Back to Journals » Patient Preference and Adherence » Volume 16
Adherence to Inhaled Corticosteroid Therapy and Its Clinical Impact on Asthma Control in Adults Living with Asthma in Northwestern Ethiopian Hospitals
Authors Belachew EA , Netere AK , Sendekie AK
Received 14 March 2022
Accepted for publication 13 May 2022
Published 25 May 2022 Volume 2022:16 Pages 1321—1332
DOI https://doi.org/10.2147/PPA.S365222
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Eyayaw Ashete Belachew, Adeladlew Kassie Netere, Ashenafi Kibret Sendekie
Department of Clinical Pharmacy, University of Gondar, Gondar, Ethiopia
Correspondence: Eyayaw Ashete Belachew, Department of Clinical Pharmacy, University of Gondar, PO Box 196, Gondar, Ethiopia, Email [email protected]
Background: Inhaled corticosteroids (ICS) are the backbone of and primary choice for long-term asthma control therapy; however, the level of adherence to this regimen has not yet been investigated, particularly in the study area of Northwest Ethiopia. Therefore, this study aimed to examine the level of adherence to ICS treatment and its impact on adults living with asthma in Northwestern Ethiopian hospitals.
Materials and Methods: A multicenter institution-based survey was conducted in asthma patients treated with ICS-based regimens in public hospitals in Northwest Ethiopia. Adherence to ICS was measured with the Medication Adherence Report Scale for Asthma. Descriptive statistics were used to present respondents’ characteristics, and logistic regression was used to test associations between predictors and outcome variables. A level of p< 0.05 was used as a cut-off point for a significant association.
Results: Of a total of 422 approached subjects, 96.7% completed the survey. The mean (±SD) age of the participants was 49.82 (± 16.1) years. The majority of participants (86.1%) had a low level of adherence to ICS treatment. A significant proportion (42%) of the respondents reported that they utilized ICS only before performing exercises that made them breathless. Around two-fifths of the participants used ICS either when they needed it or when they felt breathless. Furthermore, one-third of the study subjects reported that they either avoided or forgot to take ICS. Participants who had access to free healthcare services had better adherence to ICS (p=0.01), and non-adherence to ICS therapy was significantly associated with poor levels of asthma control (p≤ 0.001).
Conclusion: This study found that adult patients with asthma had low levels of adherence to ICS therapy. Future prospective research in a larger population, focusing on identifying the obstacles to ICS adherence in patients living with asthma and creating successful intervention options, is recommended.
Keywords: adherence, asthma control, inhaled corticosteroid, hospitals, Ethiopia
Introduction
Asthma is the leading cause of chronic inflammatory airway diseases, characterized as restricted airflow followed by wheezing, shortness of breath, chest tightness, and cough, which affects daily activities.1 In 2018, the World Health Organization (WHO) reported that over 339 million people were affected by asthma.2,3 In Africa, in the past two decades, asthma has increased significantly, from 74.4 to 119.3 million.4 Similarly, in sub-Saharan African countries, and specifically in Ethiopia, the prevalence rate ranged from 10.2% to 13%.5 Asthma contributes to significant levels of disabilities in millions of people in all age groups; this burden has continued for years and has remained unchanged since 1990,2 resulting in thousands of deaths per annum.6 The rate of hospital admissions has been increasing, and is associated with severe attacks, poor disease management, and poverty.4 Lack of optimum management results in disease progression, depreciation in the quality of life, increased healthcare costs, and frequent deaths outside hospital.4,7
Although asthma leads to several complications, it can be controlled with long-term antiasthma medications, such as anti-inflammatory agents. Hence, patients’ adherence to their medications is very important and crucial.8 The prevalence of non-adherence to inhaled corticosteroids (ICS) among asthma patients in both industrialized and developing countries has been studied in different regions of the world. The WHO reported that the average rate of treatment adherence among patients with chronic diseases in developed countries was about 50%,9 and in the USA it ranges from 22% to 50%.10–14 Similarly, it was suboptimal in the UK,15,16 with 65% of patients taking less than 80% of ICS and 35% of patients taking less than 50% of ICS prescriptions. Poor adherence was also observed in Iran (55%),17 Egypt (21%),18 and Kuwait (17.4%).19 However, this level of adherence was estimated to be lower in developing countries, where access to healthcare and medications is limited.9
As a result, measuring the adherence levels to ICS therapy in these patient groups has significant importance. Previously, different tools have been used to measure adherence levels to ICS therapy, but each strategy has its own set of benefits and drawbacks. Despite its limitations, hair analysis was chosen to measure adherence levels in a study on prescribed drugs.20 Alternatively, indirect measurement of adherence can be classified as objective or subjective; electronic medication monitoring, medication event monitoring systems (MEMS), electronic devices, medication refill monitoring, and canister weighing are known objective techniques.21,22 On the other hand, self-report is a subjective method for determining adherence. The use of validated scales or questionnaires to determine adherence to ICS is an example of this strategy. Although these scales are widely used, they may overestimate true adherence. Furthermore, these scores are susceptible to recall errors and can be influenced by poor memory.23,24 Therefore, carefully conducted self-report measures of adherence can help identify potential problems associated with poor adherence and determine the prevalence of adherence in patients with asthma.23
In Ethiopia, most studies have been conducted in single-center settings and few of them have focused on non-adherence to ICS;25 these studies lack both public health and methodological significance, which our study will potentially emphasize. Concerning the public health perspective, this study is the first of its kind to assess the level of adherence to ICS and its impact in the study areas. Also, this study produces evidence for planning interventional strategies, creating important clues to characterize and stratify patients at follow-up care clinics and to optimize the therapeutic outcomes of the patients at large. In general, identifying the barriers to adherence with ICS therapy, and measuring the relationship between adherence and level of asthma control using the Medication Adherence Rating Scale for Asthma (MARS-A) measurement tool has meaningful importance in terms of health outcomes for patients with asthma. Therefore, the aim of this paper was to determine the level of adherence to ICS therapy using a validated instrument (MARS-A) in adult asthma patients in Northwestern Ethiopia. The findings from this study may be used as a benchmark for future research in the study area.
Method
Study Design and Setting
This institution-based multicenter cross-sectional survey was conducted over a period of three consecutive months from October to December 2021. Three public comprehensive specialized Northwestern Ethiopian hospitals, namely University of Gondar Comprehensive Specialized Hospital (UoGCSH), Felege-Hiwot Comprehensive Specialized Hospital (FHCSH), and Tibebe-Ghion Comprehensive Specialized Hospital (TGCSH), were included in this study.
Study Population and Sampling
Patients living with asthma and aged 18 years and above who attended for follow-up at the outpatient department of the selected hospitals were eligible for this study. In addition, the study subjects should have received ICS therapy for past 3 months. Patients who were unable to communicate, critically ill, or admitted to inpatient departments, and those with incomplete medical records, were excluded. Sample size was determined as per the single population proportion rules. Owing to the lack of published articles concerning the proportion of ICS therapy of adherence in the study area, 50% of the prevalence was considered as proportion (P). The marginal error of 5% (w=0.05) at the 95% confidence level (Z α/2=1.96) was used and a number of about 384 participants was calculated; however, allowing for possible non-responses and missing data, a 10% contingency was considered, and eventually a sample size of 422 was included in the final analysis. A systematic random sampling technique was applied to select the study subjects. According to University of Gondar asthma ambulatory care records, on average 300 hundred asthma patients visited per month; regarding FHCSH chest clinic records, on average 190 patients visited per month; and according to TGCSH ambulatory care records, on average 75 patients visited per month (bearing in mind that all asthma patients would have attended for 1–3 months in all hospitals).
Asthma patients are recommended to visit the ambulatory care clinic for a minimum of one month and a maximum of three months. Therfore, the total numbers of patients visited the hospitals within a three month peroid had yielded a total of 900 visits at UoGCSH, 570 at FHCSH, and 225 at TGCSH hospitals, and the overall number of patients over 3 months in all hospitals was 1695. Finally, by proportional allocation, 224, 141, and 57 patients from UoGCSH, FHCSH, and TGCSH, respectively, were approached to participate in the study. Taking into consideration that the sample was collected within 3 months, this makes the sampling fraction (k-interval) 1695/422=4 (approximately). Therefore, the initial study subject was selected by the lottery method and then study individuals were chosen as every fourth person; their corresponding medical records were collected and relevant data were extracted. In addition, each selected respondent was interviewed. The medical records of study subjects who met the inclusion criteria were considered and whenever one medical record was not eligible, the next one was selected, and the same approach was followed throughout the entire data collection procedure.
Data Collection Instruments and Procedures
After reviewing various related literature, the data collection tool was first prepared in the English language. It was then translated into the local language (Amharic), then back-translated into English to ensure consistency. The instrument consisted of three sections: (I) the first section included socio-demographic characteristics of the study participants; (II) the second section contained the clinical characteristics and measurement of asthma control; and (III) the third section contained the instrument to measure ICS treatment adherence (MARS-A). Data collectors had received training about the aims of the study, data collection instruments and producers, and ethical issues. Relevant data were obtained by interviewing participants and reviewing their medical records. However, if one of the available medical records was not eligible, the next one was considered, and the same approach was followed throughout data collection. Adherence to ICS therapy was assessed using the Medication Adherence Report Scale-Asthma (MARS-A) in a face-to-face interview. Asthma severity was measured based on the Global Initiative Asthma Network (GIAN, 2018).26 The Asthma Control Test (ACT) was employed in this study to measure the levels of asthma control. The tool is standardized and applicable across populations worldwide.27 For this population, the data collectors administered the questionnaires to participants in a questionnaire-guided interview, mitigating against such obstacles as language barriers and low literacy levels. A separate questionnaire that collected information on the determinants of asthma control was administered. The ACT tool is a simple test for asthmatic patients aged 12 years and above, and measures the level of asthma control. It contains five questions on a five-point scale depicting the frequency of asthma symptoms and use of rescue medication by participants in the previous 4 weeks. The overall score is in the range of 5 (worse control) to 25 (total control).27 Participants who score greater than 19 are categorized as having controlled asthma, and those who score less than or equal to 19 as having uncontrolled asthma.27
Measurement of Adherence to ICS
The MARS-A, a previously validated 10-item questionnaire, was used to measure patient adherence to ICS therapy.28 The instrument has demonstrated good internal validity, construct validity, and criterion validity in English.29 The MARS-A was used as a self-report instrument, and showed good test–retest reliability (r=0.65, p<0.001) and internal consistency, with acceptable levels of reliability, sensitivity, and specificity of 0.85, 0.82, and 0.69, respectively. Responses were recorded on a five-point Likert scale (1 = Never, 2 = Rarely, 3 = Sometimes, 4 = Often, 5 = Always). Self-reported level of adherence was reported as the average score of the 10 items (each item ranges from 1 to 5), with patients who had a MARS-A score of ≥4.5 being categorized as having higher adherence,28 and the patients were categorized as having high or low self-reported levels of adherence.
Data Quality Management
The data collection instruments were prepared after reviewing similar literature, and amendments were performed by considering local clinical settings. The face validity of the questionnaire was checked by three language experts for clarity of the questions. Subsequently, the survey was pretested for content, design, readability, and comprehensibility in 5% of study participants, who were excluded from the final analysis. Socio-cultural adaptation was performed using recommendations from the WHO, and changes were made based on responses. Thus, the survey was easy to understand and respond to, while still providing accurate data. The data collectors and supervisors checked the data quality at every point of data collection. Cronbach’s alpha was calculated for MARS-A and ACT to test the internal consistency of the tools, and found to be α=0.90 and α=0.83, respectively.
Data Entry and Analysis
After the cleanness and consistency of the data had been checked, data were coded and entered into SPSS version 26. Both descriptive and inferential analyses were performed. Results are presented as means (±SD) and numbers with percentages. Univariable logistic regression was performed to determine the association between each experimental variable and self-reported level of adherence to ICS therapy. Variables with p≤0.25 in the univariable analysis were included in the multivariable logistic regression to see whether the predictor variable was associated with expected outcomes.
Ethical Consideration
Ethical approval was obtained from the Institutional Review Board (IRB) of the University of Gondar (approval number SOP/131/2021). Then, an official letter from the clinical director of each hospital was also received before the study began in their facilities. The purpose of the study was explained to the study participants and informed consent, both verbally and in written form, was given by the participants, indicating their willingness to participate. Participants who were unwilling to participate in the study and those who wished to drop out at any point during the interview were informed that they could do so without any restriction. Confidentiality was also ensured by anonymizing the collected data. All rules and regulations were followed according to the ethical principles of the Declaration of Helsinki.
Results
Socio-Demographic and Clinical Characteristics of Participants
Of the total 422 subjects approached, 409 completed the questionnaire, resulting in a response rate of 96.7%. The mean age (±SD) of the study participants was 49.82 (±16.1) years and most (60.1%) were female. Nearly three-quarters of the respondents were married (71.9%) and city residents (73.3%). Significant numbers of participants used biomass as a fuel for cooking food (88.5%) and were non-smokers (87.5%). The mean score on the ACT was 16.46 (±4.1). Out of the 409 participants, 118 (28.9%, 95% CI 24.7, 33.5) had controlled asthma and 291 (71.1%) had uncontrolled asthma (Table 1).
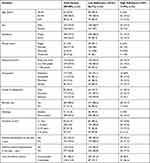 |
Table 1 Socio-Demographic and Clinical Characteristics of the Participants and Their Level of Adherence |
Level of Adherence to ICS Therapy in the Participants
The overall mean (±SD) adherence level to the ICS therapy was 3.8 (±0.81) out of 5 points (from 1 to 5) on the five-point Likert scale. The majority of respondents (86.1%) scored low adherence to ICS therapy. A lower rate of adherence was also noted in almost all MARS-A items. More than 45% of the participants reported that they used the ICS therapy only when needed, with an average adherence level of 3.8 (±1.4), or only when they felt breathless [mean 3.5 (±1.1)], out of 5 points on the five-point Likert scales. Likewise, many study subjects (>30%) confessed that they tried to avoid using the ICS therapy [mean 3.8 (±1.0)] or forgot to take it (32.5%) [mean 3.8 (±0.91)]. In addition, approximately one-quarter of study participants had suspended taking ICS for a period (24.5%) [mean 3.9 (±1.0)]. In general, the average score for adherence was less than 4 for the majority of the 10 items used to assess the level of adherence to ICS therapy (Table 2 and Figure 1).
 |
Table 2 Percentage of Patients Who Engaged in Each Act of Non-Adherence and the Mean Value for Each Item |
 |
Figure 1 Distribution of participants based on the mean score of each item. |
Relationship Between Overall Adherence and Participants’ Characteristics
The multivariable logistic regression model showed that participants who had received free healthcare services were 3.4 times more likely to adhere to ICS therapy (AOR=3.4, 95% CI 1.2, 5, p=0.01) (Table 3). Regarding the association between adherence to ICS therapy and degree of asthma control, as measured by the multiple variable logistic regression, non-adherence to ICS therapy was significantly associated with a lower degree of asthma control, by 82% compared to adherence to therapy (AOR=0.18, 95% CI 0.09, 0.35, p≤0.001). Adherence to antiasthmatic medication was the only predictor of asthma control identified in the multivariable logistic model (Table 4).
 |
Table 3 Association Between Overall Adherence and Participants’ Characteristics |
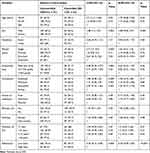 |
Table 4 Determinants of Asthma Control Among Asthmatic Patients in Northwestern Ethiopia |
Discussion
To the best of the authors’ knowledge, no other authors have reported on the degree of adherence to ICS therapy in asthmatic patients and its impacts on asthma control using the MARS-A tool for this patient group. This multicenter institution-based survey highlighted the levels of adherence to the ICS-based regimen and its determinants in patients with asthma treated in Northwestern Ethiopian hospitals. The adherence levels, measured with the MARS-A adherence scale, proved that significant numbers of patients had low adherence levels to the ICS therapy. On the other hand, the overall mean adherence score was marginally good, and only less than 15% of the patients rigorously adhered to the ICS therapy. This level was lower compared with findings reported from other studies19,28 and a previous study conducted in Ethiopia.25 In addition, the overall mean score for adherence to ICS therapy in the current study was also lower than previous findings,28,29 but higher than in a study from Kuwait.19 The difference in the level of adherence to ICS therapy may due to socio-cultural differences, with different attitudes towards medications and the use of different instruments to measure adherence. Patients with different socio-cultural identities potentially have different levels of medication adherence. In addition, inhalational steroids require technical administration techniques, which may lead to patients being reluctant to undertake these procedures, especially in a population with a low educational background, resulting in poor medication adherence.
On the MARS-A rating scale of the individual adherence assessment items, the mean level for each item was low, and the participants’ responses were unsatisfactory on almost all items. More respondents responded that they use the ICS medication as a back-up if their other inhalers fail, and this result was inconsistent with the findings from a published study by Horne.29 In addition, about two-fifths of respondents admitted that they use their ICS therapy only when they are breathless. This result was significantly higher than that reported in the previous study.29 Moreover, Williams et al reported that non-adherence to ICS therapy was associated with lower use of emergency medications, suggesting that these patients may have a lower perception of their need for ICS therapy owing to the milder nature of their disease. This could be due to a lack of understanding of the nature of asthma.12 This is in line with another study, in which the investigators reported that a greater proportion of patients with asthma believed that their asthma was acute and episodic,30 resulting in patients being non-adherent on purpose and inadvertently.
The current study showed that a high proportion of participants chose to skip an ICS dose, which is in contrast to a study conducted in the UK, where a lower proportion of participants reported that they tried to avoid taking their ICS.29 However, our finding revealed that a significant number of participants attempted to avoid taking their ICS. The current study also found that the need for dose adjustment by the patient was higher than in the study by Laforest et al.29,31 A potential reason for the higher rate of non-adherence regarding dosage in the current study may be a lower degree of understanding of the nature of the disease and treatment, so that the participants may not perceive that they should adhere to taking their asthmatic medications. This finding suggests that healthcare providers should be highly involved in providing clear information for patients about the medications and the maintenance management patterns. Moreover, the low economic status of the patients may have meant that they could not afford to cover the full dosage regimen, thus hampering the continual availability of the medications and leading to doses being skipped. Another potential reason for skipping ICS doses may be because of adverse effects. A previous study showed that patients’ concerns about ICS side effects were mentioned as a reason for non-adherence to ICS.32
The current study demonstrated that a significant number of participants were involved with unintentional non-adherence, which is inconsistent with and higher than the results of Horne and Laforest et al.29,31 This finding indicates that a high proportion of patients were forgetting to take their medication on time. In such situations, reminder methods should be discussed with patients.10,33 A previous study found that patients who integrated the ICS therapy use into their daily activities had better adherence than those who used other techniques or did not use any strategy at all.34
Even though ICS is the main therapy for asthma patients, a high proportion of participants in the present study used ICS as a back-up in case other treatments failed. However, a lower proportion of such participants was observed in the previous literature.29 In addition, almost half of the participants in the present study used ICS before engaging in activities that could render them breathless. This could be related to patients misunderstanding the role of control medications such as ICS and the role of reliever therapy, such as short-acting beta-2 agonists. This suggests that healthcare providers should provide clear information to patients regarding the medication differences and treatment modalities.
Another important finding of the current study is the association between different variables and levels of adherence; it was found that there was no association between reported adherence and patient demographic factors, except for sources of income for healthcare access, which is consistent with prior studies.29,35 Also consistent with a previous study,36 patients who had free access to healthcare were more likely to adhere to their ICS therapy than patients who used out-of-pocket expenses to cover their healthcare expenditures. The study may indicate that patients who pay out-of-pocket to cover healthcare access suffer from a high burden of unplanned expenditure, and this may lead to poor medication adherence.
In this study, the level of asthma control was suboptimal and adherence to ICS was identified as the single independent predictor of asthma control. This result is supported by other studies.37,38 The findings of this study are interesting because poor ICS adherence has previously been connected with negative health outcomes. Consistently with a study conducted in Brazil,39 non-compliance with ICS medication was found to be substantially linked to poor asthma control. However, the level of adherence to ICS therapy had no significant relationship with asthma exacerbation. In contrast, the impact of ICS adherence on asthma exacerbations was studied in the UK,13 where non-adherence to ICS therapy was associated with asthma exacerbation in a significant number of patients. This disparity could be due to socio-demographic differences in the study sites as well as discrepancies in the study methodology.
In general, this research has highlighted the level of adherence to ICS therapy and factors associated with non-adherence to ICS medication in patients with chronic asthma in Northwest Ethiopia. Non-adherence is a direct cause of therapy failure in patients with chronic disorders. It is essential to effectively communicate with patients in order to identify any potential individual barriers that may prevent them from engaging in successful self-management. Furthermore, clinicians play an important role in improving drug adherence by carefully considering patients’ awareness of the purpose of ICS therapy in asthma and emphasizing the distinction between preventer and reliever medications in asthma management. Future research could look at the barriers to ICS adherence in a larger group of asthma patients, as well as testing other interventions for overcoming non-adherence through longitudinal studies.
Strengths and Limitations of the Study
The strengths of this study are that it was a real-life multicenter study, using well-validated tools to measure adherence to ICS therapy in adult patients with asthma. The present study also has a few limitations. Owing to the nature of the data collection, the responses depend on the honesty and faith of the respondents, which could affect the results either by underreporting or by exaggeration. Nevertheless, the current study aimed to assess the level of adherence to ICS therapy and its impact on asthma control among patients with asthma in selected public hospitals in Northwest Ethiopia, and it was adequately powered and compared well with other similar studies conducted worldwide. We hope that the study will fill the existing gap in the literature in this area.
Conclusion
In the present study, there was a high overall rate of non-adherence to ICS therapy, with a lower rate of adherence to almost all MARS-A items used to assess medication adherence in asthma patients. Patients who had free healthcare services were more likely to adhere to their ICS therapy than patients who paid for healthcare out of pocket. The level of asthma control in this study was suboptimal, and non-adherence to ICS therapy was identified as the single predictor of asthma control. Future research is recommended in large populations, prospectively focusing on identifying the obstacles to ICS adherence in patients with asthma and creating effective intervention options.
Abbreviations
ACT, Asthma Control Test; FHCSH, Felege-Hiwot Comprehensive Specialized Hospital; GINA, Global Initiative for Asthma; ICS, inhaled corticosteroids; MARS-A, Medication Adherence Rating Scale for Asthma; SD, standard deviation; TGCSH, Tibebe-Ghion Comprehensive Specialized Hospital; UoGCSH, University of Gondar Comprehensive Specialized Hospital; WHO, World Health Organization.
Data Sharing Statement
The data sets supporting the conclusions of this article are available on reasonable request to the corresponding author.
Consent to Publication
Consent to publication from participants was not required because confidentiality was maintained according to the rules and regulations.
Acknowledgments
We thank the clinical directors at all sites for allowing us to conduct this study. We especially thank the study participants for their voluntary participation.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest related to this work.
References
1. Asthma GIF. Pocket guide for asthma management and prevention; 2019.
2. Network GA. Global asthma report; 2018.
3. World Health Organization. Asthma fact sheets; 2017.
4. Adeloye D, Chan KY, Rudan I, et al. An estimate of asthma prevalence in Africa: a systematic analysis. Croat Med J. 2013;54(6):519–531. doi:10.3325/cmj.2013.54.519
5. To T, Stanojevic S, Moores G, et al. Global asthma prevalence in adults: findings from the cross-sectional world health survey. BMC Public Health. 2012;12(1):1–8.
6. Rothe T, Spagnolo P, Bridevaux P-O, et al. Diagnosis and management of asthma–the Swiss Guidelines. Respir Int Rev Thoracic Dis. 2018;95(5):364–380. doi:10.1159/000486797
7. Turpin K, Rockers PC, Vian T, et al. Prevalence and treatment of hypertension, diabetes and asthma in Kenya: a representative household survey in eight counties in 2016. East Afr Med J. 2018;7:45.
8. Al Qasem A, Smith F, Clifford S. Adherence to medication among chronic patients in Middle Eastern countries: review of studies. East Mediterr Health J. 2011;17:356–363.
9. World Health Organization. Adherence to long-term therapies: evidence for action. World Health Organization; 2003.
10. Apter AJ, Reisine ST, Affleck G, et al. Adherence with twice-daily dosing of inhaled steroids: socioeconomic and health-belief differences. Am J Respir Crit Care Med. 1998;157(6):1810–1817. doi:10.1164/ajrccm.157.6.9712007
11. Bender BG, Pedan A, Varasteh LT, et al. Adherence and persistence with fluticasone propionate/salmeterol combination therapy. J Allergy Clin Immunol. 2006;118(4):899–904.
12. Williams LK, Joseph CL, Peterson EL, et al. Patients with asthma who do not fill their inhaled corticosteroids: a study of primary nonadherence. J Allergy Clin Immunol. 2007;120(5):1153–1159.
13. Williams LK, Peterson EL, Wells K, et al. Quantifying the proportion of severe asthma exacerbations attributable to inhaled corticosteroid nonadherence. J Allergy Clin Immunol. 2011;128(6):1185–1191. e2. doi:10.1016/j.jaci.2011.09.011
14. Williams LK, Pladevall M, Xi H, et al. Relationship between adherence to inhaled corticosteroids and poor outcomes among adults with asthma. J Allergy Clin Immunol. 2004;114(6):1288–1293. doi:10.1016/j.jaci.2004.09.028
15. Gamble J, Stevenson M, McClean E, et al. The prevalence of nonadherence in difficult asthma. Am J Respir Crit Care Med. 2009;180(9):817–822. doi:10.1164/rccm.200902-0166OC
16. Murphy AC, Proeschal A, Brightling CE, et al. The relationship between clinical outcomes and medication adherence in difficult-to-control asthma. Thorax. 2012;67(8):751–753.
17. Rahimi A, Sulong S, Maimaiti N, et al. Asthma treatment adherence among asthmatic patients in Yazd. BMC Public Health. 2012;12. doi:10.1186/1471-2458-12-S2-A39
18. Rifaat N, Abdel-Hady E, Hasan AA, et al. The golden factor in adherence to inhaled corticosteroid in asthma patients. Egypt J Chest Dis Tuberc. 2013;62(3):371–376.
19. Albassam A, Alharbi A, Awaisu A, et al. Assessing adherence to inhaled corticosteroids among adults with asthma in Kuwait using the medication adherence report scale for asthma. Patient Prefer Adherence. 2020;14:963. doi:10.2147/PPA.S248655
20. Hassall D, Brealey N, Wright W, et al. Hair analysis to monitor adherence to prescribed chronic inhaler drug therapy in patients with asthma or COPD. Pulm Pharmacol Ther. 2018;51:59–64. doi:10.1016/j.pupt.2018.07.001
21. Foster J, Smith L, Bosnic-Anticevich SZ, et al. Identifying patient‐specific beliefs and behaviours for conversations about adherence in asthma. Internal Med J. 2012;42(6):e136–e144. doi:10.1111/j.1445-5994.2011.02541.x
22. O’Dwyer S, Greene G, MacHale E, et al. Personalized biofeedback on inhaler adherence and technique by community pharmacists: a Cluster Randomized Clinical Trial. J Allergy Clin Immunol. 2020;8(2):635–644. doi:10.1016/j.jaip.2019.09.008
23. Jentzsch NS. Methods of assessing adherence to inhaled corticosteroid therapy in children and adolescents: adherence rates and their implications for clinical practice. Jornal Brasileiro de Pneumologia. 2008;34(8):614–621.
24. Rand CS. Measuring adherence to asthma medication regimens. Am J Respir Crit Care Med. 1994;149(2):69–76.
25. Aberhe W, Hailay A, Zereabruk K, et al. Non-adherence to inhaled medications among adult asthmatic patients in Ethiopia: a systematic review and meta-analysis. Asthma Res Pract. 2020;6(1):1–8.
26. Ong KY. What’s new in the global initiative for asthma 2018 report and beyond. Allergo J Int. 2019;28(2):63–72.
27. Jia CE, Zhang HP, Lv Y, et al. The Asthma Control Test and Asthma Control Questionnaire for assessing asthma control: systematic review and meta-analysis. J Allergy Clin Immunol. 2013;131(3):695–703. doi:10.1016/j.jaci.2012.08.023
28. Cohen JL, Mann DM, Wisnivesky JP, et al. Assessing the validity of self-reported medication adherence among inner-city asthmatic adults: the Medication Adherence Report Scale for Asthma. Ann Allergy, Asthma Immunol. 2009;103(4):325–331.
29. Horne R. Self-regulation and self-management in asthma: exploring the role of illness perceptions and treatment beliefs in explaining non-adherence to preventer medication. Psychol Health. 2002;17(1):17–32.
30. Halm EA, Mora P, Leventhal HJC. No symptoms, no asthma: the acute episodic disease belief is associated with poor self-management among inner-city adults with persistent asthma. Chest. 2006;129(3):573–580. doi:10.1378/chest.129.3.573
31. Laforest L, El Hasnaoui A, Pribil C, et al. Asthma patients’ self-reported behaviours toward inhaled corticosteroids. Respir Med. 2009;103(9):1366–1375. doi:10.1016/j.rmed.2009.03.010
32. Cooper V, Metcalf L, Versnel J, et al. Patient-reported side effects, concerns and adherence to corticosteroid treatment for asthma, and comparison with physician estimates of side-effect prevalence: a UK-wide, cross-sectional study. NPJ Prim Care Respir Med. 2015;25(1):1–6.
33. Menckeberg TT, Bouvy ML, Bracke M, et al. Beliefs about medicines predict refill adherence to inhaled corticosteroids. J Psychosom Res. 2008;64(1):47–54.
34. Brooks TL, Leventhal H, Wolf MS, et al. Strategies used by older adults with asthma for adherence to inhaled corticosteroids. J Gen Intern Med. 2014;29(11):1506–1512.
35. Horne R, Weinman J. Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res. 1999;47(6):555–567. doi:10.1016/s0022-3999(99)00057-4
36. Nittala A, Nahmens I, Ikuma L, et al. Effects of medication adherence on healthcare services use among asthma patients. J Healthcare Qual Res. 2019;34(6):301–307. doi:10.1016/j.jhqr.2019.06.007
37. AL-Jamal M, AObeidat L, AL-Huneity SZ, et al. Adherence and nonadherence to inhaled corticosteroids in asthma patients factors and consequences. Int J Med Investig. 2015;4(1):173–179.
38. Lim T, Kowalski S, Tan K, et al. Impact of asthma counseling by pharmacist on asthma control and medication adherence in Asia. J Allergy Clin Immunol. 2012;129(2):AB125.
39. Lasmar L, Camargos P, Champs NS, et al. Adherence rate to inhaled corticosteroids and their impact on asthma control. Allergy. 2009;64(5):784–789. doi:10.1111/j.1398-9995.2008.01877.x
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
