Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 16
Adherence to an eHealth Self-Management Intervention for Patients with Both COPD and Heart Failure: Results of a Pilot Study
Authors Sloots J , Bakker M , van der Palen J , Eijsvogel M, van der Valk P, Linssen G, van Ommeren C, Grinovero M, Tabak M , Effing T, Lenferink A
Received 29 December 2020
Accepted for publication 19 April 2021
Published 15 July 2021 Volume 2021:16 Pages 2089—2103
DOI https://doi.org/10.2147/COPD.S299598
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Joanne Sloots,1 Mirthe Bakker,1 Job van der Palen,1,2 Michiel Eijsvogel,1 Paul van der Valk,1 Gerard Linssen,3 Clara van Ommeren,1 Martijn Grinovero,4 Monique Tabak,5,6 Tanja Effing,7 Anke Lenferink1,8
1Department of Pulmonary Medicine, Medisch Spectrum Twente, Enschede, the Netherlands; 2Department of Research Methodology, Measurement & Data Analysis, University of Twente, Enschede, the Netherlands; 3Department of Cardiology, Hospital Group Twente, Almelo and Hengelo, the Netherlands; 4Amiko Digital Health Limited, London, UK; 5eHealth Group, Roessingh Research and Development, Enschede, the Netherlands; 6Department of Biomedical Signals and Systems, Faculty of Electrical Engineering, Mathematics and Computer Science, University of Twente, Enschede, the Netherlands; 7College of Medicine and Public Health, Flinders University, Adelaide, South Australia, Australia; 8Department of Health Technology and Services Research, Faculty of Behavioural, Management and Social sciences, Technical Medical Centre, University of Twente, Enschede, the Netherlands
Correspondence: Joanne Sloots
Department of Pulmonary Medicine, Medisch Spectrum Twente, Koningsplein 1, Enschede, 7512 KZ, the Netherlands
Tel +31651660063
Email [email protected]
Background: Chronic obstructive pulmonary disease (COPD) and chronic heart failure (CHF) often coexist and share periods of symptom deterioration. Electronic health (eHealth) might play an important role in adherence to interventions for the self-management of COPD and CHF symptoms by facilitating and supporting home-based care.
Methods: In this pilot study, an eHealth self-management intervention was developed based on paper versions of multi-morbid exacerbation action plans and evaluated in patients with both COPD and CHF. Self-reporting of increased symptoms in diaries was linked to an automated decision support system that generated self-management actions, which was communicated via an eHealth application on a tablet. After participating in self-management training sessions, patients used the intervention for a maximum of four months. Adherence to daily symptom diary completion and follow-up of actions were analyzed. An add-on sensorized (Respiro®) inhaler was used to analyze inhaled medication adherence and inhalation technique.
Results: In total, 1148 (91%) of the daily diaries were completed on the same day by 11 participating patients (mean age 66.8 ± 2.9 years; moderate (55%) to severe (45%) COPD; 46% midrange left ventricular function (LVF) and 27% reduced LVF). Seven patients received a total of 24 advised actions because of increased symptoms of which 11 (46%) were followed-up. Of the 13 (54%) unperformed advised actions, six were “call the case manager”. Adherence to inhaled medication was 98.4%, but 51.9% of inhalations were performed incorrectly, with “inhaling too shortly” (< 1.25 s) being the most frequent error (79.6%).
Discussion: Whereas adherence to completing daily diaries was high, advised actions were inadequately followed-up, particularly the action “call the case manager”. Inhaled medication adherence was high, but inhalations were poorly performed. Future research is needed to identify adherence barriers, further tailor the intervention to the individual patient and analyse the intervention effects on health outcomes.
Keywords: telemedicine, chronic conditions, self-treatment, disease management, dry powder inhalers
Introduction
Chronic obstructive pulmonary disease (COPD) and chronic heart failure (CHF) are both progressive diseases that share periods of acute deterioration of symptoms. They are associated with each other (7.5% to 31.3% of patients with COPD also have CHF) which adds to the high burden of both diseases.1,2 This association may result from shared risks factors (eg smoking) and overlapping symptoms. Dyspnea could, for example, be related to either a COPD exacerbation or a sudden deterioration of CHF.3 Overlapping symptoms can easily delay the start of appropriate treatment as it complicates differentiation of both diseases,4 and may lead to a further increase in patient burden and healthcare costs.5,6
Self-management interventions become more significant in the usual care of patients with COPD and CHF.7 They consist of multiple components (eg optimizing physical activity, self-treatment of exacerbations and improving inhaled medication adherence and technique)8 and aim to positively change the patient’s health behavior and improve patients’ self-management skills by a personalized, structured approach.8 Evidence shows that COPD self-management interventions lead to improved health-related quality of life, respiratory-related hospitalization rate and dyspnea.9–11 Self-management interventions for patients with CHF have shown beneficial effects on heart failure hospitalization duration for younger patients (<65 years), and time to CHF-related hospitalizations and all-cause death for patients of all ages.12 Incorporating multi-morbid exacerbation action plans into a self-management intervention tailored for COPD patients with at least one common comorbidity (CHF, ischemic heart disease, anxiety, depression, diabetes mellitus) reduces COPD exacerbation duration and the risk of having at least one respiratory-related hospitalization during follow-up.13
Adherence to and uptake of self-management interventions are essential for effective self-management in patients with COPD.14,15 Studies have shown that adherence to action plans is associated with a reduced exacerbation duration and hospitalization rate in patients with COPD.14,15 However, previous randomized controlled trials, involving paper-based COPD self-management interventions, have shown poor results with regard to action plan adherence. In the study of Schrijver et al, only 38% of the patients showed (sub)optimal adherence to paper COPD exacerbation action plans16 and Bischoff et al found similar results on adherence (40%).14 Adherence to self-management interventions in patients with chronic diseases like COPD and CHF might be influenced by factors, such as age, social support, disease perception and knowledge, the role of the healthcare provider and case manager, (digital) health literacy, and to what level an intervention fits a patient’s needs and competences.17–25
Electronic health (eHealth) could play an important role in improving adherence, as it makes it easier to provide tailored information, give reminders, and adapt to patients’ needs.21,26–29 Self-management interventions are increasingly provided to patients with COPD or CHF by using eHealth technology at home to support patients in health communication (eg teleconsultation), self-monitoring (eg symptom diary, wearables), and their medical treatment (eg self-treatment with prednisolone).30–33 The different intervention components have, however, yet to be robustly combined into a comprehensive eHealth intervention for patients with both COPD and CHF.
In this study, we developed an eHealth self-management intervention for patients with both COPD and CHF that was based on an already evaluated paper-based self-management intervention.13 The aim of this pilot study was to evaluate patient adherence to this eHealth self-management intervention during a follow-up of a maximum of four months. In addition, adherence to inhaled medication and patients’ inhalation technique was assessed.
Materials and Methods
Study Design
This is a prospective pilot study in which patients with both COPD and CHF were recruited from two hospitals (Medisch Spectrum Twente (MST) Enschede and Ziekenhuisgroep Twente (ZGT) Almelo and Hengelo) in the Netherlands.
In September and October 2018, patients participated in three self-management training sessions. After the first session, patients started to use an eHealth self-management intervention on a tablet for a period of a maximum of four months. The study was approved by the Medical Ethical Committee Twente and registered with the Netherlands Trial Register (NL6480). The study was conducted in accordance with the Declaration of Helsinki.
Selection of Patients
In hospital MST, electronic health record data of in- and outpatients from the respiratory department were screened for patient eligibility. In hospital ZGT, outpatients attending the heart failure clinics of the involved nurse practitioner CHF were screened for eligibility. Subsequently, the patient’s pulmonary physician and/or cardiologist were asked for permission for inclusion of their patient. Furthermore, patients received information about the study from the involved researchers face-to-face and/or by phone. Written informed consent to participate in the study was obtained from all patients prior to data collection.
Patients had to fulfill the following inclusion criteria: 1) a clinical diagnosis of COPD defined according to the GOLD criteria;7 2) a clinical diagnosis of CHF defined according to the current (2016) ESC guidelines;3 3) ≥2 COPD and/or CHF exacerbations, defined as deterioration of symptoms for which treatment with oral corticosteroids and/or antibiotics (for COPD) or diuretics (for CHF) were necessary, in the two years preceding study entry, and/or ≥1 hospitalization for COPD and/or CHF in the two years preceding study entry; 4) age ≥ 40 years; 5) ≥ 1 week after exacerbation of COPD and/or CHF; 6) ≥ 1 week after hospitalization; 7) ≥ 4 weeks post-rehabilitation; 8) able to understand and read the Dutch language; and 9) able to use a tablet.
Exclusion criteria were as follows: 1) end stage of a disease; 2) other serious lung disease (eg α1-antitrypsin deficiency; interstitial lung diseases); 3) expected cardiovascular intervention within three months; 4) currently enrolled in a (randomized controlled) trial; 5) waiting for a heart or lung transplantation; 6) renal dialysis; 7) diabetes mellitus type I; 8) Hospital Anxiety and Depression Scale (HADS)-score34,35 of ≥11 for anxiety and/or depression domain symptom scores.
Self-Management Intervention
Self-Management Training Sessions
Self-management training sessions consisted of two group sessions (two hours per session) and a one-hour individual session at weekly intervals. These sessions were led by trained case managers (one nurse practitioner COPD, one nurse practitioner CHF). The sessions were based on self-management training sessions that were provided in a previous self-management study in patients with COPD and comorbidities.13 During these sessions, the significance of early recognition of symptom deterioration and prompt and appropriate treatment was emphasized. First, the patient’s individual symptom level in a stable health state was discussed and described on a “what are my ‘usual’ symptoms” card. Subsequently, patients were trained on how to recognize symptom deterioration by comparing their symptoms over the last 24 hours to their “usual” symptoms. Monitoring of symptoms over a longer period of time (eg recovery from exacerbation to a stable health state) was trained using a specific “monitoring module” on the eHealth application (see details in the paragraph “monitoring module”). During the self-management training sessions, patients received a tablet and were instructed on how to connect it to WiFi internet at home. They also received an add-on sensor for inhaled medication (if applicable), a Fitbit® for measuring step count, and a digital weighing scale. Study technicians assured that all devices were connected to the tablet via Bluetooth. Two weeks after the self-management training sessions, a follow-up phone call with the patients was performed by the case managers to verify usage of and problems with the different components of the self-management intervention. The content of the self-management training sessions is detailed in Table 1.
 |
Table 1 Content of Self-Management Training Sessions |
eHealth Self-Management Application Modules
The eHealth self-management intervention was offered through a tablet application for patients and a website accessible via PC for the case managers and researchers. Patients required accessibility to the internet to be able to use the self-management application. The different modules of the eHealth self-management application for patients are shown in Table 2, and some are explained in more detail below.
 |
Table 2 Content of the eHealth Self-Management Intervention Modules |
Self-Management Module
The digital daily symptom diary included symptoms that were related to COPD (eg dyspnea, cough) and CHF (eg weight by digital weighing scale, edema). All diaries included symptoms regarding ischemic heart disease, anxiety and depression, irrespective of formal diagnosis. This was done for safety reasons, as exacerbations of these comorbidities can be triggered by and may show similar symptoms as COPD and CHF exacerbations (eg dyspnea, fatigue).3,7,36 The latter may confuse patients with proper differentiation and self-treatment of diseases. Every day, participants had to detail whether their symptoms in the last 24 hours were the same, slightly more, or significantly more compared to their “usual” symptoms (defined on their “what are my ‘usual’ symptoms” card). However, for some symptoms, they only had to differentiate between “normal” or “increased”. If necessary, an automated decision support system launched a message with an action for the patient to take (eg initiate self-treatment, perform relaxation exercises, call the case manager). The automated decision support system was based on a paper version of an exacerbation action plan for patients with COPD and comorbidities.13 It was personalized by establishing per patient the type and dose of diuretics and antibiotics that should be used during symptom deterioration and whether they needed to have nitroglycerine at home (in case of diagnosed ischemic heart disease). The type and dose of diuretics and antibiotics were established in agreement with the patient’s healthcare providers from the cardiology and pulmonology department. Patients received prescriptions for the action plan medication from the case manager at the start of the study. The automated decision support system advised patients to call the case manager (or general practitioner outside office hours) when symptoms did not improve after two days of self-treatment. If dyspnea did not improve two days after the start of self-treatment, patients were asked to have NT-proBNP measured at a local laboratory to be able to distinguish between symptoms of COPD or CHF and subsequently call the case manager for their results and further advice. Once a week, patients were asked if they had visited a doctor or started medication. A link to this weekly questionnaire, daily symptom diary, advised actions and a reminder for weighing were listed in the patient self-management application.
For safety reasons, case managers would check online whether patients reported an improvement in symptoms the days after patients had received advice on self-treatment. If symptoms did not improve, case managers would call the patient to provide further advice.
Monitoring Module
The monitoring module showed individual patients’ health status and self-reported symptoms per day, advised and performed actions, and a weight graph.
Inhaler Module
Inhaled medication adherence and inhalation technique, including duration of flow, peak inspiratory flow, orientation of the inhaler, and closing of the cap, were monitored by an add-on inhaler sensor (Respiro®37 by Amiko Digital Health Limited, London, UK), which was compatible with the Ellipta® (GlaxoSmithKline BV, UK) inhaler. At inclusion, each patient’s pulmonary physician was asked whether the patient was eligible for a switch to the Ellipta® inhaler. The patient’s daily medication schedule was established collaboratively by patient and healthcare provider. The add-on inhaler sensors transferred all data collected via Bluetooth to a paired app installed on the tablet device, which in turn uploaded the data to the cloud. The self-management application showed these data and indicated whether the inhalations were performed correctly. On-sensor audio-visual signs reminded the patients of scheduled inhaled medication doses.
Outcomes
The primary outcome of this study was adherence to different components of the eHealth self-management intervention: completing digital daily symptom diaries, following the advised actions, and using inhaled medication. Adherence was measured starting from the patient’s individual self-management training session (first two weeks of October 2018), till their last completed diary (last week of January/first week of February 2019). Adherence to daily diary completion was reported per month and per patient, calculated by dividing the total number of diary completions by the total number of follow-up days. Patient’s adherence to the advised actions was calculated by dividing the number of performed actions by the total number of actions that were advised by the automated decision support system. Inhaled medication adherence levels were calculated by dividing the number of times patients used their inhaler by the number of times they should have used their inhaler according to their physician’s prescription.
The patient’s inhalation technique, evaluated by the frequency of correct inhalations and by the different types of errors that were made per patient, was a secondary outcome parameter of this study. The correct inhalation technique was defined as duration of flow >1.25 seconds, Peak Inspiratory Flow > 30 L/min, orientation of the inhaler between 45° and 135°, and closing the cap of the Ellipta® inhaler properly. These cut-off values were based on literature and expert opinion.37 In addition, paper questionnaires at baseline and after a maximum of four months of follow-up were used to assess other secondary outcomes: health-related quality of life (St. George Respiratory Questionnaire,39 Minnesota Living with Heart Failure Questionnaire40), COPD self-management behavior and knowledge (Partners in Health questionnaire41), COPD self-efficacy (COPD Self-Efficacy Score42) and anxiety/and depression score (HADS35). Due to the low number of participants and the short follow-up time of this pilot study, we decided a-priori not to analyze differences between baseline and follow-up data using statistical tests.
Statistical Analyses
Descriptive statistics were used to report numbers and percentages, means and standard deviations (SD) and medians and interquartile ranges (IQR) of baseline characteristics. Numbers and percentages were calculated for adherence to daily symptom diary completion, following up of advised actions and inhaled medication, number of correct inhalations and types of inhalation errors. These analyses were performed using SPSS version 22. Daily data on inhalation adherence and inhalation technique were delivered by Amiko and subsequently analyzed by SPSS version 22.
Results
Patient Characteristics
Fifty patients of hospital MST met the study criteria. Of these patients, 40 were excluded for reasons detailed in Figure 1,Figure 2. Three patients of hospital ZGT were included. Unfortunately, data on screened patients in hospital ZGT were not assessed and are therefore not available. In total, thirteen patients signed informed consent. One patient deceased before the start of the study and another patient decided to withdraw from participation after the first group self-management training session. The remaining 11 patients that completed the training and follow-up had a mean age of 66.8 ± 2.9 years, moderate (55%, n=6) to severe (45%, n=5) COPD, five (46%) patients had midrange left ventricular function (LVF) and three (27%) patients had reduced LVF (Table 3). Three (27.3%) patients had ischemic heart disease and two (18.2%) had diabetes mellitus as comorbid diseases. Eight patients (72.7%) had at least one COPD exacerbation and six patients (54.5%) had increased the dose of diuretics because of decompensated CHF in the previous year. Three patients (27.3%) had a low digital health literacy score (eHealth Literacy Scale (eHEALS) <26). Six patients (54.5%) were familiar with using a tablet at home.
 |
Table 3 Baseline Characteristics of Participants Who Completed Follow-Up |
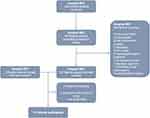 |
Figure 1 Flowchart of patient enrollment. Notes: aFor hospital ZGT detailed information on screened patients is not available. |
Adherence to Daily Symptom Diary
1148 (91.0%) out of 1261 daily diary entries were completed on the actual day (Table 4). Nine patients showed total adherence rates above 95%, and the other two patients completed 59–64% of the diaries on the same day. Because of a system failure, 23 diary days were excluded from adherence analyses as patients were not able to adhere to their intervention for 2 days in a row (for one patient: 3 days). Because of another system failure, diaries were completed twice for 119 diary days. Only one set of these double daily entrees was included in adherence analyses.
 |
Table 4 Adherence to Daily Symptom Diary per Month and in Total |
Action Plan Adherence
Seven patients received a total of 24 messages with advised actions because of symptom deterioration (see Table 5). Eleven (46%) of these advised actions were actually performed by the patients. Thirteen actions (54%) were not performed, of which six were “call the case manager”. In five cases, patients decided not to perform the action that was advised by the self-management application after having contact with a healthcare provider and/or because they were hospitalized. An overview of action plan adherence differentiated per advised action and reasons for non-adherence is shown in Table 5.
 |
Table 5 Action Plan Adherence During Follow-Up Time |
In six cases (patients n=4) patients increased the dose of diuretics in the absence of an automated advice by the eHealth self-management application. These patients reported significantly increased symptoms during one day instead of two days (n=1), slightly increased instead of significantly increased symptoms (n=4) and/or a gradual instead of a significant weight increase (n=4). In six cases (patients n=5), patients started a course of prednisolone and/or antibiotics, while not being advised by the self-management application to do this. It should be noted that this was always done after consulting the case manager or general practitioner. Symptoms of chest pain occurred twelve times in two patients. Because of a fault in the self-management application, the patients were unable to see the advised action “take nitroglycerine” after reporting their chest pain. No adverse effects (eg hospitalization, death) were reported as a result of this system failure.
Inhalations
Adherence to taking inhaled medication and using the correct inhalation technique was measured in seven patients. Three of these patients had already used an Ellipta® Inhaler before the start of this study. Prior to study participation, four patients were switched to Ellipta® in agreement with their respiratory physician. All seven patients had to use their inhaled maintenance medication once a day according to their physician’s prescription.
Inhalation Adherence and Technique
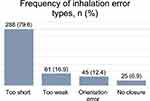
Patients showed an inhaled medication adherence of 98.4% (752 out of 764 days). The range of performed inhalations between patients was 96.3–100%. 390 (51.9%) inhalations were performed correctly. The percentage of correct inhalations varied between and within patients (Table 6). Inhaling too shortly (<1.25 s) was by far the most made inhalation error (79.6%). Inhalation errors are detailed in Figure 1. The add-on sensor of two patients had a low battery for a total of 32 days. Adherence and inhalation technique could therefore not be measured during these days. Double doses (n=10, 1.3% of the total inhalations) were not taken into account in our analyses.
 |
Table 6 Inhaled Medication Adherence and Technique per Patient During Follow-Up |
Secondary Outcomes
The mean and median scores of baseline and follow-up assessments of the secondary outcomes are presented in Table 7.
 |
Table 7 Quality of Life at Baseline and After Follow-Up |
Discussion
In this pilot study, we have tested an eHealth self-management intervention for patients with both COPD and CHF. Whereas our study results showed high patient adherence to completing daily symptom diaries, more than half of the advised actions were not performed by patients, especially the action “call the case manager”. Patients showed high adherence to taking inhaled medication, but their inhaled medication technique was poor.
Patient adherence to completing a symptom diary on a daily basis was high (91%). This was in line with previous findings from studies on eHealth self-management interventions in patients with only COPD.31,33 Despite including a more complex patient group in our study, the adherence to the diary remained high. Real-time reminders to complete the diary might have positively influenced adherence to symptom diaries. Overall patient adherence to advised actions was relatively low, especially “call the case manager for support”. Non-adherence to actions as self-initiating prednisolone could, however, often be explained for valid reasons (eg, patients already started treatment after consultation with the case manager or patients already received in-hospital treatment). Most patients ignored the advice to call the case manager because of dizziness. Whereas patients did report their dizziness in the diary, it might not have felt serious enough for them to call the case manager. Emphasizing when patients with dizziness problems should call a case manager (eg, the severity of dizziness that might suggest health deterioration or medication side effects) during the self-management training sessions and providing this information on the eHealth self-management application, might improve patients’ adherence to contact the case manager for dizziness problems. Moreover, reducing the threshold to call the case manager by improving the availability of case managers’ support (eg by using a chat service) might improve adherence. In our study, some patients also initiated actions without an advice from the self-management application, showing their willingness to self-manage their disease. For example, doses of diuretics were increased by patients themselves, while CHF symptoms were only slightly increased or present for only one day. Furthermore, prednisolone and antibiotics were initiated after having contact with a healthcare provider because of increased symptoms. We know that patients use experiential knowledge to recognize exacerbations, we could therefore assume that decisions to start self-treatment without being prompted by the eHealth application may have been driven by previous experiences with exacerbations.45 This might suggest that, to improve adherence to action plans and adequate timing of self-treatment, action plans for the self-treatment of symptoms should be further tailored to previous experiences of the individual patient. For example, for specific patients, self-treatment could be started after one day instead of two days of significantly increased symptoms, taking into account the risk of side effects as low blood pressure, with diuretics.
Previous studies showed that more frequent follow-up contact initiated by the case manager increases adherence to action plans by improving disease awareness.21,22 These contacts with a case manager could be beneficial for the patients in our study as well. However, it might influence the degree to which patients self-manage their disease as patients might become too reliant on their case manager and thereby eliminate the effect of the self-management intervention. However, this was not assessed in our study. The low adherence to advised actions also raises the question whether patients felt completely confident with following up the advice of an eHealth application. This is supported by the findings of a qualitative study on perceptions of patients and healthcare providers on using mobile Health (mHealth) for the self-management of COPD, showing that some patients had limited trust in the advice of mHealth interventions.46 Moreover, there is a growing body of literature that suggests that the individual patient’s needs of COPD self-management (depending on, eg culture, norms and values) misalign with the healthcare providers expectations of behavior change of the patient, which may influence patient adherence.17,47 Furthermore, the adoption of the self-management intervention by the healthcare provider is highly important and is dependent on the particular healthcare provider’s perceived value and needs, which should be aligned with the patient’s needs and preferences before implementing.48
Adherence to inhaled medication was high for all patients (98.4%). This is in contrast to literature,49–51 possibly because in our study patients received reminders via the Embodied Conversational Agent (ECA) and via audio-visual signs by the sensor to take their inhaled medication. Despite the inhalation instruction provided by the case manager, also on the use of the add-on inhaler sensors, the patient’s inhalation technique was poor, similar to other studies.52,53 More time should therefore be taken for an individual inhalation instruction at the start of the study.54 This should be repeated during follow-up within the self-management application and/or by the case manager. Also, feedback of the ECA on inhalation medication was not specified on error type and, because of technical problems with the sensor, not always correct (eg a performed inhalation was wrongly registered by the sensor as unperformed, leading to incorrect feedback to the patient by the ECA). In the future, prompt, specified and correct information about individual inhalations that is recorded by the sensor, may be helpful to improve inhalation technique.
In addition to the quantitative results we obtained, this pilot study taught us more about how to further develop the eHealth self-management intervention for patients with multiple chronic conditions, such as COPD and CHF. We experienced that, especially at the start of the study, patients were focused on how to use the tablet and various devices that were connected to this tablet. This might have distracted them from (learning how to) self-report symptoms and follow-up advices.55 It suggests that some patients need more intensive training and/or an adaptation period before starting the actual self-management intervention. Before the start of a self-management intervention, it is important to assess patient readiness to change behavior, as it is related to adherence to the intervention.8,56 Motivational interviewing could be used to improve patient readiness.57 Further, we feel that for patients that suffer from severe COPD and/or CHF, more intensive case manager support (by phone or face-to-face) might be necessary to differentiate between symptoms of COPD and/or CHF. In case of significantly increased symptoms in these patients, the automated decision support system should probably give advice towards case manager contact instead of self-treatment. Further, we aimed to support patients in differentiating between COPD and CHF breathlessness symptoms in case breathlessness symptoms did not improve after starting self-treatment by adding the laboratory test NT-proBNP to the action plan. During this study, patients were only twice advised to have the lab-test NT-proBNP measured. In both cases, these actions were not performed. A longer follow-up time and a larger group of patients are therefore needed to evaluate the usefulness of NT-proBNP as part of a multi-morbid exacerbation action plan. Moreover, an easy-to-use point-of-care test for measuring NT-proBNP at home in these patients with health deterioration could be advantageous to improve adherence to measuring NT-proBNP.
The limitations of our study also gave us insight into how to further develop the eHealth self-management intervention. First, although including a small patient group fits to the pilot phase of our study, we included less patients than expected. This has limited the generalizability of our results. Many patients in hospital MST declined participation because of logistic issues (eg not being able to visit self-management training because of immobility and transport problems), not being familiar with using a tablet, and because patients presumed it was too much effort. Unfortunately, for ZGT patients this information was not reported. Offering digital and/or face-to-face self-management training sessions at home for a specific group of patients who are immobile or who have no experience in using a tablet might increase the number of patients willing to participate in eHealth self-management interventions. Second, we did not assess (e)health literacy and cognitive impairment during patient enrollment. Future studies should not only measure (e)health literacy and cognitive impairment at enrollment but ideally eHealth self-management interventions should also be adjustable to (e)health literacy and cognitive impairment. This will increase its applicability and uptake in patients with limited (e)health literacy and cognitive impairment.24,55,56,58 For example, more and tailored self-management training sessions and a simplified version of the eHealth self-management intervention could be offered to these patients. Third, the use of home monitoring devices (add-on sensors for an inhaler device, a smart weighing scale and an activity sensor) led to connection and low-battery problems. In addition, due to system failures, the self-management application could not be used for two days, some diaries were completed double, and the action “take nitroglycerine” in case of chest pain did not appear. Whereas this was recognized as a serious safety issue in this pilot study, fortunately no adverse events were reported related to this. Although the technical issues correspond to the pilot phase of the technology and technology readiness level,59,60 it led to some frustrations and distrust amongst patients regarding the eHealth self-management intervention. In follow-up studies, the technology should therefore be intensively tested upfront together with its end users (ie patients, technicians, healthcare providers) to ensure its suitability for larger-scale summative evaluation.61 Finally, a physical activity program could be added to our future eHealth self-management intervention to optimize and preserve physical health.8,62
Our results indicate that the eHealth self-management intervention should be further adapted to the needs and competences of the individual patient, which is also suggested by several qualitative studies.8,46,55,63 This will increase patient adherence, and thereby potentially improve individual health outcomes. Also, the eHealth self-management application itself should be further developed towards an application with a higher technology readiness level, suitable for larger-scale follow-up studies to investigate the clinical added value.59,60 In addition, further analysis of both clinical and home monitoring data of eHealth self-management interventions can increase our understanding of the development and onset of disease progression and exacerbations of COPD and CHF. It can provide insight into day-to-day fluctuations in COPD and CHF symptoms and behavior (eg adherence), and thereby work towards preventive chronic disease self-management for the patient with COPD and CHF.64–66
Conclusion
Whereas adherence to completing daily diaries was high as part of our eHealth self-management intervention for patients with both COPD and CHF, advised actions were inadequately followed-up, particularly the action “call the case manager”. Inhaled medication adherence was also high, but inhalations were poorly performed. This pilot study gives insight into how to further develop the eHealth self-management intervention. For this, personalizing and tailoring to an individual patient’s needs and competences are essential. Future quantitative and qualitative analyses are necessary to unravel patient adherence and evaluate the effects of a further developed eHealth self-management intervention on health outcomes.
Abbreviations
eHEALS, eHealth Literacy Scale; FEV1%, FEV1 percentage of predicted; FVC, Forced Vital Capacity; GOLD, Global Initiative For Chronic Obstructive Lung Disease; mMRC, modified Medical Research Council; COPD, Chronic Obstructive Pulmonary Disease; CHF, Chronic Heart Failure; ECA, Embodied Conversational Agent; eHealth, electronic Health; HADS, Hospital Anxiety and Depression Scale; IQR, interquartile range; LVEF, Left Ventricular Ejection Fraction; n, number; IQR, interquartile range.
Data Sharing Statement
Deidentified participant data are stored in archived datasets for a total of 15 years and can be requested from the corresponding author.
Ethics Approval and Informed Consent
This study was approved by the Medical Ethical Committee of Twente (NL17-17). Informed consent for participation was given by all participants.
Consent for Publication
All authors have seen the content of this article and have given consent for publication.
Acknowledgments
We would like to thank all patients who participated in this study. Furthermore, we would like to thank A. Kleberger for her involvement as case manager cardiology, B. van Schooten, D. Hofs and S. ter Stal for the technical part of the development of the eHealth self-management application. Lastly, we would like to thank the nurse practitioners cardiology of Medisch Spectrum Twente and pulmonary physicians of ZiekenhuisGroep Twente for their insights regarding self-treatment for the individual patient.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in study conception and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision and review of the manuscript or acquisition of funding. All authors gave final approval of the version to be published, have agreed on the journal to which the article has been submitted, and agree to be accountable for all aspects of the work.
Funding
This study was funded by the Pioneers in Health Care Innovation Fund (2016), a cooperation between Medisch Spectrum Twente (hospital), ZiekenhuisGroep Twente (hospital), University of Twente and Saxion Academy, all in The Netherlands. The aim of this fund is to stimulate the introduction of innovative technology in healthcare.
Disclosure
Martijn Grinovero is an employee (CCO) at Amiko Digital Health Limited. Dr Anke Lenferink reports grants from Pioneers In Healthcare Innovation Fund 2016, during the conduct of the study. The other authors did not report any conflicts of interest related to this work.
References
1. Schnell K, Weiss CO, Lee T, et al. The prevalence of clinically-relevant comorbid conditions in patients with physician-diagnosed COPD: a cross-sectional study using data from NHANES 1999–2008. BMC Pulm Med. 2012;12(1):26. doi:10.1186/1471-2466-12-26
2. Mannino DM, Thorn D, Swensen A, Holguin F. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur Respir J. 2008;32(4):962–969. doi:10.1183/09031936.00012408
3. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failureThe Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–2200. doi:10.1093/eurheartj/ehw128
4. Vanfleteren LEGW, Spruit MA, Wouters EFM, Franssen FME. Management of chronic obstructive pulmonary disease beyond the lungs. Lancet Respir Med. 2016;4(11):91–924. doi:10.1016/S2213-2600(16)00097-7
5. Chen W, FitzGerald JM, Sin DD, Sadatsafavi M. Excess economic burden of comorbidities in COPD: a 15-year population-based study. Eur Respir J. 2017;50(1):1700393. doi:10.1183/13993003.00393-2017
6. Hillas G, Perlikos F, Tsiligianni I, Tzanakis N. Managing comorbidities in COPD. Int J COPD. 2015;10:95–109.
7. GOLD. The Global Strategy for Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease (Updated 2021). GOLD; 2021.
8. Effing TW, Vercoulen JH, Bourbeau J, et al. Definition of a COPD self-management intervention: International Expert Group consensus. Eur Respir J. 2016;48(1):46–54. doi:10.1183/13993003.00025-2016
9. Zwerink M, Kerstjens HAM, Van Der Palen J, et al. (Cost-)effectiveness of self-treatment of exacerbations in patients with COPD: 2 years follow-up of a RCT. Respirology. 2016;21(3):497–503. doi:10.1111/resp.12697
10. Zwerink M, Brusse-Keizer M, van der Valk PD, et al. Self management for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;(3). doi:10.1002/14651858.CD002990.pub3
11. Lenferink A, Brusse-Keizer M, van der Valk PD, et al. Self-management interventions including action plans for exacerbations versus usual care in patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2017;8(8). doi:10.1002/14651858.CD011682.pub2.
12. Jonkman NH, Westland H, Groenwold RHH, et al. Do self-management interventions work in patients with heart failure? An individual patient data meta-analysis. Circulation. 2016;133(12):1189–1198. doi:10.1161/CIRCULATIONAHA.115.018006
13. Lenferink A, van der Palen J, van der Valk PD, et al. Exacerbation action plans for patients with COPD and comorbidities: a randomised controlled trial. Eur Respir J. 2019;54(5):1802134. doi:10.1183/13993003.02134-2018
14. Bischoff EWM, Hamd DH, Sedeno M, et al. Effects of written action plan adherence on COPD exacerbation recovery. Thorax. 2011;66(1):26–31. doi:10.1136/thx.2009.127621
15. Choi JY, Chung HIC, Han G. Patient outcomes according to COPD action plan adherence. J Clin Nurs. 2014;23(5–6):883–891. doi:10.1111/jocn.12293
16. Schrijver J, Effing TW, Brusse-Keizer M, van der Palen J, van der Valk P, Lenferink A. Predictors of patient adherence to COPD self-management exacerbation action plans. Patient Educ Couns. 2021;104(1):163–170. doi:10.1016/j.pec.2020.06.015
17. Sigurgeirsdottir J, Halldorsdottir S, Arnardottir RH, Gudmundsson G, Bjornsson EH. COPD patients’ experiences, self-reported needs, and needs-driven strategies to cope with self-management. Int J COPD. 2019;14:1033–1043. doi:10.2147/COPD.S201068
18. Ogunbayo OJ, Russell S, Newham JJ, et al. Understanding the factors affecting self-management of COPD from the perspectives of healthcare practitioners: a qualitative study. NPJ Prim Care Respir Med. 2017;27(1):54. doi:10.1038/s41533-017-0054-6
19. Korpershoek YJG, Vervoort SCJM, Nijssen LIT, Trappenburg JCA, Schuurmans MJ. Factors influencing exacerbation-related self-management in patients with COPD: a qualitative study. Int J COPD. 2016;11:2977–2990. doi:10.2147/COPD.S116196
20. Lenferink A, van der Palen J, Effing T. The role of social support in improving chronic obstructive pulmonary disease self-management. Expert Rev Respir Med. 2018;12(8):623–626. doi:10.1080/17476348.2018.1489723
21. Farias R, Sedeno M, Beaucage D, et al. Innovating the treatment of COPD exacerbations: a phone interactive telesystem to increase COPD Action Plan adherence. BMJ Open Respir Res. 2019;6(1). doi:10.1136/bmjresp-2018-000379.
22. Bourbeau J, Saad N, Joubert A, et al. Making collaborative self-management successful in COPD patients with high disease burden. Respir Med. 2013;107(7):1061–1065. doi:10.1016/j.rmed.2013.03.003
23. Bucknall CE, Miller G, Lloyd SM, et al. Glasgow supported self-management trial (GSuST) for patients with moderate to severe COPD: randomised controlled trial. BMJ. 2012;344(March):e1060. doi:10.1136/bmj.e1060
24. Bos-Touwen I, Schuurmans M, Monninkhof EM, et al. Patient and disease characteristics associated with activation for self-management in patients with diabetes, chronic obstructive pulmonary disease, chronic heart failure and chronic renal disease: a cross-sectional survey study. PLoS One. 2015;10(5):e0126400. doi:10.1371/journal.pone.0126400
25. Myers SL, Siegel EO, Hyson DA, Bidwell JT. A qualitative study exploring the perceptions and motivations of patients with heart failure who transitioned from non-adherence to adherence. Heart Lung. 2020;49(6):817–823. doi:10.1016/j.hrtlng.2020.09.010
26. Webb TL, Joseph J, Yardley L, Michie S. Using the Internet to promote health behavior change: a systematic review and meta-analysis of the impact of theoretical basis, use of behavior change techniques, and mode of delivery on efficacy. J Med Internet Res. 2010;12(1):e4. doi:10.2196/jmir.1376
27. Fry JP, Neff RA. Periodic prompts and reminders in health promotion and health behavior interventions: systematic review. J Med Internet Res. 2009;11(2):e16. doi:10.2196/jmir.1138
28. Kelders SM, Kok RN, Ossebaard HC, Van Gemert-pijnen JEWC. Persuasive system design does matter: a systematic review of adherence to web-based interventions. J Med Internet Res. 2012;14(6):e152. doi:10.2196/jmir.2104
29. Bourbeau J, Farias R. Making sense of telemedicine in the management of COPD. Eur Respir J. 2018;51(5):1800851. doi:10.1183/13993003.00851-2018
30. Mccabe C, Mccann M, Brady AM. Computer and mobile technology interventions for self-management in chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2017;5(5). doi:10.1002/14651858.CD011425.pub2
31. Tabak M, Brusse-Keizer M, van der Valk P, Hermens H, Vollenbroek-Hutten M. A telehealth program for self-management of COPD exacerbations and promotion of an active lifestyle: a pilot randomized controlled trial. Int J COPD. 2014;9:935–944. doi:10.2147/COPD.S60179
32. Wagenaar KP, Broekhuizen BDL, Jaarsma T, et al. Effectiveness of the European Society of Cardiology/Heart Failure Association website ‘heartfailurematters.org’ and an e-health adjusted care pathway in patients with stable heart failure: results of the ‘e-Vita HF’ randomized controlled trial. Eur J Heart Fail. 2019;21(2):238–246. doi:10.1002/ejhf.1354
33. Boer LM, van der Heijden M, van Kuijk NME, et al. Validation of ACCESS: an automated tool to support self-management of COPD exacerbations. Int J COPD. 2018;13:3255–3267. doi:10.2147/COPD.S167272
34. Spinhoven P, Ormel J, Sloekers PP, Kempen GI, Speckens AEM, Van Hemert AM. A validation study of the Hospital Anxiety and Depression Scale (HADS) in different groups of Dutch subjects. Psychol Med. 1997;27(2):363–370. doi:10.1017/S0033291796004382
35. Zigmond AS, Snaith RP. The hospital anxiety and depression scale (HADS). Acta Psychiatr Scand. 1983;67(6):361–370. doi:10.1111/j.1600-0447.1983.tb09716.x
36. Maurer J, Rebbapragada V, Borson S, et al. Anxiety and depression in COPD: current understanding, unanswered questions, and research needs. Chest. 2008;134(4):43S–56S. doi:10.1378/chest.08-0342
37. Braido F, Paa F, Ponti L, Canonica G. A new tool for inhalers ’ use and adherence monitori ng: the Amiko ® validation trial. Int J Eng Res Sci. 2016;(10):159–166.
38. Ruttkay Z, Dormann C, Noot H. Embodied conversational agents on a common ground: a framework for design and evaluation. In: From Brows to Trust. Kluwer; 2004:27–66.
39. Jones PW, Quirk FH, Baveystock CM. The St George’s respiratory questionnaire. Respir Med. 1991;85(suppl B):25–31. doi:10.1016/S0954-6111(06)80166-6
40. Rector TS, Cohn JN. Assessment of patient outcome with the Minnesota Living with Heart Failure questionnaire: reliability and validity during a randomized, double-blind, placebo-controlled trial of pimobendan. Pimobendan Multicenter Research Group. Am Heart J. 1992;124(4):1017–1025. doi:10.1016/0002-8703(92)90986-6
41. Petkov J, Harvey P, Battersby M. The internal consistency and construct validity of the partners in health scale: validation of a patient rated chronic condition self-management measure. Qual Life Res. 2010;19(7):1079–1085. doi:10.1007/s11136-010-9661-1
42. Wigal JK, Creer TL, Kotses H. The COPD self-efficacy scale. Chest. 1991;99(5):1193–1196. doi:10.1378/chest.99.5.1193
43. Van Der Vaart R, Van Deursen AJ, Drossaert CHC, Taal E, Van Dijk JA, Van De Laar MA. Does the eHealth literacy scale (eHEALS) measure what it intends to measure? Validation of a Dutch version of the eHEALS in two adult populations. J Med Internet Res. 2011;13(4):e86. doi:10.2196/jmir.1840
44. Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest. 1988;93(3):580–586. doi:10.1378/chest.93.3.580
45. Williams V, Hardinge M, Ryan S, Farmer A. Patient’s experience of identifying and managing exacerbations in COPD: a qualitative study. NPJ Prim Care Respir Med. 2014;24(1):14062. doi:10.1038/npjpcrm.2014.62
46. Korpershoek YJG, Vervoort SCJM, Trappenburg JCA, Schuurmans MJ. Perceptions of patients with chronic obstructive pulmonary disease and their health care providers towards using mHealth for self-management of exacerbations: a qualitative study. BMC Health Serv Res. 2018;18(1):757. doi:10.1186/s12913-018-3545-4
47. Russell S, Ogunbayo OJ, Newham JJ, et al. Qualitative systematic review of barriers and facilitators to self-management of chronic obstructive pulmonary disease: views of patients and healthcare professionals. NPJ Prim Care Respir Med. 2018;28(1):2. doi:10.1038/s41533-017-0069-z
48. Gaveikaite V, Grundstrom C, Lourida K, et al. Developing a strategic understanding of telehealth service adoption for COPD care management: a causal loop analysis of healthcare professionals. PLoS One. 2020;15(3):e0229619. doi:10.1371/journal.pone.0229619
49. Cecere LM, Slatore CG, Uman JE, et al. Adherence to long-acting inhaled therapies among patients with chronic obstructive pulmonary disease (COPD). COPD. 2012;9(3):251–258. doi:10.3109/15412555.2011.650241
50. Asche CV, Leader S, Plauschinat C, et al. Adherence to current guidelines for chronic obstructive pulmonary disease (COPD) among patients treated with combination of long-acting bronchodilators or inhaled corticosteroids. Int J Chron Obs Pulmon Dis. 2012;7:201–209. doi:10.2147/COPD.S25805
51. Koehorst- Ter Huurne K, Movig K, Van Der Valk P, Van Der Palen J, Brusse-Keizer M. Differences in adherence to common inhaled medications in COPD. COPD. 2015;12(6):643–648. doi:10.3109/15412555.2014.995292
52. Gregoriano C, Dieterle T, Breitenstein AL, et al. Use and inhalation technique of inhaled medication in patients with asthma and COPD: data from a randomized controlled trial. Respir Res. 2018;19(1):237. doi:10.1186/s12931-018-0936-3
53. Chrystyn H, Price DB, Molimard M, et al. Comparison of serious inhaler technique errors made by device-naïve patients using three different dry powder inhalers: a randomised, crossover, open-label study. BMC Pulm Med. 2016;16:12. doi:10.1186/s12890-016-0169-5
54. Price D, Keininger DL, Viswanad B, Gasser M, Walda S, Gutzwiller FS. Factors associated with appropriate inhaler use in patients with COPD – lessons from the REAL survey. Int J COPD. 2018;13:695–702. doi:10.2147/COPD.S149404
55. Cabrita M, Tabak M, Vollenbroek-Hutten MMR. Older adults’ attitudes toward ambulatory technology to support monitoring and coaching of healthy behaviors: qualitative study. J Med Internet Res. 2019;2(1).
56. Yadav UN, Hosseinzadeh H, Lloyd J, Harris MF. How health literacy and patient activation play their own unique role in self-management of chronic obstructive pulmonary disease (COPD)? Chron Respir Dis. 2019;16:147997311881641. doi:10.1177/1479973118816418
57. Emmons KM, Rollnick S. Motivational interviewing in health care settings: opportunities and limitations. Am J Prev Med. 2001;20(1):68–74. doi:10.1016/S0749-3797(00)00254-3
58. Blackstock FC, Lareau SC, Nici L, et al. Chronic obstructive pulmonary disease education in pulmonary rehabilitation: an official American thoracic society/thoracic society of Australia and New Zealand/Canadian thoracic society/British thoracic society workshop report. Ann Am Thorac Soc. 2018;15(7):769–784. doi:10.1513/AnnalsATS.201804-253WS
59. DeChant HK, Tohme WG, Mun SK, Hayes WS, Schulman KA. Health systems evaluation of telemedicine: a staged approach. Telemed J. 1996;2(4):303–312. doi:10.1089/tmj.1.1996.2.303
60. Jansen - Kosterink S, Vollenbroek - Hutten MM, Hermens HJ. A renewed framework for the evaluation of telemedicine. In:
61. Kushniruk A. Evaluation in the design of health information systems: application of approaches emerging from usability engineering. Comput Biol Med. 2002;32(3):141–149. doi:10.1016/S0010-4825(02)00011-2
62. Zwerink M, Van Der Palen J, Kerstjens HAM, et al. A community-based exercise programme in COPD self-management: two years follow-up of the COPE-II study. Respir Med. 2014;108(10):1481–1490. doi:10.1016/j.rmed.2014.07.016
63. Wade R, Cartwright C, Shaw K. Factors relating to home telehealth acceptance and usage compliance. Risk Manag Healthc Policy. 2012;(5):25–33. doi:10.2147/RMHP.S30204
64. van der Heijden M, Lucas PJF, Lijnse B, Heijdra YF, Schermer TRJ. An autonomous mobile system for the management of COPD. J Biomed Inform. 2013;46(3):458–469. doi:10.1016/j.jbi.2013.03.003
65. Velickovski F, Ceccaroni L, Roca J, et al. Clinical decision support systems (CDSS) for preventive management of COPD patients. J Transl Med. 2014;12(2):1–10. doi:10.1186/1479-5876-12-S2-S9
66. Merone M, Pedone C, Capasso G, Incalzi RA, Soda P. A decision support system for tele-monitoring COPD-related worrisome events. IEEE J Biomed Health Inform. 2017;21(2):296–302. doi:10.1109/JBHI.2017.2654682
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.