Yang S-J, Jeong J-O, Lim Y-M, Park J-S. Synthesis and characterization of PVP microneedle patch using metal bioelectrodes for novel drug delivery system. Mater Des. 2021;201:109485. doi:10.1016/j.matdes.2021.109485
84. Yu K, Yu X, Cao S, et al. Layered dissolving microneedles as a need-based delivery system to simultaneously alleviate skin and joint lesions in psoriatic arthritis. Acta Pharm Sin B. 2021;11(2):505–519. doi:10.1016/j.apsb.2020.08.008
85. Wang P-C, Paik S-J, Kim S-H, Allen MG. Hypodermic-needle-like hollow polymer microneedle array: fabrication and characterization. J Microelectromech Syst. 2014;23(4):991–998. doi:10.1109/jmems.2014.2307320
86. Saha I, Rai VK. Hyaluronic acid based microneedle array: recent applications in drug delivery and cosmetology. Carbohydr Polym. 2021;267:118168. doi:10.1016/j.carbpol.2021.118168
87. Wang C, Liu S, Xu J, et al. Dissolvable microneedles based on Panax notoginseng polysaccharide for transdermal drug delivery and skin dendritic cell activation. Carbohydr Polym. 2021;268:118211. doi:10.1016/j.carbpol.2021.118211
88. Pei P, Yang F, Liu J, et al. Composite-dissolving microneedle patches for chemotherapy and photothermal therapy in superficial tumor treatment. Biomater Sci. 2018;6(6):1414–1423. doi:10.1039/c8bm00005k
89. Han T, Das DB. Potential of combined ultrasound and microneedles for enhanced transdermal drug permeation: a review. Eur J Pharm Biopharm. 2015;89:312–328. doi:10.1016/j.ejpb.2014.12.020
90. Ali R, Mehta P, Arshad MS, Kucuk I, Chang MW, Ahmad Z. Transdermal microneedles-A materials perspective. AAPS PharmSciTech. 2019;21(1):12. doi:10.1208/s12249-019-1560-3
91. Wang Q, Yao G, Dong P, et al. Investigation on fabrication process of dissolving microneedle arrays to improve effective needle drug distribution. Eur J Pharm Sci. 2015;66:148–156. doi:10.1016/j.ejps.2014.09.011
92. Al Sulaiman D, Chang JYH, Bennett NR, et al. Hydrogel-coated microneedle arrays for minimally invasive sampling and sensing of specific circulating nucleic acids from skin interstitial fluid. ACS Nano. 2019;13(8):9620–9628. doi:10.1021/acsnano.9b04783
93. Gupta J, Park SS, Bondy B, Felner EI, Prausnitz MR. Infusion pressure and pain during microneedle injection into skin of human subjects. Biomaterials. 2011;32(28):6823–6831. doi:10.1016/j.biomaterials.2011.05.061
94. Strambini LM, Longo A, Scarano S, et al. Self-powered microneedle-based biosensors for pain-free high-accuracy measurement of glycaemia in interstitial fluid. Biosens Bioelectron. 2015;66:162–168. doi:10.1016/j.bios.2014.11.010
95. Sezgin B, Ozel B, Bulam H, Guney K, Tuncer S, Cenetoglu S. The effect of microneedle thickness on pain during minimally invasive facial procedures: a clinical study. Aesthet Surg J. 2014;34(5):757–765. doi:10.1177/1090820X14532941
96. Wu C, Cheng J, Li W, Yang L, Dong H, Zhang X. Programmable polymeric microneedles for combined chemotherapy and antioxidative treatment of rheumatoid arthritis. ACS Appl Mater Interfaces. 2021;13(46):55559–55568. doi:10.1021/acsami.1c17375
97. Li Y, Sun Y, Wei S, Zhang L, Zong S. Development and evaluation of tofacitinib transdermal system for the treatment of rheumatoid arthritis in rats. Drug Dev Ind Pharm. 2021;47(6):878–886. doi:10.1080/03639045.2021.1916521
98. Norman JJ, Arya JM, McClain MA, Frew PM, Meltzer MI, Prausnitz MR. Microneedle patches: usability and acceptability for self-vaccination against influenza. Vaccine. 2014;32(16):1856–1862. doi:10.1016/j.vaccine.2014.01.076
99. Guo T, Zhang Y, Li Z, Zhao J, Feng N. Microneedle-mediated transdermal delivery of nanostructured lipid carriers for alkaloids from Aconitum sinomontanum. Artif Cells Nanomed Biotechnol. 2018;46(8):1541–1551. doi:10.1080/21691401.2017.1376676
100. Chen G, Hao B, Ju D, et al. Pharmacokinetic and pharmacodynamic study of triptolide-loaded liposome hydrogel patch under microneedles on rats with collagen-induced arthritis. Acta Pharm Sin B. 2015;5(6):569–576. doi:10.1016/j.apsb.2015.09.006
101. Cui Y, Mo Y, Zhang Q, et al. Microneedle-assisted percutaneous delivery of paeoniflorin-loaded ethosomes. Molecules. 2018;23(12):3371. doi:10.3390/molecules23123371
102. Amodwala S, Kumar P, Thakkar HP. Statistically optimized fast dissolving microneedle transdermal patch of meloxicam: a patient friendly approach to manage arthritis. Eur J Pharm Sci. 2017;104:114–123. doi:10.1016/j.ejps.2017.04.001
103. Chen J, Huang W, Huang Z, et al. Fabrication of tip-dissolving microneedles for transdermal drug delivery of meloxicam. AAPS PharmSciTech. 2018;19(3):1141–1151. doi:10.1208/s12249-017-0926-7
104. Cao J, Zhang N, Wang Z, et al. Microneedle-assisted transdermal delivery of etanercept for rheumatoid arthritis treatment. Pharmaceutics. 2019;11(5):235. doi:10.3390/pharmaceutics11050235
105. Yao W, Tao C, Zou J, et al. Flexible two-layer dissolving and safing microneedle transdermal of neurotoxin: a biocomfortable attempt to treat Rheumatoid Arthritis. Int J Pharm. 2019;563:91–100. doi:10.1016/j.ijpharm.2019.03.033
106. Shende P, Salunke M. Transepidermal microneedles for co-administration of folic acid with methotrexate in the treatment of rheumatoid arthritis. Biomed Phys Eng Express. 2019;5(2):025023. doi:10.1088/2057-1976/aafbbb
107. Zhao M, Bai J, Lu Y, et al. Anti-arthritic effects of microneedling with bee venom gel. J Tradit Chin Med. 2016;3(4):256–262. doi:10.1016/j.jtcms.2016.09.005
108. Chen Z, Han B, Liao L, et al. Enhanced transdermal delivery of polydatin via a combination of inclusion complexes and dissolving microneedles for treatment of acute gout arthritis. J Drug Delivery Sci Technol. 2020;55:101487. doi:10.1016/j.jddst.2019.101487
109. Shu Z, Cao Y, Tao Y, et al. Polyvinylpyrrolidone microneedles for localized delivery of sinomenine hydrochloride: preparation, release behavior of in vitro & in vivo, and penetration mechanism. Drug Deliv. 2020;27(1):642–651. doi:10.1080/10717544.2020.1754524
110. Guo T, Cheng N, Zhao J, Hou X, Zhang Y, Feng N. Novel nanostructured lipid carriers-loaded dissolving microneedles for controlled local administration of aconitine. Int J Pharm. 2019;572:118741. doi:10.1016/j.ijpharm.2019.118741
111. Song X, Wang Y, Chen H, et al. Dosage-efficacy relationship and pharmacodynamics validation of brucine dissolving microneedles against rheumatoid arthritis. J Drug Delivery Sci Technol. 2021;63:102537. doi:10.1016/j.jddst.2021.102537
112. Hu H, Ruan H, Ruan S, et al. Acid-responsive PEGylated branching PLGA nanoparticles integrated into dissolving microneedles enhance local treatment of arthritis. Chem Eng J. 2022;431:134196. doi:10.1016/j.cej.2021.134196
113. Qiu Y, Li C, Zhang S, Yang G, He M, Gao Y. Systemic delivery of artemether by dissolving microneedles. Int J Pharm. 2016;508(1–2):1–9. doi:10.1016/j.ijpharm.2016.05.006
114. Tekko IA, Chen G, Dominguez-Robles J, et al. Development and characterisation of novel poly (vinyl alcohol)/poly (vinyl pyrrolidone)-based hydrogel-forming microneedle arrays for enhanced and sustained transdermal delivery of methotrexate. Int J Pharm. 2020;586:119580. doi:10.1016/j.ijpharm.2020.119580
115. Cao J, Su J, An M, et al. Novel DEK-targeting aptamer delivered by a hydrogel microneedle attenuates collagen-induced arthritis. Mol Pharm. 2021;18(1):305–316. doi:10.1021/acs.molpharmaceut.0c00954
116. Chong RH, Gonzalez-Gonzalez E, Lara MF, et al. Gene silencing following siRNA delivery to skin via coated steel microneedles: in vitro and in vivo proof-of-concept. J Control Release. 2013;166(3):211–219. doi:10.1016/j.jconrel.2012.12.030
117. Tan C, Wang J, Sun B. Biopolymer-liposome hybrid systems for controlled delivery of bioactive compounds: recent advances. Biotechnol Adv. 2021;48:107727. doi:10.1016/j.biotechadv.2021.107727
118. Jia M, Deng C, Luo J, et al. A novel dexamethasone-loaded liposome alleviates rheumatoid arthritis in rats. Int J Pharm. 2018;540(1–2):57–64. doi:10.1016/j.ijpharm.2018.02.001
119. Luo Y, Li J, Hu Y, et al. Injectable thermo-responsive nano-hydrogel loading triptolide for the anti-breast cancer enhancement via localized treatment based on ”two strikes” effects. Acta Pharm Sin B. 2020;10(11):2227–2245. doi:10.1016/j.apsb.2020.05.011
120. Bello AE, Perkins EL, Jay R, Efthimiou P. Recommendations for optimizing methotrexate treatment for patients with rheumatoid arthritis. Open Access Rheumatol. 2017;9:67–79. doi:10.2147/OARRR.S131668
121. Jacobse J, Ten Voorde W, Rissmann R, Burggraaf J, Ten Cate R, Schrier L. The effect of repeated methotrexate injections on the quality of life of children with rheumatic diseases. Eur J Pediatr. 2019;178(1):17–20. doi:10.1007/s00431-018-3286-8
122. Branco JC, Barcelos A, de Araujo FP, et al. Utilization of subcutaneous methotrexate in rheumatoid arthritis patients after failure or intolerance to oral methotrexate: a multicenter cohort study. Adv Ther. 2016;33(1):46–57. doi:10.1007/s12325-015-0276-3
123. Braun J, Kastner P, Flaxenberg P, et al. Comparison of the clinical efficacy and safety of subcutaneous versus oral administration of methotrexate in patients with active rheumatoid arthritis: results of a six-month, multicenter, randomized, double-blind, controlled, Phase IV trial. Arthritis Rheum. 2008;58(1):73–81. doi:10.1002/art.23144
124. Klein A, Kaul I, Foeldvari I, Ganser G, Urban A, Horneff G. Efficacy and safety of oral and parenteral methotrexate therapy in children with juvenile idiopathic arthritis: an observational study with patients from the German Methotrexate Registry. Arthritis Care Res. 2012;64(9):1349–1356. doi:10.1002/acr.21697
125. Rohr MK, Mikuls TR, Cohen SB, Thorne JC, O’Dell JR. Underuse of methotrexate in the treatment of rheumatoid arthritis: a national analysis of prescribing practices in the US. Arthritis Care Res. 2017;69(6):794–800. doi:10.1002/acr.23152
126. Nguyen HX, Banga AK. Delivery of methotrexate and characterization of skin treated by fabricated PLGA microneedles and fractional ablative laser. Pharm Res. 2018;35(3):68. doi:10.1007/s11095-018-2369-6
127. Lu Y, Xiao T, Lai R, et al. Co-delivery of loxoprofen and tofacitinib by photothermal microneedles for rheumatoid arthritis treatment. Pharmaceutics. 2023;15(5):1500. doi:10.3390/pharmaceutics15051500
128. Cai L, Chen WN, Li R, Hu CM, Lei C, Li CM. Therapeutic effect of Acetazolamide, an aquaporin 1 inhibitor, on adjuvant-induced arthritis in rats by inhibiting NF-kappaB signal pathway. Immunopharmacol Immunotoxicol. 2018;40(2):117–125. doi:10.1080/08923973.2017.1417998
129. da Fonseca LJS, Nunes-Souza V, Goulart MOF, Rabelo LA. Oxidative stress in rheumatoid arthritis: what the future might hold regarding novel biomarkers and add-on therapies. Oxid Med Cell Longev. 2019;2019:7536805. doi:10.1155/2019/7536805
130. Kocabas H, Kocabas V, Buyukbas S, Salli A, Ugurlu H. Relationship of cellular oxidant and antioxidant status with disease activity in patients with rheumatoid arthritis. Turk J Rheumatol. 2010;25(3):141–146. doi:10.5152/tjr.2010.18
131. Centre for Human Drug Research, Netherlands. NCT03607903: Adalimumab Microneedles in Healthy Volunteers. ClinicalTrials.gov. Available from: https://clinicaltrials.gov/study/NCT03607903.
132. Larrañeta E, Lutton REM, Woolfson AD, Donnelly RF. Microneedle arrays as transdermal and intradermal drug delivery systems: materials science, manufacture and commercial development. Mater Sci Eng R Rep. 2016;104:1–32. doi:10.1016/j.mser.2016.03.001
133. Luo Z, Wang Y, Li J, Wang J, Yu Y, Zhao Y. Tailoring hyaluronic acid hydrogels for biomedical applications. Adv Funct Mater. 2023. doi:10.1002/adfm.202306554
134. Li S, Wang X, Yan Z, et al. Microneedle patches with antimicrobial and immunomodulating properties for infected wound healing. Adv Sci. 2023;10(22):e2300576. doi:10.1002/advs.202300576
135. Chen BZ, Liu JL, Li QY, et al. Safety evaluation of solid polymer microneedles in human volunteers at different application sites. ACS Appl Bio Mater. 2019;2(12):5616–5625. doi:10.1021/acsabm.9b00700
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.