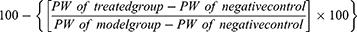Back to Journals » Research and Reports in Urology » Volume 14
Administration of Caesalpinia bonduc Seed Extracts Ameliorates Testosterone-Induced Benign Prostatic Hyperplasia (BPH) in Male Wistar Rats
Authors Sasidharan S , KP S, Bhaumik A, Kanti Das S, Nair J H
Received 8 March 2022
Accepted for publication 7 May 2022
Published 26 May 2022 Volume 2022:14 Pages 225—239
DOI https://doi.org/10.2147/RRU.S365598
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Panagiotis J Vlachostergios
Shan Sasidharan,1,2 Srinivasakumar KP,1 Amiya Bhaumik,1 Sreemoy Kanti Das,1 Hareebndran Nair J3
1Department of Pharmacy, Lincoln University College, Petaling Jaya, Malaysia; 2Small Animal Research Centre, Department of Toxicology and Pharmacology, CARe KERALA, Thrissur, Kerala, India; 3Department of R&D, Pankajakasthuri Herbal Research Foundation, Thiruvananthapuram, Kerala, India
Correspondence: Shan Sasidharan, Department of Pharmacy Lincoln University College, Petaling Jaya, Malaysia, Email [email protected]
Introduction: Benign prostatic hyperplasia (BPH) is a major chronic disease affecting men, and the therapeutic agents currently used to manage it have significant side effects. As a result, an alternative medicine with improved therapeutic properties with no side effects is desperately needed. The current investigation aims to study whether the Caesalpinia bonduc seed extracts (ethanolic-A, hydroalcoholic-B, and aqueous-C) have inhibitory potential on testosterone propionate (TP)-induced BPH in Wistar rats.
Methods: Wistar rats (male) were randomly allocated to one of five groups: control, BPH (TP-3 mg/kg, subcutaneously daily), low dose (TP + C. bonduc seed extracts – 200 mg/kg body weight), high dose (TP + C. bonduc seed extracts – 400 mg/kg body weight), and standard drug (TP + finasteride – 10 mg/kg body weight). At the end of drug treatment, the rats were sacrificed and their serum and prostates were taken for biochemical and histological studies.
Results: C. bonduc seed extracts treatment significantly decreased prostate weight and prostatic index in rats with TP-induced BPH. The seed extracts exhibited a potent inhibitory effect on dihydrotestosterone (DHT) in serum and prostate. In addition, the PSA level in the serum showed a noteworthy decrease in comparison with the BPH group. Histopathological examination also indicated that extracts improved the tissue morphology of the prostate significantly. Out of three extracts tested, ethanolic and hydroalcoholic extract recorded significant effect. Finally, liquid chromatography quadrupole time-of-flight mass spectrometry (LC/MS-QTOF) analysis showed that the major compounds present in the extracts were tocopherols, fucosterol, linoleic acid, β-amyrin, β-sitosterol, campesterol, cassane furanoditerpene, norcassane furanoditerpene and other diterpenes.
Conclusion: Thus, C. bonduc seed extracts could be a potential source for the formulation of new drug for managing BPH. To the best of our knowledge, this is the first scientific animal investigation into the use of C. bonduc seed extract for the management of BPH.
Keywords: Caesalpinia bonduc seed extracts, benign prostatic hyperplasia, testosterone, Wistar rats, prostate gland
Introduction
Benign prostatic hyperplasia (BPH) is the topmost prominent disease affecting the prostate gland in older men and approximately one-half of all men aged above 50 years will have this disease.1–3 The BPH condition is characterized by an abnormally enlarged prostate gland, which induces urethral constriction, resulting in increased frequency, urgency, and hesitancy of urination, as well as disrupted urine flow, which eventually affects the quality of life of the patients.3 Thus far, the pathophysiology of BPH is not fully elucidated, although several attempts have been made in the previous years. However, previous research studies have established that a discrepancy between androgens and estrogens plays a critical role in the development and progression of BPH.4,5
Even though the pathway associated with prostatic enlargement is not fully understood, androgens are regarded as one of the most significant factors. Although androgens do not directly cause BPH, testicular androgens play an important role in the development of prostatic growth in men.6 Dihydrotestosterone (DHT) is likely to be the most vital factor associated with the enlargement of prostate. It was reported that the DHT concentrations in the serum are higher in BPH patients than in unaffected men of similar age.7 In the prostate, 5α-reductase converts testosterone into DHT.8 Because of its higher affinity for the androgen receptor, DHT is a more potent androgen than testosterone. DHT is an active metabolic product of testosterone reduction by 5α-reductase. It is essential for prostate growth because it binds to the nuclear androgen receptor, causing the synthesis of growth factors acting on prostatic epithelia and stroma, resulting in prostate enlargement.9 Experiments have also revealed that estrogen levels rise with age, potentially increasing the expression of DHT, the progenitor of BPH.10 The current use of 5α -reductase inhibitors in the treatment of BPH is based on the incrimination of DHT in the pathogenesis of BPH.
The management options available for BPH include watchful waiting, drug treatment and finally surgery. Conventional drugs that manage BPH include 5α-reductase inhibitors (finasteride and dutasteride) and α1-adrenergic antagonists (alfuzosin, doxazosin, tamsulosin and terazosin). The α1-adrenergic antagonists are the early drugs for treating BPH, and they improve lower urinary tract symptoms (LUTS) by relaxing the smooth muscle in the prostate and the neck of the bladder.11 However, the 5α-reductase inhibitors inhibit the enlargement of BPH by reducing the production of DHT.12 Even though the drugs mentioned above have ample effectiveness in treating BPH, their severe side effects should not be neglected. To put this into perspective, some of the severe adverse effects of 5α-reductase inhibitors include impotence, gynecomastia, impairment of muscle growth, and reduced libido. On the other hand, α1-adrenergic antagonists cause orthostatic hypotension, fatigue, dizziness and abnormal ejaculation. Additionally, the required long-term drug treatment to manage the condition and any surgical treatments are expensive and risky for aged men, which ignores their use as routine treatment.13 This warrants a search for new effective and safe drugs for the BPH patients. Medicinal plants are currently being researched for the treatment of BPH patients, and shallow research shows that many BPH patients use complementary and alternative medicines along with their prescriptions to manage their condition.14,15
Regardless of the several endeavors in medication therapy, there is still a need to dig out a new effectual treatment for managing this disease that can deal with its significant potency and fewer side effects in the long-term use.16 Hence, the present investigation aimed to study the effects of the seed extracts of a renowned medicinal plant called Caesalpinia bonduc. C. bonduc (L.) Roxb., one of the pantropical leguminous scandent shrubs that has been explored by numerous researchers for multiple medicinal uses worldwide.17,18 This plant belongs to the family of Caesalpiniaceae which is generally known as Fever Nut or Nicker Nut.19,20 Since ancient times, all parts of this plant have had significant therapeutic value and have been used in traditional medicine.21 Additionally, this plant is considered as an essential remedy for several major ailments because of its significant therapeutical effects.21
In folklore medicine, the seeds of C. bonduc have been used as antiinflammatory agents. The seed oil and the kernel extracts have been screened for antiinflammatory and antipyretic activity. The steroids and terpenes present in the seed are responsible for the antiinflammatory property.22 Anticancer and cytotoxic activities of various parts of C bonduc23,24 and its isolated phytoconstituents25,26 have been reported earlier. Furthermore, in a study, the anti-androgenic potential of C. bonduc seed extract was highly evidenced in male Wistar rats.27 The seed is known to contain fatty oil, starch, sucrose, phytosterols, stearic, palmitic, oleic, linoceric, linolenic and a mixture of unsaturated acid of low molecular weights17 and this clearly indicates the presence of various bioactive metabolites. By considering all these facts, an effort was made to evaluate the amelioration of C. bonduc seed extract on testosterone propionate (TP) inducted BPH in experimental animals.
In our study, therefore, we investigated the inhibitory effects of the seed extracts of C. bonduc on the TP-induced BPH rat model by measuring changes in prostate weight and the inhibition of DHT, and prostate-specific antigen (PSA) as well as prostate histopathology. The results from the present clearly indicated that seed extract of C. bonduc might be a novel candidate medication for treating BPH shortly.
Materials and Methods
Study Plant – C. bonduc
In this study, we have used dried seeds of C. bonduc to prepare the extracts. The C. bonduc seeds were collected from the Ernakulam region of Kerala State, India, and identified by Dr. Dan Mathew, Senior scientist at Jawaharlal Nehru Tropical Botanic Garden and Research Institute (JNTBGRI), Palode, Trivandrum, Kerala, India. The voucher specimens were also deposited at the same institute.
Preparation of Seed Extract
The C. bonduc seeds were thoroughly washed in distilled water and dried to a constant weight at 50°C in a hot air oven for 1 h. After that, the seeds were crushed using a herb crusher or choppers to smash them into a colloidal mass. The ethanolic, hydroalcoholic and aqueous extracts of the seed materials were prepared separately by macerating 200 gm of powdered seed sample in 1 L of ethanol, water: alcohol (1:1) and water for 72 h, after which the suspended materials were carefully filtered. The extraction procedure was repeated twice, and all filtrates were combined and separately concentrated with a rotary evaporator at 40℃ in vacuum. The residual solvent was evaporated to dryness with the help of a water bath. The extracts thus obtained were stored in the refrigerator for future studies.
Ethical Approval
The experimental protocol of the present study was reviewed thoroughly and approved with reference no. CKL/TOX/IAEC/2017-3/89 by the Institutional Animal Ethical Committee of CARe KERALAM Ltd., KINFRA Park P.O, Koratty, Thrissur, Kerala 680309 (Reg. No.1620/PO/RcBi/S/12/CPCSEA). The rats were kept in accordance with the guidelines published by CPCSEA (The Committee for the Purpose of Control and Supervision of Experiments on Animals), Government of India.
Study Animals
The candidates chosen for this study were seven-week-old healthy male Wistar rats (175–250 g). All rats were housed in clean plastic cages with a 12-hour light/dark cycle (temperature 22℃, relative humidity 55%). The rats were allowed to access water and food freely throughout the experiment tenure. Rats were also allowed to acclimatize before the commencement of the study. All the animal experiments were strictly conducted conforming to the protocols approved by the Institutional Animal Care Committee of CARe KERALAM Ltd., Koratty, Thrissur, Kerala, India.
Induction of BPH in the Experimental Rats
BPH was induced in rats through daily subcutaneous injections (s.c.) of TP (3 mg/kg, dissolved in corn oil) for 28 days.
Study Design
The rats were randomized into five groups; Group 1 received distilled water (control), Group 2 received TP injection along with distilled water and acted as a BPH induced control, Group 3 (low dose) was divided into three subgroups and each subgroup separately received 200 mg/kg body weight of ethanolic, hydroxyethanolic and aqueous seed extract of C. bonduc along with TP injection, Group 4 (high dose) also divided into three subgroups and each subgroup separately received 400 mg/kg body weight of ethanolic, hydroxyethanolic and aqueous seed extract of C. bonduc along with TP injection. Finally, Group 5 (standard drug) received TP injection along with finasteride treatment (10 mg/kg body weight, orally). The animal grouping and treatment process are summarized in Table 1.
 |
Table 1 Grouping of Rats and the Treatment Details |
The weights of the rats were carefully recorded before the initiation of the experiments and consequently every week until the termination of the experiment. All the test materials (C. bonduc seed extracts) were administered to the rats in the morning for 28 days. Following the final administration of test material and overnight fasting, the rats were anesthetized using pentobarbital (50 mg/kg, intraperitoneal injection). After that, blood samples were taken for examination and prostate tissues were dissected and weighed right away. The sections prostate lobe was fixed with paraformaldehyde (4%) for histopathological analysis, and the leftover prostate sections were stored at −80℃ for further examination.
Ratio of Body and Prostate Weights
At the terminal sacrifice of the study, the body weight (BW) and prostate weight (PW) of each animal were recorded. Following this, group mean body weights were calculated. For each individual study group animal, the prostate weight (P) to body weight (BW) ratio was calculated by dividing the prostate weight by the animal’s body weight.
Percentage of Recovery
We have calculated the percentage recovery in P/BW ratio by test group versus control group based on mean prostatic weight and P/BW ratios. The dilation caused by TP alone was assumed to be 100%, and the values of all other test groups were compared to this reference. The formula used for the calculation is as follows:
Percent recovery by the test sample = [A – B]
Where A is the percentage increase in prostatic weight caused by TP, and B is the percentage increase in prostatic weight caused by test materials.
Percentage of Inhibition of Increase in Prostate Weight
The percentage inhibition (%) of the increase in prostate weight caused by the treatment of C. bonduc seed extracts was calculated using the formula below:28
Determination of DHT Levels in Serum and Prostate
The rats underwent fasting overnight on the 28th day following the final administration of C. bonduc seed extracts and sacrificed the next day. Blood samples were carefully collected in test tubes, kept for 1 h and then rotated for 15 min at 3000 rpm to obtain serum, which was used for biochemical investigation. After that, approximately 100 mg of prostate tissue was rinsed with 1× phosphate buffered saline (PBS), homogenized in 1 mL of 1× PBS and stored overnight at −20℃. Following this, the homogenates were centrifuged for 5 minutes at 5000 rpm in 4℃. The supernatant was removed and assayed immediately. The levels of DHT in serum and the prostate were determined using an enzyme-linked immunosorbent assay (ELISA) kit following the manufacturer’s instructions (Cusabio Biotech Co., Ltd. China). The absorbance was measured using a microplate ELISA reader at 450 nm. Values are expressed as pg/mL tissue homogenate for the prostate and pg/mL for serum.
Determination of the Levels of Prostate-Specific Antigen (PSA) in the Serum
The serum PSA levels were measured using an ELISA kit following the manufacturer’s instructions (Cusabio Biotech Co., Ltd., China). The absorbance of the color intensity was measured at 450 nm. After that, the concentration of PSA in the samples was expressed as ng/mL in the serum.
Histopathological Studies Using Hematoxylin and Eosin (H&E) Staining
The histological examination of the prostate tissue was performed according to the method of Bianchi-Frias.29 The prostate tissue was fixed in 10% neutral buffered formalin. The fixed prostate tissues were dehydrated with subsequent 50%, 70%, 90%, and 100% ethanol and then cleared in xylene. After incubation in paraffin at 60℃ in an incubator, they were embedded and blocked-in paraffin at the same temperature. Fine serial sections (5 μm thick) obtained by cutting the embedded tissue using a microtome then mounted on gelatinous water coated slides and dried for 24 h at room temperature. The sections on the slides were deparaffinized using xylene, rehydrated in a descending series of alcohol and water, before stained with hematoxylin and eosin dyes. After that, the slides were dried and mounted on a light microscope for microscopic examination at 200x magnification for histopathological analysis.
Characterization of Active Compounds Through LC/MS-QTOF Analysis
The characterization of bioactive compounds was carried out by LC-QTOF (Agilent Technologies, Santa Clara, CA, USA, Model no: 6545 QTOF).
Statistical Analysis
The data were expressed as mean± standard deviation. The averages were analyzed using a one-way analysis. The post-test analysis was performed using Dunnett’s multiple comparison tests to determine the comparison between the treatment and control groups. Values were considered significantly different when P value <0.05. The analyses were done using the GraphPad Prism software version 5.00 (GraphPad Prism Software, Inc., San Diego, California, 2007).
Results
Effect of C. bonduc Seed Extract on the Prostrate Weight
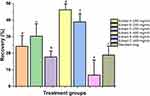
Relative prostate weight is normally used to evaluate the growth of BPH. Rats in the TP-induced BPH group recorded significantly greater absolute and relative prostate weights than those of rats in the normal control group. Prostate weights in the ethanolic (A) and hydroalcoholic (B) extracts treated groups, at 400 mg/kg, were decreased appreciably (P < 0.05) and exhibited significant decrease in relative prostate weights or PW index compared to the BPH group (P < 0.01 and P < 0.05 respectively) (Figure 1). In addition, C. bonduc seed extracts inhibited the testosterone-induced increase in prostate weight by 41.74% [ethanolic (A) - 41.94%, hydroalcoholic (B) - 52.47% and aqueous (C) - 30.79%] in the 200 mg/kg dose group and by 53.18% [ethanolic - 80.22%, hydroalcoholic - 67.46% and aqueous - 11.87%] in the 400 mg/kg dose group (Figure 2). The C. bonduc seed extracts also recorded a significant percentage recovery of prostate. At 400 mg/kg dose, ethanolic extract recorded 46.27% recovery of the prostate, whereas hydroalcoholic and aqueous extract recorded 38.91% and 6.84% recovery of the prostate when compared to the untreated group (Figure 3). Surprisingly, the finasteride treated groups recorded only 18.92% recovery of the prostate. In the present study, the finasteride treated group did not show any significant treatment-related changes in the prostatic parameters. We could not find any statistically significant differences in body weight changes between the groups throughout the study.
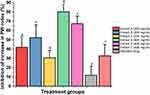 |
Figure 2 The percentage inhibition of the increase in prostate weight induced by treatments. The values with different letter (a–g) are significantly different (p < 0.05) for each group. |
Effect of C. bonduc Seed Extract on the Levels of DHT in Serum and Prostate Tissue
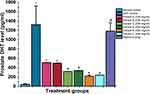
Prostate enlargement is dependent on the prostatic androgen DHT. In the prostate gland 5α-reductase metabolizes testosterone to DHT which in turn binds to receptors in the cell, resulting in BPH. The BPH induced control group recorded a significant increase in serum DHT level (1599.33±305.934 pg/mL, P < 0.001) compared with the normal control group (153.667±52.413 pg/mL) (Figure 4). However, the high dose of C. bonduc seed extracts (A, B & C) treated groups recorded a remarkably reduced serum DHT level (561.50±23.50, 563.00±24.00 and 563.50±1.50 pg/mL, P < 0.05) compared with the BPH group (Figure 4). The extract treatment showed significant reduction in prostate DHT levels (A-510.00±2.00, B - 496.500±4.50 pg/mL, P < 0.01 and C-320.00±1.00 pg/mL, P < 0.001) in the 200 mg/kg group and (A-339.00±2.00, B-202.50±2.50 and C-240.00±2.00 pg/mL) in the 400 mg/kg group (P < 0.001) compared to the BPH group (Figure 5). There was no treatment related significant reductions in serum and prostate DHT levels in the finasteride treated groups in the present study.
Prostate-Specific Antigen (PSA) Levels in Serum
There was a rise in PSA level to 4.017±0.075 ng/mL in BPH induced rats, which indicated a significant increase (P < 0.001) in the value when compared to that of the normal rats (1.517±0.142 ng/mL). The PSA level in the ethanolic and hydroalcoholic extract treatment groups (200 and 400 mg/kg) showed a marked decrease of 39.41% (2.43 ng/mL) and 49.17% (1.98 ng/mL), respectively (Figure 6).
Histopathological Evaluations
On gross examination, marked enlargement of the prostates without any visible lesions was observed in the different experimental groups.
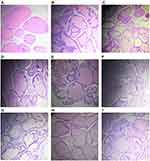
The prostate of rats generally consists of three distinct lobes, including dorsal, lateral and ventral lobes. We have analyzed the ventral lobe of prostate tissue for various histopathological changes.30,31 Histological sections from the control group showed a normal architecture of the ventral prostate lobe characterized by columnar epithelium with round-shaped nuclei adjacent to the intact basal membrane and regular acini. Furthermore, the lumen contains only a small number of papillary folds. The lumens were filled with prostatic secretions in connective tissue and further the matrix was observed normal. Overall, histopathology revealed a normal prostate gland (Figure 7A).
In contrast, the BPH-induced group showed marked epithelial hyperplasia with numerous intraluminal folds, resulting in the lumen narrowing. These animals exhibited greatly increased prostatic epithelial thickness compared to those from the normal control group. In addition to this, the walls of tubules were observed thickened when compared to the control group (Figure 7B). Nearly every tubule developed large involutions which project into the lumen, resulting in the reduction of lumen volume compared with the control group. Overall histopathology for this group revealed BPH condition (Figure 7B).
The animals from the group treated with the extracts at 200 mg/kg recorded only a mild reduction in the epithelial thickness compared with those from the BPH group (Figure 7C–E). However, rats treated with C. bonduc seed extracts (A, B and C) at 400 mg/kg displayed remarkably reduced hyperplasia symptoms compared with those from the BPH group. Furthermore, this group demonstrated a significant reduction in the thickness of the epithelium wall of their prostatic tubules, implying a significant reduction in histoarchitectural disruption of the prostate (Figure 7F–H). The finasteride treated group also showed only a mild reduction in the epithelial thickness when compared to the BPH group (Figure 7I).
Identification of Compounds in the Extracts of C. bonduc
The LC/MS-QTOF spectral results and database search tentatively confirmed 16, 17 and 9 major compounds from the ethanolic, hydroxyethanolic and aqueous extracts of C. bonduc, respectively (Table 2). The LC/MS-QTOF analysis recorded that the major compounds present in the extracts were cassane furanoditerpene (Caesalpinin), Norcassane furanoditerpene (Norcaesalpinin) and other diterpenes (Caesaldekarin) (Table 2).
 |
Table 2 The Compounds from the C. bonduc Seed Extracts |
Discussion
Irrespective of the progression in the diagnosis and management of BPH, it remains the most dominant urologic health problem troubling elderly men worldwide.11 The current study was aimed to evaluate the inhibitory or antiproliferative effects of C. bonduc seed extracts on the development of BPH using a TP-induced model in rats. The rats with TP-induced BPH displayed elevated absolute and relative prostate weights, increased DHT and PSA levels and prostatic epithelial hyperplasia compared to normal control rats. We found that C. bonduc seed extracts reduced the testosterone-induced increment in prostate weight and then improved the histology of prostate tissue. Further, the extracts decreased the expression of DHT and PSA.
A handful of research studies have proven that an increase in the prostate is a vital indicator of the development of BPH,32–34 and our study reconfirmed this since BPH rats recorded significant enlargement in the size of prostate gland. It is well understood that when the prostate enlarges, the urethral canal constricts, resulting in partial or complete urinary canal obstruction. According to Veeresh et al,35 increased relative prostate weight is used as one of the significant marker indicating the development of BPH and in earlier studies, where experimental rats with BPH have shown an increased relative prostate weight. BPH is characterized by epithelial and stromal hyperplasia of the prostate, which results in an increase in prostate weight. When the prostate dilates, it results in the constriction of urethral canal, causing partial or complete obstruction. Due to these reasons, many studies have tested the inhibitory effects of many compounds, especially phytochemicals, on the development of BPH by measuring prostate weights.36 In the present study also, oral administration of ethanolic (A) and hydroxyalcoholic (B) extracts of C. bonduc seed, at 400 mg/kg, resulted in substantial reductions in absolute and relative prostate weights. Hence, the ability of C. bonduc seed extracts to reduce the prostate weight of BPH-induced rats clearly pointed the ameliorative potential of this plant.
Testosterone and DHT are involved in the pathogenesis of BPH and play an important role in the development of male reproductive organs.37,38 The serum concentrations of testosterone and DHT may vary with age.39 The levels of DHT in the serum of BPH patients are significantly higher than those of unaffected men of comparable age.40 DHT is primarily synthesized from circulating testosterone in the prostate, hair follicles, and testes via the enzymatic action of 5α-reductase. Interestingly, DHT binds to androgen receptors more strongly than testosterone and adrenal androgens. This is due to the greater affinity of DHT towards androgen receptors when compared to that of testosterone and adrenal androgens.37 As a result, a number of studies have been conducted to investigate how 5α-reductase regulates DHT levels. Finasteride is a known 5α-reductase inhibitor commonly used to manage BPH conditions. It reduces testosterone and DHT levels in serum and the prostate gland, contracting prostate size and BPH-related symptoms like LUTS.41,42 Scientists, however, are vigorously investigating to find an alternative medicine for finasteride to treat and manage BPH because long-term use of this drug has serious adverse effects.43 In our studies, the testosterone-induced increase in the production of serum DHT was significantly inhibited by oral administration of C. bonduc seed extracts in a dose-dependent manner. This suggests that the inhibitory potential of C. bonduc seed extracts in our TP-induced rat model was due to the downregulation of DHT production in serum and prostate. These results suggest that C. bonduc seed extracts may be a viable alternative to finasteride to manage BPH conditions in the future.
Enlargement of the prostate gland is viewed as a histological diagnosis characterized by the proliferation of the prostate’s cellular elements, including stromal and epithelial components.44 In our study, histopathological data clearly suggested the overall histological improvement in the C. bonduc seed extracts group compared to the BPH control. The histomorphological changes in prostate tissue sections of the C. bonduc seed extract treated with the features earlier reported.45 The observed hyperplasia mechanism may be associated with DHT accumulation in the prostate, which binds to nuclear hormone receptors and stimulates cellular growth. The majority of hyperplasia is caused by glandular proliferation, but the stroma is also thickened. Histopathology of the prostate in rats with BPH revealed epithelial hyperplasia with an increase in epithelial thickness when compared to normal control animals in this study. In contrast, the C. bonduc seed extracts treated rats recorded mild prostatic epithelial hyperplasia with decreased epithelial thickness compared with BPH-induced animals. These results totally agreed with the data of prostate weight. Thus, the C. bonduc seed extracts were highly effective in attenuating prostatic hyperplasia. The histopathological observations further support the results of serum analyses. Hence, the BPH-induced rats with C. bonduc seed extracts normalize aberrations associated with BPH.
PSA is a glycoprotein that is produced by the secretory epithelium of the prostate gland and it has been reported to be mildly increased in BPH.46 PSA level will significantly increase in both benign and malignant lesions of the prostate but is usually marked in case of prostatic cancer.47,48 Thus, the enhanced serum PSA levels of the disease control group suggest the development of BPH for this group of rats and a decrease in PSA is linked to a reduction in prostatic hyperplasia due to inhibition of prostatic 5α-reductase, the enzyme that converts DHT.49 Therefore, a reduction in PSA level after the administration of any test materials displays the effectiveness of the treatment of BPH.50 In the present study, PSA levels were increased in BPH-induced rats but reduced markedly in all three C. bonduc seed extract treated rats (200 and 400 mg/kg) compared with those in the BPH/vehicle control group. Adding to this, the decreased serum PSA levels of the BPH rats following administration of C. bonduc seed extracts (both doses) further underscores the potential of this plant in the management of BPH.
Because of its numerous pharmacological relevant properties, C. bonduc has been used traditionally by several people for managing various alignments. Numerous research studies have been conducted to demonstrate its anti-diabetic, anti-cancer, anti-inflammatory, and anti-pyretic properties with recent studies concentrating on its antiestrogenic and anti-androgenic properties.51 Gupta et al52 reported that C. bonduc seeds demonstrated a significant anti-cancer property when studied using the Ehrlich ascites carcinoma model in Swiss albino mice. In this study, ethanol seed extract treated mice showed a substantial decrease in tumor size and a higher life span than the non-treated control tumor-induced mice. It is also reported that androgen stimulates the growth of prostate cells, testosterone and related hormones that are shown to play a vital role in the development of BPH.53 Meerwal and Jain27 established the anti-androgenic potential of C. bonduc ethanolic seed extract (200 mg and 400 mg/kg body weight) in male Wistar rats, resulting in a significant decrease in sperm count, viability, and motility, as well as lower testosterone levels.
C. bonduc has been found to exert a soothing anti-inflammatory action, making it particularly beneficial for improving an enlarged prostate.54 Furthermore, this plant is used for the treatment of prostatic adenoma. A 90-day oral toxicity study of ethanolic root extract of C. bonduc conducted in Wistar rats significantly decreased absolute prostate weight,55 and this result clearly indicated that the phytochemicals present in the extract might have anti-BPH properties. Thus, this plant was selected for investigating the anti-BPH properties. The screening of phytochemicals from the C. bonduc seeds recorded the presence of diverse bioactive metabolites like flavonoids, alkaloids, sterols, saponins, phenols, glycosides/cardiac glycosides, tannins, and resins.56 Tocopherol derivatives purified and identified from seed kernels include α-tocopherol, γ-tocopherol, and δ-tocopherol. The major sterols reported were β-sitosterol, campesterol, and stigmasterol. In addition to this, seed kernel oil was rich with linoleic acid.57
In the present study, C. bonduc seed extracts inhibited BPH progression, which was indicated by significant reductions in absolute and relative prostate weights. These results were further supported by the declines in the serum and prostate DHT levels and PSA levels in the serum. Histological changes indicated that C. bonduc seed extract treatment ameliorated prostatic epithelial hyperplasia. The anti-BPH property of the extracts from C. bonduc detected in our investigation may be due to the occurrence of various bioactive compounds, especially phenolics and flavonoids. It was reported that a variety of flavonoids derived from various plants possessed α1-adrenergic receptor antagonists and reduced the contraction of the prostate smooth muscles of experimental animals.58 In this study, besides the efficacy of flavonoids presented in seed extracts, the phytosterols in the extracts from these plants may have acted as α1-blockers and alleviated the prostate contractions produced by adrenaline.58 A phytochemical investigation conducted by us found the presence of sterols in the seed extracts. β -sitosterol is also known as plant sterol, which is richly found in the seed extract of C. bonduc. The LC/MS-QTOF analysis reveals the presence of high amounts of three phytosterols, campesterol, stigmasterol and β-sitosterol in the seed extracts. The structure of β-sitosterol is very similar to that of cholesterol. Moreover, β -sitosterol might help to reduce cholesterol levels by limiting the amount of cholesterol entering the body. In addition to this, it can also help to reduce swelling in the prostate and other tissues and thus improve the symptoms associated with the enlargement of the prostate.14 Besides, sitosterol recorded significant anticancer activity.14 An earlier study confirmed the potential role of β-sitosterol in managing BPH in human and animal models. Pagano et al reported that β-sitosterol could improve BHP men’s urological symptoms and urine flow rate.59
γ-tocopherol is one of the major compounds identified in the seed extract, which is reported to have a protective effect against prostate cancer.60 Another important compound identified in the extract was linoleic acid. Linoleic acid effectively inhibits 5α-reductase present in the prostatic epithelium and stroma homogenates.61 Adaramoye et al reported that the hexane fraction of Annona muricata seed ameliorates testosterone-induced benign prostatic hyperplasia in rats, and the phytochemical analysis of the hexane extract reveals the presence of β -amyrin in a significant quantity, which may be one of the major phytochemicals contributing to the activity.62 Similarly, in our study, β -amyrin was one of the major phytochemicals present in all the extracts. According to our best knowledge, the anti-BPH properties of caesalpinine, caesalpinins, caesalmin, norcaesalpinins and caesaldekarins were not reported. But our structural activity relationship studies clearly revealed that these compounds also exert significant anti-BPH activities. As said earlier, the seed extracts of C. bonduc recorded significant anti-BPH effects, especially reducing the serum and prostate DHT levels and serum PSA levels. Finally, more research at the precise cellular and molecular levels is still needed to elucidate the underlying mechanisms of seed extract in the treatment of BPH.
Conclusions
To the best of our knowledge, this is the first animal study on BPH management with C. bonduc seed extracts. Considering the potent side effects associated with the conventional drugs currently being used in the management of BPH, the need for a new therapeutic drug for meliorating BPH is obvious. The results of our study clearly demonstrated that the C. bonduc seed extracts would be a novel candidate drug for managing BPH in the future. However, more advanced research is needed to identify bioactive components and the associated molecular mechanisms for managing BPH.
Data Sharing Statement
All the data supporting the current study were included in the article. The raw data used to support the findings of this study are available from the corresponding author upon request.
Statements of Ethics
Our research did not include any human subjects. All animal experiments were approved by the Animal Ethics Committee of CARe KERALAM Ltd., KINFRA Park PO, Koratty, Thrissur, Kerala-680309 and carried out in harmony with guidelines CPCSEA, Government of India.
Acknowledgments
The authors give adored pranams to “Aadhyathma Chinthalayesan„, Chinthalaya Ashram, Pothencode, Trivandrum, Kerala, India for his blessings. The authors would like to thank Pankajakasthuri Herbals India Pvt. Ltd., Poovachal, Kattakada, Trivandrum, India, for providing the necessary infrastructure, amenities, and financial support for this study. The authors would also like to thank Dr. Suresh Kumar C MD (Ayurveda), Professor, Department of Shalyatantra, Pankajakasthuri Ayurveda Medical College & P.G. Centre, Killy, Kattakada, Thiruvananthapuram, Kerala, India, and Prof. (Dr.) K.G. Revikumar, Former Chief & Head, Clinical Pharmacy, Govt. Medical College Hospital, Trivandrum, Kerala, Kerala, India, for their valuable contributions. The authors would like to thank Dr. Sithara MS (MVSc.), Small Animal Research Centre, Department of Toxicology and Pharmacology, CARe KERALA, Koratty, Thrissur, Kerala, India, for her invaluable technical assistance in carrying out the animal experiments.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Thorpe A, Neal D. Benign prostatic hyperplasia. Lancet. 2003;361(9366):
2. Lee SWH, Chan EMC, Lai YK. The global burden of lower urinary tract symptoms suggestive of benign prostatic hyperplasia: a systematic review and meta‑analysis. Sci Rep. 2017;7:7984. doi:10.1038/s41598-017-06628-8
3. Lee YJ, Lee JW, Park J, et al. Nationwide incidence and treatment pattern of benign prostatic hyperplasia in Korea. Investig Clin Urol. 2016;57:424–430. doi:10.4111/icu.2016.57.6.424
4. Chughtai B, Forde JC, Thomas DD, et al. Benign prostatic hyperplasia. Nat Rev Dis Primers. 2016;2:16031. doi:10.1038/nrdp.2016.31
5. Henry G, Malewska A, Mauck R, et al. Molecular pathogenesis of human prostate basal cell hyperplasia. Prostate. 2017;77:1344–1355.
6. McConnell JD. Prostatic growth: new insights into hormonal regulation. Br J Urol. 1995;76:5–10.
7. Tong Y, Zhou RY. Review of the roles and interaction of androgen and inflammation in benign prostatic hyperplasia. Mediators Inflamm. 2020;2020:7958316.
8. Da Silva MHA, De Souza DB. Current evidence for the involvement of sex steroid receptors and sex hormones in benign prostatic hyperplasia. Res Rep Urol. 2019;11:1–8.
9. Nahata A, Dixit KV. Sphaeranthus indicus attenuates testosterone induced prostatic hypertrophy in albino rats. Phytother Res. 2011;25:1839–1848.
10. Cooke PS, Nanjappa MK, Ko C, Prins GS, Hess RA. Estrogens in male physiology. Physiol Rev. 2017;97(3):995–1043. doi:10.1152/physrev.00018.2016
11. Yu Z-J, Yan H-L, Xu F-H, et al. Efficacy and side effects of drugs commonly used for the treatment of lower urinary tract symptoms associated with benign prostatic hyperplasia. Front Pharmacol. 2020;11:658. doi:10.3389/fphar.2020.00658
12. Gravas S, Oelke M. Current status of 5alpha-reductase inhibitors in the management of lower urinary tract symptoms and BPH. World J Urol. 2010;28:9–15. doi:10.1007/s00345-009-0493-y
13. Dhingra N, Bhagwat D. Benign prostatic hyperplasia: an overview of existing treatment. Indian J Pharmacol. 2011;43(1):6–12. doi:10.4103/0253-7613.75657
14. Salehi B, Fokou PVT, Yamthe LRT, et al. Phytochemicals in prostate cancer: from bioactive molecules to upcoming therapeutic agents. Nutrients. 2019;11(7):1483. doi:10.3390/nu11071483
15. Ejike CE, Ezeanyika LUS. Management of experimental BPH in rats using a food-based therapy containing Telfairia occidentalis seeds. Afr J Tradit Complement Altern Med. 2011;8(4):340–398. doi:10.4314/ajtcam.v8i4.9
16. Akbari F, Azadbakht M, Megha K, et al. Evaluation of Juniperus communis L. seed extract on benign prostatic hyperplasia induced in male Wistar rats. Afr J Urol. 2021;27(1):48. doi:10.1186/s12301-021-00137-x
17. Subbiah V, Nagaraja P, Narayan P, Nagendra HG. Evaluation of pharmacological properties of Caesalpinia bonducella seed and shell extract. Pharmacogn J. 2019;11(1):150–154. doi:10.5530/pj.2019.1.25
18. Murugesan BM, Muralidharan P, Hari R. Effect of ethanolic seed extract of Caesalpinia bonducella on hormones in mifepristone induced PCOS rats. J Appl Pharmaceut Sci. 2020;10:72–76.
19. Iheagwam FN, Ogunlana OO, Ogunlana OE, Isewon I, Oyelade J. Potential anti-cancer flavonoids isolated from Caesalpinia bonduc young twigs and leaves: molecular docking and in silico studies. Bioinform Biol Insights. 2019;13:1–16. doi:10.1177/1177932218821371
20. Patel DV, Joshi M. Antidiabetic activity of aqueous extract of Caesalpinia bonducella leaves in streptozotocin induced diabetic rats. Eur J Pharmaceut Med Res. 2020;7:362–367.
21. Anil VD, Chikane DR. Seed germination studies in Caesalpinia bonduc (L) Roxb. J Emerg Technol Innov Res. 2020;7:1074–1077.
22. Kannur DM, Paranjpe MP, Sonavane LV, Dongre PP, Khandelwal KR. Evaluation of Caesalpinia bonduc seed coat extract for anti-inflammatory and analgesic activity. J Adv Pharm Technol Res. 2012;3(3):171–175. doi:10.4103/2231-4040.101010
23. Ubhenin A, Uwakwe A, Falodun A, Engel N, Onwuka F, Langer P. Antiproliferative and pro-apoptotic effects of Caesalpinia bonduc extract and its fractions in estrogen-sensitive human breast adenocarcinoma cell line. J Herbs Spices Med Plants. 2013;19:159–167. doi:10.1080/10496475.2012.762725
24. Pillai PG, Suresh P, Mishra G. Induction of apoptosis and inhibitory potential of the methanol extract of Caesalpinia bonduc (L.) Roxb against breast cancer. Ann Plant Sci. 2013;2:284–291.
25. Yadav PP, Maurya R, Sarkar J, et al. Cassane diterpenes from Caesalpinia bonduc. Phytochem. 2009;70(2):256–261. doi:10.1016/j.phytochem.2008.12.008
26. Wu L, Luo J, Zhang Y, Wang X, Yang L, Kong L. Cassane-type diterpenoids from the seed kernels of Caesalpinia bonduc. Fitoterapia. 2014;93:201–208. doi:10.1016/j.fitote.2014.01.011
27. Meerwal P, Jain GC. Antifertility effect of Caesalpinia bonducella (L.) Fleming in male Wistar rat. Int J Pharmacogn. 2016;3:265–275.
28. Vyas BA, Desai NY, Patel PK, Joshi SV, Shah DR. Effect of Boerhaavia diffusa in experimental prostatic hyperplasia in rats. Indian J Pharmacol. 2013;45(3):264–269. doi:10.4103/0253-7613.111946
29. Liu TT, Thomas S, Mclean DT, et al. Prostate enlargement and altered urinary function are part of the aging process. Aging. 2019;11(9):2653–2669. doi:10.18632/aging.101938
30. Brandli A, Simpson JS, Ventura S. Isoflavones isolated from red clover (Trifolium pratense) inhibit smooth muscle contraction of the isolated rat prostate gland. Phytomedicine. 2010;17(11):895–901. doi:10.1016/j.phymed.2010.05.006
31. Choo SH, Sung HH, Chae MR, et al. Effects of Schisandra chinensis extract on the relaxation of isolated human prostate tissue and smooth muscle cell. J Ethnopharmacol. 2014;156(2014):271–276. doi:10.1016/j.jep.2014.08.025
32. Morcos MA, Afifi NM. Effect of doxazocin on experimentally induced prostatic hyperplasia in adult male albino rats: a histological and immunohistochemical study. Egypt J Histol. 2011;34(4):870–882. doi:10.1097/01.EHX.0000407624.42021.3b
33. Akanni OO, Owumi SE, Olowofela OG, Adeyanju AA, Abiola OJ, Adaramoye OA. Protocatechuic acid ameliorates testosterone-induced benign prostatic hyperplasia through the regulation of inflammation and oxidative stress in castrated rats. J Biochem Mol Toxicol. 2020;34(8):e22502. doi:10.1002/jbt.22502
34. Huang JJ, Cai Y, Huang MY, Zhu L, He F, Liu XW. Pharmaceutical evaluation of naftopidil enantiomers: rat functional assays in vitro and estrogen/androgen induced rat benign prostatic hyperplasia model in vivo. Eur J Pharmacol. 2016;791:473–481. doi:10.1016/j.ejphar.2016.09.009
35. Veeresh BSV, Veeresh B, PatilL AA, Warke A. Lauric acid and myristic prevent testosterone induced prostatic hyperplasiain rats. Euro J Pharmacol. 2010;625:262–265. doi:10.1016/j.ejphar.2009.09.037.
36. An YJ, Lee JY, Kim Y, Jun W, Lee YH. Cranberry powder attenuates benign prostatic hyperplasia in rats. J Med Food. 2020;23(12):1296–1302. doi:10.1089/jmf.2020.4779
37. Hwangbo H, Kwon DH, Choi EO, et al. Corni Fructus attenuates testosterone-induced benign prostatic hyperplasia by suppressing 5α-reductase and androgen receptor expression in rats. Nutr Res Pract. 2018;12(5):378–386. doi:10.4162/nrp.2018.12.5.378
38. Xin Q, Kwon MJ, Lee JW, et al. Gamma irradiated rhodiola sachalinensis extract ameliorates testosterone-induced benign prostatic hyperplasia by downregulating 5-alpha reductase and restoring testosterone in rats. Molecules. 2019;24(21):3981. doi:10.3390/molecules24213981
39. Izumi K, Mizokami A, Lin WJ, Lai KP, Chang C. Androgen receptor roles in the development of benign prostate hyperplasia. Am J Pathol. 2013;182:1942–1949. doi:10.1016/j.ajpath.2013.02.028
40. Cannarella R, Condorelli RA, Barbagallo F, La Vignera S, Calogero AE, La Vignera S and Calogero AE. endocrinology of the aging prostate: current concepts. Front Endocrinol. 2021;12:554078. doi:10.3389/fendo.2021.554078
41. Arena AC, Kassuya CAL, Fernandes GSA, Scarano WR. Toxic versus therapeutic effects of natural products on reproductive disorders. Evid Based Complement Alternat Med. 2019;2019:9791506. doi:10.1155/2019/9791506
42. Paba S, Frau R, Godar S, Devoto P, Marrosu F, Bortolato M. Steroid 5a-reductase as a novel therapeutic target for schizophrenia and other neuropsychiatric disorders. Curr Pharm Design. 2011;17:151–167. doi:10.2174/138161211795049589
43. Cauci S, Chiriacò G, Cecchin E, et al. Androgen Receptor (AR) Gene (CAG)n and (GGN)n length polymorphisms and symptoms in young males with long-lasting adverse effects after finasteride use against androgenic alopecia. Sex Med. 2017;5(1):e61–e71. doi:10.1016/j.esxm.2016.11.001
44. Seftel AD, Rosen RC, Rosenberg MT, Sadovsky R. Benign prostatic hyperplasia evaluation, treatment and association with sexual dysfunction: practice patterns according to physician specialty. Int J Clin Pract. 2008;62(4):614–622. doi:10.1111/j.1742-1241.2008.01699.x
45. Mbaka G, Anunobi C, Ogunsina S, Osiagwu D. Histomorphological changes in induced benign prostatic hyperplasia with exogenous testosterone and estradiol in adult male rats treated with aqueous ethanol extract of Secamone afzelii. Egypt J Basic Appl Sci. 2017;4:15–21.
46. Han C, Zhu L, Liu X, Ma S, Liu Y, Wang X. Differential diagnosis of uncommon prostate diseases: combining mpMRI and clinical information. Insights Imaging. 2021;12(1):79.
47. Levitt JM, Slawin KM. Prostate-specific antigen and prostate-specific antigen derivatives as predictors of benign prostatic hyperplasia progress. Curr Urol Rep. 2007;8:269–274.
48. Andriole GL, Crawford ED, Grubb RL, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360(13):1310–1319.
49. Hu X, Wang YH, Yang ZQ, Shao YX, Yang WX, Li X. Association of 5-alpha-reductase inhibitor and prostate cancer incidence and mortality: a meta-analysis. Transl Androl Urol. 2020;9(6):2519–2532.
50. Rick FG, Schally AV, Block NL, et al. LHRH antagonist Cetrorelix reduces prostate size and gene expression of proinflammatory cytokines and growth factors in a rat model of benign prostatic hyperplasia. Prostate. 2011;71:736–747.
51. Kandasamy V, Balasundaram U. Caesalpinia bonduc (L.) Roxb. as a promising source of pharmacological compounds to treat Poly Cystic Ovary Syndrome (PCOS): a review. J Ethnopharmacol. 2021;279:114375.
52. Gupta M, Mazumder UK, Kumar RS, Sivakumar T, Vamsi MLM. Antitumor activity and antioxidant status of Caesalpinia bonducella against Ehrlich ascites carcinoma in Swiss albino mice. J Pharmacol Sci. 2004;94:177–184.
53. Mirone V, Fusco F, Verze P, Schulman C, Debruyne F, Imbimbo C. Androgens and benign prostatic hyperplasia. Eur Urol Suppl. 2006;5:410–417.
54. Shrivastava A, Gupta VB. Various treatment options for benign prostatic hyperplasia: a current update. J Midlife Health. 2012;3(1):10–19. doi:10.4103/0976-7800.98811
55. Sindete M, Gbankoto A, Osseni R, et al. A 90-day oral toxicity study of an ethanolic root extract of Caesalpinia bonduc (L.) Roxb. in Wistar rats. Evid Based Complement Alternat Med. 2021;2021:6620026.
56. Shukla S, Mehta P, Mehta A, Vyas SP, Bajpai VK. Preliminary phytochemical and antifungal screening of various organic extracts of Caesalpinia bonducella seeds rom. Biotechnol Lett. 2011;16:6384–6389.
57. Sultana R, Saleem R, Sultana N, Afshan F, Gulzar T. Characterization of the composition of Caesalpinia bonducella seed grown in temperate regions of Pakistan. J Am Oil Chem Soc. 2012;89:1021–1027.
58. Buncharoen W, Supap S, Kanokporn S. Relaxant activities of extracts from Uvaria rufa Blume and Caesalpinia sappan L. on excised rat’s prostate strips. J Pharmaceut Res Int. 2019;29(1):1–12.
59. Pagano E, Laudato M, Griffo M, Capasso R. Phytotherapy of benign prostatic hyperplasia. A minireview. Phytother Res. 2014;28(7):949–955.
60. Sato C, Kaneko S, Sato A, Virgona N, Namiki K, Yano T. Combination effect of δ-Tocotrienol and γ-Tocopherol on prostate cancer cell growth. J Nutr Sci Vitaminol. 2017;63(5):349–354.
61. Kwon Y. Use of saw palmetto (Serenoa repens) extract for benign prostatic hyperplasia. Food Sci Biotechnol. 2019;28(6):1599–1606.
62. Adaramoye OA, Oladipo TD, Akanni OO, Abiola OJ. Hexane fraction of Annona muricata (sour sop) seed ameliorates testosterone-induced benign prostatic hyperplasia in rats. Biomed Pharmacother. 2019;111:403–413.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.