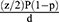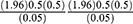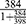Back to Journals » Breast Cancer: Targets and Therapy » Volume 13
Adherence to Adjuvant Hormonal Therapy and Associated Factors Among Women with Breast Cancer Attending the Tikur Anbessa Specialized Hospital, Addis Ababa Ethiopia, 2019: A Cross-sectional Study
Authors Wako Z , Mengistu D , Dinegde NG , Asefa T , Wassie M
Received 16 March 2021
Accepted for publication 21 May 2021
Published 9 June 2021 Volume 2021:13 Pages 383—392
DOI https://doi.org/10.2147/BCTT.S311445
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pranela Rameshwar
Zerko Wako, 1 Daniel Mengistu, 2 Negalign Getahun Dinegde, 2 Tseganesh Asefa, 1 Mulugeta Wassie 1
1Department of Medical Nursing, School of Nursing, College of Medicine and Health Science, University of Gondar, Gondar, Ethiopia; 2School of Nursing and Midwifery, College of Health Science, Addis Ababa University, Addis Ababa, Ethiopia
Correspondence: Zerko Wako Tel +251918728268
Email [email protected]
Background: In breast cancer patients, adherence to adjuvant hormone therapy (AHT) is uncertain. Seven in every 10 patients were hormone receptor positive and adjuvant hormone therapy (AHT) is prescribed for 5– 10 years for a woman with breast cancer. Therefore, the aim of this research was to examine adherence to adjuvant hormone therapy and related factors among women with breast cancer attending the Tikur Anbessa Specialized Hospital Oncology Center.
Methods: An institutional-based cross-sectional study was conducted from March to April, 2019. Systematic random sampling technique was used to select participants. A semi-structured questionnaire was used. Medication possession ratio (MPR) was used where score ≥ 80% was adherence. Data were entered in EpiData version 4.4.2.1 and transferred to SPSS version 25, and analyzed using binary logistic regression.
Results: Out of 216 women with breast cancer 209 participated in the study with a response rate of 97%. The overall adherence in this study was 77.5%. Getting social support (OR=3.959, 95%CI: 1.570– 9.980), being on anastrozole (OR=0.139, 95%CI: 0.040– 0.485), getting a thorough therapeutic communication about treatment (OR=4.590, 95%CI: 1.061– 19.863), undergoing mastectomy (OR=0.215, 95%CI: 0.059– 0.788), having side effects (OR=0.210, 95%CI: 0.085– 0.517) were found to be significantly associated with adherence to AHT.
Conclusion: In general, the overall adherence to AHT was 77.5% for women with breast cancer. Factors such as types of adjuvant hormone therapy, lack of side effects, mastectomy, getting social support, and thorough therapeutic communication were strongly linked with adherence to them.
Keywords: adherence, adjuvant hormone therapy, breast cancer, Ethiopia
A Letter to the Editor has been published for this article.
A Response to Letter has been published for this article.
Introduction
Breast cancer has remained a significant public health concern worldwide, including in Ethiopia. It accounts for around one-fourth of all cancer cases and one-sixth of all deaths associated with cancer. Breast cancer is the principal cause of cancer mortality, according to the Addis Ababa cancer registry, which precedes cervical cancer. In Ethiopia, nevertheless, hormone receptor staining is sometimes not available. However, some studies showed that in every 10 women with breast cancer seven of them were hormone receptor-positive (HR+).1–4In countries such as Kenya, Tanzania, and Uganda, the prevalence of hormone receptor-positive (ER+/PR+) among women with breast cancer was 27%, 40%, and 51%. Tamoxifen is used to treat ER+ BC cancers, in sub-Saharan Africa it is administered without knowledge of receptor status. Besides countries without these facilities blindly administer hormonal therapies.2,5–9
These therapies include a selective modulator of the estrogen receptor (tamoxifen 20 mg po daily) works by blocking the effects of estrogen and aromatase inhibitors (anastrozole 1 mg po daily) that inhibit the biosynthesis of estrogen. Adjuvant tamoxifen and anastrozole reduce the risk of recurrence in women with ER+ BC by 40% and 12%, and mortality by 33% and 9%, respectively.10,11 As patients are expected to take the drug for almost 5–10 years, the duration of adjuvant hormonal therapy is relatively extended. Thus, this lengthy oral therapy duration stressed whether or not the patient complied with the prescribed regimen. Despite its efficacy in treating breast cancer only one in two patients adhered to the medicated AHT regimen. Consequently, it then accelerates their mortality risk.10,12–18
Adherence is a multidimensional problem that is strongly linked with adherence to tamoxifen and AIs linked to the patient, therapy, health-care system, sociodemographic, disease, and behavioral factors.18–20 In the Nigeria study, financial burdens and side effects were the most factors responsible for not complying with the regimen.21 The excellence or incapability to conform to adjuvant hormone therapy was the duration, while the increase in medication duration resulted in a reduction in adherence owing to intolerable adverse effects. For example, tamoxifen and anastrozole adherence rates in the first to fifth years were around 79% to 65% and 80% to 72%, respectively.13,22–24
Different efforts have been made to mitigate the concern of adherence by educating patients on medication and also its adverse effects, skills on self-management of side effects, strengthening connections between patient and provider and securing health-care coverage, providing connections to social support and designing policies and practices to overcome factors that influenced adherence to AHT. Consequently, this improved breast cancer outcomes amongst survivors of breast cancer care.11,25–30 There is, however, a paucity of reporting on the adherence status of Ethiopian women receiving adjuvant hormonal therapy and its relevant factors in the study area. This research was therefore aimed at determining adherence status and associated factors for adjuvant hormone therapy among patients with female breast cancer attending TASH oncology center.
Methods and Materials
Study Design, Period, and Setting
An institutional based cross sectional study was conducted from March to April, 2019 at the oncology center of TASH. The hospital is found in Addis Ababa Lideta Sub City. This hospital worked as the nation’s sole cancer referral center and has given radiotherapy, chemotherapy, concurrent cancer treatment services and palliative care for patients with cancer. According to the registry in the unit, more than 10,000 cancer cases were seen in this hospital per year. The most common cancer cases seen in this hospital were breast, cervical, and sarcomas.1
Population
All women diagnosed with breast cancer in the TASH oncology center were the source population and those are available during the data collection period were the study population. Patients who were severely ill and incompetent to interview were excluded.
Sample Size Determination and Sampling Procedure
To determine the sample size assuming number of the study subjects as n, the standardized normal distribution curve value for 95% confidence level (1.96), taking 50% of proportion because no previous similar study on adherence to adjuvant hormonal therapy and associated factors among women with breast cancer. By applying single population proportion formula for a cross-sectional survey, the sample size was 384.
where Z=1.96; P=0.5; d=0.05.
Since the flow of patients during data collection period was less than 10,000, then a correction formula was applied.
where nf=desired sample size; n=the calculated sample size; N=total population (during data collection period).
After adding a 10% nonresponse rate the final sample size was 216. Then, data was collected by using a systematic sampling method. Every two patients were interviewed.
Operational Definition
Adherence: those who score medication possession ratio ≥80%.
Nonadherence: Those whose score medication possession ratio <80%.2
Adjuvant hormonal therapy: includetamoxifen 20 mg po/day and anastrozole 1 mg po/day in this study.
Data Collection Tools
Data was collected using a pretested and semi-structured face-to-face interview and patient health records were used. The question was translated in to Amharic by experts in both languages before, and translated back to English after data collection to ensure its consistency.
The interview-guided questionnaire was used to collect information on patients’ sociodemographics, clinical and health-care system related characteristics. Patient charts review guide and prescription paper were used to identify date and days of supply and types of the AHT. Adherence was defined by the medication possession ratio (≥80%).2,3 Medication possession ratio (MPR), defined as the days’ supply of medication during the period from the first dispensing to the last dispensing in the time period of three months, divided by number of days.
Data Quality Control
Data quality assurance was applied using different techniques. Firstly, a content validity test was conducted. Then the pretest was done at Hawassa University Specialized Hospital by taking 5% of the study sample. Findings and experiences from the pretest were utilized in modifying the data collection tool. Training was given by the principal investigator for one day for data collectors and supervisors. The supervisor and principal investigator reviewed each questionnaire daily and checked for completeness and were then edited; the necessary feedback was given to the data collectors the next morning.
Data Processing and Analysis
Data were first checked, coded and entered using EpiData version 4.4.2.1 and transferred to SPSS version 25. Description of frequency, mean, median, proportion, and SD was used. Significance was determined using odds ratios with 95% confidence intervals. To assess the association between the different predictor variables with the dependent variable, first bivariate analysis were conducted between each independent variable and outcome variable, those eligible with P-value <0.2 were then entered in multivariate analysis. Those variables with a P-value <0.05 and a 95% confidence interval were regarded as significant factors using an binary logistic regression.
Results
Sociodemographic Characteristics of the Study Participants
From 216 selected participants, 209 were involved in the study with a response rate of 97%. Of 209, 88 (42.1%) were in the age group 45–65 years, 121 (57.9%) were from urban, 103 (49.3%) were high school and above, 154 (73.7%) of the participant were married, 139 (66.5%) were Orthodox by faith, 82 (39.7%) were Amhara by ethnicity, 117 (56%) were housewives (Table 1).
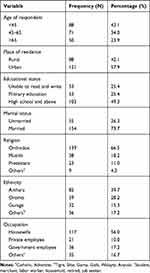 |
Table 1 Sociodemographic Characteristics of Women with Breast Cancer Attending Oncology Center at TASH, Addis Ababa, Ethiopia, 2019 (N=209) |
Patient-related Factors
Of 209, 207 (99.1%) of the study participants had no smoking habit, 205 (98.1%) never drank alcohol before and 140 (67%) were socially supported (Table 2).
 |
Table 2 Patient-related Variables of Women with Breast Cancer Attending Oncology Center at TASH, Addis Ababa, Ethiopia, 2019 (N=209) |
Disease-related Factors
Of 209, 190 (90.9%) had no family history, 187 (89.5%) were ductal carcinoma, 151 (72.2%) with advanced stage, 143 (68.4%) were postmenopause women, 186 (89.0%) with unknown receptor status, 205 (98.1%) were with one breast unilateral, respondents who received chemotherapy, surgery and radiotherapy were 194 (92.8%), 188 (90.0%) and 51 (28.2%), respectively, 189 (90.4%) undergoing mastectomy, 159 (76.1%) have no comorbidity, Among 50 (23.9%) those who had comorbidity; 24 (48%) were diabetic, 12 (24%) were HIV/AIDS, 8 (16%) had a heart problem and 6 (12%) had a respiratory problem (Table 3).
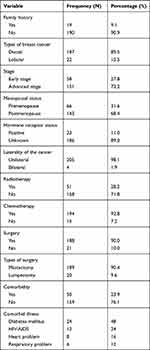 |
Table 3 Disease-related Variables of Women with Breast Cancer Attending Oncology Center at TASH, Addis Ababa, Ethiopia, 2019 (N=209) |
Medication-related Factors
Of 209, 118 (56.5%) were on tamoxifen, 62 (29.7%) initiated in the year 2019, 122 (58.4%) had not experienced side effects. Among 87 those who reported side effects; nausea and vomiting 25 (28.7%), tiredness and fatigue 23 (26.4%), joint and muscle pain 20 (23.0%), weight gain 9 (10.3%), sleep disorder 7 (8.0%), hot flash 3 (3.4%) (Figure 1, Figure 2). One hundred and twelve (53.1%) were getting their medication free of charge, 110 (52.6%) had follow-up of one year (Table 4).
 |
Figure 1 Side effects of adjuvant hormonal therapy’s reported by the respondents among women with breast cancer attending oncology center at TASH, Addis Ababa, Ethiopia, 2019 (N=209). |
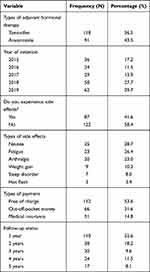 |
Table 4 Medication-Related Variables of Women with Breast Cancer Attending Oncology Center at TASH, Addis Ababa, Ethiopia, 2019 (N=209) |
Health Care System-related Factors
Among the respondents, 136 (65.1%) reported adjuvant hormonal therapy is not available, 151 (72.2%) have had thorough therapeutic communication from a health-care provider, 102 (48.8%) took more than seven hours to reach hospital (Table 5).
 |
Table 5 Health-care System-Related Factors of Women with Breast Cancer Attending Oncology Center at TASH, Addis Ababa, Ethiopia, 2019 (N=209) |
Prevalence of Adherence to Adjuvant Hormonal Therapy
The overall adherence to adjuvant hormonal therapy in the current study was 77.5% (95%CI; 71.3–83.3) (Figure 1).
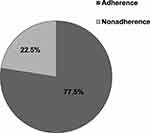 |
Figure 2 AHT adherence among women with breast cancer attending oncology center at TASH, Addis Ababa, Ethiopia, 2019 (N=209). |
Factors Associated with Adherence to Adjuvant Hormonal Therapy
A binary logistic regression model was used to identify factors that affect the outcome variable. In bivariable analysis; place of residence, educational status, getting social support, family history of breast cancer, types of breast cancer, stage of breast cancer, types of hormonal therapy, side effects, types of surgery and types of payment were fitted at P-value <0.2. But in multivariable analysis, getting support, types of hormonal therapy, side effects, thorough therapeutic communication, types of surgery were significantly associated with the outcome at P-value <0.05 with 95%CI. Being socially supported (OR=3.959, 95%CI: 1.570–9.980), Being on anastrozole (OR=0.139, 95%CI: 0.040–0.485), Getting a thorough therapeutic communication (OR=4.590, 95%CI: 1.061–19.863), undergoing mastectomy (OR=0.215, 95%CI: 0.059–0.788); having side effects (OR=0.210, 95%CI: 0.085–0.517), were found to be significantly associated in multivariate analysis (Table 6).
 |
Table 6 Bivariate and Multivariate Analysis of Factors Associated with Adherence to AHT Among Women with Breast Cancer Attending Oncology Center at TASH, Addis Ababa, Ethiopia, 2019 |
Discussion
A central component of breast cancer patients who are undergoing hormone therapy is adherence to adjuvant hormonal therapy. However, little is known about the status of women adhering to this therapy in Africa, including Ethiopia. Therefore, the purpose of this study was to assess adherence to adjuvant hormone therapy and the associated factor of TASH breast cancer among women. This study showed that the prevalence of adherence was 77.5%. The current study is in agreement with studies conducted in Brazil 76.3%,32 Sweden 79%,33 New York 76%,34 North Carolina 78%,35 another study in North Carolina 81.7%,34 and Nigeria 75%.21 The similarities may be attributed to the adherence measurement method, sample size, study nature, study population. Except in Nigeria, where their socioeconomic status and sample size may be almost similar. The current study finding is higher than studies conducted in Rome 60%,36 Ireland 67.8%,37 China 63.1%,38 Singapore 40.8%,25 and Texas 44%.39 The difference could be the population of the study, different tools for measuring adherence, sample size, and design of the study. However, our results were lower than studies in Sweden 92%40 and South Carolina 91.4%.41 Sample size, study design, different socioeconomic status, and study duration could differ. In multivariate analysis the following factors were associated with adherence to adjuvant hormonal therapy. In the current study, those who were getting social support, being on tamoxifen, getting thorough therapeutic communication about treatment, undergoing lumpectomy, lack of side effects were found to be significantly associated in multivariate analysis.
A significant variable that affected adherence was obtaining social support in the current study. Those getting social support from others were nearly four times more committed than those who did not. This finding is consistent with the study conducted in northern California,42 UK,43 and a study from Los Angeles, California, Houston, and Texas.44 These similarities might be attributed to supportive social reinforcement, emotional support, as well as comforting them to comply with the medication and providing a better influence on adherence to the medication.
In the current study, those who had thorough therapeutic communications with the health-care provider about their treatment are 4.6 times more likely to be adherent than those who did not. This finding is supported by the studies conducted in California,45 and the UK.43 These similarities could be patient–provider interaction and effective communication about the use of medicines had a positive impact on AHT adherence. In this study, compared to those who had no side effects, of those who had encountered side effects around 79% were apt to become nonadherent. This result is consistent with the result of different studies in North America, Europe, and Australia,17 China,38 California,45 Ireland,37 New Zealand,46 and Nigeria.21 The similarities might be due to the unbearable side effects such as hot flash, nausea, mood swings and muscle or joint pain. In this study, those who were on anastrozole were 86% less likely to have adhered than those who were on tamoxifen. This finding is in agreement with studies conducted in Singapore,25 North America,47 and Canada.48 These similarities might be because those on anastrozole appear to complain of more muscle pain and joint stiffness and/or pain and a higher risk of fracture, as they have different side effect levels. In the current study, those who have to undergo lumpectomy were about 78.5% likely to be adherent compared to those who had a mastectomy. This finding is supported by the study conducted in Texas.49 The similarities may be attributed to the fact that they are less at risk of being disregarded because their entire breast has been removed. Those undergoing lumpectomy, however, are also afraid of residue during surgery, that is why they tend to be more adherent than those undergoing mastectomy.
Limitations
Even though this study involved a relatively small sample size, the establishment of a causal relationship could not be possible since it is a cross-sectional study. In addition, the study was conducted in one setting which could not represent the whole of the country.
Conclusion
In general, the overall adherence to AHT was good for women with breast cancer. Factors such as types of adjuvant hormone therapy, lack of side effects, types of surgery, getting social support, and thorough therapeutic communication were strongly linked with adherence to them. Much has to be achieved by resolving the abovementioned factors and ensuring the relevance of recommended therapies to scaling up medication adherence. Side effects of the drugs, good social support and effective communication with a patient should be given great emphasis. Adherence counseling instructions on adjuvant hormone therapy for breast cancer should also be created. In addition, they can work on promoting AHT adherence, including the distribution of leaflets, as an alert, using mobile message technology. Finally, various adherence assessment tools should be used by future researchers and different study designs should be warranted, ie longitudinal study. In addition, they need to evaluate the impact of adherence to AHT on the outcome of breast cancer.
Abbreviations
AAU, Addis Ababa University; AET, adjuvant endocrine therapy; AHT, adjuvant hormonal therapy; AIs, aromatase inhibitors; OR, odds ratio; BC, breast cancer; CI, confidence interval; COR, crude odds ratio; DC, data collector; ER+, estrogen receptor positive; ETB, Ethiopian Birr; HCP, health care provider; HR+, hormone receptor-positive; PgR+, progesterone receptor positive; SD, standard deviation; SERMs, selective estrogen receptor modulators; SPSS, Statistical Package for the Social Sciences; TAM, tamoxifen; TASH, Tikur Anbessa Specialized Hospital.
Data Sharing Statement
Data will be available upon request from the corresponding author.
Ethics Approval and Consent to Participate
Ethical approval for this study was obtained from the Institutional Review Board of the School of Nursing and Midwifery, Addis Ababa University and approved with IRB protocol number: 012/19/SNM. The letter of permission was written from the School of Nursing and Midwifery to the cancer centre of TASH. Then, the cancer centre chief administrator allowed collection of the data from breast cancer patients. Written informed consent was obtained from individual patients. In the data collection questionnaire, the names of the patients, as well as their medical record numbers, were not included for the sake of their privacy. All the processes of the research were performed and secured as per the Declaration of Helsinki.
Acknowledgments
Our gratitude goes to the School of Nursing and Midwifery, College of Health Sciences, Addis Ababa University for giving us the chance to conduct this research. The authors duly acknowledge the University of Gondar for allowing us to attend the Masters of Clinical Oncology Nursing Program in addition to a paid study leave. The authors’ thank also goes to Tikur Anbessa Specialized Hospital Managers and all cancer center staff.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Weiner CM, Mathewos A, Addissie A, et al. Characteristics and follow-up of metastatic breast cancer in Ethiopia: a cohort study of 573 women. Breast. 2018;42:23–30.
2. Indrojit Roy M. Breast Carcinoma in Uganda. Arch Pathol Lab Med. 2011;135.
3. Timotewos G, Solomon A, Mathewos A, et al. First data from a population based cancer registry in Ethiopia. Cancer Epidemiol. 2018;53:93–98.
4. Eber-Schulz P, Tariku W, Reibold C, et al. Survival of breast cancer patients in rural Ethiopia. Breast Cancer Res Treat. 2018;170(1):111–118.
5. Kingham TP, Alatise OI, Vanderpuye V, et al. Treatment of cancer in sub-Saharan Africa. Lancet Oncol. 2013;14(4):e158–e67.
6. Condorelli R, Vaz-Luis I. Managing side effects in adjuvant endocrine therapy for breast cancer. Expert Rev Anticancer Ther. 2018;18(11):1101–1112.
7. Burson AM, Soliman AS, Ngoma TA, et al. Clinical and epidemiologic profile of breast cancer in Tanzania. Breast Dis. 2010;31(1):33–41.
8. Wata DE, Osanjo GO, Oluka M, Guantai AN. Predictors of breast cancer treatment outcomes in Kenyan women. African J Pharmacol Ther. 2013;2(4):109–115.
9. Condorelli R, Vaz-Luis I. Managing side effects in adjuvant endocrine therapy for breast cancer. Expert Rev Anticancer Ther. 2018;18(11):1101.
10. Brett J, Boulton M, Fenlon D, et al. Adjuvant endocrine therapy after breast cancer: a qualitative study of factors associated with adherence. Patient Prefer Adherence. 2018;12:291.
11. Farias AJ, Hansen RN, Zeliadt SB, Ornelas IJ, Li CI, Thompson B. Factors associated with adherence to adjuvant endocrine therapy among privately insured and newly diagnosed breast cancer patients: a quantile regression analysis. J Managed Care Specialty Pharmacy. 2016;22(8):969–978.
12. Lambert LK, Balneaves LG, Howard AF, Gotay CC. Patient-reported factors associated with adherence to adjuvant endocrine therapy after breast cancer: an integrative review. Breast Cancer Res Treat. 2018;1–19.
13. Chlebowski RT. Adherence to oral endocrine therapy for breast cancer: a nursing perspective. Clin J Oncol Nursing. 2008.
14. Iacorossi L, Gambalunga F, Fabi A, et al. Adherence to hormone therapy in women with breast cancer: a quantitative study. Prof Inferm. 2016;69:2.
15. Chlebowski RT, Geller ML. Adherence to endocrine therapy for breast cancer. Oncology. 2006;71(1–2):1–9.
16. Lam WY, Fresco P. Medication adherence measures: an overview. Biomed Res Int. 2015;2015:217047.
17. Lambert LK, Balneaves LG, Howard AF, Gotay CC. Patient-reported factors associated with adherence to adjuvant endocrine therapy after breast cancer: an integrative review. Breast Cancer Res Treat. 2018;167(3):615–633.
18. Sawesi S, Carpenter JS, Jones J. Reasons for nonadherence to tamoxifen and aromatase inhibitors for the treatment of breast cancer: a literature review. Clin J Oncol Nurs. 2014;18(3):E50–7.
19. Touchette DR, Shapiro NL. Medication compliance, adherence, and persistence: current status of behavioral and educational interventions to improve outcomes. J Manag Care Pharm. 2008;14(6):S2–S10.
20. Wickersham K, Sereika SM, Bender CM. Pretreatment predictors of short-term nonadherence to oral hormonal therapy for women with breast cancer. Nurs Res. 2013;62(4):243.
21. Oguntola AS, Adeoti ML, Akanbi O. Non-adherence to the use of tamoxifen in the first year by the breast cancer patients in an African population. East Cent Afr j Surg. 2011;16(1). .
22. Group EBCTC. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomized trials. Lancet. 2005;365:1687–1717.
23. Farias AJ, Hansen RN, Zeliadt SB, Ornelas IJ, Li CI, Thompson B. Factors associated with adherence to adjuvant endocrine therapy among privately insured and newly diagnosed breast cancer patients: a quantile regression analysis. J Managed Care Specialty Pharmacy. 2016;22(8):969–978.
24. Huiart L, Bardou V, Giorgi R. L’adhésion thérapeutique aux traitements oraux: enjeux en oncologie-l’exemple du cancer du sein. Bull Cancer. 2013;100(10):1007–1015.
25. Ali EE, Cheung KL, Lee CP, Leow JL, Yap KY-L, Chew L. Prevalence and determinants of adherence to oral adjuvant endocrine therapy among breast cancer patients in Singapore. Asia-Pacific j Oncol Nursing. 2017;4(4):283.
26. Anyanwu SN, Egwuonwu OA, Ihekwoaba EC. Acceptance and adherence to treatment among breast cancer patients in Eastern Nigeria. Breast. 2011;20:S51–S3.
27. Liu Y, Malin JL, Diamant AL, Thind A, Maly RC. Adherence to adjuvant hormone therapy in low-income women with breast cancer: the role of provider–patient communication. Breast Cancer Res Treat. 2013;137(3):829–836.
28. Murphy CC, Bartholomew LK, Carpentier MY, Bluethmann SM, Vernon SW. Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: a systematic review. Breast Cancer Res Treat. 2012;134(2):459–478.
29. Wheeler SB, Roberts MC, Bloom D, et al. Oncology providers’ perspectives on endocrine therapy prescribing and management. Patient Prefer Adherence. 2016;10:2007.
30. Brett J, Boulton M, Fenlon D, et al. Adjuvant endocrine therapy after breast cancer: a qualitative study of factors associated with adherence. Patient Prefer Adherence. 2018;12:291–300.
31. Osterberg L, Blaschke T. Adherence to medication. N Eng j Med. 2005;353(5):487–497.
32. Brito C, Portela MC, de Vasconcellos MTL. Adherence to hormone therapy among women with breast cancer. BMC Cancer. 2014;14(1):397.
33. Wulaningsih W, Garmo H, Ahlgren J, et al. Determinants of non-adherence to adjuvant endocrine treatment in women with breast cancer: the role of comorbidity. Breast Cancer Res Treat. 2018;172(1):167–177.
34. Hershman DL, Shao T, Kushi LH, et al. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat. 2011;126(2):529–537.
35. Kroenke CH, Hershman DL, Gomez SL, et al. Personal and clinical social support and adherence to adjuvant endocrine therapy among hormone receptor-positive breast cancer patients in an integrated health care system. Breast Cancer Res Treat. 2018;1–9.
36. Doggrell SA. Adherence to oral endocrine therapy in women with breast cancer: a mixed method study. Imperial j Interdisciplinary Res. 2016;2(8).
37. Quinn EM, Fleming C, O’Sullivan MJ. Endocrine therapy adherence: a cross-sectional study of factors affecting adherence and discontinuation of therapy. Ir J Med Sci. 2016;185(2):383–392.
38. Gao P, You L, Wu D, et al. Adherence to endocrine therapy among Chinese patients with breast cancer: current status and recommendations for improvement. Patient Prefer Adherence. 2018;12:887–897.
39. Philipovskiy A, Campbell A, Heydarian R, et al. Adherence to Adjuvant aromatase inhibitor therapy among postmenopausal Hispanic/Latino women with breast cancer. Anticancer Res. 2020;40(2):857–864.
40. Lundgren C, Lindman H, Rolander B, Ekholm M. Good adherence to adjuvant endocrine therapy in early breast cancer–a population-based study based on the Swedish Prescribed Drug Register. Acta Oncologica. 2018;1–6.
41. Heiney SP, Truman S, Babatunde OA, et al. Racial and geographic disparities in endocrine therapy adherence among younger breast cancer survivors. Am J Clin Oncol. 2020;43(7):504–509.
42. Kroenke CH, Hershman DL, Gomez SL, et al. Personal and clinical social support and adherence to adjuvant endocrine therapy among hormone receptor-positive breast cancer patients in an integrated health care system. Breast Cancer Res Treat. 2018;170(3):623–631.
43. Moon Z, Moss-Morris R, Hunter MS, Carlisle S, Hughes LD. Barriers and facilitators of adjuvant hormone therapy adherence and persistence in women with breast cancer: a systematic review. Patient Prefer Adherence. 2017;11:305–322.
44. Toledo G, Ochoa CY, Farias AJ. Exploring the role of social support and adjuvant endocrine therapy use among breast cancer survivors. Supportive Care Cancer. 2020;28(1):271–278.
45. Liu Y, Malin JL, Diamant AL, Thind A, Maly RC. Adherence to adjuvant hormone therapy in low-income women with breast cancer: the role of provider-patient communication. Breast Cancer Res Treat. 2013;137(3):829–836.
46. Robinson B, Dijkstra B, Davey V, Tomlinson S, Frampton C. Adherence to adjuvant endocrine therapy in Christchurch women with early breast cancer. Clin oncol. 2018;30(1):e9–e15.
47. Ganz PA, Cecchini RS, Julian TB, et al. Patient-reported outcomes with anastrozole versus tamoxifen for postmenopausal patients with ductal carcinoma in situ treated with lumpectomy plus radiotherapy (NSABP B-35): a randomised, double-blind, Phase 3 clinical trial. Lancet. 2016;387(10021):857–865.
48. Cheung WY, Lai EC, Ruan JY, Chang JT, Setoguchi S. Comparative adherence to oral hormonal agents in older women with breast cancer. Breast Cancer Res Treat. 2015;152(2):419–427.
49. Zhao H, Hei N, Wu Y, et al. Initiation of and adherence to tamoxifen and aromatase inhibitor therapy among elderly women with ductal carcinoma in situ. Cancer. 2017;123(6):940–947.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.