Back to Journals » International Journal of Nanomedicine » Volume 19
Advances in Ferroptosis-Inducing Agents by Targeted Delivery System in Cancer Therapy
Authors Xiang D, Zhou L, Yang R, Yuan F, Xu Y, Yang Y , Qiao Y, Li X
Received 9 November 2023
Accepted for publication 16 February 2024
Published 5 March 2024 Volume 2024:19 Pages 2091—2112
DOI https://doi.org/10.2147/IJN.S448715
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor R.D.K. Misra
Debiao Xiang,1– 3 Lili Zhou,4 Rui Yang,1,4 Fang Yuan,1– 3 Yilin Xu,4 Yuan Yang,1,4 Yong Qiao,1– 3 Xin Li1– 3
1Department of Pharmacy, The Third Hospital of Changsha, Changsha, Hunan Province, People’s Republic of China; 2Hunan Provincial Key Laboratory of Anti-Resistance Microbial Drugs, Changsha, Hunan Province, People’s Republic of China; 3The Clinical Application Research Institute of Antibiotics in Changsha, Changsha, Hunan Province, People’s Republic of China; 4College of Pharmacy, Hunan University of Chinese Medicine, Changsha, Hunan Province, People’s Republic of China
Correspondence: Xin Li, 176 Laodong West Road, Tianxin District, Changsha, Hunan Province, People’s Republic of China, Tel +86 0731-85171320, Email [email protected]
Abstract: Currently, cancer remains one of the most significant threats to human health. Treatment of most cancers remains challenging, despite the implementation of diverse therapies in clinical practice. In recent years, research on the mechanism of ferroptosis has presented novel perspectives for cancer treatment. Ferroptosis is a regulated cell death process caused by lipid peroxidation of membrane unsaturated fatty acids catalyzed by iron ions. The rapid development of bio-nanotechnology has generated considerable interest in exploiting iron-induced cell death as a new therapeutic target against cancer. This article provides a comprehensive overview of recent advancements at the intersection of iron-induced cell death and bionanotechnology. In this respect, the mechanism of iron-induced cell death and its relation to cancer are summarized. Furthermore, the feasibility of a nano-drug delivery system based on iron-induced cell death for cancer treatment is introduced and analyzed. Secondly, strategies for inducing iron-induced cell death using nanodrug delivery technology are discussed, including promoting Fenton reactions, inhibiting glutathione peroxidase 4, reducing low glutathione levels, and inhibiting system Xc−. Additionally, the article explores the potential of combined treatment strategies involving iron-induced cell death and bionanotechnology. Finally, the application prospects and challenges of iron-induced nanoagents for cancer treatment are discussed.
Keywords: cancers, ferroptosis, nano-drug delivery system, target
Introduction
Over the past decade, various malignant tumor treatment methods have been applied in the clinic, including traditional treatment strategies such as surgery, chemotherapy, radiotherapy, and emerging immunotherapy.1 However, due to the inherent limitations of conventional treatment approaches and the heterogeneity of tumors, their clinical efficacy remains dismal.2,3 Although immunotherapy has become a promising cancer treatment in recent years, its narrow anti-tumor spectrum, potential toxicity, and autoimmune suppression have greatly limited its clinical application.4,5 Additionally, drug delivery barriers within solid tumors account for the poor efficacy of tumor immunotherapy.6,7 Therefore, in the face of the serious threat to human health posed by malignant tumors, there is an urgent need for a safer and more effective treatment approach.
In recent years, emerging biomedical developments and technological innovations have provided more possibilities for effectively treating cancer.8 An increasing body of literature suggests that inducing iron death in tumor cells represents a potential new anti-tumor strategy.9,10 Iron is one of the essential elements of the human body. It exists in the form of hemoglobin, myoglobin, and other compounds, and the rest is stored as ferritin in the liver, spleen, and bone marrow.11,12 Iron is reportedly involved in metabolic processes and various life activities, including oxygen transport, cell respiration, electron transfer, DNA synthesis, and immune regulation.13,14 The abnormal metabolism of iron leads to many physiological disorders. In this regard, the presence of Fe2+ in cells greatly accelerates the lipid peroxidation of saturated fat.15 During mitochondrial oxidative phosphorylation, where iron is involved, cells generate both adenosine triphosphate (ATP) and reactive oxygen species (ROS).16 If the production of ROS surpasses the cellular antioxidant capacity, it can trigger oxidative stress reactions.17 Consequently, macromolecules like proteins, nucleic acids, and lipids may suffer damage, ultimately leading to cell impairment or death.18–20 This novel form of regulated cell death is called ferroptosis. In recent years, research on ferroptosis has increased exponentially.21 It is widely thought that the induction of ferroptosis in tumor cells represents a feasible research direction for tumor treatment.22,23 The compounds that trigger ferroptosis in tumor cells and the underlying mechanisms leading to this process represent crucial aspects of anti-tumor drug development.24,25
With the development of nanotechnology and biomaterials, biological nanotechnology has been widely applied in designing nanomedicine delivery systems.26,27 This system offers significant advantages in drug delivery, such as improving water solubility, prolonging circulation time, enhancing tumor accumulation, increasing cell uptake, and reducing toxicity.28–30 Various nanoformulations, such as doxorubicin liposomes (Doxil) and paclitaxel albumin nanoparticles (Abraxan), have been applied in clinical practice.31 Therefore, biological nanotechnology holds significant implications for the delivery of hydrophobic small-molecule anticancer drugs, leading to enhanced treatment efficiency and minimized adverse reactions.32–34 This concept opens up innovative possibilities for the in vivo delivery of ferroptosis inducers that suffer from poor water solubility, high systemic toxicity, and limited tumor delivery efficiency.35 As a result, it paves the way for the development of novel, efficient, and low-toxicity cancer therapeutics. Interestingly, nanoformulations can use endogenous substances from tumors as substrates to drive catalytic reactions and regulate their microenvironment to achieve anti-tumor effects.36,37 They can also enable targeted delivery and controlled release of clinical chemotherapy drugs, achieving site-specific therapy of malignant tumors. In recent years, nanotherapy in the context of ferroptosis has received extensive attention. The synergistic combination of ferroptosis inducers and biological nanotechnology for tumor treatment holds tremendous potential for various applications.9 This article provides a systematic introduction to the process of ferroptosis, elucidates the mechanism of nanoparticle-induced ferroptosis, and highlights the latest advancements in combined anti-tumor therapy based on ferroptosis.
Ferroptosis and Its Process
Overview of Ferroptosis
In 2003, Dolma et al discovered a new compound called erastin, which could induce RAS mutations and cause tumor cells to die differently from traditional cell apoptosis.38 In 2008, Yang et al discovered two new compounds (RSL3 and RSL5) that yielded the same effect as erastin and determined that this novel form of cell death could be inhibited by iron chelating agents, ferroamine B methanesulfonate, and antioxidant vitamin E, which captures peroxyl radicals, confirming that this form of cell death is related to intracellular iron and ROS levels.39,40 The term “Ferroptosis” was coined by Dixon et al in 2012 to describe a specific type of cell death that occurs due to the accumulation of iron and lipid reactive oxygen species.41,42
The common forms of cell death include apoptosis, necrosis, necrotic apoptosis, autophagy, pyroptosis, copper death, etc. (1) Apoptosis refers to the autonomous and programmed death of cells controlled by genes to maintain the stability of the internal environment.43 It involves the activation, expression, and regulation of a series of genes, which is physiological and selective.44 The main morphological changes include cell atrophy, increased cytoplasmic density, decreased mitochondrial membrane potential (MMP), permeability changes, and the appearance of complete apoptotic bodies.45 (2) Cell necrosis is cell damage and death caused by extreme physical and chemical factors or severe pathological stimulation and represents an unnatural form of cell death.46 When apoptosis cannot occur under normal circumstances, necrosis is employed as an alternative mechanism for cell death.47 (3) Necrotic apoptosis is also a form of programmed cell death (PCD) mediated by various cytokines and pattern recognition receptors (PRR), leading to cytological changes, including swelling, membrane rupture, chromosomal condensation, and the release of damage-related molecular patterns (DAMPs), inflammatory cytokines, and chemokines, mediating extreme inflammatory responses.48 (4) Autophagy refers to the process of degrading dysfunctional cell components through lysosomes.49 Its main morphological features include autophagic vacuole accumulation, cytoplasm vacuolization, and the absence of chromatin condensation.50 It is well-established that autophagy can degrade and digest damaged and denatured organelles, proteins, nucleic acids, and other biological macromolecules, providing raw materials for cell regeneration and repair and achieving the recycling and utilization of intracellular substances.51 Autophagy also promotes cellular senescence and cell surface antigen presentation and prevents genomic instability and necrosis, accounting for its key role in preventing cancer, neurodegeneration, cardiomyopathy, diabetes, liver disease, and other diseases.52 (5) Pyroptosis is a newly discovered mode of programmed cell death, characterized by the continuous expansion of cells until cell membrane rupture, leading to the release of cell contents and then activating a strong inflammatory reaction.53 Pyroptosis is vital to the body’s innate immune response, serving as a crucial defense mechanism against infections and endogenous danger signals.54 (6) Cuproptosis is caused by the direct combination of Cu2+ with the fatty acid component of the citric acid cycle in mitochondrial respiration, inducing the aggregation of fatty acylated proteins and the instability of iron-sulfur proteins, leading to proteotoxic stress and inducing cell death independent of the apoptosis pathway.55 The manifestation of copper death includes mitochondrial shrinkage and mitochondrial membrane rupture.
In terms of morphological and biochemical characteristics, ferroptosis is different from other forms of cell death. Morphologically, ferroptosis is characterized by mitochondrial contraction and increased mitochondrial membrane density.56,57 Unlike other cell death types, there is no cell swelling or membrane rupture, and no changes in the nucleus or chromatin contraction are observed. Biochemically, ferroptosis involves the aggregation of reactive oxygen species and iron ions, activation of the mitogen-activated protein kinase (MAPK) system, reduced cystine uptake, depletion of glutathione, and inhibition of system Xc−, among others.58,59 At the gene level, ferroptosis is mainly regulated by specific genes, including Ribosome L8 (RPL8), iron response element binding protein (IREB2), ATP synthase F0 complex subunit C3 (ATP5G3), tritetrapeptide repeat domain 35 (TTC35), citrate synthase (CS), acyl-coenzyme A synthase family member 2 (ACSF2), and metabolic and storage genes such as transferrin receptor protein 1 (TFRC), iron-sulfur cluster assembly enzyme (ISCU), ferritin heavy chain 1 (FTH1), ferritin light chain (FTL), and solute carrier family 11 member 2 (SLC11A2).60
The Process of Ferroptosis
It is well-established that lipoxygenase and ROS can oxidize the polyunsaturated fatty acid (PUFA) chain on the cell membrane lipid to form lipid peroxide (L-OOH). In the presence of iron, lipid peroxides turn into toxic lipid free radicals, resulting in the fragmentation of polyunsaturated fatty acids of cell membrane lipids and cell death.61 The glutathione-dependent lipid repair enzyme glutathione peroxidase 4 (GPX4) can prevent the development of this process by reducing the iron-dependent lipid peroxidation into highly active lipid free radicals, thereby reducing the accumulation of lipid reactive oxygen species (L-ROS).62 The synthesis of GSH requires cystine-glutamate reverse transporter system (system Xc−, an important antioxidant that imports cystine (Cys2) into the cell), while the Xc− transporter transports glutamate out of the cell.63 The inhibition of system Xc− transporter function reduces glutathione (GSH levels in cells, inhibits GSH-dependent GPX4 enzyme activity, and increases the iron content in cells, leading to the accumulation of L-ROS and ferroptosis.
Regulation of Intracellular Biochemical Processes on Ferroptosis
Ferroptosis refers to the process whereby excessive iron in cells participates in the Fenton reaction, which leads to the production of highly active hydroxyl radicals, promotes the peroxidation of PUFA, induces the production of many lipid reactive oxygen species, and leads to cell death.64 The excessive production of reactive oxygen species, the massive accumulation of iron ions, and lipid peroxidation are the three major characteristics of ferroptosis. These key aspects highlight the central role of lipid metabolism, iron metabolism, and amino acid metabolism in driving the occurrence of ferroptosis. However, it should be borne in mind that other pathways and processes can also regulate this form of cell death.
Lipid Metabolism
The essential reaction substrates for lipid peroxidation, a central process in ferroptosis, are provided by fatty acids. Among fatty acids, polyunsaturated fatty acids (PUFAs), particularly arachidonic acid and adrenic acid found in phosphatidylethanolamine (PE) and phosphatidylcholine (PC) are the preferred targets for lipid peroxidation.65,66 This process affects the cell membrane’s function by disrupting the lipid bilayer. Free PUFA can be converted within cells to polyunsaturated fatty acids coenzyme A (PUFA CoA) catalyzed by long-chain acyl-coenzyme A synthetase 4 (ACSL4).67 Subsequently, PUFA CoA enters the endoplasmic reticulum phospholipids via lysophosphatidylcholine acyltransferase 3 (LPCAT3), leading to the generation of polyunsaturated fatty acid phospholipids (PUFA PLs). Under the dual influence of reactive oxygen species and lipoxygenase, these PUFA PLs undergo oxidation, forming peroxidized PUFA PLs. This process destroys the lipid bilayer membrane, affecting membrane function, and ultimately triggers ferroptosis.68 In contrast, monounsaturated fatty acid (MUFA), like Saturated fat palmitate (SFA) through stearoyl CoA desaturase-1 (SCD1), are incorporated into phospholipids in a manner dependent on ACSL3. This incorporation interferes with the formation of PUFA phospholipids and protects cells from iron-induced apoptosis.69
During lipid peroxidation, intracellular lipid peroxidation products primarily accumulate through non-enzymatic and enzymatic lipid peroxidation. During the non-enzymatic autooxidation process, free ferrous reacts with hydrogen peroxide (H2O2) to produce triiron and hydroxyl radicals (•OH) through the Fenton reaction.70 On the other hand, the enzymatic mechanism of Lipid peroxidation involves the involvement of NADPH oxidase (NOX) and Lipoxygenase (LOXs), which are closely linked to the imbalance of lipid oxidation metabolism.71 Overexpression of NADPH oxidase (NOX) can lead to NADPH depletion, causing a rapid increase in oxidative free radicals in cells and significantly enhancing the cell’s susceptibility to ferroptosis.72 Several pathways, including peptidyl peptidase 4 (DPP4) and p53 pathway, Arachidonic acid and Protein kinase C pathway, and HIPPO signal pathway, have been found to regulate NOX function.73 Lipoxygenase (LOX) is a group of non-heme iron-containing enzymes. In the ferroptosis pathway, LOX is crucial in peroxidizing the plasma membrane and promoting ferroptosis in tumor cells.74 Among the LOX family members, LOX5, LOX12, and LOX15 have been established to significantly impact lipid metabolism.75
Iron Metabolism
Ferroptosis primarily occurs due to abnormal cell iron metabolism, especially excess iron. Transferrin (TF) is a glycoprotein with a molecular weight of 76 kDa, responsible for tightly and reversibly binding to iron in the blood, facilitating its transport to various tissues and organs.76 The binding of transferrin to iron is pH-dependent.77 At pH 7.4, transferrin efficiently binds with Fe3+, but under acidic pH conditions, they separate, releasing iron from TF.78 On the cell surface, TF interacts with Fe3+ to form a total iron-transferrin (Tf) complex, which then binds to the Transferrin receptor (TFR) and enters the endosome via endocytosis.79 Within the acidic endosomes, Fe3+ separates from TF. Simultaneously, iron oxidoreductase 3 (STEAP3) reduces Fe3+ to Fe2+, which is transported to the cytoplasm by the divalent metal ion transporter 1 (DMT1).80 The TF-TFR complex releases Fe3+ and returns to the cell surface through exocytosis. Iron in cells can be stored in two ways: either stored harmlessly in Ferritin or forming an unstable iron pool in the form of free Fe.2+81 Ferritin is a cytoplasmic iron storage protein comprising Ferritin heavy chain 1 (FTH1) and Ferritin light chain (FTL) subunits.82 FTH1 exhibits iron oxidase activity, converting excess Fe2+ to Fe3+, while FTL allows for Fe3+ storage.83 Fe2+ is mainly excreted from the cell membrane by the solute carrier family 40 member 1 (SLC40A1) or can be expelled as Ferritin via exosomes.84 The protein prominin 2 promotes the formation of polyvesicles and exosomes, facilitating iron excretion from cells and maintaining the dynamic equilibrium of intracellular iron levels.85 Furthermore, Nuclear receptor coactivator 4 (NCOA4) selectively recognizes ferritin within cells and transports it to lysosomes for degradation, releasing free Fe.2+86 This process is known as ferritinophagy, a special type of autophagy involved in the regulation of iron levels within cells.
Finally, the instability and heightened reactivity of excess Fe2+ results in the Fenton reaction, generating highly reactive hydroxyl radicals.87 These radicals directly interact with polyunsaturated fatty acids present in the cell membrane, leading to a substantial production of lipid reactive oxygen species and cell ferroptosis.
Amino Acid Metabolism
Cells experiencing ferroptosis usually exhibit an imbalance between the antioxidant defense system and lipid peroxidation.88 The cellular stability of cells may be compromised when the intracellular antioxidant defense system fails to sufficiently limit the extent of lipid peroxidation. The GSH-GPX4 antioxidant system plays a pivotal role in the ferroptosis process.89 GSH, serving as the substrate of GPX4, is a vital component of the intracellular antioxidant system and a critical factor influencing the occurrence of ferroptosis.90 GSH synthesis relies on amino acid metabolism, where the specific transporter cystine/glutamate reverse transporter (system Xc−) facilitates the entry and exit of amino acids.91 Comprising SLC7A11 and SLC3A2, system Xc− transports glutamic acid out of cells while importing cystine into cells.92 Once inside the cell, cystine is oxidized to cysteine, which, under the influence of glutamate cysteine ligase and glutathione synthetase, combines with glycine to produce glutathione (GSH).93 Glutathione exists in two forms: oxidized and reduced, and Glutathione reductase catalyzes their interconversion, enabling them to regulate the intracellular oxidative balance.94 GSH serves as a cofactor for GPX4, allowing GPX4 to reduce hydrogen peroxide or peroxidized PUFA PLs to water and alcohol, respectively. This action inhibits the process of lipid peroxidation within cells and consequently hinders the occurrence of ferroptosis.95
When there is an increase in extracellular glutamate, or specific inhibitors hinder the function of the cystine-glutamate reverse transporter system, the transport of cystine and glutamate into cells becomes impaired, resulting in reduced intracellular GSH synthesis.96 Since GSH is a crucial antioxidant in cells, its reduction leads to the accumulation of a large number of lipid reactive oxygen species, consequently promoting the occurrence of ferroptosis and causing cell death. GPX4 is a significant marker of ferroptosis and one of the GSH peroxidases. It is a Selenoprotein, and its functionality depends on the selenium level in the body.97 When selenium is deficient, the catalytic activity of GPX4 can decrease dramatically, up to 1000 times.97 Research has indicated that both the iron inducer Erastin and RSL3 can inhibit the activity of GPX4. Erastin may promote ferroptosis by depleting the GPX4 substrate GSH, while RSL3 directly inhibits the activity of GPX4.39 Consequently, the inactivation or reduction of GPX4 becomes a crucial factor in promoting tumor cell death, which is also an important condition for inducing ferroptosis in tumor cells.
Others
In addition to iron ion metabolism, lipid metabolism, and amino acid metabolism, ferroptosis is regulated by other regulatory factors, including ferroptosis suppressor protein 1 (FSP1), NRF2, p53, and mitochondria.98,99
Fsp1
FSP1 is a recently discovered significant regulatory protein crucial in ferroptosis inhibition. Initially known as mitochondrial apoptosis-inducing factor 2, it was renamed FSP1 in 2019 to highlight its unique function in preventing cell ferroptosis.100 FSP1 functions as the Oxidoreductase of Coenzyme Q10 (CoQ10).101 Its N-terminal undergoes lipid modification through myristoylation, promoting FSP1’s localization to the plasma membrane that enables FSP1 to facilitate NADH-dependent CoQ reduction on the plasma membrane, inhibiting CoQ10 activity.102 Ultimately, through the FSP1-CoQ10-NAD(P)H pathway, which runs parallel to the classic glutathione (GSH) - GPX4 pathway, FSP1 hinders phospholipid peroxidation and ferroptosis by interfering with MDM2-MDMX.103
Nrf2
Nuclear factor E2-related factor 2 (NRF2) is a key regulatory factor in the endogenous antioxidant defense system of cells.104 It controls the expression of antioxidant and electrophilic stress genes and maintains cellular metabolism, redox, and protein homeostasis balance. NRF2 can reportedly induce the expression of numerous protective and detoxifying genes and is widely thought to act as a negative regulator of ferroptosis.105,106 In hepatoma cell lines, upregulation of NRF2 was found to promote the expression of antioxidant genes, such as heme oxygenase-1 and quinone oxidoreductase 1, leading to the inhibition of ferroptosis.107 Conversely, downregulation of NRF2 can accelerate ferroptosis induced by substances like erastin or Sorafenib.25 The NRF2 target genes not only regulate the key process of iron metabolism but also control the catabolism of foreign organisms and reactive aldehydes, as well as the synthesis of GSH and regeneration of nicotinamide adenine dinucleotide phosphate (NADPH), making it a versatile regulator of cellular resistance to iron degradation.108,109
P53
The transcription factor p53 plays a crucial role in inhibiting tumor cell growth. In recent years, its involvement in ferroptosis has become a new research hotspot. Studies indicate that p53 exhibits a dual role in regulating ferroptosis.110 On one hand, it can promote the occurrence of ferroptosis by inhibiting the expression of solute vector family 7 member 11 (SLC7A11) or by promoting the expression of spermidine/spermine N1 acetyltransferase 1 (SAT1) and glutaminase 2 (GLS2).111 On the other hand, it can inhibit ferroptosis by inhibiting dipeptidyl peptidase 4 (DPP4) activity or by promoting the expression of cell cyclin-dependent kinase inhibitor 1A (CDKN1A/p21).112
Vdac
Voltage-dependent anion channel (VDAC) is an ion channel in the outer mitochondrial membrane that mediates and controls molecular and ion exchange between mitochondria and the cytoplasm.113 Yagoda et al revealed that cells activated by the mitosis-activated protein kinase pathway (RAS-RAF-MEK pathway) could bind with certain ligands of VDAC, resulting in increased sensitivity to erastin.114 Erastin can prevent and reverse the blocking of VDAC by free Tubulin in the cytoplasm in vivo and in vitro, thus opening VDAC and leading to non-apoptotic cell death.115 VDAC can be upregulated in response to the RAS-RAF-MEK pathway, leading to mitochondrial dysfunction and ROS accumulation.116 Additionally, drugs can alter the permeability of VDAC, resulting in disrupted mitochondrial metabolism, leading to an influx of substances into the mitochondria and excessive ROS production.117
The Mechanism of Tumor Cell Ferroptosis Mediated by Nanoparticles
With the rapid advancement of nanotechnology and biomaterials, loading ferroptosis inducers onto nanocarriers is feasible, offering significant advantages.62,118 It enhances the accumulation and controlled release of ferroptosis inducers at tumor sites, effectively reducing the toxicity of drug delivery systems, overcoming drug resistance in tumor cells, and notably enhancing the ferroptosis effect on tumor cells. Ferroptosis can be induced by various drugs and strategies that lead to the accumulation of reactive oxygen species and lipid peroxides within cells. To achieve efficient ferroptosis induction in tumor cells, novel nanopreparations adopt the following strategies: (1) Increasing the content of intracellular iron ions to trigger the Fenton reaction, thereby elevating ROS levels.119 (2) Inhibiting system Xc− activity to reduce glutathione (GSH) synthesis or suppressing GPX-4 to increase intracellular ROS and lipid peroxide accumulation.120 (3) Exogenously regulating Lipid peroxidation in tumor cells.121 By employing these ferroptosis-induction strategies, researchers have significantly improved the efficacy of tumor treatment, offering the potential for developing new, efficient, and low-toxicity nano agents (Figure 1).
Iron Ion-Based Fenton Reaction Induces Ferroptosis
It is now understood that the process of tumor cell ferroptosis is dependent on the presence of iron, with a significant increase in ferrous ion (Fe2+) levels observed during ferroptosis. The elevated Fe2+ triggers the Fenton reaction [Fe2+ + H2O2 → Fe3+ + (OH)− + •OH], generating toxic hydroxyl radicals that react with polyunsaturated fatty acids in cells, resulting in the production of lipid peroxides and inducing ferroptosis. However, the concentration of hydrogen peroxide and iron ions in tumor cells is generally low, making it less effective in inducing ferroptosis.122 To address this, researchers have devised various nanotherapy strategies to trigger Fenton reactions in tumor cells. One strategy involves delivering high-performance nanocatalysts based on nanodrug delivery systems or directly delivering reactants of Fenton reactions, such as iron ions and H2O2 (Table 1). For instance, Xu et al123 designed a highly efficient and multifunctional nano drug delivery system by encapsulating Piperine nano MOF within a pH-sensitive lipid layer and modifying it with transferrin (Tf-LipoMof@PL) (Figure 2). This system regulates intracellular iron by using iron-containing MOF and transferrin, while Piperine, an inducer of ferroptosis, provides H2O2. Combining these two triggers the Fenton reaction, increasing intracellular ROS and inducing cell ferroptosis. In vitro and in vivo experiments demonstrated that Tf-LipoMof@PL had a highly effective cytotoxic effect, with the Tf-LipoMof@PL group showing the strongest anti-tumor effect. Furthermore, due to their strong hydrophobicity, good biological stability, and low toxicity, Ferrocene and its derivatives can act as stable sources of Fe2+ and catalyze the Fenton reaction. Lin et al successfully prepared amphiphilic polymer nanoparticles with selective release of doxorubicin (mPEG-b-PPLGFc@Dox).124 When entering the body, mPEG-b-PPLGFc@Dox undergoes oxidation by endogenous H2O2, leading to a hydrophilic-to-hydrophobic transition of hydrophobic Ferrocene molecules and releasing Dox. This process disrupts the redox balance and increases H2O2. Additionally, the Fe2+ from Ferrocene can transform H2O2 into hydroxyl radicals (•OH), subsequently inducing ferroptosis. To improve the utilization of iron sources and enhance the induction of Lipid peroxidation in cells,125 FeOCl nanosheets functionalized with unsaturated phospholipids and polyacrylic acid (FeOCl@PAA-Lip) were prepared by He et al.126 These nanosheets enter tumor cells through endocytosis, reacting with H2O2 in tumor cells to generate •OH via the Fenton reaction. The •OH attacks the unsaturated phospholipids on the FeOCl@PAA-Lip, converting them into phospholipid free radicals (L •) and lipid peroxidation free radicals (LOO •), effectively avoiding their annihilation. The FeOCl@PAA-Lip particles exhibit good dispersion and physiological stability. Apart from organic nanocatalytic medicines based on iron ions, inorganic nanocatalytic medicines using iron ions, such as Fe2O3 and Fe3O4, are also essential components of nanotherapy strategies.127
 |
Table 1 Nanoparticles for Ferroptosis in Tumor Cells |
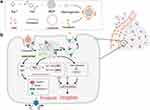 |
Figure 2 Formation of the Tf-LipoMof@PL nanoparticles (a) and the illustration of the mechanism of the nanoparticles (b). Reprinted with permission of the Royal Society of Chemistry from Xu R, Yang J, Qian Y, et al. Ferroptosis/pyroptosis dual-inductive combinational anti-cancer therapy achieved by transferrin decorated nanoMOF. Nanoscale Horiz. 2021;6(4):348–356; permission conveyed through Copyright Clearance Center, Inc.123 |
Inhibition of GPX-4 Activity Induces Ferroptosis
GPX-4 is a crucial regulator of ferroptosis, a process characterized by iron-dependent lipid peroxidation and reactive oxygen species generation. GPX-4 catalyzes the conversion of R-OOH to R-OH, effectively preventing the production of iron-dependent lipid ROS and inhibiting ferroptosis. In recent years, nanotherapy approaches that target GPX-4 activity have been harnessed to induce ferroptosis, involving the targeted delivery of GPX-4 small molecule inhibitors and the rational design of nanocarrier materials with GPX-4 inhibitory function. One such inhibitor is RSL3, which directly targets GPX4 to trigger ferroptosis. By inhibiting the expression of GPX4, RSL3 disrupts cysteine and glutamate ester transporters, ultimately leading to cell ferroptosis (Table 1). Liang et al developed magnetic Fe3O4 nanocubes loaded with the small molecule ferroptosis inducer RSL3, modified with Polyethyleneimine (PEI) and Hyaluronic acid (HA) (Fe3O4-PEI@HA-RSL3).128 This drug delivery system actively targets tumor cells using an external magnetic field in combination with HA-CD44. Cell experiments revealed that Fe3O4-PEI@HA-RSL3 nanoparticles effectively suppressed the proliferation of liver cancer cells without harming normal liver cells. An increase in the concentration of Fe3O4-PEI@HA-RSL3 nanoparticles was found to activate the ferroptosis signal transduction pathway by inhibiting the expression of Lactoferrin, FACL4, GPX4, and Ferrin protein in Huh 7 cancer cells, leading to an enhanced formation of ROS. Sorafenib, another agent, has been reported to inhibit GPX4 activity by reducing GSH synthesis and inhibiting the activity of the SLC7A11 transporter, thus promoting ROS and lipid peroxide accumulation and inducing ferroptosis to achieve a therapeutic effect. Studies have demonstrated that Manganese dioxide nanoparticles loaded with Sorafenib effectively deplete GSH in cells, resulting in reduced GPX4 activity, enhanced lipid ROS accumulation, and the induction of ferroptosis in hepatocellular carcinoma (HCC). Iron nanoparticles loaded with Sorafenib (MIL-101(Fe)@sor NPs) significantly inhibit tumor growth, reduce GPX-4 expression levels, and exhibit negligible long-term toxicity.131 When MIL-101(Fe)@sor NPs and iRGD peptide are combined, they notably promote lipid ROS accumulation and accelerate ferroptosis occurrence in HCC.129
Reduction of Glutathione Level Induces Ferroptosis
Glutathione, composed of glutamic acid, cysteine, and glycine, exerts antioxidant effects and regulates cellular redox balance. The elevated expression of GSH in tumor cells, typically 7–10 times higher than normal cells, poses challenges to various cancer treatment strategies, given that GSH also acts as a cofactor of GPX-4.136 Consequently, depleting GSH can disrupt the redox balance in tumor cells, making it a targeted strategy for tumor treatment (Table 1). Wang et al reported manganese-doped silicon nanoparticles (AMSNs) modified with arginine to consume intracellular GSH.130 The arginine (Arg) ligands on AMSNs’ outer surface allowed tumor targeting after intravenous administration. AMSNs contained a manganese oxygen bond (- Mn-O -) sensitive to the tumor microenvironment rich in GSH. Upon breaking one molecule of - Mn-O -, two molecules of GSH were consumed, demonstrating efficient GSH clearance ability and facilitating ferroptosis induction. Similarly, Tang et al developed manganese-doped mesoporous silica nanoparticles loaded with Sorafenib by a one-pot method (MMSNs@SO).131 MMSNs@SO degraded in the tumor microenvironment due to the breakage of - Mn-O -, leading to glutathione depletion in cells. Additionally, the degradation of MMSNs released Sorafenib, dysregulating the function of the Xc− transport system. As a result, GSH content rapidly decreased in HCC cells, and the synthesis of GSH was inhibited upon incubation with MMSNs@SO. Another approach designed by Zhang et al involved constructing nanoparticles (PCDF) with doxorubicin and Fe3+, combined with a ROS-sensitive ligand (TCA) binding cinnamaldehyde (CA).132 PCDF internalized by tumor cells released CA upon exposure to intracellular ROS, increasing ROS levels and consuming glutathione through thiol Michael addition reaction, ultimately inducing iron apoptosis and leading to significant and sustained tumor suppression in in vivo experiments. Moreover, He et al designed anti-HER2-modified arsenic nanosheets loaded with cisplatin (Her2-ANs@CDDP).133 These nanosheets could overcome cisplatin drug resistance by consuming intracellular GSH and inducing ferroptosis, thus showing promising application potential in treating chemotherapy-insensitive tumors and expanding the usage of arsenic in solid tumor treatment.
Inhibitory System Xc− Activity Induces Ferroptosis
Current evidence suggests that the intracellular cysteine concentration is closely related to the initial step of glutathione production, which is regulated by the membrane Na+-dependent cysteine-glutamic acid exchange transporter known as system Xc−. This transporter is responsible for transporting glutamic acid out of the cell and NADPH into the cell. Intracellular cystine is further converted into cysteine, which promotes GSH synthesis. Thus, downregulation of system Xc− expression can reduce intracellular cysteine concentration, limiting GSH synthesis efficiency, which increases the level of intracellular ROS and the accumulation of lipid peroxides, ultimately inducing ferroptosis. Erastin, an inhibitor of system Xc−, induces cell ferroptosis by inhibiting its activity, activating P53, and acting on voltage-dependent anion channels, highlighting its significance as an important anti-tumor strategy. Exosomes are microbubbles secreted by cells that effectively transfer internal substances to surrounding cells through interactions between rich adhesion proteins and cell membranes. However, Erastin faces challenges due to its poor water solubility and low bioavailability. To overcome these issues, Yu et al134 designed a system to load Erastin into FA-modified exosomes (erastin@FA-exo), which enabled the rapid and massive accumulation of Erastin in triple-negative breast cancer cells, significantly increasing ROS production and GSH consumption in cells. Similarly, Gai et al developed a nanodelivery system based on folate-modified liposomes (FA-LP) to co-deliver MT1DP (a lncRNA) and Erastin (E/M@FA-LPs).135 In vitro and in vivo experiments demonstrated that E/M@FA-LPs exhibited a potent killing effect on tumor cells (Table 1).
Exogenous Regulation of Lipid Peroxidation of Tumor Cells Induces Ferroptosis
Lipid peroxides primarily originate from PUFAs produced by membrane phospholipids under oxidative stimulation. Supplementing exogenous lipids can enhance the accumulation of lipid peroxides within cells. Researchers conducted screenings of various unsaturated fatty acids and discovered that oral administration of conjugated linolenic acid effectively induces ferroptosis in triple-negative breast cancer cells, demonstrating its potent activity.137 This finding supports the possibility of using exogenous lipid supplementation to induce ferroptosis for cancer treatment, providing the foothold for the design of relevant nanodrug delivery systems. Zhou et al developed a nano drug delivery system based on a Fenton-like reaction. They modified the surface of iron oxide nanoparticles with hydrophobic peroxidized linoleic acid (LAHP) and hydrophilic oligomers.138 In an acidic tumor microenvironment, metastable iron(II) oxide and ferric tetroxide release Fe2+ on demand. Subsequently, the release of Fe2+ and LAHP significantly generates singlet oxygen (1O2). These LAHP-modified iron oxide nanoparticles induce ferroptosis of tumor cells by producing tumor-specific ROS. Moreover, Gao et al designed polymer micelles with modified unsaturated lipid side chains, encapsulating the ferroptosis inducer RSL3.139 Notably, modifying the unsaturated fatty acid side chain enhanced the level of lipid peroxidation in vivo and synergistically induced ferroptosis.
Combined Treatment Scheme Based on Ferroptosis
The combination of two or more therapies has been found to enhance their interaction and produce significant superadditive (“1+1>2”) therapeutic effects.140 As a result, tumor therapy has gradually shifted towards multimodal approaches with flexible and adjustable advantages. Numerous studies have demonstrated that compared to single treatment strategies, combining multiple therapies offers distinct advantages in clinical cancer treatment, including efficient synergistic effects,141 reduced adverse reactions,142 and addressing drug resistance.143 In recent years, the development of nano-drug delivery technology has provided a versatile platform for co-loading two or more drugs, opening up promising possibilities for combined tumor therapy. This paper reviews the latest research progress on combining ferroptosis with chemotherapy, phototherapy, sonodynamic therapy, immunotherapy, hunger therapy, and other therapies based on nanotechnology. These advancements present a new nano-combined delivery approach for effective cancer treatment (Table 2 and Figure 3).
 |
Table 2 Combination Therapy Regimens Based on Ferroptosis |
Ferroptosis Combined with Chemotherapy
Chemotherapy (CT) is a crucial cancer treatment method, often indicated for managing tumor recurrence and metastasis.151,152 However, most anti-tumor chemotherapy drugs suffer from drawbacks like poor water solubility, low specificity, and severe organ toxicity, which limit their clinical use.153 Using nanodrug delivery systems to load chemotherapy drugs has significantly improved drug delivery efficiency and therapeutic effectiveness.154 Nevertheless, the efficacy of single-drug chemotherapy remains poor for most tumors. Hence, combining chemotherapy with other therapies has become a prominent trend in tumor treatment. Doxorubicin (DOX) is a common chemotherapy drug often combined with ferroptosis therapy. DOX typically induces cell apoptosis through a gene-regulated process involving complex signal transduction within cells.155 However, this slow process leads to weak inhibitory effects on tumors. Additionally, tumor cells often develop resistance to apoptosis-inducing drugs by enhancing gene mutations. Long-term exposure to apoptosis inducers can also invalidate apoptosis mechanisms. DNA damage reactions further contribute to resistance, undermining the efficacy of chemotherapy drugs. To address these challenges, Lin et al synthesized amphiphilic polymer nanoparticles that can be oxidized by ROS and selectively release DOX (mPEG-b-PPLGFc@Dox).124 When these nanoparticles enter tumor cells and undergo oxidation by endogenous H2O2, Fe (II) ions and DOX are released, and Fe(II) derived from Ferrocene triggers the Fenton reaction, generating ·OH and inducing ferroptosis. Furthermore, DOX stimulates cancer cells to produce more H2O2, leading to significant apoptosis. In vivo anti-tumor experiments with mPEG-b-PPLGFc@Dox demonstrated lower systemic toxicity and higher combined treatment efficiency of chemotherapy and ferroptosis, effectively inducing tumor cell death and inhibiting tumor growth in mice with volumes of approximately 200 mm3. Similarly, Celia Nieto et al developed a method to simultaneously load Fe3+ and DOX by examining the adsorption of PDA NPs on Fe3+ under different pH conditions (PD NPs@Fe/DOX).156 This approach led to a synergistic therapeutic effect of ferroptosis and DOX chemotherapy, significantly reducing the viability of breast cancer cells without affecting the survival rate of normal cells to the same extent. In another study, Ji et al designed a tumor-selective nanoreactor (DOX) based on MOF/ART@LiMOFs, where Artesunate (ART) and DOX were wrapped in the porous structure of MOF and coated with a lipid bilayer on the surface.144 LiMOFs exhibited excellent pH response, and in an acidic tumor cell environment, they self-degraded, providing ferrous ions and inducing ART to carry out redox reactions in cells, producing ROS to trigger Lipid peroxidation and induce ferroptosis. Simultaneously, DOX slowly permeated outward, activating the apoptotic pathway and achieving a binding function of cell apoptosis and iron apoptosis. Apart from doxorubicin, other chemotherapy drugs like cisplatin (DDP)157,158 and camptothecin (CPT)159 are also often used in combination with ferroptosis to enhance tumor treatment.
Ferroptosis Combined with Optical Therapy
Optical therapy encompasses photodynamic therapy and photothermal therapy. Photothermal therapy (PTT) employs photothermal agents to harness energy from external light sources, typically near-infrared light, and convert it into heat, raising the surrounding environment’s temperature and inducing cancer cell death.160 PTT offers several advantages, including low invasiveness, high spatiotemporal accuracy, and low biological toxicity. When tissue reaches 41°C, cells upregulate heat shock proteins (HSPs) to resist initial heat damage. At temperatures exceeding 42°C, irreversible tissue damage and cell necrosis occur. Additionally, at 46–52°C, microvascular thrombosis and ischemia can arise. Beyond 60°C, protein denaturation and plasma membrane damage cause rapid cell death.161 The insufficient blood supply in tumor tissue results in lower heat resistance than normal tissue, making photothermal therapy an appropriate approach for treating solid tumors.162 PTT typically uses near-infrared light ranging from 650 nm to 900 nm as the light source since tissue absorption in this range is weak, resulting in deep tissue penetration and minimal tissue damage.163 However, the heterogeneity of tumors and the low efficiency of deep tissue photothermal conversion have limited the efficacy of single photothermal therapy in eradicating tumors.164 Therefore, combining multiple drugs or treatment modes to construct a multimodal collaborative treatment system is important to improve treatment effectiveness and reduce chemotherapy drug toxicity.165 Jiang et al145 synthesized Bi2Te3 NPs with high-performance photothermal electrocatalytic effects through hydrothermal reactions. Under near-infrared light irradiation (808 nm and 1064 nm), Bi2Te3 NPs induced PTT temperatures to increase to 55.6°C and 63.3°C, respectively, effectively inhibiting the expression of MTH1. The temperature changes caused by heating and cooling led to Bi2Te3 NPs generating free charges and electron transfer, generating ROS, damaging heat shock proteins, reducing the heat tolerance of tumor cells, and synergistically enhancing PTT. Moreover, the hole (H+) with strong oxidizing capacity on Bi2Te3 NPs can oxidize GSH to GSSG, leading to GSH depletion, further improving the ROS level, and ultimately reducing Glutathione peroxidase 4 activity, inducing ferroptosis of tumor cells. In vivo anti-tumor results showed that Bi2Te3 NPs exhibited significant tumor inhibitory effects in both ectopic and in situ eye tumor models. Among the reported PTT materials, the polymer polydopamine (PDA) is an excellent photothermal conversion material with biocompatibility. Additionally, its molecular structure’s high content of primary and secondary amines and catechol (3,4-dihydroxybenzene) provides it with high adhesion and excellent drug release ability. Researches166,167 prepared (PDA)-hemin PEG ferrocene (Fc) (PHPF) nanomotors and Fe3O4@PDA-HCPT (10-hydroxycamptothecin) NPs, and in vivo and in vitro experiments demonstrated that the combination of photothermal ferroptosis and photothermal chemotherapy ferroptosis significantly inhibited tumor growth, making nanoparticles a promising cancer treatment strategy.
Photodynamic therapy (PDT) is a non-invasive and highly selective treatment method.168 Its principle involves sending a specific photosensitizer (PS) to an excited state, and the excited state photosensitizer transmits energy to the surrounding oxygen, generating reactive oxygen species, including singlet oxygen, which exerts toxicity on surrounding tissues and cells.169 PDT has been widely used in treating tumors such as esophageal cancer and skin cancer.170 Although PDT offers many advantages, the tumor site’s hypoxic microenvironment often limits its ability to produce 1O2 due to the large amount of O2 involved in its production process. Simultaneously, PDT’s characteristic consumption of O2 further exacerbates hypoxia at the tumor site, inhibiting its therapeutic effect.171 Generally, ferroptosis requires two factors, ROS production and GSH depletion. Therefore, there may be some interaction and mutual promotion between ferroptosis and PDT.172 Zhao et al173 found that the highly active 1O2 produced by PDT can stimulate ferroptosis by consuming GSH. In vivo anti-tumor experiments showed that the novel metal-organic framework loaded with Chlorin e6 (Ce6) significantly inhibited tumor growth through PDT-induced ferroptosis. However, ferroptosis induced by PDT (mainly to promote GSH depletion) may be inhibited by iron droop inhibitor protein 1 (FSP1). To overcome this limitation, researchers used the light-responsive nanocomposite self-assembled by BODIPY-modified PAMAM (BMP) to stably encapsulate the FSP1 inhibitor (iFSP1) and Ce6.146 This nanosystem can promote intracellular transmission, penetration, and accumulation of the iron death inducer in tumors under light radiation. Both in vitro and in vivo, they demonstrated a high ability to trigger ferroptosis and immunogenic cell death (ICD).
Ferroptosis Combined with Acoustic Dynamic Therapy
Sonodynamic therapy (SDT) was first proposed by the Japanese scientist Yumita as a tumor treatment strategy that combines low-frequency focused ultrasound irradiation with sound-sensitive substances.174,175 Traditional sound sensitizers mainly originate from photosensitive substances, known as photosensitizers, which possess special porphyrin rings, anthracene rings, and other structures that respond well to light and sound.176 When stimulated by radiation, they can rapidly produce highly oxidizing substances such as reactive oxygen species to kill tumor cells. The main chemical dynamic process in which ferroptosis occurs is the Fenton reaction. Research has shown that Fenton catalysis can be mediated by external energy stimulation. For instance, light Fenton relies on a reaction solution of metal ions, where ultraviolet light stimulates and guides ions to generate redox reactions, producing H2O2 and adjusting the iron valence, thus initiating an electron exchange process.177,178 Similarly, ultrasound can mediate Fenton catalysis, and the sound energy addresses the unevenness of light irradiation, resulting in more comprehensive energy conduction. Bai et al147 synthesized Fe-doped TiO2 nanoparticles (Fe TiO2 NDs) through a thermal decomposition strategy, which can serve as a sound-sensitive agent for the combined treatment of tumors with SDT and CDT. The experimental results indicate that with increased Fe doping, the Fenton reaction of Fe TiO2 NDs becomes more pronounced. However, to optimize the balance between US irradiation and Fenton reaction-induced ROS generation, the authors chose Fe TiO2 NDs with a Fe:Ti feed ratio of 1:4 for subsequent experiments. Upon intravenous injection, Fe TiO2 NDs efficiently accumulate in tumors and exert significant anti-tumor therapeutic effects through the combined action of SDT and CDT. Moreover, due to the extremely small size of Fe TiO2 NDs (average size 2.49 ± 0.74 nm), they can be excreted from the body, and their long-term toxicity to mice can be disregarded. Therefore, incorporating metal ions into TiO2 nanostructures boosts the production of reactive oxygen species by ultrasound-triggered nanodroplets and confers Fenton catalytic capabilities to generate ROS from endogenous H2O2 within tumors. This offers promising prospects for the application of combined sonodynamic therapy and catalytic therapy in cancer treatment. This offers promising prospects for the application of combined sonodynamic therapy and catalytic therapy (SDT/CDT) in cancer treatment. Similarly, Hu et al148 designed iron (Fe) and manganese (Mn) co-doped zinc oxide nanosensitizers, which can synergistically induce ferroptosis in addition to the sono-Fenton catalysis mediated by nano SDT. Nie et al179 synthesized HSA-Ce6 IrO2 (HCIr) nanoclusters using a mild biomineralization method. HCIr produces a large amount of 1O2 under US stimulation, promoting the accumulation of lipid peroxidation (LPO) in cells. Additionally, HCI can consume GSH by accelerating the conversion of Ir (IV) to Ir (III), inhibiting GPX4 activity, thus enhancing SDT-induced ferroptosis. Zhu et al149 combined biomimetic technology with SDT and ferroptosis to construct multifunctional cancer homologous targeting biomimetic nanoparticles encapsulating Fe3O4 and Ce6 (PIOC@CMNPs) as a nanosound sensitizer. The unique tumor-specific surface antigen on the C6 cell membrane (CM) of gliomas endows PIOC@CMNPs with innate immune escape and homologous cell targeting advantages, enabling targeted delivery to tumor tissue. Under ultrasound irradiation, the reduction of ROS and the consumption of GSH lead to the loss of GPX4 activity, promoting SDT and ferroptosis to kill glioma C6 cells. Mitochondria are potential therapeutic targets crucial in regulating tumor cell death and metabolism. Wang et al22 proposed a new mitochondrial targeting nanosystem (“Mito Bob”) for SDT promoted by ferroptosis. The sound-sensitive agent IR780 and the ferroptosis activator RSL-3 are encapsulated in biocompatible PLGA nanoparticles to form “Mito Bob” (IRP NPs). IR780 mediates mitochondrial targeting of SDT in this nanosystem, while RSL-3 inhibits GPX4 activity in the antioxidant system and induces ferroptosis of tumor cells, thereby altering tumor metabolism and enhancing the sensitivity of tumor cells to SDT-induced apoptosis. Additionally, the good biosafety of IRP NPs makes them more valuable for clinical applications.
Combined Immunotherapy for Ferroptosis
In recent years, there has been growing interest in using immunotherapy (IMT) as an innovative approach to cancer treatment.180 Specifically, the use of PD-1 antibodies has gained FDA approval for clinical treatment.181 At the same time, researchers have been exploring the potential of ferroptosis, a process that regulates intracellular lipid and oxygen metabolism and is associated with immune response, as a complementary method to enhance cancer treatment outcomes.182 Traditional tumor immunotherapy primarily focuses on four approaches:183 immunomodulatory therapy, immune checkpoint blockade therapy, adoptive immunotherapy, and tumor vaccine immunotherapy. However, these therapies still face certain limitations, including effectiveness being limited to a small group of patients,184 potential adverse reactions, and low efficiency due to insufficient immunogenicity of tumor cells.185 The combination of immunotherapy with ferroptosis has emerged as a promising and effective strategy for cancer treatment. Ferroptosis can effectively inhibit tumor growth and increase the immunogenicity of tumor cells. When ferroptosis occurs, tumor cells release tumor-associated antigens (TAAs) and damage-associated molecular patterns (DAMPs), which induce the maturation and antigen presentation of dendritic cells (DCs). This, in turn, initiates a T-cell-mediated anti-tumor immune response.186,187 Ferroptosis also triggers immunogenic cell death (ICD) in tumor cells, activating T-cell immune responses.188–190 The release of interferon-gamma (IFN-γ) further enhances the immune response and inhibits tumor growth by reducing cystine uptake by tumor cells, leading to the inhibition of GSH synthesis and promoting ferroptosis.191,192
Researchers150 have conducted experiments using ferroptosis inducer Cannabidiol nanoparticles (CBD NPs) and the immune enhancer Poly (I: C) (CP nanoparticles) delivered via a Pluronic F127 thermosensitive injection hydrogel. The study demonstrated that CBD NPs promote ferroptosis in tumor cells and induce lipid peroxidation and ferroptosis. Additionally, CP nanoparticles enhance the immune response, inhibiting distant tumors, reducing the burden of metastatic tumors, and improving the survival rate in a lung metastasis mouse model. This approach of combining CP nanoparticles to induce tumor ferroptosis and immune activation proves to be a promising strategy for controlling tumor development. Researchers such as Chin193 and Wang et al194 have observed similar successful designs and conclusions. Furthermore, using an FSP1 inhibitor (iFSP1) in mice has shown increased numbers of DCs, macrophages, and T cells in tumors. This novel finding suggests that iFSP1 can cooperate with various immunotherapies to improve the survival rate in mice with liver cancer, presenting a new avenue for the combined application of ferroptosis and immunotherapy in cancer treatment.195
Combination of Ferroptosis and Hunger Therapy
Organisms possess an inherent enzyme called glucose oxidase (GOx), which efficiently catalyzes the conversion of β-D-glucose into gluconic acid and H2O2. Subsequently, the H2O2 interacts with Fe2+ to induce ferroptosis in tumor cells. Tumor growth relies on glucose to generate ATP for energy, but GOx can deplete a substantial amount of glucose within the tumor, cutting off the nutrient supply and impeding the growth of tumor blood vessels, a process referred to as “hunger therapy” for tumors.196 Wan et al197 introduced a nanoreactor utilizing an iron metal-organic framework (MOF) and glucose oxidase (GOx) modified cancer cell membrane coating. Upon reaching the tumor site, the high levels of GSH trigger the reduction of Fe3+, leading to the collapse of the MOF structure and releasing Fe2+. Simultaneously, GOx catalyzes glucose oxidation to generate H2O2. The interaction between H2O2 and Fe2+ via the Fenton reaction produces hydroxyl radicals (· OH), achieving a combined effect of ferroptosis and “starvation therapy”(ST).
Ferroptosis Combined with Multiple Treatment Strategies
Due to tumor heterogeneity, the induction of ferroptosis in combination with other monotherapies has not yet achieved the desired therapeutic effect. Researchers have explored multi-functional nanoplatforms to combine multiple treatment methods with ferroptosis, aiming to achieve a synergistic and enhanced tumor treatment effect. Liang et al198 constructed a novel TME activated metal organic framework by involving Fe & Cu ions bridged by disulfide bonds (FCSP@DOX MOFs). FCSP@DOX MOFs could accumulate in the tumor site through the EPR effect and break disulfide linkers in the TME of overexpressed GSH, consuming GSH. This nanoparticle not only inhibits GPX4 expression and induces ferroptosis, but also simultaneously releases Fe3+, Cu2+ and DOX. The release of double ions serves as a catalyst for the Fenton reaction of overexpressed H2O2, whereas DOX can induce chemotherapy by inhibiting topoisomerase II and additionally enhance the therapeutic impact of ferroptosis through the production of H2O2. Meanwhile, due to the inherent photothermal property of the released Cu2+and FCSP MOFs, more ROS could be generated through the acceleration of Fenton reaction. This system is specifically activated in TME, achieving a synergistic therapeutic effect based on ferroptosis through chemodynamic/photothermal/chemo therapy. In another recent study, Wang et al199 reported a NIR-triggered and ROS-boosted nanoplatform [MnO2-SOR-Ce6@PDA-PEGFA (MSCPF)] for enhancing the chemo/PDT/PTT synergistic therapy of sorafenib (SOR/SRF) in HCC treatment. MSCPF could generate excessive ROS to alleviate tumor hypoxia, and the production of ROS further enhances Ce6-mediated PDT and PDA-mediated PTT. In vitro and in vivo experiments have shown that MSCPF could inhibit P-gp expression and induce ferroptosis through the inactivation of GPX4 and SLC7A11, ultimately enhancing the synergistic therapeutic effect of ferroptosis in HCC treatment. In this research, the constructed MSCPF not only demonstrates great potential as a multifunctional nanoplatform to improve the efficiency of HCC treatment and reduce drug resistance, but also provides new ideas for loading other anti-tumor drugs and synergistic PTT/PDT therapy of other types of tumors. These combinations have shown relatively efficient anti-tumor treatment outcomes, presenting advantages over single therapies. The development of multi-strategy synergistic anti-tumor therapy based on ferroptosis holds great promise and significance. However, these novel combined multi-strategy therapies still have certain drawbacks and potential risks that hinder their further clinical application. Challenges include the complexity of nanocomposite systems, difficulty in achieving industrial production, accurate calculation of synergistic effects between multiple therapies, and concerns about drug toxicity and side effects resulting from the combination of different drugs or methods.
Summary and Outlook
Ferroptosis is a novel cell death mechanism induced by iron-dependent lipid peroxides that has garnered increasing attention in tumor biology and anti-tumor treatment as a novel target for cancer therapy. Nanotechnology and emerging nanomaterials have provided a foundation for delivering ferroptosis inducers and combining them with multiple therapies, capitalizing on their unique advantages. This review summarizes the latest research on nanotechnology-induced tumor ferroptosis and highlights the design of nanotherapy methods based on ferroptosis inducers and their combined applications with other treatments. Albeit significant progress has been made in cancer treatment based on ferroptosis, the clinical implementation of ferroptosis-based nanotherapy remains challenging. It is essential to further study the potential differences in this non-apoptotic cell death mode between animals and humans. Additionally, research on potential adverse reactions of ferroptosis inducers is crucial to ensure tumor-specific ferroptosis induction while avoiding off-target toxicity to normal tissues. The design of combined nanotherapy schemes based on ferroptosis requires careful evaluation of potential superimposed toxicity and industrial feasibility. Thus, more research is needed to deepen our understanding and exploration of ferroptosis, considering various factors, to design safe and effective nano preparations capable of inducing ferroptosis.
Acknowledgments
This work was supported by “Changsha Anti-Infective Drugs Engineering Technology Research Center” [No. kq1801120], Changsha Municipal Natural Science Foundation [No. kq2208463, No. kq2208464]and the Hunan Provincial Science and Technology Department Foundation, China [No. 2016SK4008, No. 2020SK52901].
Disclosure
The authors report no conflicts of interest in this work.
References
1. Machado V, Morais M. Hyaluronic acid-based nanomaterials applied to cancer: where are we now? Pharmaceutics. 2022;14:10.
2. Xie C, Gu A, Khan M, et al. Opportunities and challenges of hepatocellular carcinoma organoids for targeted drugs sensitivity screening. Front Oncol. 2022;12:1105454. doi:10.3389/fonc.2022.1105454
3. Ma M, Liang J, Zhang D, et al. Monitoring treatment efficacy of antiangiogenic therapy combined with hypoxia-activated prodrugs online using functional MRI. Front Oncol. 2021;11:672047. doi:10.3389/fonc.2021.672047
4. Le Y, Gao H, Bleday R, Zhu Z. The homeobox protein VentX reverts immune suppression in the tumor microenvironment. Nat Commun. 2018;9(1):2175. doi:10.1038/s41467-018-04567-0
5. Wu Z, Li S, Zhu X. The mechanism of stimulating and mobilizing the immune system enhancing the anti-tumor immunity. Front Immunol. 2021;12:682435. doi:10.3389/fimmu.2021.682435
6. Hont AB, Cruz CR, Ulrey R, et al. Immunotherapy of relapsed and refractory solid tumors with ex vivo expanded multi-tumor associated antigen specific cytotoxic t lymphocytes: a phase I study. J Clin Oncol. 2019;37(26):2349–2359. doi:10.1200/JCO.19.00177
7. Zhang X, Lin A, Han QY, et al. Intratumor heterogeneity of HLA-G expression in cancer lesions. Front Immunol. 2020;11:565759. doi:10.3389/fimmu.2020.565759
8. Raniolo S, Unida V, Vindigni G, Stolfi C. Combined and selective miR-21 silencing and doxorubicin delivery in cancer cells using tailored DNA nanostructures. Cell Death Dis. 2021;12(1):7. doi:10.1038/s41419-020-03339-3
9. Ding Y, Chen X, Liu C, et al. Identification of a small molecule as inducer of ferroptosis and apoptosis through ubiquitination of GPX4 in triple negative breast cancer cells. J Hematol Oncol. 2021;14(1):19. doi:10.1186/s13045-020-01016-8
10. Jiang Z, Li J, Feng W, Sun Y, Bu J. A ferroptosis-related lncRNA model to enhance the predicted value of cervical cancer. J Oncol. 2022;2022:6080049. doi:10.1155/2022/6080049
11. Zhao X, Zhang X, Xu T, Luo J. Comparative effects between oral lactoferrin and ferrous sulfate supplementation on iron-deficiency anemia: a comprehensive review and meta-analysis of clinical trials. Nutrients. 2022;14:3.
12. Rozema J, van Asten I, Kwant B, et al. Clinical view versus guideline adherence in ferritin monitoring and initiating iron chelation therapy in patients with myelodysplastic syndromes. Europ J Haematol Europ J Haematol. 2022;109(6):772–778. doi:10.1111/ejh.13865
13. Collatuzzo G, Teglia F, Pelucchi C. Inverse association between dietary iron intake and gastric cancer: a pooled analysis of case-control studies of the stop consortium. Nutrients. 2022;14(12):2555. doi:10.3390/nu14122555
14. Shao B, Mao L, Tang M, Yan ZY, Shao J. Caffeic acid phenyl ester (CAPE) protects against iron-mediated cellular DNA damage through its strong iron-binding ability and high lipophilicity. Antioxidants. 2021;10(5):798. doi:10.3390/antiox10050798
15. Li X, Si W, Li Z, et al. miR‑335 promotes ferroptosis by targeting ferritin heavy chain 1 in in vivo and in vitro models of Parkinson’s disease. Int J Mol Med. 2021;47(4). doi:10.3892/ijmm.2021.4894
16. Tang X, Chen W, Liu H, et al. Research progress on SLC7A11 in the regulation of cystine/cysteine metabolism in tumors. Oncol Lett. 2022;23(2):47. doi:10.3892/ol.2021.13165
17. Zhao T, Wang H, Liu Z, et al. Recent perspective of lactobacillus in reducing oxidative stress to prevent disease. Antioxidants. 2023;12(3):769. doi:10.3390/antiox12030769
18. Sun W, Zeng C, Yue D, et al. Ageratina adenophora causes spleen toxicity by inducing oxidative stress and pyroptosis in mice. Royal Soc Open Sci. 2019;6(7):190127. doi:10.1098/rsos.190127
19. Hamblin MR, Abrahamse H. Inorganic salts and antimicrobial photodynamic therapy: mechanistic conundrums? Molecules. 2018;23(12):3190. doi:10.3390/molecules23123190
20. El-Garawani IM, Khallaf EA, Alne-Na-Ei AA, et al. The effect of neonicotinoids exposure on Oreochromis niloticus histopathological alterations and genotoxicity. Bull Environm Contaminat Toxicol. 2022;109(6):1001–1009. doi:10.1007/s00128-022-03611-6
21. Yang Z, Huang R, Wang Y, et al. SIRT6 drives sensitivity to ferroptosis in anaplastic thyroid cancer through NCOA4-dependent autophagy. Am J Can Res. 2023;13(2):464–474.
22. Wang J, Zhao Z, Liu Y, et al. ‘Mito-Bomb’: a novel mitochondria-targeting nanosystem for ferroptosis-boosted sonodynamic antitumor therapy. Drug Delivery. 2022;29(1):3111–3122. doi:10.1080/10717544.2022.2126027
23. Cheng Z, Chen Y, Huang H. Identification and validation of a novel prognostic signature based on ferroptosis-related genes in ovarian cancer. Vaccines. 2023;11(2):205. doi:10.3390/vaccines11020205
24. Huang S, Cao B, Zhang J. Induction of ferroptosis in human nasopharyngeal cancer cells by cucurbitacin B: molecular mechanism and therapeutic potential. Cell Death Dis. 2021;12(3):237. doi:10.1038/s41419-021-03516-y
25. Zhu JF, Liu Y, Li WT, et al. Ibrutinib facilitates the sensitivity of colorectal cancer cells to ferroptosis through BTK/NRF2 pathway. Cell Death Dis. 2023;14(2):151. doi:10.1038/s41419-023-05664-9
26. Li Q, Chen Q, Yang X, et al. Cocktail strategy based on a dual function nanoparticle and immune activator for effective tumor suppressive. J Nanobiotechnol. 2022;20(1):84. doi:10.1186/s12951-022-01241-y
27. Peng P, Chen Z, Wang M, Wen B, Deng X. Polysaccharide-modified liposomes and their application in cancer research. Chem Biol Drug Des. 2023;101(4):998–1011. doi:10.1111/cbdd.14201
28. Liu J, Mu W, Gao T, Fang Y, Zhang N, Liu Y. CD13-mediated pegylated carboxymethyl chitosan-capped mesoporous silica nanoparticles for enhancing the therapeutic efficacy of hepatocellular carcinoma. Pharmaceutics. 2023;15(2):426. doi:10.3390/pharmaceutics15020426
29. Wan Z, Xie F, Wang L, Zhang G, Zhang H. Preparation and evaluation of cabazitaxel-loaded bovine serum albumin nanoparticles for prostate cancer. Int j Nanomed. 2020;15:5333–5344. doi:10.2147/IJN.S258856
30. Domiński A, Domińska M, Skonieczna M, Pastuch-Gawołek G, Kurcok P. Shell-sheddable micelles based on poly(ethylene glycol)-hydrazone-poly[R,S]-3-hydroxybutyrate copolymer loaded with 8-hydroxyquinoline glycoconjugates as a dual tumor-targeting drug delivery system. Pharmaceutics. 2022;14(2):290. doi:10.3390/pharmaceutics14020290
31. Wu D, Vogus D, Krishnan V, Broto M, Pusuluri A, Zhao Z. Optimized 5-fluorouridine prodrug for co-loading with doxorubicin in clinically relevant liposomes. Pharmaceutics. 2021;13(1):107. doi:10.3390/pharmaceutics13010107
32. Dai C, Zhang D, Li J, Li J. Effect of colistin exposure on calcium homeostasis and mitochondria functions in chick cortex neurons. Toxicol Mech Methods. 2013;23(4):281–288. doi:10.3109/15376516.2012.754533
33. Zhao M, van Straten D, Broekman MLD, Préat V, Schiffelers RM. Nanocarrier-based drug combination therapy for glioblastoma. Theranostics. 2020;10(3):1355–1372. doi:10.7150/thno.38147
34. Piao X, Yin H, Guo S, Wang H, Guo P. RNA nanotechnology to solubilize hydrophobic antitumor drug for targeted delivery. Advan Sci. 2019;6(22):1900951. doi:10.1002/advs.201900951
35. Zhang X, Wang L, Li H, Zhang L, Zheng X, Cheng W. Crosstalk between noncoding RNAs and ferroptosis: new Dawn for overcoming cancer progression. Cell Death Dis. 2020;11(7):580.
36. Kong F, He H, Bai H, et al. A biomimetic nanocomposite with enzyme-like activities and CXCR4 antagonism efficiently enhances the therapeutic efficacy of acute myeloid leukemia. Bioact Mater. 2022;18:526–538. doi:10.1016/j.bioactmat.2022.03.022
37. Yang G, Tian J, Chen C. An oxygen self-sufficient NIR-responsive nanosystem for enhanced PDT and chemotherapy against hypoxic tumors. Chem Sci. 2019;10(22):5766–5772. doi:10.1039/C9SC00985J
38. Zuo S, Yu J, Pan H, Lu L. Novel insights on targeting ferroptosis in cancer therapy. Biomarker Res. 2020;8:50. doi:10.1186/s40364-020-00229-w
39. Hu Y, Guo N, Yang T, Yan J, Wang W. The Potential mechanisms by which artemisinin and its derivatives induce ferroptosis in the treatment of cancer. Oxid Med Cell Longev. 2022;2022:1458143. doi:10.1155/2022/1458143
40. Li S, Wang R, Wang Y, et al. Ferroptosis: a new insight for treatment of acute kidney injury. Front Pharmacol. 2022;13:1065867. doi:10.3389/fphar.2022.1065867
41. Chen X, Yu C, Kang R, Tang D. Iron metabolism in ferroptosis. Front Cell Develop Biol. 2020;8:590226. doi:10.3389/fcell.2020.590226
42. Akiyama H, Carter BZ, Andreeff M, Ishizawa J. Molecular mechanisms of ferroptosis and updates of ferroptosis studies in cancers and leukemia. Cells. 2023;12(8). doi:10.3390/cells12081128
43. Shen Z, Shao J, Zhang J, Qu W. Ultrasound cavitation enhanced chemotherapy: in vivo research and clinical application. Experim Biol Med. 2020;245(14):1200–1212. doi:10.1177/1535370220936150
44. Sun J, Wang J, Hu L, Yan J. K-3-Rh protects against cerebral ischemia/reperfusion injury by anti-apoptotic effect through PI3K-Akt signaling pathway in rat. Neuropsychiatr Dis Treat. 2020;16:1217–1227. doi:10.2147/NDT.S233622
45. Do Carmo AL, Bettanin F, Oliveira Almeida M, et al. Competition between phenothiazines and BH3 peptide for the binding site of the antiapoptotic BCL-2 protein. Front Chem. 2020;8:235. doi:10.3389/fchem.2020.00235
46. Duan PY, Ma Y, Li XN, et al. Inhibition of RIPK1-dependent regulated acinar cell necrosis provides protection against acute pancreatitis via the RIPK1/NF-κB/AQP8 pathway. Exp Mol Med. 2019;51(8):1–17. doi:10.1038/s12276-019-0278-3
47. Jia Y, Wang F, Guo Q, et al. Curcumol induces RIPK1/RIPK3 complex-dependent necroptosis via JNK1/2-ROS signaling in hepatic stellate cells. Redox Biol. 2018;19:375–387. doi:10.1016/j.redox.2018.09.007
48. Wang Y, Kanneganti TD. From pyroptosis, apoptosis and necroptosis to PANoptosis: a mechanistic compendium of programmed cell death pathways. Comput Struct Biotechnol J. 2021;19:4641–4657. doi:10.1016/j.csbj.2021.07.038
49. Wang J, Yu Y, Lu K, et al. Silica nanoparticles induce autophagy dysfunction via lysosomal impairment and inhibition of autophagosome degradation in hepatocytes. Int j Nanomed. 2017;12:809–825. doi:10.2147/IJN.S123596
50. Debnath J, Gammoh N, Ryan KM. Autophagy and autophagy-related pathways in cancer. Nat Rev. 2023;24(8):560–575. doi:10.1038/s41580-023-00585-z
51. Rivera Vargas T, Cai Z, Shen Y, et al. Selective degradation of PU.1 during autophagy represses the differentiation and antitumour activity of T(H)9 cells. Nat Commun. 2017;8(1):559. doi:10.1038/s41467-017-00468-w
52. Kang C, Elledge SJ. How autophagy both activates and inhibits cellular senescence. Autophagy. 2016;12(5):898–899. doi:10.1080/15548627.2015.1121361
53. Mouri A, Kaira K, Yamaguchi O, et al. Efficacy and feasibility of programmed death-1/programmed death ligand-1 blockade therapy in non-small cell lung cancer patients with high antinuclear antibody titers. Front Oncol. 2021;11:610952. doi:10.3389/fonc.2021.610952
54. Du T, Gao J, Li P, et al. Pyroptosis, metabolism, and tumor immune microenvironment. Clin Translat Med. 2021;11(8):e492. doi:10.1002/ctm2.492
55. Tang D, Chen X, Kroemer G. Cuproptosis: a copper-triggered modality of mitochondrial cell death. Cell Res. 2022;32(5):417–418. doi:10.1038/s41422-022-00653-7
56. Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1005–L1028. doi:10.1152/ajplung.2000.279.6.L1005
57. Pan F, Xu W, Ding J, Wang C. Elucidating the progress and impact of ferroptosis in hemorrhagic stroke. Front Cell Neurosci. 2022;16:1067570. doi:10.3389/fncel.2022.1067570
58. Yang Y, Zhu T, Wang X, et al. ACSL3 and ACSL4, distinct roles in ferroptosis and cancers. Cancers. 2022;14(23):5896. doi:10.3390/cancers14235896
59. Chen S, Zhang Z, Zhang B, et al. CircCDK14 promotes tumor progression and resists ferroptosis in glioma by regulating PDGFRA. Int J Bio Sci. 2022;18(2):841–857. doi:10.7150/ijbs.66114
60. Zhang J, Sheng S, Wang W, et al. Molecular mechanisms of iron mediated programmed cell death and its roles in eye diseases. Frontiers in Nutrition. 2022;9:844757. doi:10.3389/fnut.2022.844757
61. Liu B, Wang W, Shah A, et al. Sodium iodate induces ferroptosis in human retinal pigment epithelium ARPE-19 cells. Cell Death Dis. 2021;12(3):230. doi:10.1038/s41419-021-03520-2
62. Liang C, Zhang X, Yang M, Dong X. Recent progress in ferroptosis inducers for cancer therapy. Advan Mater. 2019;31(51):e1904197. doi:10.1002/adma.201904197
63. Chen Y, Li L, Lan J, et al. CRISPR screens uncover protective effect of PSTK as a regulator of chemotherapy-induced ferroptosis in hepatocellular carcinoma. Mol Cancer. 2022;21(1):11. doi:10.1186/s12943-021-01466-9
64. Chaudhary K, Promsote W, Ananth S, et al. Iron overload accelerates the progression of diabetic retinopathy in association with increased retinal renin expression. Sci Rep. 2018;8(1):3025. doi:10.1038/s41598-018-21276-2
65. Lu Y, Chan YT, Tan HY, et al. Epigenetic regulation of ferroptosis via ETS1/miR-23a-3p/ACSL4 axis mediates sorafenib resistance in human hepatocellular carcinoma. J Experiment Clin Can Res. 2022;41(1):3. doi:10.1186/s13046-021-02208-x
66. Yan N, Zhang JJ. The emerging roles of ferroptosis in vascular cognitive impairment. Front Neurosci. 2019;13:811. doi:10.3389/fnins.2019.00811
67. He F, Huang X, Wei G, et al. Regulation of ACSL4-catalyzed lipid peroxidation process resists cisplatin ototoxicity. Oxid Med Cell Longev. 2022;2022:3080263. doi:10.1155/2022/3080263
68. Wiernicki B, Dubois H, Tyurina YY, Hassannia B, Bayir H, Kagan VE. Excessive phospholipid peroxidation distinguishes ferroptosis from other cell death modes including pyroptosis. Cell Death Dis. 2020;11(10):922. doi:10.1038/s41419-020-03118-0
69. Zhang Q, Li N, Deng L. ACSL1-induced ferroptosis and platinum resistance in ovarian cancer by increasing FSP1 N-myristylation and stability. Cell Death Discovery. 2023;9(1):83. doi:10.1038/s41420-023-01385-2
70. Yun HR, Jo YH, Kim J, Shin Y, Kim SS, Choi TG. Roles of autophagy in oxidative stress. Int J Mol Sci. 2020;21(9):3289. doi:10.3390/ijms21093289
71. Ng SW, Norwitz SG, Norwitz ER. The impact of iron overload and ferroptosis on reproductive disorders in humans: implications for preeclampsia. Int J Mol Sci. 2019;20(13):3283. doi:10.3390/ijms20133283
72. Yao W, Liao H, Pang M, et al. Inhibition of the NADPH oxidase pathway reduces ferroptosis during septic renal injury in diabetic mice. Oxid Med Cell Longev. 2022;2022:1193734. doi:10.1155/2022/1193734
73. Trzaskalski NA, Vulesevic B, Nguyen MA, et al. Hepatocyte-derived DPP4 regulates portal GLP-1 bioactivity, modulates glucose production, and when absent influences NAFLD progression. JCI Insight. 2023;8(2). doi:10.1172/jci.insight.154314
74. Chen C, Xie B, Li Z, Chen L, Chen Y, Zhou J. Fascin enhances the vulnerability of breast cancer to erastin-induced ferroptosis. Cell Death Dis. 2022;13(2):150. doi:10.1038/s41419-022-04579-1
75. Xue X, Ma L, Zhang X, et al. Tumour cells are sensitised to ferroptosis via RB1CC1-mediated transcriptional reprogramming. Clin Translat Med. 2022;12(2):e747. doi:10.1002/ctm2.747
76. Wang K, Shang F, Chen D, et al. Protein liposomes-mediated targeted acetylcholinesterase gene delivery for effective liver cancer therapy. J Nanobiotechnol. 2021;19(1):31. doi:10.1186/s12951-021-00777-9
77. Zhou Y, Que KT, Zhang Z, et al. Iron overloaded polarizes macrophage to proinflammation phenotype through ROS/acetyl-p53 pathway. Cancer Med. 2018;7(8):4012–4022. doi:10.1002/cam4.1670
78. Wan Q, Liao Z, Rao Y, et al. Transferrin receptor 1-associated iron accumulation and oxidative stress provides a way for grass carp to fight against reovirus infection. Int J Mol Sci. 2019;20(23):5857. doi:10.3390/ijms20235857
79. Liu X, Chen C, Han D, Zhou W. SLC7A11/GPX4 inactivation-mediated ferroptosis contributes to the pathogenesis of triptolide-induced cardiotoxicity. Oxid Med Cell Longev. 2022;2022:3192607. doi:10.1155/2022/3192607
80. Mou Y, Wang J, Wu J, et al. Ferroptosis, a new form of cell death: opportunities and challenges in cancer. J Hematol Oncol. 2019;12(1):34. doi:10.1186/s13045-019-0720-y
81. Amaral C, Vicente CT, Caetano SM, Gaspar-Cordeiro A. An internal promoter drives the expression of a truncated form of CCC1 capable of protecting yeast from iron toxicity. Microorganisms. 2021;9(6):1337. doi:10.3390/microorganisms9061337
82. Zhu L, Chen D, Zhu Y, et al. GPX4-regulated ferroptosis mediates S100-induced experimental autoimmune hepatitis associated with the Nrf2/HO-1 signaling pathway. Oxid Med Cell Longev. 2021;2021:6551069. doi:10.1155/2021/6551069
83. Kasztura M, Kiczak L. Hemosiderin accumulation in liver decreases iron availability in tachycardia-induced porcine congestive heart failure model. Oxid Med Cell Longev. 2022;23:3.
84. Pei Z, Qin Y, Fu X, et al. Inhibition of ferroptosis and iron accumulation alleviates pulmonary fibrosis in a bleomycin model. Redox Biol. 2022;57:102509. doi:10.1016/j.redox.2022.102509
85. Liu D, Yang M, Yao Y, et al. Cardiac fibroblasts promote ferroptosis in atrial fibrillation by secreting exo-miR-23a-3p targeting SLC7A11. Oxid Med Cell Longev. 2022;2022:3961495. doi:10.1155/2022/3961495
86. Guo J, Duan L, He X, et al. A combined model of human iPSC-derived liver organoids and hepatocytes reveals ferroptosis in DGUOK Mutant mtDNA depletion syndrome. Adv Sci. 2021;8(10):2004680. doi:10.1002/advs.202004680
87. Lee J, Hyun DH. The interplay between intracellular iron homeostasis and neuroinflammation in neurodegenerative diseases. Antioxidants. 2023;12:4.
88. Chen Y, Mi Y, Zhang X, et al. Dihydroartemisinin-induced unfolded protein response feedback attenuates ferroptosis via PERK/ATF4/HSPA5 pathway in glioma cells. J Experiment Clin Can Res. 2019;38(1):402. doi:10.1186/s13046-019-1413-7
89. Wei Y, Lv H, Shaikh AB, et al. Directly targeting glutathione peroxidase 4 may be more effective than disrupting glutathione on ferroptosis-based cancer therapy. Biochim Biophys Acta Gen Subj. 2020;1864(4):129539. doi:10.1016/j.bbagen.2020.129539
90. Chen L, Wu D, Zhou L, Ye Y. Platelet-rich plasma promotes diabetic ulcer repair through inhibition of ferroptosis. Ann Translat Med. 2022;10(20):1121. doi:10.21037/atm-22-4654
91. Gao L, Hua W, Tian L. Molecular mechanism of ferroptosis in orthopedic diseases. Cells. 2022;11(19):2979. doi:10.3390/cells11192979
92. Shao J, Bai Z, Zhang L, Zhang F. Ferrostatin-1 alleviates tissue and cell damage in diabetic retinopathy by improving the antioxidant capacity of the Xc(-)-GPX4 system. Cell Death Discovery. 2022;8(1):426. doi:10.1038/s41420-022-01141-y
93. Xu W, Sun T, Wang J, et al. GPX4 alleviates diabetes mellitus-induced erectile dysfunction by inhibiting ferroptosis. Antioxidants. 2022;11:10.
94. Jellusova J. The role of metabolic checkpoint regulators in B cell survival and transformation. Immunol Rev. 2020;295(1):39–53. doi:10.1111/imr.12855
95. Honarpisheh M, Desai J, Marschner JA, et al. Regulated necrosis-related molecule mRNA expression in humans and mice and in murine acute tissue injury and systemic autoimmunity leading to progressive organ damage, and progressive fibrosis. Biosci Rep. 2016;36(6). doi:10.1042/BSR20160336
96. Roberts JA, Varma VR, Huang CW, An Y, Oommen A. Blood metabolite signature of metabolic syndrome implicates alterations in amino acid metabolism: findings from the Baltimore longitudinal study of aging (BLSA) and the tsuruoka metabolomics cohort study (TMCS). Int J Mol Sci. 2020;21(4):1249. doi:10.3390/ijms21041249
97. Liu C, Li B, Yan Q. Protective effects and mechanisms of recombinant human glutathione peroxidase 4 on isoproterenol-induced myocardial ischemia injury. Oxid Med Cell Longev. 2021;2021:6632813. doi:10.1155/2021/6632813
98. Yuan F, Sun Q, Zhang S, et al. The dual role of p62 in ferroptosis of glioblastoma according to p53 status. Cell Biosci. 2022;12(1):20. doi:10.1186/s13578-022-00764-z
99. Zhou Y, Lin W, Rao T, et al. Ferroptosis and its potential role in the nervous system diseases. J Inflamm Res. 2022;15:1555–1574. doi:10.2147/JIR.S351799
100. Kitakata H, Endo J. Therapeutic targets for DOX-induced cardiomyopathy: role of apoptosis vs. ferroptosis. Int J Mol Sci. 2022;23(3). doi:10.3390/ijms23031414
101. Santoro MM. The antioxidant role of non-mitochondrial CoQ10: mystery solved! Cell Metab. 2020;31(1):13–15. doi:10.1016/j.cmet.2019.12.007
102. Arslanbaeva L, Tosi G, Ravazzolo M, et al. UBIAD1 and CoQ10 protect melanoma cells from lipid peroxidation-mediated cell death. Redox Biol. 2022;51:102272. doi:10.1016/j.redox.2022.102272
103. Xu S, Min J, Wang F. Ferroptosis: an emerging player in immune cells. Sci Bull. 2021;66(22):2257–2260. doi:10.1016/j.scib.2021.02.026
104. Zhang P, Wang X, Peng Q. Four-octyl itaconate protects chondrocytes against H(2)O(2)-induced oxidative injury and attenuates osteoarthritis progression by activating Nrf2 signaling. Oxid Med Cell Longev. 2022;2022:2206167. doi:10.1155/2022/2206167
105. Ismail MB, Rajendran P, AbuZahra HM, et al. Mangiferin inhibits apoptosis in doxorubicin-induced vascular endothelial cells via the Nrf2 signaling pathway. Int J Mol Sci. 2021;22(8):4259. doi:10.3390/ijms22084259
106. Hanus J, Anderson C, Sarraf D, Ma J, Wang S. Retinal pigment epithelial cell necroptosis in response to sodium iodate. Cell Death Discovery. 2016;2:16054. doi:10.1038/cddiscovery.2016.54
107. Xu Y, Li Y, Li J, Chen W. Ethyl carbamate triggers ferroptosis in liver through inhibiting GSH synthesis and suppressing Nrf2 activation. Redox Biol. 2022;53:102349. doi:10.1016/j.redox.2022.102349
108. Cai B, Zhong L, Liu Y, Xu Q, Chen T. δ-opioid receptor activation inhibits ferroptosis by activating the Nrf2 pathway in MPTP-induced Parkinson disease models. Evidence Bas Complem Altern Med. 2023;2023:4130937. doi:10.1155/2023/4130937
109. Liu Y, Myojin T, Li K, et al. A major intestinal catabolite of quercetin glycosides, 3-hydroxyphenylacetic acid, protects the hepatocytes from the acetaldehyde-induced cytotoxicity through the enhancement of the total aldehyde dehydrogenase activity. Int J Mol Sci. 2022;23:3.
110. Vatner SF, Zhang J, Oydanich M, Berkman T, Naftalovich R, Vatner DE. Healthful aging mediated by inhibition of oxidative stress. Ageing Res Rev. 2020;64:101194. doi:10.1016/j.arr.2020.101194
111. Qi W, Li Z, Xia L, et al. LncRNA GABPB1-AS1 and GABPB1 regulate oxidative stress during erastin-induced ferroptosis in HepG2 hepatocellular carcinoma cells. Sci Rep. 2019;9(1):16185. doi:10.1038/s41598-019-52837-8
112. Da Q, Ren M, Huang L, Qu J, Yang Q, Xu J. Identification and validation of a ferroptosis-related signature for predicting prognosis and immune microenvironment in papillary renal cell carcinoma. Int J Gene Med. 2022;15:2963–2977. doi:10.2147/IJGM.S354882
113. Nowak G, Megyesi J, Craigen WJ. Deletion of VDAC1 hinders recovery of mitochondrial and renal functions after acute kidney injury. Biomolecules. 2020;10(4):585. doi:10.3390/biom10040585
114. Shi Z, Zheng J, Tang W, et al. Multifunctional nanomaterials for ferroptotic cancer therapy. Front Chem. 2022;10:868630. doi:10.3389/fchem.2022.868630
115. Xiao FJ, Zhang D, Wu Y, et al. miRNA-17-92 protects endothelial cells from erastin-induced ferroptosis through targeting the A20-ACSL4 axis. Biochem Biophys Res Commun. 2019;515(3):448–454. doi:10.1016/j.bbrc.2019.05.147
116. Xia X, Fan X, Zhao M, Zhu P. The relationship between ferroptosis and tumors: a novel landscape for therapeutic approach. Current Gene Therapy. 2019;19(2):117–124. doi:10.2174/1566523219666190628152137
117. Cierluk K, Szlasa W, Rossowska J, et al. Cepharanthine induces ROS stress in glioma and neuronal cells via modulation of VDAC permeability. Saudi Pharma J. 2020;28(11):1364–1373. doi:10.1016/j.jsps.2020.08.026
118. Yi J, Minikes AM, Jiang X. Aiming at cancer in vivo: ferroptosis-inducer delivered by nanoparticles. Cell Chem Biol. 2019;26(5):621–622. doi:10.1016/j.chembiol.2019.05.002
119. Semerad J, Pacheco NIN, Grasserova A. In vitro study of the toxicity mechanisms of nanoscale zero-valent iron (nZVI) and released iron ions using earthworm cells. Nanomaterials. 2020;10(11):2189. doi:10.3390/nano10112189
120. Zhou Y, Shen Y, Chen C, et al. The crosstalk between autophagy and ferroptosis: what can we learn to target drug resistance in cancer? Can Biol Med. 2019;16(4):630–646. doi:10.20892/j.issn.2095-3941.2019.0158
121. Zhang TT, Xu J, Wang YM, Xue CH. Health benefits of dietary marine DHA/EPA-enriched glycerophospholipids. Prog Lipid Res. 2019;75:100997. doi:10.1016/j.plipres.2019.100997
122. Ranji-Burachaloo H, Gurr PA, Dunstan DE, Qiao GG. Cancer treatment through nanoparticle-facilitated Fenton reaction. ACS nano. 2018;12(12):11819–11837. doi:10.1021/acsnano.8b07635
123. Xu R, Yang J, Qian Y, et al. Ferroptosis/pyroptosis dual-inductive combinational anti-cancer therapy achieved by transferrin decorated nanoMOF. Nanoscale Horiz. 2021;6(4):348–356. doi:10.1039/D0NH00674B
124. Lin J, Yang H, Zhang Y, et al. Ferrocene-based polymeric nanoparticles carrying doxorubicin for oncotherapeutic combination of chemotherapy and ferroptosis. Small. 2023;19(2):e2205024. doi:10.1002/smll.202205024
125. Li H. Extraction, purification, characterization and antioxidant activities of polysaccharides from Ramaria botrytis (Pers.) Ricken. Chem Cent J. 2017;11:24. doi:10.1186/s13065-017-0252-x
126. He Z, Guo Y, Chen J, et al. Unsaturated phospholipid modified FeOCl nanosheets for enhancing tumor ferroptosis. J Mat Chem B. 2023;11(9):1891–1903. doi:10.1039/D2TB01854C
127. Huang Z, Xu B, Huang X, et al. Metabolomics reveals the role of acetyl-l-carnitine metabolism in γ-Fe(2)O(3) NP-induced embryonic development toxicity via mitochondria damage. Nanotoxicology. 2019;13(2):204–220. doi:10.1080/17435390.2018.1537411
128. Liang Z, Wang Y, Wang J, et al. Multifunctional Fe(3)O(4)-PEI@HA nanoparticles in the ferroptosis treatment of hepatocellular carcinoma through modulating reactive oxygen species. Colloids Surf B. 2023;227:113358. doi:10.1016/j.colsurfb.2023.113358
129. Liu X, Zhu X, Qi X, Meng X, Xu K. Co-Administration of iRGD with sorafenib-loaded iron-based metal-organic framework as a targeted ferroptosis agent for liver cancer therapy. Int j Nanomed. 2021;16:1037–1050. doi:10.2147/IJN.S292528
130. Wang S, Li F, Qiao R, et al. Arginine-rich manganese silicate nanobubbles as a ferroptosis-inducing agent for tumor-targeted theranostics. ACS nano. 2018;12(12):12380–12392. doi:10.1021/acsnano.8b06399
131. Tang H, Chen D, Li C, et al. Dual GSH-exhausting sorafenib loaded manganese-silica nanodrugs for inducing the ferroptosis of hepatocellular carcinoma cells. Int J Pharm. 2019;572:118782. doi:10.1016/j.ijpharm.2019.118782
132. Zhang Z, Pan Y, Cun JE, et al. A reactive oxygen species-replenishing coordination polymer nanomedicine disrupts redox homeostasis and induces concurrent apoptosis-ferroptosis for combinational cancer therapy. Acta Biomater. 2022;151:480–490. doi:10.1016/j.actbio.2022.07.055
133. He P, Xu S, Miao Z, et al. Anti-Her2 affibody-decorated arsenene nanosheets induce ferroptosis through depleting intracellular GSH to overcome cisplatin resistance. J Nanobiotechnol. 2023;21(1):203. doi:10.1186/s12951-023-01963-7
134. Yu M, Gai C, Li Z, Ding D, Zheng J. Targeted exosome-encapsulated erastin induced ferroptosis in triple negative breast cancer cells. Can Sci. 2019;110(10):3173–3182. doi:10.1111/cas.14181
135. Gai C, Liu C, Wu X, et al. MT1DP loaded by folate-modified liposomes sensitizes erastin-induced ferroptosis via regulating miR-365a-3p/NRF2 axis in non-small cell lung cancer cells. Cell Death Dis. 2020;11(9):751. doi:10.1038/s41419-020-02939-3
136. Khalid HMB, Rasul A, Shah S, Abbas G, Mahmood A. Disulfide bridged nanoparticles of thiolated sodium alginate and eudragit RS100 for oral delivery of paclitaxel: in vitro and in vivo evaluation: in vitro and in vivo evaluation. ACS omega. 2023;8(10):9662–9672. doi:10.1021/acsomega.3c00400
137. Beatty A, Singh T, Tyurina YY, Nicolas E, Peterson JR. Conjugated linolenic fatty acids trigger ferroptosis in triple-negative breast cancer. Cold Spring Harbor Lab. 2019;2019:1.
138. Zhou Z, Song J, Tian R, et al. Activatable singlet oxygen generation from lipid hydroperoxide nanoparticles for cancer therapy. Angew Chem. 2017;56(23):6492–6496. doi:10.1002/anie.201701181
139. Gao M, Deng J, Liu F, et al. Triggered ferroptotic polymer micelles for reversing multidrug resistance to chemotherapy. Biomaterials. 2019;223:119486. doi:10.1016/j.biomaterials.2019.119486
140. Cheng W, Zeng X, Chen H. Versatile polydopamine platforms: synthesis and promising applications for surface modification and advanced nanomedicine. ACS nano. 2019;13(8):8537–8565. doi:10.1021/acsnano.9b04436
141. Yang X, Lan T, Zhong H, et al. To systematically evaluate and analyze the efficacy and safety of transcatheter arterial chemoembolization (TACE) in the treatment of primary liver cancer. J Health Engin. 2022;2022:8223336. doi:10.1155/2022/8223336
142. Henkin JS, Botton CE, Simon MS, et al. Telehealth multicomponent exercise and health education in breast cancer patients undergoing primary treatment: rationale and methodological protocol for a randomized clinical trial (ABRACE: telehealth). Trials. 2023;24(1):42. doi:10.1186/s13063-022-07015-z
143. Kashfi K. Nitric oxide in cancer and beyond. Biochem Pharmacol. 2020;176:114006. doi:10.1016/j.bcp.2020.114006
144. Ji P, Wang X, Yin J, Yao Y, Du W. Amplification of ferroptosis with a liposomal nanoreactor cooperates with low-toxicity doxorubicin apoptosis for enhanced tumor chemotherapy. Biomater Sci. 2022;10(6):1544–1553. doi:10.1039/D2BM00079B
145. Jiang X, Yang M, Fang Y, et al. A photo-activated thermoelectric catalyst for ferroptosis-/pyroptosis-boosted tumor nanotherapy. Adv Healthcare Mater. 2023;12(24):e2300699. doi:10.1002/adhm.202300699
146. Zhou Y, Chen K, Lin WK, et al. Photo-enhanced synergistic induction of ferroptosis for anti-cancer immunotherapy. Adv Healthcare Mater. 2023;12:27.
147. Bai S, Yang N, Wang X. Ultrasmall iron-doped titanium oxide nanodots for enhanced sonodynamic and chemodynamic cancer therapy. ACS nano. 2020;14(11):15119–15130. doi:10.1021/acsnano.0c05235
148. Hu Z, Song X, Ding L, et al. Engineering Fe/Mn-doped zinc oxide nanosonosensitizers for ultrasound-activated and multiple ferroptosis-augmented nanodynamic tumor suppression. Mater Today Bio. 2022;16:100452. doi:10.1016/j.mtbio.2022.100452
149. Zhu M, Wu P, Li Y, Zhang L, Zong Y, Wan M. Synergistic therapy for orthotopic gliomas via biomimetic nanosonosensitizer-mediated sonodynamic therapy and ferroptosis. Biomater Sci. 2022;10(14):3911–3923. doi:10.1039/D2BM00562J
150. Yang Q, Liu T, Zheng H, et al. A nanoformulation for immunosuppression reversal and broad-spectrum self-amplifying antitumor ferroptosis-immunotherapy. Biomaterials. 2023;292:121936. doi:10.1016/j.biomaterials.2022.121936
151. Zhu X, Gong Y, Liu Y, et al. Ru@CeO(2) yolk shell nanozymes: oxygen supply in situ enhanced dual chemotherapy combined with photothermal therapy for orthotopic/subcutaneous colorectal cancer. Biomaterials. 2020;242:119923. doi:10.1016/j.biomaterials.2020.119923
152. Yang Q, Wang J, Zhong P. The clinical prognostic value of lncRNA FAM83H-AS1 in cancer patients: a meta-analysis. Can Cell Inter. 2020;20:72. doi:10.1186/s12935-020-1148-8
153. Liu L, Wang R, Wang C, Wang J, Chen L, Cheng J. Light-triggered release of drug conjugates for an efficient combination of chemotherapy and photodynamic therapy. Biomater Sci. 2018;6(5):997–1001. doi:10.1039/C7BM01114H
154. Zhang S, Zheng F, Liu K, Liu S, Xiao T. Mitochondria-targeting polymer micelles in stepwise response releasing gemcitabine and destroying the mitochondria and nucleus for combined antitumor chemotherapy. Int J Mol Sci. 2022;23:20.
155. Lin Y, Li C, Liu A, Zhen X, Gao J, Wu W. Responsive hyaluronic acid-gold cluster hybrid nanogel theranostic systems. Biomater Sci. 2021;9(4):1363–1373. doi:10.1039/D0BM01815E
156. Nieto C, Vega MA, Martín Del Valle EM. Tailored-made polydopamine nanoparticles to induce ferroptosis in breast cancer cells in combination with chemotherapy. Int J Mol Sci. 2021;22(6):3161. doi:10.3390/ijms22063161
157. Bao W, Liu X, Lv Y, et al. Nanolongan with multiple on-demand conversions for ferroptosis-apoptosis combined anticancer therapy. Int J Mol Sci. 2019;13(1):260–273.
158. Shen Z, Liu T, Li Y, Lau J, Yang Z. Fenton-reaction-acceleratable magnetic nanoparticles for ferroptosis therapy of orthotopic brain tumors. ACS nano. 2018;12(11):11355–11365. doi:10.1021/acsnano.8b06201
159. Chen Y, Yao Z, Liu P, et al. A self-assembly nano-prodrug for triple-negative breast cancer combined treatment by ferroptosis therapy and chemotherapy. Acta Biomater. 2023;159:275–288. doi:10.1016/j.actbio.2023.01.050
160. Liu Y, Bhattarai P, Dai Z, Chen X. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem Soc Rev. 2019;48(7):2053–2108. doi:10.1039/c8cs00618k
161. Wang Y, Meng HM, Li Z. Near-infrared inorganic nanomaterial-based nanosystems for photothermal therapy. Nanoscale. 2021;13(19):8751–8772. doi:10.1039/D1NR00323B
162. Hader M, Streit S, Rosin A. In vitro examinations of cell death induction and the immune phenotype of cancer cells following radiative-based hyperthermia with 915 MHz in combination with radiotherapy. Cells. 2021;10(6):1436. doi:10.3390/cells10061436
163. Lee WT, Yoon J, Kim SS, et al. Combined antitumor therapy using in situ injectable hydrogels formulated with albumin nanoparticles containing indocyanine green, chlorin e6, and perfluorocarbon in hypoxic tumors. Pharmaceutics. 2022;14(1). doi:10.3390/pharmaceutics14010148
164. Kim D, Kim H. Optimization of photothermal therapy treatment effect under various laser irradiation conditions. Int J Mol Sci. 2022;23:11.
165. Tan X, Pang X, Lei M, et al. An efficient dual-loaded multifunctional nanocarrier for combined photothermal and photodynamic therapy based on copper sulfide and chlorin e6. Int J Pharm. 2016;503(1–2):220–228. doi:10.1016/j.ijpharm.2016.03.019
166. Zhang Y, Zhang K, Yang H, Hao Y, Zhang J, Zhao W. Highly penetrable drug-loaded nanomotors for photothermal-enhanced ferroptosis treatment of tumor. ACS Appl Mater Interfaces. 2023;8:3.
167. Chen Y, Su M, Jia L, Zhang Z. Synergistic chemo-photothermal and ferroptosis therapy of polydopamine nanoparticles for esophageal cancer. Nanomedicine. 2022;17(16):1115–1130. doi:10.2217/nnm-2022-0064
168. Chang MH, Pai CL, Chen YC, Yu HP, Hsu CY, Lai PS. Enhanced antitumor effects of epidermal growth factor receptor targetable cetuximab-conjugated polymeric micelles for photodynamic therapy. Nanomaterials. 2018;8(2):121. doi:10.3390/nano8020121
169. Liu Z, Xie Z, Li W, et al. Photodynamic immunotherapy of cancers based on nanotechnology: recent advances and future challenges. J Nanobiotechnol. 2021;19(1):160. doi:10.1186/s12951-021-00903-7
170. Zhou M, Zheng M, Zhou X, et al. The roles of connexins and gap junctions in the progression of cancer. Cell Commun Signal. 2023;21(1):8. doi:10.1186/s12964-022-01009-9
171. Wang D, Wu H, Phua SZF, et al. Self-assembled single-atom nanozyme for enhanced photodynamic therapy treatment of tumor. Nat Commun. 2020;11(1):357. doi:10.1038/s41467-019-14199-7
172. Shui S, Zhao Z, Wang H, Conrad M, Liu G. Non-enzymatic lipid peroxidation initiated by photodynamic therapy drives a distinct ferroptosis-like cell death pathway. Redox Biol. 2021;45:102056. doi:10.1016/j.redox.2021.102056
173. Meng X, Deng J, Liu F, et al. Triggered all-active metal organic framework: ferroptosis machinery contributes to the apoptotic photodynamic antitumor therapy. Nano Lett. 2019;19(11):7866–7876. doi:10.1021/acs.nanolett.9b02904
174. Hu C, Hou B, Xie S. Application of nanosonosensitizer materials in cancer sono-dynamic therapy. RSC Adv. 2022;12(35):22722–22747. doi:10.1039/D2RA03786F
175. Martins YA, Fonseca MJV, Pavan TZ, Lopez RF. Bifunctional therapeutic application of low-frequency ultrasound associated with zinc phthalocyanine-loaded micelles. Int j Nanomed. 2020;15:8075–8095. doi:10.2147/IJN.S264528
176. Yuan M, Liang S, Zhou Y. A robust oxygen-carrying hemoglobin-based natural sonosensitizer for sonodynamic cancer therapy. Int j Nanomed. 2021;21(14):6042–6050.
177. Yang C, Zhang C, Luo X, Liu X, Cao F, Zhang YL. Isomerization and degradation of levoglucosan via the photo-Fenton process: insights from aqueous-phase experiments and atmospheric particulate matter. Environ Sci Technol. 2020;54(19):11789–11797. doi:10.1021/acs.est.0c02499
178. Lagori G, Fornaini C, Rocca JP, Merigo E. Use of photo-Fenton’s reaction by 400-nm LED light for endodontic disinfection: a preliminary in vitro study on Enterococcus faecalis. J Photochem Photobiol B Biol. 2017;171:85–89. doi:10.1016/j.jphotobiol.2017.04.033
179. Nie T, Zou W, Meng Z, et al. Bioactive iridium nanoclusters with glutathione depletion ability for enhanced sonodynamic-triggered ferroptosis-like cancer cell death. Advan Mater. 2022;34(45):e2206286. doi:10.1002/adma.202206286
180. Zong S, Li J, Ye Z, et al. Lachnum polysaccharide suppresses S180 sarcoma by boosting anti-tumor immune responses and skewing tumor-associated macrophages toward M1 phenotype. Int J Biol Macromol. 2020;144:1022–1033. doi:10.1016/j.ijbiomac.2019.09.179
181. Qin Z, Zhang W, Liu S, Wang Y, Peng X, Jia L. PVT1 inhibition stimulates anti-tumor immunity, prevents metastasis, and depletes cancer stem cells in squamous cell carcinoma. Cell Death Dis. 2023;14(3):187. doi:10.1038/s41419-023-05710-6
182. Cronin SJF, Woolf CJ, Weiss G, Penninger JM. The role of iron regulation in immunometabolism and immune-related disease. Front Mol Biosci. 2019;6:116. doi:10.3389/fmolb.2019.00116
183. Chen X, Lan H, He D, et al. Analysis of autophagy-related signatures identified two distinct subtypes for evaluating the tumor immune microenvironment and predicting prognosis in ovarian cancer. Front Oncol. 2021;11:616133. doi:10.3389/fonc.2021.616133
184. Li M, Xu Y, Liang J, et al. USP22 deficiency in melanoma mediates resistance to T cells through IFNγ-JAK1-STAT1 signal axis. Molecular Thera. 2021;29(6):2108–2120. doi:10.1016/j.ymthe.2021.02.018
185. Saleh T, Shojaosadati SA. Multifunctional nanoparticles for cancer immunotherapy. Hum Vaccines Immunother. 2016;12(7):1863–1875.
186. Ding B, Zheng P, Jiang F, et al. MnO(x) nanospikes as nanoadjuvants and immunogenic cell death drugs with enhanced antitumor immunity and antimetastatic effect. Angew Chem. 2020;59(38):16381–16384. doi:10.1002/anie.202005111
187. Tang D, Kang R, Berghe TV, Vandenabeele P, Kroemer G. The molecular machinery of regulated cell death. Cell Res. 2019;29(5):347–364. doi:10.1038/s41422-019-0164-5
188. Jiang Q, Wang K, Zhang X, et al. Platelet membrane-camouflaged magnetic nanoparticles for ferroptosis-enhanced cancer immunotherapy. Small. 2020;16:22.
189. Wen Q, Liu J, Kang R, Zhou B, Tang D. The release and activity of HMGB1 in ferroptosis. Biochem Biophys Res Commun. 2019;510(2):278–283. doi:10.1016/j.bbrc.2019.01.090
190. Zhang F, Li F, Lu GH, et al. Engineering magnetosomes for ferroptosis/immunomodulation synergism in cancer. ACS nano. 2019;13(5):5662–5673. doi:10.1021/acsnano.9b00892
191. Lang X, Green MD, Wang W. Radiotherapy and immunotherapy promote tumoral lipid oxidation and ferroptosis via synergistic repression of SLC7A11. Cancer Discovery. 2019;9(12):1673–1685. doi:10.1158/2159-8290.CD-19-0338
192. Zhao L, Zhou X, Xie F, et al. Ferroptosis in cancer and cancer immunotherapy. Cancer Communic. 2022;42(2):88–116. doi:10.1002/cac2.12250
193. Chin YC, Yang LX, Hsu FT, et al. Iron oxide@chlorophyll clustered nanoparticles eliminate bladder cancer by photodynamic immunotherapy-initiated ferroptosis and immunostimulation. J Nanobiotechnol. 2022;20(1):373. doi:10.1186/s12951-022-01575-7
194. Wang Y, Chen Q, Song H, et al. A triple therapeutic strategy with antiexosomal iron efflux for enhanced ferroptosis therapy and immunotherapy. Small. 2022;18:41.
195. Cheu JW, Lee D, Li Q, et al. Ferroptosis suppressor protein 1 inhibition promotes tumor ferroptosis and anti-tumor immune responses in liver cancer. CMGH. 2023;16(1):133–159. doi:10.1016/j.jcmgh.2023.03.001
196. Zhu H, Li Y, Ming Z, Liu W. Glucose oxidase-mediated tumor starvation therapy combined with photothermal therapy for colon cancer. Biomater Sci. 2021;9(16):5577–5587. doi:10.1039/D1BM00869B
197. Wan X, Song L, Pan W, Zhong H, Li N, Tang B. Tumor-targeted cascade nanoreactor based on metal-organic frameworks for synergistic ferroptosis-starvation anticancer therapy. ACS nano. 2020;14(9):11017–11028. doi:10.1021/acsnano.9b07789
198. Liang Y, Zhang L, Peng C, et al. Tumor microenvironments self-activated nanoscale metal-organic frameworks for ferroptosis based cancer chemodynamic/photothermal/chemo therapy. Acta pharmaceutica Sinica B. 2021;11(10):3231–3243. doi:10.1016/j.apsb.2021.01.016
199. Wang C, Cheng X, Peng H, Zhang Y. NIR-triggered and ROS-boosted nanoplatform for enhanced chemo/PDT/PTT synergistic therapy of sorafenib in hepatocellular carcinoma. Nanoscale Res Lett. 2022;17(1):1–16. doi:10.1186/s11671-022-03729-w
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.