Back to Journals » International Journal of Nanomedicine » Volume 18
Advancements in Macrophage-Targeted Drug Delivery for Effective Disease Management
Authors Liu H, Lv H, Duan X , Du Y , Tang Y , Xu W
Received 9 August 2023
Accepted for publication 8 November 2023
Published 23 November 2023 Volume 2023:18 Pages 6915—6940
DOI https://doi.org/10.2147/IJN.S430877
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor R.D.K. Misra
Hanxiao Liu,1– 3 Hui Lv,2,3 Xuehui Duan,3 Yan Du,3 Yixuan Tang,3 Wei Xu1– 3
1School of Pharmacy, Weifang Medical University, Weifang, Shandong, 261053, People’s Republic of China; 2Department of Pharmacy, the First Affiliated Hospital of Shandong First Medical University & Shandong Provincial Qianfoshan Hospital, Jinan, Shandong, 250014, People’s Republic of China; 3School of Pharmaceutical Sciences & Institute of Materia Medica, National Key Laboratory of Advanced Drug Delivery System, Medical Science and Technology Innovation Center, Key Laboratory for Biotechnology Drugs of National Health Commission, Shandong First Medical University & Shandong Academy of Medical Sciences, Jinan, Shandong, 250117, People’s Republic of China
Correspondence: Yixuan Tang; Wei Xu, Email [email protected]; [email protected]
Abstract: Macrophages play a crucial role in tissue homeostasis and the innate immune system. They perform essential functions such as presenting antigens, regulating cytokines, and responding to inflammation. However, in diseases like cancer, cardiovascular disorders, and autoimmune conditions, macrophages undergo aberrant polarization, which disrupts tissue regulation and impairs their normal behavior. To address these challenges, there has been growing interest in developing customized targeted drug delivery systems specifically designed for macrophage-related functions in different anatomical locations. Nanomedicine, utilizing nanoscale drug systems, offers numerous advantages including improved stability, enhanced pharmacokinetics, controlled release kinetics, and precise temporal drug delivery. These advantages hold significant promise in achieving heightened therapeutic efficacy, specificity, and reduced side effects in drug delivery and treatment approaches. This review aims to explore the roles of macrophages in major diseases and present an overview of current strategies employed in targeted drug delivery to macrophages. Additionally, this article critically evaluates the design of macrophage-targeted delivery systems, highlighting limitations and discussing prospects in this rapidly evolving field. By assessing the strengths and weaknesses of existing approaches, we can identify areas for improvement and refinement in macrophage-targeted drug delivery.
Keywords: macrophages, nanomedicine, drug delivery, tumors, autoimmune disease
Introduction
Macrophages, discovered by Metchnikoff, are crucial immune cells that maintain overall health by engulfing and digesting harmful microorganisms, dead cells, and foreign substances. They assist in tissue clearance and homeostasis.1 Macrophages differentiate into tissue-resident macrophages (RTMs) or monocyte-derived macrophages based on the tissue environment and demand. RTMs establish long-term residence in tissues, while monocyte-derived macrophages are recruited to inflamed sites and exhibit functional differences.2,3 Macrophages have diverse functions, including tissue homeostasis, immune response induction and resolution, and tissue development and repair.4–6 They can be activated into distinct cellular states in response to stimuli from different tissues or cues.7 Typically, macrophages can differentiate into classically activated M1 macrophages or alternatively activated M2 macrophages. M1-type macrophages kill pathogens, while M2-type macrophages contribute to tissue remodeling and healing. Dysregulated expression of M1 or M2 is implicated in diseases such as tumors and rheumatoid arthritis.8,9
Targeting macrophages using drug delivery systems holds promise for regulating disease progression and improving therapeutic efficacy. These targeted delivery systems enable high selectivity in delivering nanomedicines, reducing distribution in healthy tissues, and minimizing toxicity.10 Various nanomaterials have been employed to target macrophages through different routes.11 The intense phagocytic activity of macrophages leads to the passive targeting of conventional nanoscale carriers due to their surface materials or serum conditioning-mediated effects.12 The liver, which contains a significant number of macrophages as part of the reticuloendothelial system (RES), often accumulates a substantial proportion of nanoscale carriers, which are subsequently recognized and metabolized by macrophages.13 However, if targeted macrophages are not resident in the liver, the carriers must cross the RES to reach the lesion site and bind with specific macrophages.14–18 For example, clodronate, a specific macrophage-toxic drug, has been encapsulated in liposomes and shown promise in modulating various diseases, including cancer, cardiovascular diseases, and autoimmune diseases.19 Although the concept of targeting macrophages with personalized drug delivery systems for immune-related diseases is still in its early stages, it holds great long-term potential.20
This article summarizes active and passive targeting techniques for interaction between different carriers and macrophages, elucidating the role of macrophages in specific diseases. Additionally, the article examines the obstacles encountered by macrophage-targeted nanomedicine, including issues related to poor selectivity. Furthermore, it explores the potential prospects of nanocarriers in modulating macrophage activity.
Influence of Physiological Environment on Macrophage-Nanomedicine Interactions
Proteins, Biological Molecules
Proteins and other biomolecules play an important role in regulating the interactions between nanomaterials and macrophages. Plasma is the most important biological medium, in which the total plasma protein concentration is about 60–80 g/L.21 When the nanomedicine enters into the blood vessel for about 30s, it adsorbs a large amount of proteins on its surface and forms a biocrown.22 The composition of the biocrown significantly affects the in vivo biological properties of the carrier. First, the binding mode of nanodrugs to plasma proteins is a key factor affecting their recognition by macrophages. It has been shown that adsorption of non-modulin proteins such as serum albumin and apolipoprotein J (Apo J) reduces the carrier-cell membrane interaction and macrophage uptake, and incubation with serum albumin significantly inhibits uptake by dendritic cells.23 On the contrary, when more conditioning agents, such as complement and immunoglobulin, were adsorbed on the carrier surface, phagocytes could rapidly recognize the nanomedicine, which in turn accelerated its clearance. Secondly, the adsorbed protein crowns can significantly affect the targeting of nanocarriers such as liposomes, eg, Guan et al showed that liposomes modified with cation-rich peptides adsorbed higher amounts of IgG and IgM, which inhibited the targeting of the formulations and enhanced the interaction with immune cells.24 Therefore, reducing the content of protein crowns or adjusting the adsorbed species can effectively regulate the interaction between carriers and macrophages. One approach is to modify nanomedicine with polyethylene glycol (PEG).25–27 PEG, as a hydrophilic long-chain polymer, effectively reduces protein adsorption on the surface of nanocarriers.28 Increasing the density of PEG on gold nanoparticles was shown to significantly reduce serum protein adsorption and uptake by macrophages.29 However, Schottler et al discovered that the adsorption of a specific quantity of serum proteins could actually enhance the carrier’s ability to inhibit macrophage uptake.30 This finding introduces complexity and raises questions about how protein corona formation influences long-circulation properties. In addition to PEG modification, biomimetic modifications have gained attention. Incorporating cell membranes or exosomes into liposomes during preparation allows retention of self-recognition properties while achieving targeted delivery and high drug loading capacity.31–33 CD47, which interacts with SIRPα proteins on macrophages, is considered a basis for achieving biocompatibility and efficient drug delivery in biomimetic systems. Interestingly, our preliminary validation confirms that this modification strategy does not decrease accessibility for cell recognition and internalization, and thus, there was no significant reduction in the distribution of carriers in the liver or on cell membrane surfaces.34
In conclusion, proteins and other biomolecules play a key regulatory role in modulating the interactions between nanomaterials and macrophages. They can influence the interaction of nanomaterials with macrophages by way of adsorption, immunomodulation and biocompatibility, which can have a significant impact on biological effects.
Physiological Fluids
Physiological fluids play an important influence in regulating the interaction of nanomaterials with macrophages. First, the presence of physiological fluids changes the way nanomaterials move in biological systems. Nanomaterials exhibit different behaviors in fluids due to the mechanics of fluid motion, such as shear and turbulence. In blood, different tissues and organs in the body often have their own unique physiological microenvironments, allowing nanocarriers to have different motions. For example, in the hepatic sinusoids of the liver, the flow rate of the nanocarriers is reduced by ~1000 times relative to the blood vessels outside the liver, allowing them to passively aggregate in the liver, thus enhancing the interaction with macrophages in the liver.35 However, as the particle size of the carriers increases to the micrometer scale, they can significantly break through the limitations of the hepatic endothelial system to achieve aggregation in the lungs.36 Lymph is a colorless, transparent fluid composed of plasma, lymphocytes, and other solutes. Its circulation rate is significantly lower than in the blood. As a result, the interaction between nanomedicines and macrophages in lymph is significantly enhanced. For example, based on the role of lymph in immune defense, Wang et al constructed a nano vaccine targeting lymphatic sinus-associated SIGNR1+ macrophages, which could effectively present antigens to B cells after interaction with macrophages and promote the killing of chronic hepatitis B.37
Second, physiological fluids can modulate macrophage response to nanomaterials. Through hydrodynamic stimulation, physiological fluids can affect macrophage morphology, degree of activation, and functional expression. One of the key effects is that mechanical stimulation under physiological flow conditions induces macrophages to secrete specific cytokines that modulate their interaction with nanomaterials. The work of Hyojae Son et al showed that shear stress induces monocyte/macrophage to undergo M1-type polarization and increase secretion of pro-inflammatory factors such as CCL2, TNF-α, and IL-1β. and IL-1β.38 Yazdimamaghani et al produced four types of silica nanoparticles (NPs) with different densities and surface charges and tested the cytotoxicity and uptake on RAW 264.7 macrophage cells after 24 hours of incubation with the cells, in static or under flow conditions. They found that the cell viability is enhanced under flow conditions, compared to static culture. Authors also found that, cellular uptake of silica NPs was more in static conditions compared to dynamic conditions. Furthermore, low-density particles, showed lower uptake under flow conditions compared to high-density particles.39
Local Pathology on Nanomedicine-Macrophages Interactions
When exposed to various microenvironmental stimuli, macrophages are highly plastic and differentiate predominantly into pro-inflammatory M1 and anti-inflammatory M2 types, both of which almost always have completely opposite functions. Due to this feature, macrophages perform different functions at different stages of immunity and inflammation.
The inflammatory state is a complex physiological response that is a nonspecific defense and repair process of the body against injury, infection, or other stimuli.40 Undue chemokines, cytokines, and proinflammatory mediators from macrophages are closely associated with many human diseases, such as pneumonia and rheumatoid arthritis.41 In an inflammatory environment, macrophages often present as pro-inflammatory M1-type macrophages. They constitute the first line of defense against intracellular pathogens and promote Th1 polarization of CD4 cells, in addition to releasing inflammatory mediators such as cytokines, chemokines, and oxygen free radicals, affecting the surface properties and stability of nanomaterials and promoting their interaction with macrophages.42–45
M2-like polarized populations are particularly important during parasitic, helminthic, and fungal infections, where they are induced in response to a Th2 response. Macrophage colony-stimulating factor (M-CSF), IL-4, IL-10, IL-13, or a combination of these factors are able to polarize macrophages toward an M2-like phenotype.46,47 M2-like macrophages are identified primarily based on the expression of CD64 and CD209 (a C-type lectin).45 In contrast to classically activated M1-like macrophages, M2-like macrophages have negatively regulated pro-inflammatory cytokines and induce the production of anti-inflammatory mediators such as IL-4, IL-10, and TGF-β.48,49 Indeed, they are highly endocytosed and partially phagocytosed and are involved in a wide range of functions, including repair mechanisms, homeostasis, metabolic processes, and pathogenesis.50
Toxicity of Nanomaterials
The toxicity of macrophage-targeted nanoparticles (NPs) is an important consideration in their clinical application. Macrophages play a crucial role in the immune response and are responsible for the phagocytosis of foreign substances, including nanoparticles. However, the interaction of nanoparticles with macrophages may lead to potential toxicity issues. First, when macrophages phagocytose nanoparticles, they may trigger cellular stress and inflammatory responses, which in turn produce reactive oxygen radicals (ROS) and pro-inflammatory cytokines. Excessive ROS production may lead to oxidative stress and damage cellular structures, while high levels of pro-inflammatory cytokines may lead to tissue damage and immune dysregulation. For example, Aaron Lee et al demonstrated that polylysine modification increased TNF-α secretion.51
In addition, macrophages are important regulators of the immune system. The interaction of nanoparticles with macrophages may modulate their normal immune functions, such as antigen presentation, cytokine production, and phagocytic activity. Depending on the nature of the nanoparticles, this altered immune response may have systemic effects and influence overall immune homeostasis. For example, inhalation of carbon nanotubes can suppress the humoral immune response through the production of transforming growth factor β (TGF-β) by alveolar macrophages and the subsequent production of prostaglandins by splenocytes, leading to systemic immunosuppression.52
Targeting Strategy
Nanomedicine targeted delivery involves precise drug transportation to specific body sites by manipulating nanoscale drug carriers. It allows drugs to bypass tissue barriers and accumulate selectively in diseased tissues or organs. Targeted delivery operates on two main mechanisms: the affinity between target receptors and drug carriers for precise drug localization, and strategies like surface modification to enhance targeting delivery. This approach enhances drug efficacy, reduces side effects, and improves patient quality of life. As a result, targeted delivery has emerged as a major focus of research in the field of drug delivery. In this context, we aim to explore the interaction between nanocarriers and macrophages from both passive and active targeting perspectives.
Passive Targeting
Passive targeting is one of the important strategies for nanodrug-targeted therapy. It is a non-specific targeting approach that does not require specific targeting molecules. Instead, Passive targeting relies on the preferential accumulation of NPs modified with specific physical properties (such as size, shape, and charge), which significantly impact the rate and range of macrophage uptake.53 Therefore, we will provide an overview of these specific conditions (Figure 1).
Particle Size
The shape of nano-carriers plays a significant role in macrophage uptake, influencing the mechanism and rate of macrophage engulfment. Generally, particles with sizes ranging from 30 nm to 3 µm can be specifically recognized and phagocytosed by macrophages, while outside this range, the phagocytic activity towards the particles decreases.54–56 Moreover, within a certain range, macrophages seem to preferentially internalize larger-sized carriers. For instance, a previous study demonstrated that in cultured primary peritoneal macrophages, they exhibited higher uptake of approximately 500 nm polymer nanoparticles compared to those sized 150 or 300 nm.57 In other studies, J774A.1 macrophages showed the highest uptake of nanoparticles with a diameter of 1.9 μm compared to sizes of 430 nm and 4.8 μm.58
Macrophages are capable of internalizing and engulfing particles of various sizes through different mechanisms. For larger particles, their micrometer scale allows for active binding and phagocytosis, while smaller particles can be taken up by macrophages through various mechanisms. Therefore, the particle size of nanocarriers not only affects their uptake efficiency but also determines the internalization mechanisms of nanoparticles. Experimental studies have compared the absorption mechanisms of particles by macrophages. Kuhn et al conducted cellular experiments using different-sized polystyrene particles in macrophages (J774A.1).59 In J774A.1 cells, 1 μm particles were internalized via phagocytosis/macropinocytosis, while 40 nm polystyrene particles were taken up through clathrin-mediated or caveolin-mediated endocytosis as well as phagocytosis. Furthermore, Yue et al demonstrated that macrophages solely employ the lysosomal pathway when engulfing nanosized carriers.58 These findings underscore the significance of considering particle size in comprehending the mechanisms of nanoparticle uptake by macrophages.
Particle Shape
The shape of nanocarriers is a factor that is easily overlooked, as conventional nanocarriers such as liposomes, polymer micelles, and polymer nanoparticles are spherical in shape. However, emerging nanocarrier preparation technologies, such as lipid nanodiscs and membrane-encapsulated carriers, offer the possibility to modulate carrier morphology and optimize carrier performance.
In general, spherical particles are more readily phagocytosed by macrophages compared to elliptical, rod-shaped, or cylindrical particles. When the curvature of a particle exceeds 45 degrees, it cannot be completely internalized by cells.60,61 Some studies have found that worm-like elongated nanoparticles or ellipsoids can reduce or inhibit the rate of phagocytosis, allowing them to evade immune responses.62,63 For instance, Champion et al conducted studies on the phagocytic mechanisms using different shapes of polystyrene nanoparticles, including spheres, ellipsoids, and oval or rectangular disks.64 The results showed that, the curvature of the particles plays a crucial role in the internalization process, independent of their size. Furthermore, other studies indicate that a high aspect ratio leads to reduced internalization. Different morphologies of carriers may have different hemodynamic parameters, and their behavior in the vasculature can influence their interactions with macrophages.35 Indirectly, the interaction between carriers and organ macrophages in the hemodynamic environment of the liver is also affected. Additionally, highly curved regions such as carbon nanotubes or graphene edges have penetrating abilities and can puncture the cell membrane to enter the cell. These differences in uptake mechanisms enable macrophages to selectively internalize particles of different shapes.65,66
Particle Stiffness
Stiffness can affect the mechanical properties and rigidity of nanoparticles, thereby influencing their interactions with cells.67 For example, Aaron Lee et al evaluated the uptake of poly(N-isopropylacrylamide) with different degrees of crosslinking by primary human monocyte-derived macrophages (MDM) and observed a preference of MDM for removing highly crosslinked (stiffer) particles over less crosslinked (softer) particles.51 Furthermore, there are variations in the response of different shapes of carriers to stiffness. In reducing macrophage internalization of spherical particles, increasing size was more effective than reducing the stiffness of immunoglobulin G-functionalized poly(acrylic acid) (PAA) particles. On the other hand, the internalization of rod-shaped but non-spherical PAA particles critically depended on stiffness.64 In addition, Palomba et al demonstrate that compared to rigid nanostructures, softer nanostructures achieved up to five-fold higher efficiency in evading cellular uptake by bone marrow-derived monocytes. Soft circular and square nanostructures were similarly absorbed by professional phagocytic cells (<15%); however, soft elliptical particles were more easily internalized (<60%), possibly due to their larger size and elongated shape, while any shape and size of rigid nanostructures were taken up by over 70% of macrophages.68
Particle Charge
The charge associated with nanoparticles, which arises from diverse surface chemistries, influences opsonization, circulation times, and interactions with macrophages in organs. Typically, cationic nanoparticles are more easily taken up by phagocytes compared to neutral or negatively charged nanoparticles. The positive charge on nanoparticles can interact with the negatively charged phospholipid compositions of cell membranes, leading to increased nonspecific interactions with biological cells and circulating proteins. However, it is crucial to acknowledge that the positive charge of nanoparticles can also induce adverse biological reactions including enhanced complement system activation and cytotoxicity. Therefore, careful consideration must be given to balancing the benefits of improved cellular uptake against potential harmful effects when utilizing cationic nanoparticles for macrophage targeting.69–71 The role of charge in macrophage uptake remains a topic of debate. For instance, Oleg Lunov et al conducted an analysis involving THP-1 cells and macrophages differentiated from human monocytes using nanoparticles with different surface charges: PS-COOH (negative charge) and PS-NH2 (positive charge). After incubation with these nanoparticles in a buffer for 3 to 6 hours, both types of nanoparticles were taken up by the cells. However, THP-1 cells absorbed approximately 38% more PS-COOH nanoparticles than macrophages did, whereas both cell types displayed similar levels of cellular internalization with PS-NH2 nanoparticles. Interestingly, when the analysis was conducted in HBSS solution, both cell types exhibited lower uptake of PS-NH2 nanoparticles compared to PS-COOH nanoparticles.72 Additionally, in the case of RAW 264.7 cells, a specific type of consuming cells, the intake level of negatively charged AuNPs is similar to that of positively charged AuNPs.73
Active Target
Active targeted therapy relies on specific molecular recognition to deliver substances to specific tissues and cells while minimizing accumulation in healthy tissues. In the case of targeting macrophages, challenges arise due to varying receptor expression among different macrophage phenotypes. For example, in tumor-associated macrophages (TAMs), it is essential to target M2-type TAMs specifically. However, distinguishing receptor expression across macrophages throughout the body can be difficult. Research has identified highly expressed receptors in macrophage populations, providing insights into ligands used for macrophage targeting and the receptors involved (Figure 2 and Table 1). These findings contribute to understanding the strength and specificity of receptor-ligand interactions.
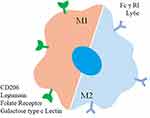 |
Figure 2 Graphical representation of surface proteins utilized for macrophage targeting. |
 |
Table 1 Affinity Analysis Between Receptors and Ligands |
Antibody Antigen Interaction
Antibodies are highly specific and diverse protein molecules produced by the immune system. They play a crucial role in targeted therapy for macrophages in two ways: 1) Regulating macrophage signaling pathways: Antibodies selectively bind to receptors or antigens on macrophages, modulating their functions including inflammation regulation, immune responses, phagocytosis, and cytokine production. 2) Serving as targeting ligands: Antibodies recognize and bind to specific antigens on macrophages, enhancing drug delivery specificity and allowing efficient and targeted delivery of therapeutic agents. However, challenges such as systemic toxicity and limited specificity exist with current antibody-based targeting strategies.
CSF-1 is a signaling protein that binds to its receptor CSF-1R and plays a crucial role in regulating macrophages.85 Inhibiting CSF-1R has shown promise in preclinical cancer models but can cause systemic toxicity.86–88 The CD47/ signal regulatory proteins-α (SIRP α) axis is another therapeutic target where blocking CD47 using anti-CD47 antibodies enhances macrophage function against cancer cells.89–92 Antibodies targeting macrophage surface proteins facilitate targeted delivery of nanoparticles, but their efficacy can be hindered by overlapping expression and non-specific uptake by macrophages.93–95 Nanobodies, smaller fragments without the Fc domain, have been developed to overcome limitations but still face challenges.96,97 Considering these limitations, research is focused on finding alternative ligands that are more efficient and specific in enhancing drug delivery to macrophages.
Peptide Ligands
Peptide ligands offer specific recognition of their corresponding receptors and come with several advantages over antibodies, including smaller size, lower immunogenicity, and reduced manufacturing costs. These ligands often target macrophage surface receptors that are highly expressed. Cieslewicz et al conducted a study where they discovered a unique peptide sequence called M2pep. This peptide demonstrated a preference for binding to M2 macrophages rather than M1/M0 macrophages or other white blood cells.98 In a subsequent study, Conde et al conjugated M2pep to gold nanoparticles to achieve targeted delivery of small interfering RNA (siRNA) for the inhibition of VEGF expression. The researchers demonstrated the high selectivity of this delivery system towards M2 macrophages in lung tissue and lavage fluid.99 Qian et al also utilized M2pep as a targeting ligand for TAMs by delivering siRNA against anti-CSF-1R. This approach resulted in anti-tumor activity in mouse tumor models.100 Furthermore, Ngambenjawong et al synthesized bivalent and tetravalent display bodies ([M2pep]2-Biotin and [M2pep]4-Biotin) based on M2pep. Compared to the monovalent form, [M2pep]2-Biotin showed higher affinity and selective binding to M2 macrophages. However, [M2pep]4-Biotin decreases the binding activity and selectivity. Interestingly, both the bivalent and tetravalent forms of M2pep displayed selective toxicity towards M2 macrophages without any cytotoxic drug loading, while this effect was not observed in monovalent M2pep treatment.101
In the case of CD206, also known as the Mannose Receptor (MMR), the peptide UNO has shown selective binding to this receptor and has been used for targeted drug delivery. The UNO-mediated delivery system has exhibited significant therapeutic efficacy in various tumor models such as breast cancer, melanoma, glioblastoma, and gastric cancer.75 This system outperforms other CD206-targeting peptides or mannose-like compounds by avoiding binding to additional receptors like SIRPα or CD209. Another peptide, CRV, which interacts with the extracellular retinoid X receptor β (RXRB) found on CD11b+F4/80+ macrophages, specifically binds to macrophages and can rapidly extravasate into tumors.83 Nevertheless, peptide delivery systems face challenges such as reduced binding affinity compared to antibodies and increased susceptibility to protein degradation.102
Carbohydrate Ligands
Carbohydrate-targeting ligands indeed offer advantages such as high specificity, high affinity, high water solubility, and low cost. The macrophage mannose receptor (MMR/CD206) is prominently expressed on M2 macrophages, making it an attractive target for specific delivery. Modifying the surface of carriers with mannose can effectively facilitate recognition by M2 macrophages, leading to significant colocalization of mannose-modified carriers with M2 macrophages.103,104 Besides monosaccharides, polysaccharides related to mannose can also exhibit similar recognition effects. These longer chain polysaccharides can effectively reduce plasma protein-mediated inhibition of carrier targeting. An example is Beta-glucan from Grifola frondosa (BSP) and its conjugates, which have been shown to reduce tumor angiogenesis through their effects on macrophages, exhibiting inhibition of sarcoma development.78 In addition, we have reported the targeting of galactose-type C-type lectins, which are highly expressed receptors on M2 macrophages. By utilizing galactose-modified liposomes, the recognition of M2 macrophages can be enhanced.105
However, it’s important to note that carbohydrates can be recognized by various lectins, and their antibody counterparts exhibit high specificity towards their homologous receptors. For instance, the mannose fraction can be recognized by other mannose-binding receptors, including DC-SIGN, L-SIGN, Endo180, or mannose-binding lectin.106 While carbohydrate ligands offer advantages such as high solubility and low cost, they may lack the specificity exhibited by other ligands toward their target receptors.
Nucleic Acid Aptamers
Nucleic acid aptamers, including both RNA and DNA oligonucleotides, exhibit unique secondary structures that enable them to interact with target molecules effectively. These aptamers possess distinct advantages such as simple structure, easy synthesis, and good chemical and biological stability.107,108 Sylvestre et al, identified a specific aptamer selectively bound to CD14+ monocytes rather than CD16+ monocytes and was rapidly internalized by these cells.109 Additionally, Qian et al constructed a DNA tetrahedral framework nucleic acid (tFNA) nano-delivery system to convert TAMs from an M2 phenotype to an M1 phenotype within the tumor microenvironment, thereby exerting significant anti-tumor effects.110 Moreover, they also proposed an aptamer-engineered M1 macrophage (ApEn-M1) which demonstrated outstanding anti-tumor efficacy.111
Interactions Between Different Carriers and Macrophages
In this context, we delve into the complex interplay between carriers and macrophages, with a primary focus on the diverse types of carriers employed in drug delivery research (Figure 3).
 |
Figure 3 Overview of commonly used carrier types: classified as organic or inorganic nanoparticles. Created with BioRender.com. |
Liposomes
Liposomes are artificial vesicles that mimic the structure of cell membranes and can effectively deliver drugs.25,112,113 The interaction between liposomes and macrophages is mainly related to the nature of their carrier materials. Tang et al compared the effects of liposome size, charge, and lipid composition on their interaction with macrophages, and their results showed that compared with 100-nm neutral 1,2-dipalmitoyl-sn-glucose-3-phosphatidylcholine (DPPC) liposomes, macrophages internalized the cationic liposomes and 1.2-di olive acyl-sn-glucose-3-phosphatidylcholine (DSPC) liposomes to a greater extent, and the primary mechanisms were phagocytosis and lattice protein-mediated endocytosis.114 In addition, phosphatidylserine (PS) is a common major cell membrane component and acts as a “phagocytic signal” for macrophages. Therefore, phosphatidylserine-constructed liposomes can mimic this exposed phosphatidylserine and trigger phagocytosis by macrophages.115 However, Riki Toita et al showed that Phosphatidylserine liposome has the potential to promote the polarization of M1-type macrophages to M2-type macrophages in bone regeneration and repair.116
Exosomes
Cellular communication primarily occurs through chemical messengers, among which extracellular vesicles (EVs) are a common form. Exosomes are the smallest type of EVs, with diameters ranging from approximately 30 to 150 nm, and they play important roles in intercellular interactions.117 Based on the protein composition anchored on the surface of exosomes, they can achieve various biological functions. Firstly, exosomes can achieve long-circulating delivery through the expression of immune recognition proteins such as CD47 on their surfaces.118 Furthermore, although both liposomes and exosomes consist of phospholipid bilayers, liposomes are often composed of synthetic materials, which have been found to have a potential risk of inducing hypersensitivity reactions.119 In addition, exosomes derived from endogenous cells can maintain the drug-loading capabilities of liposomes while achieving targeted delivery, thus improving the efficiency of targeted drug delivery.
In targeted delivery to macrophages, exosomes can play two main roles: 1) direct targeting of macrophages, and 2) targeting the communication processes between macrophages and other cells (eg, tumor cells). Engineered therapeutic exosomes developed by researchers can provide targeted control of macrophage phenotypes through transcription factor ASO (exoASO-STAT6), effectively reprogramming tumor-associated macrophages (TAMs) into an M1 phenotype.120,121 Another approach involves bacterial outer membrane vesicles (OMVs), which are highly immunogenic nanovesicles that can stimulate macrophages. To create a “tunable bidirectional adapter” based on OMVs, Feng et al fused CD47 nanobody onto the surface of OMVs and encapsulated them with polyethylene glycol (PEG) containing diselenide bonds (PEG/Se). They named this construct PEG/Se @ OMV-CD47nb. Through various stimulation pathways, this adapter activates tumor-associated macrophages by inducing M1 polarization or blocking the “do not eat me” signal. Consequently, TAMs are stimulated to engulf tumor cells.122
Micelles
Micelles prepared from amphiphilic block copolymers have become a research hotspot in drug delivery due to their unique core-shell structure. In recent years, there have been numerous studies utilizing hydrophilic polymers as shell materials for micelles. Among these, polyethylene glycol (PEG) has emerged as the most commonly used hydrophilic polymer due to its excellent biocompatibility, stealth behavior associated with its molecular structure, and ability to bind a large amount of water through hydrogen bonding.123,124
Similar to other nanoparticles, the micelles themselves interact with macrophages mainly originating from the carrier potential. Faezeh Sabzehei et al showed that conjugated branched PEI-2k and oleic acid (OL) as the building blocks of the cationic micelle significantly increased uptake in RAW264.7 cells.125 Hou et al evaluated the effect of PCL Chain Length on the interaction of Poly(ε-Caprolactone)-Methoxypolyethylene Glycol (PCL-MPEG)-based micelles with macrophages, confirming that compared to PCL-MPEG, the uptake of the cationic micelle was significantly increased. The effect of chain length on the interaction between PCL-MPEG-based micelles and macrophages confirmed that protein adsorption was affected and dependent on chain conformation compared to PCL40-MPEG and PCL20-MPEG, with the lowest adsorption and the least uptake in RAW264.7 cells.126 Similar to liposomes, the incorporation of phosphatidylserine into the carrier material can contribute to enhanced macrophage recognition.127
In addition, the micellar can be simultaneously modified with the corresponding ligand to improve targeting efficiency. Sun et al developed hemoglobin-conjugated doxorubicin-loaded micelles (Hb-DOXM) that could bind with endogenous plasma thrombin and achieve specific targeting of M2 TAMs.128 Liu et al developed zinc protoporphyrin IX (ZnPP)-grafted poly(l-lysine)-b-poly(ethylene glycol) peptide micelles (ZnPP-PM) for galactose-functionalized targeting of macrophages.129
Polymer Nanoparticles
Polymer nanoparticles (P-NPs) are nanoscale particles that can encapsulate or adsorb active compounds. They offer advantages such as easy manufacturing, surface functionalization, biodegradability, and biocompatibility.130–132 Moreover, P-NPs have shown promise as carriers for photodynamic agents, photothermal agents, and chemotherapeutic drugs.133–135
Some materials can modulate macrophage polarization, and their effects depend on various modifications and residues. Carboxyl-modified and amino-modified polystyrene nanoparticles could inhibit M2 macrophage polarization by downregulating CD200R, CD163, and IL-10 expression without affecting M1 markers.136 Similarly, hydrophilic polyurethane nanoparticles could suppress M1 macrophage polarization by reducing inflammatory mediators like TNF-α and IL-1β.137
Inorganic Nanoparticles
As mentioned previously, some nanoparticles have the ability to reprogram macrophages, and one notable example is iron oxide nanoparticles. In a study by Saeid Zanganeh et al, it was discovered that clinically approved iron oxide nanoparticles, specifically ferumoxytol, could polarize macrophages towards the M1 phenotype, leading to the inhibition of tumor growth.138 Interestingly, subsequent research revealed that both feracarbotran and ferumoxytol, two clinically approved iron oxide nanoparticles, could induce autophagy in macrophages and trigger inflammation through TLR4-mediated signaling and oxidative stress.139 Additionally, other iron oxide nanoparticles have been reported to effectively convert macrophages into anti-tumor phenotypes.140–145 Furthermore, studies have indicated that iron-related nanoparticles and calcium ions, could promote the production of pro-inflammatory cytokines such as IL-1β.146 Consequently, the use of calcium-based nanoparticle drugs may have a positive impact on M1 repolarization. For instance, He et al successfully increased the expression of several M1 markers in J774A.1 macrophages by delivering plasmid DNA using peptide-modified calcium carbonate nanoparticle.147 Nanomedicines have demonstrated the ability to induce repolarization of bone marrow-derived macrophages, leading to an increased expression of M1 markers and a decreased expression of M2 markers.148 Additionally, another study achieved similar effects in macrophage repolarization by coating hyaluronic acid (HA) onto manganese dioxide nanoparticles.103 Furthermore, black phosphorus nanoparticles and mesoporous Prussian blue have been utilized to polarize M2 macrophages through the loading of low molecular weight HA.149
The Regulatory Role of Macroscopic Targeted Nanomedicines in Different Diseases
Macrophages have a pivotal role in maintaining physiological balance within the body, and any dysregulation or impairment of their functions can contribute to the development of systemic diseases.5,150 For instance, in non-alcoholic fatty liver disease, M1-polarized macrophages create an inflammatory microenvironment by releasing cytokines like TGF-β, thereby promoting hepatocyte steatosis and liver fibrosis. Conversely, in tumors, M2-polarized tumor-associated macrophages are known to support tumor growth by participating in angiogenesis and aiding in tumor cell metastasis. Therefore, Effective therapeutic interventions can be devised by understanding the state of macrophages in diverse disease contexts. Additionally, we also summarize some of the nanomedicines that have entered clinical trials from the relevant literature and the US National Library of Medicine151 (Table 2).
 |
Table 2 Examples of Clinical Trials on Macrophage-Targeted Nanomedicine for Disease Treatment and Diagnosis |
Tumors
Tumors are a severe disease, causing harmful effects on organs, mortality, and societal implications.152 Tumor-associated macrophages (TAMs) play a critical role in cancer progression.153,154 They are classified into M1 and M2 subgroups. M1 TAMs promote an inflammatory response and exhibit anti-tumor effects during early stages, while M2 TAMs possess anti-inflammatory properties that contribute to tumor growth and invasion. Targeting M2 TAMs can offer therapeutic benefits (Figure 4). Therefore, targeting M2 TAMs offers therapeutic benefits and can be achieved through strategies such as inhibiting recruitment, eliminating or depleting TAMs, and reprogramming them into an M1 phenotype.153,155,156
Monocytes/macrophages play diverse roles in immune regulation, angiogenesis, tumor metastasis, and invasion.153 Macrophages in different tumor regions have distinct origins. Classical monocytes are the main source of macrophages in tumor epithelial, perivascular, and hypoxic areas, while non-classical monocytes contribute predominantly to macrophages in perivascular regions of tumors.157 Recruitment of monocytes/macrophages by tumors and other tissues is facilitated by cytokines and chemokines such as CCL2, CCL5, VEGF, CSF-1.153 Modulating monocytes through a lipid nanoparticle platform that silences CCR2 with siRNA has shown therapeutic effects in mouse cancer models.158 Macrophage development relies on the interaction between macrophage colony-stimulating factor (M-CSF) and its receptor M-CSFR or CD115, with recruitment dependent on the CCL2-CCR2 axis.159 Drugs like PF-04136309, a CCR2 antagonist, could block the migration of CCR2+ monocytes from bone marrow to tumors, resulting in reduced numbers of M2 TAMs.160
Targeted clearance of macrophages is another major modality, and modification of the surface of the carrier with ligands specifically expressed on the surface of M2-type macrophages is effective in increasing the selectivity of nanomedicines.161 However, carriers targeting M2-type macrophages also have high selectivity for M1-type macrophages. To address this issue, we propose to combine “eat me” and “don’t eat me” signaling to improve selectivity for M2 TAMs. We have confirmed that “do not eat me” signaling is more inclined to suppress the uptake of M1 macrophages compared to M2 macrophages. Therefore, surface modification of macrophages with CD47 and the utilization of macrophage galactose c-type lectin as targeting ligands have shown promise in achieving precise selection of M2 macrophages (Figure 5).42,105,162 In addition, while the non-specific uptake of nanoparticles by macrophages has traditionally been considered a ‘drawback’ in circulation. Macrophages could actively migrate towards and engulf nanoparticles extravasating from blood vessels, contributing to their accumulation within the tumor stroma.163
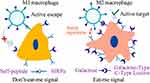 |
Figure 5 Enhancing precise recognition of M2 macrophages through combined Eat Me and Do not Eat Me signals. The interaction between the CD47 protein-derived Self peptide and the SIRPα protein on macrophage surfaces activates the Do not Eat Me signaling pathway, resulting in the inhibition of macrophage phagocytosis. Furthermore, the Do not Eat Me signal exhibits a tendency to suppress phagocytosis specifically in M1 macrophages. Conversely, the recognition of lactose by the highly expressed lactose receptor (MGL) on M2 macrophages triggers the Eat Me signal. The recognition between receptors and ligands shows selective range specificity, wherein the interaction between lactose and MGL indirectly creates spatial hindrance against the Self peptide-SIRPα protein interaction, consequently inhibiting the response of M2 macrophages to the Do not Eat Me signal. Reprinted from Tang Y, Tang Z, Li P et al. Precise delivery of nanomedicines to M2 macrophages by combining “eat me/don’t eat me” signals and its anticancer application. ACS Nano. 2021; 15(11): 18,100–18,112. Copyright 2021, with permission from ACS publications.105 |
Targeting tumor-associated macrophages (TAMs) by repolarizing M2 anti-inflammatory macrophages into M1 pro-inflammatory macrophages is an effective strategy for tumor therapy. This process, known as TAM reprogramming or education, can convert the pro-tumor phenotype into an anti-tumor one. Small molecules and nanoparticle formulations, such as toll-like receptor agonists, cytokines, antibodies, and RNA, have been investigated to achieve macrophage repolarization. For example, β-cyclodextrin nanoparticles loaded with R848 (CDNP-R848) can activate TLR7 and TLR8 in TAMs, converting them into an M1 phenotype.164 Some of the vectors can also assist in realizing the reprogramming of M2 TAM by nanomedicines, such as biomimetic nanovesicles called phagocytosable cell wall-like (PCCW) systems which could target TAMs and promote immune destruction of tumor cells.161,165
Obesity
Obesity is a metabolic disorder characterized by excessive accumulation of white adipose tissue (WAT), leading to low-grade systemic inflammation and associated complications. The presence of macrophages within WAT contributes to this inflammation, with increased pro-inflammatory M1-like macrophages in obese individuals.166–168
M1-type macrophages contribute to tissue inflammation and insulin resistance, while M2-type macrophages are more prevalent in lean individuals. Su et al, utilizing a combined treatment approach involving PHB P3 peptide-modified RHDL to enhance the expression of brown adipocyte markers, reduce adipocyte size, and inhibit weight gain caused by a high-fat diet.169 Additionally, ASXL2 gene plays an important role in regulating macrophage response to dietary factors. Therefore, deletion of the ASXL2 gene has been shown to increase caloric expenditure in obese mice.170 In a study by Liang et al, using polysaccharide nanocarriers spliced with anti-inflammatory drugs (D-70-drug) and demonstrated that more than 63% of the administered nanoparticles accumulated in visceral adipose tissue and then taken up by M1 macrophages through receptor-mediated endocytosis (Figure 6).171
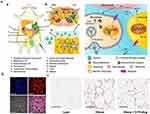 |
Figure 6 (A) Proposed mechanism of dextran-dexamethasone conjugate accumulation in obese Visceral Adipose Tissue (VAT), macrophage uptake, and uncoupling of the paracrine loop between M1 macrophages and adipocytes: (a) In obese mice following intraperitoneal left-side injection, dextran conjugates (highlighted in green) accumulate in the left perirenal adipose tissue (AT) and left gonadal AT. The anatomical depiction shows mice with different fat depots: one mesenteric, two perirenal, and two gonadal AT depots. (b) A transverse cross-section of the mouse abdomen illustrates the localization of the green dextran solution after administration into the peritoneal cavity. (cInflamed adipose tissue enables rapid association of dextran conjugates with M1 macrophages by transport across the peritoneum to directly access interstitial cells. (B) Dextran nanocarrier uptake by myeloid cells in the adipose tissue of obese mice. Obese mice were intraperitoneally injected with D-70-fluor. Twenty-four hours later, the left gonadal adipose tissue was isolated, sectioned, and stained with Hoechst dye. Confocal laser scanning microscopy images show the Hoechst channel (top left), the D-fluor channel (top right), bright-field microscopy (bottom left), and the overlay (bottom right). D-70 was observed within the interstitial space between adipocytes. Scale bars: 200 μm. (C) In vivo anti-inflammatory effects of D-70-drug in obese mouse VAT. Representative hematoxylin-and-eosin-stained images of paraffin-embedded gonadal adipose tissue isolated from each mouse group. Scale bar: 100 μm. Reprinted from Ma L, Liu TW, Wallig MA et al. Efficient targeting of adipose tissue macrophages in obesity with polysaccharide nanocarriers. ACS Nano. 2016;10(7): 6952–6962. Copyright 2016, with permission from ACS publications.171 |
Non-Alcoholic Fatty Liver Disease
NAFLD is a prevalent metabolic syndrome in obese populations, with non-alcoholic fatty liver (NAFL) and non-alcoholic steatohepatitis (NASH) as its subtypes. Hepatic resident macrophages have an important role in the progression of NAFLD. First, MCP-1 secreted by non-parenchymal liver cells recruits monocytes, which differentiate into macrophages and promote fibroblast activation.172–174 Secondly, inflammation plays a crucial role, triggered by LPS binding to Kupffer cell receptors, resulting in the release of cytokines and chemokines like TGF-β and IL-1β that contribute to NAFLD pathogenesis.175–177 Therefore, targeting lipid metabolism, inflammation, and macrophages can offer effective treatment strategies for NAFLD (Figure 7).178,179
 |
Figure 7 PLGA nanoparticle-based delivery of SYK pathway inhibitors for NASH treatment. This study utilized R406, a small molecule inhibitor, and synthesized PLGA nanoparticles to block the Fc receptor signaling pathway, specifically inhibiting the SYK pathway and reducing immune complex-mediated inflammation. Reprinted from Kurniawan DW, Jajoriya AK, Dhawan G et al. Therapeutic inhibition of spleen tyrosine kinase in inflammatory macrophages using PLGA nanoparticles for the treatment of non-alcoholic steatohepatitis. J Control Release. 2018; 288: 227–238. Creative Commons.180 |
Since liver macrophages are the largest group of phagocytes in the body, nanoparticles tend to co-localize with these cells. This characteristic can be utilized to deliver therapeutic agents specifically to the liver and macrophages, potentially improving the treatment outcomes for NAFLD.35,178,179 For example, the results of Muralidhara et al showed that liposome-encapsulated lipophilic curcumin significantly attenuated the inflammatory polarization of macrophages.181 And results from Celia Martínez-Sánchez et al also show that the treatment with dextran–dexamethasone reduced the secretion of inflammatory chemokines and directly attenuated the pro-fibrogenic response in HSCs and liver spheroids.182 In addition, in the context of liver iron deposition, a nano-antioxidant named Snoman-HSA has been developed to target Kupffer cells. By delivering nitric oxide (NO) and thiol groups to the liver, this nanodrug exhibits hepatoprotective effects comparable to combined treatment with other nano-antioxidants and NO donors.183,184 Traber and Zomer used galactin-3 inhibitors to target inflammatory macrophage functions in liver diseases in mice. The treatment with galectin-3 resulted in regression of NASH and fibrosis.185 In conclusion, targeting macrophages holds promise as a treatment option for the management of NAFLD and steatohepatitis.
Rheumatoid Arthritis (RA)
Rheumatoid arthritis (RA) is a chronic inflammatory autoimmune disease characterized by macrophage aggregation in synovial tissue, autoantibody production, synovitis, joint swelling, and cartilage and bone destruction.186,187 The increased number of macrophages in the synovium of RA patients sustains chronic inflammation by releasing pro-inflammatory cytokines (TNF-α, IL-1β, IL-6) and mediators like prostaglandins and chemokines. This process leads to pain, increased infiltration of inflammatory cells, synovial angiogenesis, and joint destruction.188 Targeting macrophages in the treatment of RA can be achieved through two strategies: directly killing macrophages or reprogramming pro-inflammatory M1 macrophages into anti-inflammatory M2 macrophages.189,190
One approach involves using clodronate, a bisphosphonate that induces apoptosis in macrophages. To target macrophages specifically, clodronate can be encapsulated in liposomes. Animal experiments using low-dose liposomal clodronate injections have shown a temporary reduction in joint swelling and slowed the progression of RA. However, clodronate may cause synovitis, and rapid clearance of nanoparticles by macrophages in the reticuloendothelial system (RES) can lead to reduced drug availability. Overcoming these challenges in selectively delivering nanodrugs to targeted organs remains a significant task.19,191
Alternatively, modulation of the inflammatory microenvironment within joints can convert M1 macrophages to anti-inflammatory M2 macrophages to alleviate inflammation. Delivering IL-10 plasmid DNA nanoparticles can promote macrophage polarization from an M1 to an M2 phenotype (Figure 8).192 Folate-modified silver nanoparticles (FA AgNPs) have been developed to actively target M1 macrophages and induce their reversion and polarization to M2 macrophages, effectively treating RA.193 pH/ROS dual-responsive carriers loaded with glucocorticoids and surface-modified with RGD peptide have also been used and demonstrated that these nanomedicines were readily phagocytosed by macrophages and synovial cells, promoting the transformation of M1 macrophages into an M2 phenotype, thereby reducing inflammation, swelling, and joint destruction in mice.194
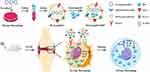 |
Figure 8 Engaging molecularly engineered M2 macrophage-derived exosomes with inflammation tropism and anti-inflammatory capabilities for co-delivery of IL-10 pDNA and GCs to achieve rheumatoid arthritis (RA) treatment via M1-to-M2 macrophage repolarization. Reprinted from J Control Release, 341, Li H, Feng Y, Zheng X et al. M2-type exosomes nanoparticles for rheumatoid arthritis therapy via macrophage re-polarization. 16–30. Copyright 2022, with permission from Elsevier.195 |
Atherosclerosis
Atherosclerosis is a progressive inflammatory disease characterized by lipid accumulation and macrophage infiltration in the arterial wall. It can lead to the formation of atherosclerotic plaques, narrowing the arteries. Macrophages play a crucial role in atherosclerosis by absorbing lipoproteins, contributing to plaque formation, and secreting inflammatory mediators.196,197
Nanomaterial-based therapies targeting macrophages in atherosclerosis show promise. Lowering lipid levels can promote regression of atherosclerosis by inducing an M2-like phenotype in plaques. Therefore, strategies that induce macrophages to transition into an M2 polarization state may be beneficial in treating atherosclerosis.198,199 Such as amino-functionalized hyaluronic acid nanoparticles (HA-NPs) could selectively target macrophages associated with atherosclerotic plaques, reducing inflammation, and transforming M1-type macrophages into M2-type macrophages.200 In addition, inducing M2 polarization by upregulating fatty acid oxidation (FAO) has shown the potential to reduce atherosclerosis occurrence. Targeting scavenger receptor B type I (SR-BI) using a peptide ligand and a macrophage-targeted lipid nanoparticle loaded with an inhibitor of the CD40-induced inflammatory NF-kB pathway could suppress inflammation in plaques.201,202 In addition, specific drug delivery systems have been developed to promote cholesterol efflux in macrophages/foam cells. This strategy could reduce cellular cholesterol accumulation, improving mobility, decreasing lipid storage, and redirecting macrophages towards an M2 phenotype.203
The design of biomimetic nanocarriers based on cellular biological actions also has great potential in atherosclerosis treatment. Gao et al designed ROS-responsive nanoparticles with lovastatin as a model drug and macrophage membranes as the outer layer, which could target atherosclerosis based on macrophage inflammatory chemotaxis and also improve the therapeutic effect on mouse atherosclerosis (Figure 9).204 While Wang et al coated macrophage membranes (MM) on the surface of rapamycin-carrying PLGA nanoparticles (RAPNPs) to prepare bionic nanoparticles (MM/RAPNPs), MM/RAPNPs can be effectively targeted and accumulated in atherosclerotic lesions, thereby slowing down the progression of atherosclerosis.205 Second, the macrophage’s tropism for atherosclerosis makes it an effective carrier to target atherosclerosis. Hu et al constructed macrophages loaded with the Tetrapod PdH Nanoenzyme, which could be targeted to the arterial plaques, thereby decreasing the level of ROS, and significantly inhibiting inflammation-associated pathological processes. In addition, the tip-like morphology of the PdH nanoenzyme could further trigger a strong autophagic response in macrophages, which could synergistically maintain intracellular homeostasis.206
 |
Figure 9 Schematic illustration of the combinational regulation of plaque microenvironment by plaque macrophage-targeted nanosystems: 37pALNP/6877002 and 37pA-PtLNP. During atherosclerosis (AS) therapy, 37pA-PtLNP effectively scavenges intracellular reactive oxygen species (ROS) in inflammatory macrophages within the plaque. On the other hand, 37pA-LNP/6877002 inhibits the activities of TRAF6, leading to a modest decrease in the proportion of the proinflammatory macrophage phenotype (reducing the expression of CD86 and iNOS). More importantly, the combination of 37pA-PtLNP and 37pA-LNP/6877002 regulates the atherosclerotic plaque microenvironment and achieves satisfactory stabilization of plaques with minimal progression. Reprinted from Yang Q, Jiang H, Wang Y et al. Plaque Macrophage-Targeting Nanosystems with Cooperative Co-Regulation of ROS and TRAF6 for Stabilization of Atherosclerotic Plaques. Adv. Funct. Mater. 2018;33: 2,301,053. © 2023 Wiley-VCH GmbH.201 |
Inflammatory Bowel Disease (IBD)
Inflammatory bowel disease (IBD), including ulcerative colitis (UC) and Crohn’s disease (CD), is a chronic inflammatory disorder of the gastrointestinal tract. Macrophages play a protective role in the healthy intestinal epithelium by defending against pathogens. Disrupted cytokine balance leads to monocyte infiltration and differentiation into tissue-resident macrophages, releasing pro-inflammatory cytokines that exacerbate inflammation.207 During steady-state, monocytes recruited to the gut differentiate into mature tissue-resident macrophages with an anti-inflammatory M2-like phenotype. These macrophages secrete cytokines like IL-10 and TGF-β, enhancing the epithelial barrier integrity and promoting stem cell renew.207 Therefore, IBD treatment methods always impact macrophage function by inhibiting inflammatory signaling or inducing M2 polarization.208,209 Approaches like adoptive transfer of M2 macrophages,210,211 oral administration of Lupeol, tumor necrosis factor α stimulated gene 6, IL-33, and probiotic butyric acid bacteria to adoptive transfer of M2 macrophages have shown benefits for IBD treatment.212–214
Nanomedicines offer a potential solution for targeted drug delivery to macrophages in inflammatory bowel disease (IBD), effectively reducing inflammation and reversing the actions of macrophages. The interaction between nanoscale carriers and macrophages facilitates their recognition and phagocytosis, enabling targeted imaging. In the context of IBD, tumor necrosis factor-alpha (TNF-α) plays a crucial role in its pathogenesis. Zhen et al have constructed nanocomplexes using konjac glucan (cKGM) combined with anti-TNF-α antisense oligodeoxynucleotides, which effectively reduced local TNF-α levels in the colon and suppressed inflammatory symptoms. These findings demonstrate the potential of using nanocomplexes as an approach to alleviate inflammation and counteract the actions of macrophages in IBD (Figure 10).215 Another approach involves using mannose-glycosylated cationic bioreducible polymers encapsulating RNA interference (RNAi) against TNF-α. Targeting macrophages with this method significantly reduces TNF-α levels in colonic tissues.216 Hyaluronic acid (HA)-bilirubin nanomedicine (HABN) has been developed as a drug specifically targeting CD44 receptors. This nanomedicine helps clear reactive oxygen species and modulate the gut microbiota for mucosal repair.217 In addition, gelatin microspheres loaded with IL-10 have also been employed to enhance the effectiveness of IBD treatment.218
 |
Figure 10 The microspheric vehicle, 3# KPM, composed of cationic konjac glucomannan (cKGM), phytagel, and an antisense oligonucleotide against TNF-α, showed anti-colitis activity. (A) Body weight changes were observed over time. (B) Survival analysis. (C) Disease activity index was calculated to evaluate the severity of colitis. (D) myeloperoxidase (MPO) activity determination. (E and F) Colon length was measured as an indicator of inflammation and tissue damage. (G and H) Colon sections were stained with H&E and histopathological scoring was conducted to assess inflammatory cell infiltration and tissue damage. Scale bar: 100 μm. (I) The levels of colonic inflammatory cytokines (IL-1β, IL-6, IL-12p70, and IL-23) were determined in DSS colitic mice with different treatments. Results are expressed as the mean (SEM). *p<0.05. Reprinted from Biomaterials; 48, Huang Z, Gan J, Jia L et al. An orally administrated nucleotide-delivery vehicle targeting colonic macrophages for the treatment of inflammatory bowel disease. 26–36. Copyright 2015, with permission from Elsevier.215 |
Conclusion
In conclusion, macrophages play a pivotal role in immune responses and have emerged as crucial therapeutic targets in various diseases. Macrophages are an important part of the body’s immune system. Therefore, the development of tumors and inflammatory and immune-related diseases such as NAFLD, IBD and RA is accompanied by macrophage dysfunction and mis-polarization. Therefore, it is an important therapeutic target in these diseases. Relevant drug formulations are already undergoing clinical trials. Compared with conventional drugs, nanomedicines show unique advantages in improving the stability and solubility of drugs, and based on the characteristic of macrophage recognition of phagocytosis of foreign substances, it makes nanomedicines can easily contact with macrophages and exert therapeutic effects.
In the physiological environment, carriers will adsorb serum proteins soon after entering the body, and the conditioning elements in serum will enhance the recognition of carriers by macrophages. However, different carrier materials have different properties, so their interaction with macrophages will be different. Here we have compiled a list of the effects of physical and chemical properties of carriers such as particle size, hardness, traits and potential on their interaction with macrophages, in general, in the range of 10~5000 nm, carriers with larger particle size, softer surfaces, higher roundness and stronger positive potentials will enhance interaction with macrophages.
In addition, targeted drug delivery systems offer a promising approach to delivering drugs specifically to macrophages, thereby enhancing efficacy while minimizing toxicity. Passive targeting exploits the phagocytic activity of macrophages, allowing nanoscale carriers to accumulate in tissues abundant in macrophages. However, when macrophages need to be targeted outside these tissues, barriers must be overcome for carriers to reach the desired sites. Active targeting strategies employ macrophage-specific ligands on carrier surfaces to enhance recognition and uptake by target macrophages, thus improving drug delivery efficiency. While liposomal clodronate formulations have already found their way into clinical applications, further research is needed to fully grasp macrophage targeting and develop personalized drug delivery systems. Overcoming challenges such as heterogeneous macrophage populations, fluctuating marker expression, and real-time monitoring of changes in macrophage phenotypes and functions remains critical.219
The role of nanomedicines in modulating the role of macrophages to enhance disease treatment is generally divided into three main blocks, including 1) inhibition of macrophage recruitment, 2) direct killing of macrophages and 3) repolarization (re-education) of macrophages. For the inhibition of macrophage recruitment, some drugs have been clinically validated and have achieved some efficacy. However, the strategy is focused on the control of the disease. Direct killing of macrophages is generally carried out by liposomes encapsulating clodronate, which is effective and simple. However, there are greater safety concerns with this approach, as this killing is not selective. Re-education of macrophages, on the other hand, has better expectations and has received a lot of attention because of its higher safety profile and its ability to convert pathogenic macrophages into therapeutic macrophages. In this regard, it is worth noting that some of the nanomedicine carrier materials themselves have certain macrophage re-education properties, and thus the organic combination of carrier material design and encapsulated drugs may be a more valuable breakthrough direction in the future.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 32001001), the Natural Science Foundation of Shandong Province (No. ZR2021MH118), Shandong Province Postdoctoral Innovation Project (202102065), Special funds for Taishan Scholars Project (tsqnz20221156, tsqn202306383), China Postdoctoral Science Foundation (2022M711972), and academic promotion program of Shandong First Medical University (2019LJ003).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol. 2013;14(10):986–995. doi:10.1038/ni.2705
2. Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5(12):953–964. doi:10.1038/nri1733
3. Ginhoux F, Guilliams M. Tissue-Resident Macrophage Ontogeny and Homeostasis. Immunity. 2016;44(3):439–449. doi:10.1016/j.immuni.2016.02.024
4. Park MD, Silvin A, Ginhoux F, Merad M. Macrophages in health and disease. Cell. 2022;185(23):4259–4279. doi:10.1016/j.cell.2022.10.007
5. Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496(7446):445–455. doi:10.1038/nature12034
6. Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11(11):723–737. doi:10.1038/nri3073
7. Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000prime Rep. 2014;6:13. doi:10.12703/P6-13
8. Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23(11):549–555. doi:10.1016/S1471-4906(02)02302-5
9. Fukui S, Iwamoto N, Takatani A, et al. M1 and M2 Monocytes in Rheumatoid Arthritis: a Contribution of Imbalance of M1/M2 Monocytes to Osteoclastogenesis. Front Immunol. 2017;8:1958. doi:10.3389/fimmu.2017.01958
10. Shields CW, Wang LL, Evans MA, Mitragotri S. Materials for Immunotherapy. Adv Mater. 2020;32(13):1901633. doi:10.1002/adma.201901633
11. Jain RK, Martin JD, Stylianopoulos T. The role of mechanical forces in tumor growth and therapy. Annu Rev Biomed Eng. 2014;16:321–346. doi:10.1146/annurev-bioeng-071813-105259
12. Papini E, Tavano R, Mancin F. Opsonins and Dysopsonins of Nanoparticles: facts, Concepts, and Methodological Guidelines. Front Immunol. 2020;11:567365. doi:10.3389/fimmu.2020.567365
13. Zhang YN, Poon W, Tavares AJ, McGilvray ID, Chan WCW. Nanoparticle–liver interactions: cellular uptake and hepatobiliary elimination. J Controlled Release. 2016;240:332–348. doi:10.1016/j.jconrel.2016.01.020
14. Wei X, Ying M, Dehaini D, et al. Nanoparticle Functionalization with Platelet Membrane Enables Multifactored Biological Targeting and Detection of Atherosclerosis. ACS Nano. 2018;12(1):109–116. doi:10.1021/acsnano.7b07720
15. Zhao Y, He Z, Gao H, et al. Fine Tuning of Core–Shell Structure of Hyaluronic Acid/Cell-Penetrating Peptides/siRNA Nanoparticles for Enhanced Gene Delivery to Macrophages in Antiatherosclerotic Therapy. Biomacromolecules. 2018;19(7):2944–2956. doi:10.1021/acs.biomac.8b00501
16. Lee GY, Kim JH, Choi KY, et al. Hyaluronic acid nanoparticles for active targeting atherosclerosis. Biomaterials. 2015;53:341–348. doi:10.1016/j.biomaterials.2015.02.089
17. Poh S, Putt KS, Low PS. Folate-Targeted Dendrimers Selectively Accumulate at Sites of Inflammation in Mouse Models of Ulcerative Colitis and Atherosclerosis. Biomacromolecules. 2017;18(10):3082–3088. doi:10.1021/acs.biomac.7b00728
18. He H, Yuan Q, Bie J, et al. Development of mannose functionalized dendrimeric nanoparticles for targeted delivery to macrophages: use of this platform to modulate atherosclerosis. Transl Res. 2018;193:13–30. doi:10.1016/j.trsl.2017.10.008
19. van Rooijen N, van Kesteren-Hendrikx E. Clodronate liposomes: perspectives in research and therapeutics. J Liposome Res. 2002;12(1–2):81–94. doi:10.1081/lpr-120004780
20. He H, Lu Y, Qi J, Zhu Q, Chen Z, Wu W. Adapting liposomes for oral drug delivery. Acta Pharm Sin B. 2019;9(1):36–48. doi:10.1016/j.apsb.2018.06.005
21. Tenzer S, Docter D, Kuharev J, et al. Rapid formation of plasma protein Corona critically affects nanoparticle pathophysiology. Nat Nanotechnol. 2013;8(10):772–781. doi:10.1038/nnano.2013.181
22. Milani S, Baldelli Bombelli F, Pitek AS, Dawson KA, Rädler J. Reversible versus Irreversible Binding of Transferrin to Polystyrene Nanoparticles: soft and Hard Corona. ACS Nano. 2012;6(3):2532–2541. doi:10.1021/nn204951s
23. Nguyen VH, Lee BJ. Protein Corona: a new approach for nanomedicine design. Int J Nanomedicine. 2017;12:3137–3151. doi:10.2147/IJN.S129300
24. Guan J, Shen Q, Zhang Z, et al. Enhanced immunocompatibility of ligand-targeted liposomes by attenuating natural IgM absorption. Nat Commun. 2018;9(1):2982. doi:10.1038/s41467-018-05384-1
25. Zabielska-Koczywąs K, Lechowski R. The Use of Liposomes and Nanoparticles as Drug Delivery Systems to Improve Cancer Treatment in Dogs and Cats. Molecules. 2017;22(12):2167. doi:10.3390/molecules22122167
26. Lamichhane N, Udayakumar T, D’Souza W, et al. Liposomes: clinical Applications and Potential for Image-Guided Drug Delivery. Molecules. 2018;23(2):288. doi:10.3390/molecules23020288
27. Shaheen SM, Shakil Ahmed FR, Hossen MN, Ahmed M, Amran MS, Ul-Islam MA. Liposome as a Carrier for Advanced Drug Delivery. Pak J Biol Sci. 2006;9(6):1181–1191. doi:10.3923/pjbs.2006.1181.1191
28. Poirier VJ, Thamm DH, Kurzman ID, et al. Liposome-Encapsulated Doxorubicin (Doxil) and Doxorubicin in the Treatment of Vaccine-Associated Sarcoma in Cats. J Vet Intern Med. 2002;16(6):726–731. doi:10.1111/j.1939-1676.2002.tb02415.x
29. Walkey CD, Olsen JB, Guo H, Emili A, Chan WCW. Nanoparticle size and surface chemistry determine serum protein adsorption and macrophage uptake. J Am Chem Soc. 2012;134(4):2139–2147. doi:10.1021/ja2084338
30. Schöttler S, Becker G, Winzen S, et al. Protein adsorption is required for stealth effect of poly(ethylene glycol)- and poly(phosphoester)-coated nanocarriers. Nat Nanotechnol. 2016;11(4):372–377. doi:10.1038/nnano.2015.330
31. Cao H, Dan Z, He X, et al. Liposomes Coated with Isolated Macrophage Membrane Can Target Lung Metastasis of Breast Cancer. ACS Nano. 2016;10(8):7738–7748. doi:10.1021/acsnano.6b03148
32. He H, Guo C, Wang J, et al. Leutusome: a Biomimetic Nanoplatform Integrating Plasma Membrane Components of Leukocytes and Tumor Cells for Remarkably Enhanced Solid Tumor Homing. Nano Lett. 2018;18(10):6164–6174. doi:10.1021/acs.nanolett.8b01892
33. Belhadj Z, He B, Deng H, et al. A combined “eat me/don’t eat me” strategy based on extracellular vesicles for anticancer nanomedicine. J Extracell Vesicles. 2020;9(1):1806444. doi:10.1080/20013078.2020.1806444
34. Tang Y, Wang X, Li J, et al. Overcoming the Reticuloendothelial System Barrier to Drug Delivery with a “Don’t-Eat-Us” Strategy. ACS Nano. 2019;13(11):13015–13026. doi:10.1021/acsnano.9b05679
35. Tsoi KM, MacParland SA, Ma XZ, et al. Mechanism of hard-nanomaterial clearance by the liver. Nat Mater. 2016;15(11):1212–1221. doi:10.1038/nmat4718
36. Cheng L, Niu MM, Yan T, et al. Bioresponsive micro-to-nano albumin-based systems for targeted drug delivery against complex fungal infections. Acta Pharm Sin B. 2021;11(10):3220–3230. doi:10.1016/j.apsb.2021.04.020
37. Wang W, Zhou X, Bian Y, et al. Dual-targeting nanoparticle vaccine elicits a therapeutic antibody response against chronic hepatitis B. Nat Nanotechnol. 2020;15(5):406–416. doi:10.1038/s41565-020-0648-y
38. Son H, Choi HS, Baek SE, et al. Shear stress induces monocyte/macrophage-mediated inflammation by upregulating cell-surface expression of heat shock proteins. Biomed Pharmacother. 2023;161:114566. doi:10.1016/j.biopha.2023.114566
39. Yazdimamaghani M, Barber ZB, Hadipour Moghaddam SP, Ghandehari H. Influence of Silica Nanoparticle Density and Flow Conditions on Sedimentation, Cell Uptake, and Cytotoxicity. Mol Pharm. 2018;15(6):2372–2383. doi:10.1021/acs.molpharmaceut.8b00213
40. Steinbach EC, Plevy SE. The Role of Macrophages and Dendritic Cells in the Initiation of Inflammation in IBD. Inflamm Bowel Dis. 2014;20(1):166–175. doi:10.1097/MIB.0b013e3182a69dca
41. Singh R, Mishra MK, Aggarwal H. Inflammation, Immunity, and Cancer. Mediators Inflamm. 2017;2017:1. doi:10.1155/2017/6027305
42. Qie Y, Yuan H, von Roemeling CA, et al. Surface modification of nanoparticles enables selective evasion of phagocytic clearance by distinct macrophage phenotypes. Sci Rep. 2016;6(1):26269. doi:10.1038/srep26269
43. Schultze JL, Schmidt SV. Molecular features of macrophage activation. Semin Immunol. 2015;27(6):416–423. doi:10.1016/j.smim.2016.03.009
44. Bonilla DL, Bhattacharya A, Sha Y, et al. Autophagy Regulates Phagocytosis by Modulating the Expression of Scavenger Receptors. Immunity. 2013;39(3):537–547. doi:10.1016/j.immuni.2013.08.026
45. Tarique AA, Logan J, Thomas E, Holt PG, Sly PD, Fantino E. Phenotypic, Functional, and Plasticity Features of Classical and Alternatively Activated Human Macrophages. Am J Respir Cell Mol Biol. 2015;53(5):676–688. doi:10.1165/rcmb.2015-0012OC
46. Verreck FAW, de Boer T, Langenberg DML, et al. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proc Natl Acad Sci. 2004;101(13):4560–4565. doi:10.1073/pnas.0400983101
47. Verreck FAW, de Boer T, Langenberg DML, van der Zanden L, Ottenhoff THM. Phenotypic and functional profiling of human proinflammatory type-1 and anti-inflammatory type-2 macrophages in response to microbial antigens and IFN-γ- and CD40L-mediated costimulation. J Leukoc Biol. 2005;79(2):285–293. doi:10.1189/jlb.0105015
48. Jaguin M, Houlbert N, Fardel O, Lecureur V. Polarization profiles of human M-CSF-generated macrophages and comparison of M1-markers in classically activated macrophages from GM-CSF and M-CSF origin. Cell Immunol. 2013;281(1):51–61. doi:10.1016/j.cellimm.2013.01.010
49. Schraufstatter IU, Zhao M, Khaldoyanidi SK, DiScipio RG. The chemokine CCL18 causes maturation of cultured monocytes to macrophages in the M2 spectrum: macrophage maturation by CCL18. Immunology. 2012;135(4):287–298. doi:10.1111/j.1365-2567.2011.03541.x
50. Vogel DYS, Glim JE, Stavenuiter AWD, et al. Human macrophage polarization in vitro: maturation and activation methods compared. Immunobiology. 2014;219(9):695–703. doi:10.1016/j.imbio.2014.05.002
51. Lee A, Septiadi D, Taladriz‐Blanco P, et al. Particle Stiffness and Surface Topography Determine Macrophage‐Mediated Removal of Surface Adsorbed Particles. Adv Healthc Mater. 2021;10(6):2001667. doi:10.1002/adhm.202001667
52. Mitchell LA, Lauer FT, Burchiel SW, McDonald JD. Mechanisms for how inhaled multiwalled carbon nanotubes suppress systemic immune function in mice. Nat Nanotechnol. 2009;4(7):451–456. doi:10.1038/nnano.2009.151
53. Kelly C, Jefferies C, Cryan SA. Targeted Liposomal Drug Delivery to Monocytes and Macrophages. J Drug Deliv. 2011;2011:1–11. doi:10.1155/2011/727241
54. Ngambenjawong C, Gustafson HH, Pun SH. Progress in tumor-associated macrophage (TAM)-targeted therapeutics. Adv Drug Deliv Rev. 2017;114:206–221. doi:10.1016/j.addr.2017.04.010
55. Tabata Y, Ikada Y. Effect of the size and surface charge of polymer microspheres on their phagocytosis by macrophage. Biomaterials. 1988;9(4):356–362. doi:10.1016/0142-9612(88)90033-6
56. Inadomi JM, Vijan S, Janz NK, et al. Adherence to colorectal cancer screening: a randomized clinical trial of competing strategies. Arch Intern Med. 2012;172(7):575–582. doi:10.1001/archinternmed.2012.332
57. He C, Hu Y, Yin L, Tang C, Yin C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials. 2010;31(13):3657–3666. doi:10.1016/j.biomaterials.2010.01.065
58. Yue H, Wei W, Yue Z, et al. Particle size affects the cellular response in macrophages. Eur J Pharm Sci. 2010;41(5):650–657. doi:10.1016/j.ejps.2010.09.006
59. Kuhn DA, Vanhecke D, Michen B, et al. Different endocytotic uptake mechanisms for nanoparticles in epithelial cells and macrophages. Beilstein J Nanotechnol. 2014;5:1625–1636. doi:10.3762/bjnano.5.174
60. Gustafson HH, Holt-Casper D, Grainger DW, Ghandehari H. Nanoparticle uptake: the phagocyte problem. Nano Today. 2015;10(4):487–510. doi:10.1016/j.nantod.2015.06.006
61. Champion JA, Mitragotri S. Role of target geometry in phagocytosis. Proc Natl Acad Sci. 2006;103(13):4930–4934. doi:10.1073/pnas.0600997103
62. Champion JA, Walker A, Mitragotri S. Role of Particle Size in Phagocytosis of Polymeric Microspheres. Pharm Res. 2008;25(8):1815–1821. doi:10.1007/s11095-008-9562-y
63. Champion JA, Mitragotri S. Shape Induced Inhibition of Phagocytosis of Polymer Particles. Pharm Res. 2009;26(1):244–249. doi:10.1007/s11095-008-9626-z
64. Garapaty A, Champion JA. Tunable particles alter macrophage uptake based on combinatorial effects of physical properties: garapaty and Champion. Bioeng Transl Med. 2017;2(1):92–101. doi:10.1002/btm2.10047
65. Albanese A, Tang PS, Chan WCW. The Effect of Nanoparticle Size, Shape, and Surface Chemistry on Biological Systems. Annu Rev Biomed Eng. 2012;14(1):1–16. doi:10.1146/annurev-bioeng-071811-150124
66. Kinnear C, Moore TL, Rodriguez-Lorenzo L, Rothen-Rutishauser B, Petri-Fink A. Form Follows Function: nanoparticle Shape and Its Implications for Nanomedicine. Chem Rev. 2017;117(17):11476–11521. doi:10.1021/acs.chemrev.7b00194
67. Anselmo AC, Mitragotri S. Impact of particle elasticity on particle-based drug delivery systems. Adv Drug Deliv Rev. 2017;108:51–67. doi:10.1016/j.addr.2016.01.007
68. Palomba R, Palange AL, Rizzuti IF, et al. Modulating Phagocytic Cell Sequestration by Tailoring Nanoconstruct Softness. ACS Nano. 2018;12(2):1433–1444. doi:10.1021/acsnano.7b07797
69. de Castro CE, Ribeiro CAS, Alavarse AC, et al. Nanoparticle–Cell Interactions: surface Chemistry Effects on the Cellular Uptake of Biocompatible Block Copolymer Assemblies. Langmuir. 2018;34(5):2180–2188. doi:10.1021/acs.langmuir.7b04040
70. Verma A, Stellacci F. Effect of Surface Properties on Nanoparticle–Cell Interactions. Small. 2010;6(1):12–21. doi:10.1002/smll.200901158
71. Rattan R, Bhattacharjee S, Zong H, et al. Nanoparticle-macrophage interactions: a balance between clearance and cell-specific targeting. Bioorg Med Chem. 2017;25(16):4487–4496. doi:10.1016/j.bmc.2017.06.040
72. Lunov O, Syrovets T, Loos C, et al. Differential Uptake of Functionalized Polystyrene Nanoparticles by Human Macrophages and a Monocytic Cell Line. ACS Nano. 2011;5(3):1657–1669. doi:10.1021/nn2000756
73. Liu X, Huang N, Li H, Jin Q, Ji J. Surface and Size Effects on Cell Interaction of Gold Nanoparticles with Both Phagocytic and Nonphagocytic Cells. Langmuir. 2013;29(29):9138–9148. doi:10.1021/la401556k
74. Szolnoky G, Bata-Csörgö Z, Kenderessy AS, et al. A Mannose-Binding Receptor is Expressed on Human Keratinocytes and Mediates Killing of Candida albicans. J Invest Dermatol. 2001;117(2):205–213. doi:10.1046/j.1523-1747.2001.14071.x
75. Scodeller P, Simón-Gracia L, Kopanchuk S, et al. Precision Targeting of Tumor Macrophages with a CD206 Binding Peptide. Sci Rep. 2017;7(1):14655. doi:10.1038/s41598-017-14709-x
76. Jaynes JM, Sable R, Ronzetti M, et al. Mannose receptor (CD206) activation in tumor-associated macrophages enhances adaptive and innate antitumor immune responses. Sci Transl Med. 2020;12(530):eaax6337. doi:10.1126/scitranslmed.aax6337
77. Parker CC, Bin Salam A, Song PN, et al. Evaluation of a CD206-Targeted Peptide for PET Imaging of Macrophages in Syngeneic Mouse Models of Cancer. Mol Pharm. 2023;20(5):2415–2425. doi:10.1021/acs.molpharmaceut.2c00977
78. Zhan X, Jia L, Niu Y, et al. Targeted depletion of tumour-associated macrophages by an alendronate–glucomannan conjugate for cancer immunotherapy. Biomaterials. 2014;35(38):10046–10057. doi:10.1016/j.biomaterials.2014.09.007
79. Vyas MN, Vyas NK, Quiocho FA. Crystallographic Analysis of the Epimeric and Anomeric Specificity of the Periplasmic Transport/Chemosensory Protein Receptor for D-Glucose and D-Galactose. Biochemistry. 1994;33(16):4762–4768. doi:10.1021/bi00182a003
80. Müller C, Schibli R. Prospects in Folate Receptor-Targeted Radionuclide Therapy. Front Oncol. 2013;3. doi:10.3389/fonc.2013.00249
81. Skytthe MK, Graversen JH, Moestrup SK. Targeting of CD163+ Macrophages in Inflammatory and Malignant Diseases. Int J Mol Sci. 2020;21(15):5497. doi:10.3390/ijms21155497
82. Barclay AN. Signal regulatory protein alpha (SIRPα)/CD47 interaction and function. Curr Opin Immunol. 2009;21(1):47–52. doi:10.1016/j.coi.2009.01.008
83. Tang T, Wei Y, Kang J, et al. Tumor-specific macrophage targeting through recognition of retinoid X receptor beta. J Controlled Release. 2019;301:42–53. doi:10.1016/j.jconrel.2019.03.009
84. Ngambenjawong C, Gustafson HH, Pineda JM, Kacherovsky NA, Cieslewicz M, Pun SH. Serum Stability and Affinity Optimization of an M2 Macrophage-Targeting Peptide (M2pep). Theranostics. 2016;6(9):1403–1414. doi:10.7150/thno.15394
85. Ries CH, Hoves S, Cannarile MA, Rüttinger D. CSF-1/CSF-1R targeting agents in clinical development for cancer therapy. Curr Opin Pharmacol. 2015;23:45–51. doi:10.1016/j.coph.2015.05.008
86. Ries CH, Cannarile MA, Hoves S, et al. Targeting Tumor-Associated Macrophages with Anti-CSF-1R Antibody Reveals a Strategy for Cancer Therapy. Cancer Cell. 2014;25(6):846–859. doi:10.1016/j.ccr.2014.05.016
87. Stafford JH, Hirai T, Deng L, et al. Colony stimulating factor 1 receptor inhibition delays recurrence of glioblastoma after radiation by altering myeloid cell recruitment and polarization. Neuro-Oncol. 2016;18(6):797–806. doi:10.1093/neuonc/nov272
88. Pyonteck SM, Akkari L, Schuhmacher AJ, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013;19(10):1264–1272. doi:10.1038/nm.3337
89. Cannarile MA, Weisser M, Jacob W, Jegg AM, Ries CH, Rüttinger D. Colony-stimulating factor 1 receptor (CSF1R) inhibitors in cancer therapy. J Immunother Cancer. 2017;5(1):53. doi:10.1186/s40425-017-0257-y
90. Matlung HL, Szilagyi K, Barclay NA, van den Berg TK. The CD47-SIRPα signaling axis as an innate immune checkpoint in cancer. Immunol Rev. 2017;276(1):145–164. doi:10.1111/imr.12527
91. Chao MP, Alizadeh AA, Tang C, et al. Anti-CD47 Antibody Synergizes with Rituximab to Promote Phagocytosis and Eradicate Non-Hodgkin Lymphoma. Cell. 2010;142(5):699–713. doi:10.1016/j.cell.2010.07.044
92. Weiskopf K, Ring AM, Ccm H, et al. Engineered SIRPα Variants as Immunotherapeutic Adjuvants to Anticancer Antibodies. Science. 2013;341(6141):88–91. doi:10.1126/science.1238856
93. Chen WC, Kawasaki N, Nycholat CM, et al. Antigen Delivery to Macrophages Using Liposomal Nanoparticles Targeting Sialoadhesin/CD169. PLoS One. 2012;7(6):e39039. doi:10.1371/journal.pone.0039039
94. Al Faraj A, Sultana Shaik A, Afzal S, Al Sayed B, Halwani R. MR imaging and targeting of a specific alveolar macrophage subpopulation in LPS-induced COPD animal model using antibody-conjugated magnetic nanoparticles. Int J Nanomedicine. 2014;1491. doi:10.2147/IJN.S59394
95. Ecker DM, Jones SD, Levine HL. The therapeutic monoclonal antibody market. mAbs. 2015;7(1):9–14. doi:10.4161/19420862.2015.989042
96. Nuhn L, Bolli E, Massa S, et al. Targeting Protumoral Tumor-Associated Macrophages with Nanobody-Functionalized Nanogels through Strain Promoted Azide Alkyne Cycloaddition Ligation. Bioconjug Chem. 2018;29(7):2394–2405. doi:10.1021/acs.bioconjchem.8b00319
97. De Meyer T, Muyldermans S, Depicker A. Nanobody-based products as research and diagnostic tools. Trends Biotechnol. 2014;32(5):263–270. doi:10.1016/j.tibtech.2014.03.001
98. Cieslewicz M, Tang J, Yu JL, et al. Targeted delivery of proapoptotic peptides to tumor-associated macrophages improves survival. Proc Natl Acad Sci. 2013;110(40):15919–15924. doi:10.1073/pnas.1312197110
99. Qian Y, Qiao S, Dai Y, et al. Molecular-Targeted Immunotherapeutic Strategy for Melanoma via Dual-Targeting Nanoparticles Delivering Small Interfering RNA to Tumor-Associated Macrophages. ACS Nano. 2017;11(9):9536–9549. doi:10.1021/acsnano.7b05465
100. Conde J, Bao C, Tan Y, et al. Dual Targeted Immunotherapy via In Vivo Delivery of Biohybrid RNAi-Peptide Nanoparticles to Tumor-Associated Macrophages and Cancer Cells. Adv Funct Mater. 2015;25(27):4183–4194. doi:10.1002/adfm.201501283
101. Ngambenjawong C, Cieslewicz M, Schellinger JG, Pun SH. Synthesis and evaluation of multivalent M2pep peptides for targeting alternatively activated M2 macrophages. J Control Release. 2016;224:103–111. doi:10.1016/j.jconrel.2015.12.057
102. Otvos L, Wade JD. Current challenges in peptide-based drug discovery. Front Chem. 2014;2. doi:10.3389/fchem.2014.00062
103. Song M, Liu T, Shi C, Zhang X, Chen X. Bioconjugated Manganese Dioxide Nanoparticles Enhance Chemotherapy Response by Priming Tumor-Associated Macrophages toward M1-like Phenotype and Attenuating Tumor Hypoxia. ACS Nano. 2016;10(1):633–647. doi:10.1021/acsnano.5b06779
104. Zhu S, Niu M, O’Mary H, Cui Z. Targeting of Tumor-Associated Macrophages Made Possible by PEG-Sheddable, Mannose-Modified Nanoparticles. Mol Pharm. 2013;10(9):3525–3530. doi:10.1021/mp400216r
105. Tang Y, Tang Z, Li P, et al. Precise Delivery of Nanomedicines to M2 Macrophages by Combining “Eat Me/Don’t Eat Me” Signals and Its Anticancer Application. ACS Nano. 2021;15(11):18100–18112. doi:10.1021/acsnano.1c06707
106. Irache JM, Salman HH, Gamazo C, Espuelas S. Mannose-targeted systems for the delivery of therapeutics. Expert Opin Drug Deliv. 2008;5(6):703–724. doi:10.1517/17425247.5.6.703
107. Keefe AD, Pai S, Ellington A. Aptamers as therapeutics. Nat Rev Drug Discov. 2010;9(7):537–550. doi:10.1038/nrd3141
108. Zhou J, Rossi J. Aptamers as targeted therapeutics: current potential and challenges. Nat Rev Drug Discov. 2017;16(3):181–202. doi:10.1038/nrd.2016.199
109. Sylvestre M, Saxby CP, Kacherovsky N, Gustafson H, Salipante SJ, Pun SH. Identification of a DNA Aptamer That Binds to Human Monocytes and Macrophages. Bioconjug Chem. 2020;31(8):1899–1907. doi:10.1021/acs.bioconjchem.0c00247
110. Qian H, Zhou T, Fu Y, et al. Self-assembled tetrahedral framework nucleic acid mediates tumor-associated macrophage reprogramming and restores antitumor immunity. Mol Ther Nucleic Acids. 2022;27:763–773. doi:10.1016/j.omtn.2021.12.036
111. Qian H, Fu Y, Guo M, et al. Dual-aptamer-engineered M1 macrophage with enhanced specific targeting and checkpoint blocking for solid-tumor immunotherapy. Mol Ther. 2022;30(8):2817–2827. doi:10.1016/j.ymthe.2022.04.015
112. Johnston MJW, Semple SC, Klimuk SK, Ansell S, Maurer N, Cullis PR. Characterization of the drug retention and pharmacokinetic properties of liposomal nanoparticles containing dihydrosphingomyelin. Biochim Biophys Acta BBA - Biomembr. 2007;1768(5):1121–1127. doi:10.1016/j.bbamem.2007.01.019
113. Filipczak N, Pan J, Yalamarty SSK, Torchilin VP. Recent advancements in liposome technology. Adv Drug Deliv Rev. 2020;156:4–22. doi:10.1016/j.addr.2020.06.022
114. Tang J, Rakshit M, Chua HM, et al. Liposome interaction with macrophages and foam cells for atherosclerosis treatment: effects of size, surface charge and lipid composition. Nanotechnology. 2021;32(50):505105. doi:10.1088/1361-6528/ac2810
115. Vorselen D. Dynamics of phagocytosis mediated by phosphatidylserine. Biochem Soc Trans. 2022;50(5):1281–1291. doi:10.1042/BST20211254
116. Toita R, Kang JH, Tsuchiya A. Phosphatidylserine liposome multilayers mediate the M1-to-M2 macrophage polarization to enhance bone tissue regeneration. Acta Biomater. 2022;154:583–596. doi:10.1016/j.actbio.2022.10.024
117. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478):eaau6977. doi:10.1126/science.aau6977
118. Ben XY, Wang YR, Zheng HH, et al. Construction of Exosomes that Overexpress CD47 and Evaluation of Their Immune Escape. Front Bioeng Biotechnol. 2022;10:936951. doi:10.3389/fbioe.2022.936951
119. Szebeni J, Alving CR, Rosivall L, et al. Animal models of complement-mediated hypersensitivity reactions to liposomes and other lipid-based nanoparticles. J Liposome Res. 2007;17(2):107–117. doi:10.1080/08982100701375118
120. Kanlikilicer P, Bayraktar R, Denizli M, et al. Exosomal miRNA confers chemo resistance via targeting Cav1/p-gp/M2-type macrophage axis in ovarian cancer. eBioMedicine. 2018;38:100–112. doi:10.1016/j.ebiom.2018.11.004
121. Yang K, Xiao Q, Niu M, Pan X, Zhu X. Exosomes in atherosclerosis: convergence on macrophages. Int J Biol Sci. 2022;18(8):3266–3281. doi:10.7150/ijbs.71862
122. Feng Q, Ma X, Cheng K, et al. Engineered Bacterial Outer Membrane Vesicles as Controllable Two‐Way Adaptors to Activate Macrophage Phagocytosis for Improved Tumor Immunotherapy. Adv Mater. 2022;34(40):2206200. doi:10.1002/adma.202206200
123. Joralemon MJ, McRae S, Emrick T. PEGylated polymers for medicine: from conjugation to self-assembled systems. Chem Commun. 2010;46(9):1377. doi:10.1039/b920570p
124. Kolate A, Baradia D, Patil S, Vhora I, Kore G, Misra A. PEG — a versatile conjugating ligand for drugs and drug delivery systems. J Controlled Release. 2014;192:67–81. doi:10.1016/j.jconrel.2014.06.046
125. Sabzehei F, Taromchi AH, Danafar H, Rashidzadeh H, Ramazani A. In vitro Characterization of Polyethyleneimine–Oleic Acid Cationic Micelle as a Novel Protein Carrier. Adv Biomed Res. 2023;12(1):126. doi:10.4103/abr.abr_303_22
126. Hou Z, Zhou W, Guo X, et al. Poly(ϵ-Caprolactone)-Methoxypolyethylene Glycol (PCL-MPEG)-Based Micelles for Drug-Delivery: the Effect of PCL Chain Length on Blood Components, Phagocytosis, and Biodistribution. Int J Nanomedicine. 2022;17:1613–1632. doi:10.2147/IJN.S349516
127. Klein ME, Rieckmann M, Lucas H, Meister A, Loppnow H, Mäder K. Phosphatidylserine (PS) and phosphatidylglycerol (PG) enriched mixed micelles (MM): a new nano-drug delivery system with anti-inflammatory potential? Eur J Pharm Sci. 2020;152:105451. doi:10.1016/j.ejps.2020.105451
128. Sun JH, Liang X, Cai M, et al. Protein-Crowned Micelles for Targeted and Synergistic Tumor-Associated Macrophage Reprogramming to Enhance Cancer Treatment. Nano Lett. 2022;22(11):4410–4420. doi:10.1021/acs.nanolett.2c00901
129. Liu L, He H, Liang R, et al. ROS-Inducing Micelles Sensitize Tumor-Associated Macrophages to TLR3 Stimulation for Potent Immunotherapy. Biomacromolecules. 2018;19(6):2146–2155. doi:10.1021/acs.biomac.8b00239
130. Lombardo D, Kiselev MA, Caccamo MT. Smart Nanoparticles for Drug Delivery Application: development of Versatile Nanocarrier Platforms in Biotechnology and Nanomedicine. J Nanomater. 2019;2019:1–26. doi:10.1155/2019/3702518
131. Pinelli F, Perale G, Rossi F. Coating and Functionalization Strategies for Nanogels and Nanoparticles for Selective Drug Delivery. Gels. 2020;6(1):6. doi:10.3390/gels6010006
132. Chenthamara D, Subramaniam S, Ramakrishnan SG, et al. Therapeutic efficacy of nanoparticles and routes of administration. Biomater Res. 2019;23(1):20. doi:10.1186/s40824-019-0166-x
133. Fu X, Bai H, Lyu F, Liu L, Wang S. Conjugated Polymer Nanomaterials for Phototherapy of Cancer. Chem Res Chin Univ. 2020;36(2):237–242. doi:10.1007/s40242-020-0012-7
134. Li S, Wang X, Hu R, et al. Near-Infrared (NIR)-Absorbing Conjugated Polymer Dots as Highly Effective Photothermal Materials for In Vivo Cancer Therapy. Chem Mater. 2016;28(23):8669–8675. doi:10.1021/acs.chemmater.6b03738
135. Xiong Z, Timokhina I, Pereloma E. Clustering, nano-scale precipitation and strengthening of steels. Prog Mater Sci. 2021;118:100764. doi:10.1016/j.pmatsci.2020.100764
136. Fuchs AK, Syrovets T, Haas KA, et al. Carboxyl- and amino-functionalized polystyrene nanoparticles differentially affect the polarization profile of M1 and M2 macrophage subsets. Biomaterials. 2016;85:78–87. doi:10.1016/j.biomaterials.2016.01.064
137. Huang YJ, Hung KC, Hung HS, Hsu SH. Modulation of macrophage phenotype by biodegradable polyurethane nanoparticles: the possible relation between macrophage polarization and immune response of nanoparticles. ACS Appl Mater Interfaces. 2018;10(23):19436–19448. doi:10.1021/acsami.8b04718
138. Zanganeh S, Hutter G, Spitler R, et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat Nanotechnol. 2016;11(11):986–994. doi:10.1038/nnano.2016.168
139. Jin R, Liu L, Zhu W, et al. Iron oxide nanoparticles promote macrophage autophagy and inflammatory response through activation of toll-like Receptor-4 signaling. Biomaterials. 2019;203:23–30. doi:10.1016/j.biomaterials.2019.02.026
140. Chen L, Ma X, Dang M, et al. Simultaneous T Cell Activation and Macrophage Polarization to Promote Potent Tumor Suppression by Iron Oxide‐Embedded Large‐Pore Mesoporous Organosilica Core–Shell Nanospheres. Adv Healthc Mater. 2019;8(9):1900039. doi:10.1002/adhm.201900039
141. Guo JC, An Q, Guo M, et al. Oxygen-independent free radical generation mediated by core-shell magnetic nanocomposites synergizes with immune checkpoint blockade for effective primary and metastatic tumor treatment. Nano Today. 2021;36:101024. doi:10.1016/j.nantod.2020.101024
142. Kang H, Kim S, Wong DSH, et al. Remote Manipulation of Ligand Nano-Oscillations Regulates Adhesion and Polarization of Macrophages in Vivo. Nano Lett. 2017;17(10):6415–6427. doi:10.1021/acs.nanolett.7b03405
143. Rojas JM, Sanz-Ortega L, Mulens-Arias V, Gutiérrez L, Pérez-Yagüe S, Barber DF. Superparamagnetic iron oxide nanoparticle uptake alters M2 macrophage phenotype, iron metabolism, migration and invasion. Nanomedicine Nanotechnol Biol Med. 2016;12(4):1127–1138. doi:10.1016/j.nano.2015.11.020
144. Shah A, Dobrovolskaia MA. Immunological effects of iron oxide nanoparticles and iron-based complex drug formulations: therapeutic benefits, toxicity, mechanistic insights, and translational considerations. Nanomedicine Nanotechnol Biol Med. 2018;14(3):977–990. doi:10.1016/j.nano.2018.01.014
145. Yang Y, Tian Q, Wu S, et al. Blue light-triggered Fe2+-release from monodispersed ferrihydrite nanoparticles for cancer iron therapy. Biomaterials. 2021;271:120739. doi:10.1016/j.biomaterials.2021.120739
146. Pazár B, Ea HK, Narayan S, et al. Basic Calcium Phosphate Crystals Induce Monocyte/Macrophage IL-1β Secretion through the NLRP3 Inflammasome In Vitro. J Immunol. 2011;186(4):2495–2502. doi:10.4049/jimmunol.1001284
147. He XY, Liu BY, Xu C, Zhuo RX, Cheng SX. A multi-functional macrophage and tumor targeting gene delivery system for the regulation of macrophage polarity and reversal of cancer immunoresistance. Nanoscale. 2018;10(33):15578–15587. doi:10.1039/C8NR05294H
148. Chang -C-C, Dinh TK, Lee Y-A, et al. Nanoparticle Delivery of MnO2 and Antiangiogenic Therapy to Overcome Hypoxia-Driven Tumor Escape and Suppress Hepatocellular Carcinoma. ACS Appl Mater Interfaces. 2020;12(40):44407–44419. doi:10.1021/acsami.0c08473
149. Zhang X, Tang J, Li C, Lu Y, Cheng L, Liu J. A targeting black phosphorus nanoparticle based immune cells nano-regulator for photodynamic/photothermal and photo-immunotherapy. Bioact Mater. 2021;6(2):472–489. doi:10.1016/j.bioactmat.2020.08.024
150. Yang L, Zhang Y. Tumor-associated macrophages: from basic research to clinical application. J Hematol Oncol. 2017;10(1):58. doi:10.1186/s13045-017-0430-2
151. Nirmala MJ, Kizhuveetil U, Johnson A, B G, Nagarajan R, Muthuvijayan V. Cancer nanomedicine: a review of nano-therapeutics and challenges ahead. RSC Adv. 2023;13(13):8606–8629. doi:10.1039/D2RA07863E
152. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. doi:10.3322/caac.21708
153. Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14(7):399–416. doi:10.1038/nrclinonc.2016.217
154. Noy R, Pollard JW. Tumor-Associated Macrophages: from Mechanisms to Therapy. Immunity. 2014;41(1):49–61. doi:10.1016/j.immuni.2014.06.010
155. Gustafson HH, Pun SH. Instructing macrophages to fight cancer. Nat Biomed Eng. 2018;2(8):559–561. doi:10.1038/s41551-018-0276-0
156. Andón FT, Digifico E, Maeda A, et al. Targeting tumor associated macrophages: the new challenge for nanomedicine. Semin Immunol. 2017;34:103–113. doi:10.1016/j.smim.2017.09.004
157. DeNardo DG, Ruffell B. Macrophages as regulators of tumour immunity and immunotherapy. Nat Rev Immunol. 2019;19(6):369–382. doi:10.1038/s41577-019-0127-6
158. Leuschner F, Courties G, Dutta P, et al. Silencing of CCR2 in myocarditis. Eur Heart J. 2015;36(23):1478–1488. doi:10.1093/eurheartj/ehu225
159. Hughes R, Qian BZ, Rowan C, et al. Perivascular M2 Macrophages Stimulate Tumor Relapse after Chemotherapy. Cancer Res. 2015;75(17):3479–3491. doi:10.1158/0008-5472.CAN-14-3587
160. Yang Z, Li H, Wang W, et al. CCL2/CCR2 Axis Promotes the Progression of Salivary Adenoid Cystic Carcinoma via Recruiting and Reprogramming the Tumor-Associated Macrophages. Front Oncol. 2019;9:231. doi:10.3389/fonc.2019.00231
161. Xu X, Li R, Dong R, et al. In vitro characterization and cellular uptake profiles of TAMs-targeted lipid calcium carbonate nanoparticles for cancer immunotherapy. Acta Mater Medica. 2022;1(3). doi:10.15212/AMM-2022-0030
162. Barclay AN, van den Berg TK. The Interaction Between Signal Regulatory Protein Alpha (SIRPα) and CD47: structure, Function, and Therapeutic Target. Annu Rev Immunol. 2014;32(1):25–50. doi:10.1146/annurev-immunol-032713-120142
163. Lin ZP, Nguyen LNM, Ouyang B, et al. Macrophages Actively Transport Nanoparticles in Tumors After Extravasation. ACS Nano. 2022;16(4):6080–6092. doi:10.1021/acsnano.1c11578
164. Shime H, Matsumoto M, Oshiumi H, et al. Toll-like receptor 3 signaling converts tumor-supporting myeloid cells to tumoricidal effectors. Proc Natl Acad Sci. 2012;109(6):2066–2071. doi:10.1073/pnas.1113099109
165. Shang Y, Lu H, Liao L, Li S, Xiong H, Yao J. Bioengineered Nanospores Selectively Blocking LC3-Associated Phagocytosis in Tumor-Associated Macrophages Potentiate Antitumor Immunity. ACS Nano. 2023;17(11):10872–10887. doi:10.1021/acsnano.3c02657
166. Pan XF, Wang L, Pan A. Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol. 2021;9(6):373–392. doi:10.1016/S2213-8587(21)00045-0
167. Hernandez ED, Lee SJ, Kim JY, et al. A macrophage NBR1-MEKK3 complex triggers JNK-mediated adipose tissue inflammation in obesity. Cell Metab. 2014;20(3):499–511. doi:10.1016/j.cmet.2014.06.008
168. Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117(1):175–184. doi:10.1172/JCI29881
169. Su Y, Wang W, Xiao Q, et al. Macrophage membrane-camouflaged lipoprotein nanoparticles for effective obesity treatment based on a sustainable self-reinforcement strategy. Acta Biomater. 2022;152:519–531. doi:10.1016/j.actbio.2022.08.055
170. Zou W, Rohatgi N, Brestoff JR, et al. Myeloid-specific Asxl2 deletion limits diet-induced obesity by regulating energy expenditure. J Clin Invest. 2020;130(5):2644–2656. doi:10.1172/JCI128687
171. Ma L, Liu TW, Wallig MA, et al. Efficient Targeting of Adipose Tissue Macrophages in Obesity with Polysaccharide Nanocarriers. ACS Nano. 2016;10(7):6952–6962. doi:10.1021/acsnano.6b02878
172. Soccio RE. Galectin-3 in NAFLD: therapeutic Target or Noncausal Biomarker? J Clin Endocrinol Metab. 2021;106(9):e3773–e3774. doi:10.1210/clinem/dgab363
173. Devisscher L, Verhelst X, Colle I, Van Vlierberghe H, Geerts A. The role of macrophages in obesity-driven chronic liver disease. J Leukoc Biol. 2016;99(5):693–698. doi:10.1189/jlb.5RU0116-016R
174. Paré A, Mailhot B, Lévesque SA, et al. IL-1β enables CNS access to CCR2 hi monocytes and the generation of pathogenic cells through GM-CSF released by CNS endothelial cells. Proc Natl Acad Sci. 2018;115(6). doi:10.1073/pnas.1714948115
175. Stöger JL, Gijbels MJJ, van der Velden S, et al. Distribution of macrophage polarization markers in human atherosclerosis. Atherosclerosis. 2012;225(2):461–468. doi:10.1016/j.atherosclerosis.2012.09.013
176. Jenne CN, Kubes P. Immune surveillance by the liver. Nat Immunol. 2013;14(10):996–1006. doi:10.1038/ni.2691
177. Tosello-Trampont AC, Landes SG, Nguyen V, Novobrantseva TI, Hahn YS. Kuppfer Cells Trigger Nonalcoholic Steatohepatitis Development in Diet-induced Mouse Model through Tumor Necrosis Factor-α Production. J Biol Chem. 2012;287(48):40161–40172. doi:10.1074/jbc.M112.417014
178. Ngo W, Ahmed S, Blackadar C, et al. Why nanoparticles prefer liver macrophage cell uptake in vivo. Adv Drug Deliv Rev. 2022;185:114238. doi:10.1016/j.addr.2022.114238
179. Ouyang B, Poon W, Zhang YN, et al. The dose threshold for nanoparticle tumour delivery. Nat Mater. 2020;19(12):1362–1371. doi:10.1038/s41563-020-0755-z
180. Kurniawan DW, Jajoriya AK, Dhawan G, et al. Therapeutic inhibition of spleen tyrosine kinase in inflammatory macrophages using PLGA nanoparticles for the treatment of non-alcoholic steatohepatitis. J Controlled Release. 2018;288:227–238. doi:10.1016/j.jconrel.2018.09.004
181. Maradana MR, Yekollu SK, Zeng B, et al. Immunomodulatory liposomes targeting liver macrophages arrest progression of nonalcoholic steatohepatitis. Metabolism. 2018;78:80–94. doi:10.1016/j.metabol.2017.09.002
182. Martínez–Sánchez C, Bassegoda O, Deng H, et al. Therapeutic targeting of adipose tissue macrophages ameliorates liver fibrosis in non-alcoholic fatty liver disease. JHEP Rep. 2023;5(10):100830. doi:10.1016/j.jhepr.2023.100830
183. Maeda H, Ishima Y, Saruwatari J, et al. Nitric oxide facilitates the targeting Kupffer cells of a nano-antioxidant for the treatment of NASH. J Controlled Release. 2022;341:457–474. doi:10.1016/j.jconrel.2021.11.039
184. Song M, Schuschke DA, Zhou Z, et al. Kupffer cell depletion protects against the steatosis, but not the liver damage, induced by marginal-copper, high-fructose diet in male rats. Am J Physiol-Gastrointest Liver Physiol. 2015;308(11):G934–G945. doi:10.1152/ajpgi.00285.2014
185. Traber PG, Zomer E. Therapy of Experimental NASH and Fibrosis with Galectin Inhibitors. PLoS One. 2013;8(12):e83481. doi:10.1371/journal.pone.0083481
186. Akram MS, Pery N, Butler L, et al. Challenges for biosimilars: focus on rheumatoid arthritis. Crit Rev Biotechnol. 2021;41(1):121–153. doi:10.1080/07388551.2020.1830746
187. Ye L, Wen Z, Li Y, et al. Interleukin-10 attenuation of collagen-induced arthritis is associated with suppression of interleukin-17 and retinoid-related orphan receptor γt production in macrophages and repression of classically activated macrophages. Arthritis Res Ther. 2014;16(2):R96. doi:10.1186/ar4544
188. Cutolo M, Campitiello R, Gotelli E, Soldano S. The Role of M1/M2 Macrophage Polarization in Rheumatoid Arthritis Synovitis. Front Immunol. 2022;13:867260. doi:10.3389/fimmu.2022.867260
189. Li S, Su J, Cai W, Liu JX. Nanomaterials Manipulate Macrophages for Rheumatoid Arthritis Treatment. Front Pharmacol. 2021;12:699245. doi:10.3389/fphar.2021.699245
190. Nasra S, Bhatia D, Kumar A. Recent advances in nanoparticle-based drug delivery systems for rheumatoid arthritis treatment. Nanoscale Adv. 2022;4(17):3479–3494. doi:10.1039/D2NA00229A
191. Gomez-Barrena E, Lindroos L, Ceponis A, et al. Cartilage oligomeric matrix protein (COMP) is modified by intra-articular liposomal clodronate in an experimental model of arthritis. Clin Exp Rheumatol. 2006;24(6):622–628.
192. Jain S, Tran TH, Amiji M. Macrophage repolarization with targeted alginate nanoparticles containing IL-10 plasmid DNA for the treatment of experimental arthritis. Biomaterials. 2015;61:162–177. doi:10.1016/j.biomaterials.2015.05.028
193. Yang Y, Guo L, Wang Z, et al. Targeted silver nanoparticles for rheumatoid arthritis therapy via macrophage apoptosis and Re-polarization. Biomaterials. 2021;264:120390. doi:10.1016/j.biomaterials.2020.120390
194. Lu Y, Zhou J, Wang Q, et al. Glucocorticoid-loaded pH/ROS dual-responsive nanoparticles alleviate joint destruction by downregulating the NF-κB signaling pathway. Acta Biomater. 2023;164:458–473. doi:10.1016/j.actbio.2023.04.012
195. Li H, Feng Y, Zheng X, et al. M2-type exosomes nanoparticles for rheumatoid arthritis therapy via macrophage re-polarization. J Controlled Release. 2022;341:16–30. doi:10.1016/j.jconrel.2021.11.019
196. Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol. 2013;13(10):709–721. doi:10.1038/nri3520
197. Peled M, Fisher EA. Dynamic Aspects of Macrophage Polarization during Atherosclerosis Progression and Regression. Front Immunol. 2014;5. doi:10.3389/fimmu.2014.00579
198. Wang Y, Li L, Zhao W, et al. Targeted Therapy of Atherosclerosis by a Broad-Spectrum Reactive Oxygen Species Scavenging Nanoparticle with Intrinsic Anti-inflammatory Activity. ACS Nano. 2018;12(9):8943–8960. doi:10.1021/acsnano.8b02037
199. Jiang C, Qi Z, Tang Y, et al. Rational Design of Lovastatin-Loaded Spherical Reconstituted High Density Lipoprotein for Efficient and Safe Anti-Atherosclerotic Therapy. Mol Pharm. 2019;16(7):3284–3291. doi:10.1021/acs.molpharmaceut.9b00445
200. Beldman TJ, Senders ML, Alaarg A, et al. Hyaluronan Nanoparticles Selectively Target Plaque-Associated Macrophages and Improve Plaque Stability in Atherosclerosis. ACS Nano. 2017;11(6):5785–5799. doi:10.1021/acsnano.7b01385
201. Yang Q, Jiang H, Wang Y, et al. Plaque Macrophage‐Targeting Nanosystems with Cooperative Co‐Regulation of ROS and TRAF6 for Stabilization of Atherosclerotic Plaques. Adv Funct Mater. 2023;33(28):2301053. doi:10.1002/adfm.202301053
202. Ouimet M, Ediriweera HN, Gundra UM, et al. MicroRNA-33–dependent regulation of macrophage metabolism directs immune cell polarization in atherosclerosis. J Clin Invest. 2015;125(12):4334–4348. doi:10.1172/JCI81676
203. He J, Zhang W, Zhou X, et al. Reactive oxygen species (ROS)-responsive size-reducible nanoassemblies for deeper atherosclerotic plaque penetration and enhanced macrophage-targeted drug delivery. Bioact Mater. 2023;19:115–126. doi:10.1016/j.bioactmat.2022.03.041
204. Gao C, Huang Q, Liu C, et al. Treatment of atherosclerosis by macrophage-biomimetic nanoparticles via targeted pharmacotherapy and sequestration of proinflammatory cytokines. Nat Commun. 2020;11(1):2622. doi:10.1038/s41467-020-16439-7
205. Wang Y, Zhang K, Li T, et al. Macrophage membrane functionalized biomimetic nanoparticles for targeted anti-atherosclerosis applications. Theranostics. 2021;11(1):164–180. doi:10.7150/thno.47841
206. Hu R, Dai C, Dong C, et al. Living Macrophage-Delivered Tetrapod PdH Nanoenzyme for Targeted Atherosclerosis Management by ROS Scavenging, Hydrogen Anti-inflammation, and Autophagy Activation. ACS Nano. 2022;16(10):15959–15976. doi:10.1021/acsnano.2c03422
207. Cordes T, Wallace M, Michelucci A, et al. Immunoresponsive Gene 1 and Itaconate Inhibit Succinate Dehydrogenase to Modulate Intracellular Succinate Levels. J Biol Chem. 2016;291(27):14274–14284. doi:10.1074/jbc.M115.685792
208. Na YR, Stakenborg M, Seok SH, Matteoli G. Macrophages in intestinal inflammation and resolution: a potential therapeutic target in IBD. Nat Rev Gastroenterol Hepatol. 2019;16(9):531–543. doi:10.1038/s41575-019-0172-4
209. Ma WT, Gao F, Gu K, Chen DK. The Role of Monocytes and Macrophages in Autoimmune Diseases: a Comprehensive Review. Front Immunol. 2019;10:1140. doi:10.3389/fimmu.2019.01140
210. Haribhai D, Ziegelbauer J, Jia S, et al. Alternatively Activated Macrophages Boost Induced Regulatory T and Th17 Cell Responses during Immunotherapy for Colitis. J Immunol. 2016;196(8):3305–3317. doi:10.4049/jimmunol.1501956
211. Lissner D, Schumann M, Batra A, et al. Monocyte and M1 Macrophage-induced Barrier Defect Contributes to Chronic Intestinal Inflammation in IBD. Inflamm Bowel Dis. 2015:1. doi:10.1097/MIB.0000000000000384
212. Zhu Y, Li X, Chen J, et al. The pentacyclic triterpene Lupeol switches M1 macrophages to M2 and ameliorates experimental inflammatory bowel disease. Int Immunopharmacol. 2016;30:74–84. doi:10.1016/j.intimp.2015.11.031
213. Song WJ, Li Q, Ryu MO, et al. TSG-6 Secreted by Human Adipose Tissue-derived Mesenchymal Stem Cells Ameliorates DSS-induced colitis by Inducing M2 Macrophage Polarization in Mice. Sci Rep. 2017;7(1):5187. doi:10.1038/s41598-017-04766-7
214. Seo DH, Che X, Kwak MS, et al. Interleukin-33 regulates intestinal inflammation by modulating macrophages in inflammatory bowel disease. Sci Rep. 2017;7(1):851. doi:10.1038/s41598-017-00840-2
215. Huang Z, Gan J, Jia L, et al. An orally administrated nucleotide-delivery vehicle targeting colonic macrophages for the treatment of inflammatory bowel disease. Biomaterials. 2015;48:26–36. doi:10.1016/j.biomaterials.2015.01.013
216. Tannahill GM, Curtis AM, Adamik J, et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature. 2013;496(7444):238–242. doi:10.1038/nature11986
217. Hayashi M, Sakata M, Takeda T, et al. Induction of glucose transporter 1 expression through hypoxia-inducible factor 1α under hypoxic conditions in trophoblast-derived cells. J Endocrinol. 2004;183(1):145–154. doi:10.1677/joe.1.05599
218. Herfarth H. IL-10 therapy in Crohn’s disease: at the crossroads. Gut. 2002;50(2):146–147. doi:10.1136/gut.50.2.146
219. Bae YH, Park K. Targeted drug delivery to tumors: myths, reality and possibility. J Controlled Release. 2011;153(3):198–205. doi:10.1016/j.jconrel.2011.06.001
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


