Back to Journals » International Journal of Women's Health » Volume 8
Adherence to a flexible extended regimen for oral hormonal contraception provided in a blister packaging compared with an adherence-supporting digital tablet dispenser: historical comparison of data from two clinical studies
Authors Elliesen J, Trummer D
Received 1 March 2016
Accepted for publication 11 May 2016
Published 9 August 2016 Volume 2016:8 Pages 351—356
DOI https://doi.org/10.2147/IJWH.S107516
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Elie Al-Chaer
Jörg Elliesen, Dietmar Trummer
Bayer Pharma AG, Berlin, Germany
Background: The Clyk™ digital pill dispenser helps ensure correct and consistent administration of a flexible extended regimen of the combined oral contraceptive, ethinylestradiol (EE) 20 µg/drospirenone 3 mg (EE/drospirenoneFlex; YAZ® Flex), guiding users through the intake cycle and 4-day pill break and providing visible and acoustic daily reminders when pill intake is due. A study showed that the audible alarm function of the dispenser could help reduce the number of missed pills, but it lacked an appropriate “non-dispenser” group for a meaningful assessment of the impact of the dispenser on adherence. This study indirectly assessed the overall effect of the digital dispenser on adherence by comparing data from a treatment with standard blister packaging.
Materials and methods: One-year adherence data were compared from two similarly designed, Phase III, open-label, randomized trials of EE/drospirenoneFlex. In study 1, women used diary cards to record adherence with EE/drospirenoneFlex dispensed in blister packs (n=640), and in study 2 the dispenser was used with the alarm activated (n=250) or deactivated (n=248) in addition to using diary cards.
Results: A mean (±SD) of 4.3 (±4.24) missed pills over 1 year were recorded in diary cards among women who dispensed their pills from the blister packages (study 1) compared with 1.0 (±2.4) recorded by the alarm-activated dispenser (study 2). In study 2, a mean of 1.9 (±4.2) missed pills were reported in the diaries over 1 year compared with 4.4 (±9.1) from automatic recording by the dispenser (both arms of study 2), indicating underreporting of missed pills in diary cards vs the digital dispenser. Adjusting for this rate of underreporting, an estimated mean of ten pills were missed over 1 year by women using EE/drospirenoneFlex in blister packs, or ten times more than with the digital dispenser with activated acoustic alarm.
Conclusion: The digital dispenser helps reduce the number of missed pills and increases adherence.
Keywords: compliance, contraception, drospirenone, ethinylestradiol, flexible extended regimen, efficacy, pill dispenser
Introduction
Extended regimens of combined oral contraceptives (COCs) may be the preferred birth control option for women who prefer fewer menstruations, as well as for non-contraceptive medical reasons such as dysmenorrhea, endometriosis, abnormal uterine bleeding, hemorrhagic diathesis, and menstrual migraine.1,2 Flexible extended regimens of COCs are designed to enable extended pill intake and to reduce the number of menstrual periods per year or schedule the menstrual bleeding (withdrawal bleeding) according to the needs of the woman, while managing breakthrough bleeding associated with extended regimens.3,4 Clinical studies provide some evidence that extended regimens could be associated with better contraceptive efficacy than standard cyclic oral contraceptive regimens.3,5 Fewer hormone-free intervals reduce the risk of escape ovulation because of missed pills in the days before or after the hormonal withdrawal. The CORALIANCE study showed that, among women taking a pill involving a treatment-free interval, the rate of missing a pill was high (42%) in the first week after resuming therapy. In fact, 18% missed taking the first pill and 24% missed a pill during the first week of resuming therapy after a treatment-free interval.6 Assuming that contraceptive failure is mostly associated with low intake adherence (ie, missed pills), the adherence pattern and possible differences resulting from packaging with different levels of adherence support are worth investigating.
A flexible extended regimen of the oral hormonal contraceptive, ethinylestradiol (EE) 20 μg/drospirenone 3 mg (EE/drospirenoneFlex; YAZ® Flex), with cycle lengths that may vary between 28 days and 124 days, including a 4-day pill break, has been approved in the European Union and other countries. Comparative studies have demonstrated that EE/drospirenoneFlex is well tolerated and has good contraceptive efficacy and significantly fewer bleeding days than conventional 28-day or fixed long-cycle 124-day COC regimens.7–10
Poor compliance is a common issue among COC users,6,11,12 and it is associated with unintended pregnancy;13 a large European study demonstrated that women who missed one or more pills a month were 2.6 times more likely to have an unintended pregnancy than those who did not miss any pills.14 The digital dispenser, Clyk™, was developed for use with EE/drospirenoneFlex to help ensure correct and consistent pill administration within a flexible regimen. It guides users through the intake cycle and the 4-day pill break, providing visible and acoustic daily reminders when pill intake is due. In addition, the dispenser provides guidance on what to do if pills are missed. If too many pills have been missed, the dispenser shows a warning that contraceptive protection may be jeopardized and provides instructions on what to do to regain full contraceptive protection. It should be noted that the dispenser is battery powered with a 2-year shelf life and does not require recharging; therefore, potential loss of functionality is not a concern. The effect of the digital dispenser with the acoustic alarm function either activated or deactivated on adherence with EE/drospirenoneFlex was assessed in a 12-month open-label, randomized, European study (France, Germany, Italy, Spain, and the UK) involving 499 women.10 The acoustic alarm of the digital dispenser substantially reduced the number of missed pills.10,15 The study did not include a “non-dispenser” comparator group to allow for an assessment of the overall effect of the dispenser on adherence. However, in addition to the automatic recording of adherence data by the digital dispenser, the study used a standard method of a paper diary to collect patient-reported adherence data in order to allow historic comparisons. A previous clinical study of EE/drospirenoneFlex using standard blister packaging included a treatment group that followed the same flexible extended regimen as described earlier,10 but in this study the women used paper diary cards to track pill intake.8 As such, these two studies provide an opportunity to assess indirectly the overall effect of the digital dispenser on adherence.
The objective of this study was to assess the impact of the digital dispenser on adherence by comparing missed pill data from the dispenser alarm-activated and alarm-deactivated groups from the digital dispenser study with historical controls who received EE/drospirenoneFlex in blister packs and tracked intake using diary cards in an earlier study. If missed pills are the main reason for contraceptive failure, any reduction in the number of missed pills may help to improve contraceptive efficacy and avoid unintended pregnancies.
Materials and methods
Study design
Design and methodological details of both studies have been published previously,8,10 but are outlined briefly in this article. The studies were Phase III, open-label, randomized, parallel-group multicenter trials. Study 18 was conducted between December 2005 and October 2008 (ClinicalTrials.gov identifier: NCT00266032), and study 210 was conducted between December 2010 and September 2012 (ClinicalTrials.gov identifier: NCT01257984). Study 1 consisted of two phases: a 1-year randomized comparative phase8 and a 1-year safety extension phase.7 Only data from patients in the EE/drospirenoneFlex treatment group during the 1-year comparative phase are reported here.
Study population
Both studies enrolled women in general good health, aged 18–35 years (or up to 30 years if smokers) who requested contraception. A normal cervical smear test result at screening or within 6 months prior was also required. Women who were pregnant or lactating, continued use of other contraceptive methods, or had been sterilized were excluded, as were those with a body mass index ≥30 kg/m2, a known hypersensitivity to the study drug ingredients, or any disease or condition that could interfere with the study medication or with the conduct of the trial. All women provided written informed consent, and the studies were conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines.
Treatment
All participants received EE/drospirenoneFlex continuously for ≥24 days (mandatory phase), followed by pill intake for up to a maximum of 120 consecutive days, then a 4-day pill-free break (Figure 1). Between days 25 and 120 (flexible phase), women could decide to take a 4-day pill-free interval at any time if desired or were advised to take a 4-day pill-free interval if they experienced three consecutive days of intracyclic bleeding. The cycles were repeated for up to 1 year, with each cycle commencing with a mandatory 24-day intake phase after each 4-day pill break, regardless of any ongoing bleeding.
In study 1, medication was provided in blister packs with tablet intake documented using daily diary cards. Participants of study 2 used the Clyk™ digital dispenser to keep track of tablet intake, with the acoustic alarm either activated or deactivated (ie, with reminders given by visual symbol only). At the same time, the digital dispenser tracked the pill release so that the adherence to daily pill intake was documented automatically without any additional action by the woman. A missed pill was identified if there was an interval of 48 hours or more between two pill intakes. In addition, the subjects were asked to document the pill intake in a paper diary. This method of documentation was used to allow a comparison of the adherence data with data from a previous study (study 1), where subjects were using the flexible extended regimen in a standard blister packaging. The women were provided with instructions for use of the digital dispenser, and women in the activated alarm group were allowed to turn off the acoustic alarm if desired.
Follow-up and outcomes
Follow-up visits were conducted within 4 weeks of study medication initiation, at 3-monthly intervals thereafter, and 18–25 days after study completion or at discontinuation. Routine pregnancy tests were performed at all study visits, and the use of backup contraceptive measures was recorded. Primary and secondary outcomes for both studies were detailed in the original publications and have been fully reported.7,8,10
Statistical analysis
The analysis for this secondary comparative study was descriptive with no hypothesis testing performed. Descriptive statistics (number, mean, and standard deviation [SD]) were calculated for each quantitative variable. A correction for underreporting of missed pills was made using the observed difference between patient-reported data and dispenser-recorded data in study 2.
Results
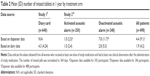
Baseline characteristics of the participants were similar between the two studies (Table 1). Table 2 summarizes the mean (±SD) number of missed pills over 1 year by the study and treatment groups. A mean of 4.3 (±4.24) missed pills was recorded in diary cards among women in study 1 who dispensed their pills from the blister packages compared with 1.0 (±2.4) recorded by the dispenser in the alarm-activated group of study 2. Among all participants in study 2, the mean (±SD) number of missed pills over 1 year recorded in diary cards was 1.9 (±4.2) compared with 4.4 (±9.1) recorded by the digital dispenser. Among women in study 2 who used the digital dispenser with the alarm deactivated, the mean number of missed pills recorded in diary cards was 2.8 (±5.3) compared with 7.8 (±11.7) recorded by the digital dispenser. It can therefore be concluded that the amount of underreporting is ~60%, ie, only ~40% of missed pills are reported by the patient in a diary-based compliance documentation (1.9/4.4=0.43). In the study arm with deactivated acoustic alarm, which showed higher frequencies of missed pills, only 36% of missed pills were reported (2.8/7.8=0.36).
Overall, adjusting for underreporting, an estimated actual mean of ten pills (ie, 4.3/0.43=10.0) were missed over 1 year by women using EE/drospirenoneFlex dispensed in blister packs (study 1). The actual mean number of missed pills estimated was based on the reported mean number of 4.3 missed pills recorded in diary cards in study 1 multiplied by the underreporting factor observed between the number of missed pills recorded by the women in diary cards (1.9) relative to that recorded by the dispenser (4.4).
Discussion
The main reason for contraceptive failure is irregular pill intake with frequent missed pills.16 Total compliance is difficult to achieve, with up to 81% of women reporting missing at least one pill per cycle, and up to 51% missing three or more pills per cycle.17 Moreover, missed pills are a source of concern; in a cross-sectional study of 17,091 pill users, 71% of pill users reported noncompliant behavior and 43.9% of the women admitted to being worried because of delayed or missed pills.11 In particular, women are unsure of what to do if they miss more than two pills;18,19 thus, a device that advises when extra protection is needed should prove valuable to pill users.
It is reasonable to assume that missed pills may be associated with increased risk of unintended pregnancy. Among the 640 historic controls from study 1, there were a total of four pregnancies during the first year of treatment (Bayer Pharma AG, data on file). Assuming that all of these pregnancies resulted from missed pills, the number of pregnancies can be expressed in relation to the number of missed pills. With 2,752 reported and 6,373 estimated (ie, 2,752×4.4/1.9) missed pills per year, the average pregnancy rate was one pregnancy per 1,593 missed pills using the estimated true rate of missed pills (ie, 6,373/4). With the use of the dispenser with the acoustic alarm activated, 3.3 missed pills per woman-year could be prevented on average, or one pregnancy in every 483 women-year of exposure.
Among the 242 women with adherence data from study 2 who received EE/drospirenoneFlex using the digital dispenser with an activated alarm with a mean of one missed pill per year of treatment (ie, a total of 242 missed pills), no contraceptive failure was reported. In addition, no failure was reported from the 246 women with adherence data from the other treatment arm using the digital dispenser with deactivated acoustic alarm with a mean of 7.8 missed pills per year (ie, a total of 1,919 missed pills). The missed-pill guidance and the warning function of the dispenser in case of significant adherence failure may have helped women with frequent missed pills to avoid an unintended pregnancy.
A recently updated Cochrane review demonstrated that compliance was similar between extended or continuous cycles of COC and various 28-day regimens, although the authors noted that measurement of compliance through participant diaries has questionable validity.5 Several studies have investigated the effects of various reminder methods on compliance in patients receiving COCs. The effectiveness of an email reminder system in improving compliance among 50 new oral contraceptive pill users has been studied.20 Subjects sent a reply email to confirm receipt of the reminder, and compliance was recorded in a diary. A median of 18% (range, 0%−65%) of email reminders were missed, and diary data showed that 20% of 40 evaluable participants missed at least one pill in each cycle.
The use of a reminder card, a credit-card-sized device that emits an audible beep daily for 21 days of a 28-day cycle at a time selected and programmed by the user, was associated with a significant improvement in adherence to oral contraceptives.21 A total of 41% of 161 reminder card users reported no missed pills during the 3-month study period compared with 19% of 264 controls. In addition, similarly to our study, comparison of an electronic monitoring device (which recorded each opening of the device) with self-reported compliance revealed underreporting of missed pills.17 Among 103 women taking oral contraceptives for the first time or resuming after at least 6 months discontinuation, diary data reported an average of 1.0-1.1 missed pills per cycle whereas electronic data reported an average of 2.6 missed pills per cycle. The number of missed pills recorded electronically increased with each cycle from 2.2-2.3 in cycles 1 and 2 to 3.5 in cycle 3.17
This trend of increasing missed pills with each cycle was also noted in a single-blind, randomized study that examined the effect of daily text message reminders on adherence to oral contraceptives as measured by electronic monitoring device.22 Over three cycles, the number of missed pills was similar between women receiving text reminders and those who did not (4.9 vs 4.6), and comparison of electronic adherence data with daily diaries showed underreporting of missed pills among the entire cohort (4.7 vs 1.2 missed pills per cycle), as well as in both the text-message (4.9 vs 1.9) and control groups (4.6 vs 1.1).22
Since both studies were conducted in a similar patient population (healthy, fertile women in Europe who are in need of contraception) with a similar methodology for adherence monitoring (paper diaries), we felt that the benefits of this historical comparison outweigh the theoretical limitations. However, it would be ideal if these results could be confirmed in a prospective study.
The use of a correction for underreporting of missed pills may also be seen as a limitation of the study, but we believe that this approach allows for a more accurate comparison of data across studies. Data retrieved from dispenser devices is more reliable than data retrieved from paper diaries (as the potential for human error is reduced), and the number of missed pills recorded by dispenser devices tends to be higher than that recorded using paper diaries. The use of a correction factor offsets this underreporting and allows the two data sets to be directly compared, giving a more realistic perspective on the issue of nonadherence.
Conclusion
This study investigates the impact of different COC presentations (digital dispenser vs standard blister pack) on adherence and compares the methods used to monitor adherence for each presentation. These results show that, compared with standard blister packs, a digital dispenser (Clyk™) reduces the number of missed pills, which may help reduce contraceptive failure rate in practice.
Acknowledgments
This study was supported by Bayer Pharma AG. Andrea Bothwell provided medical writing support on behalf of inScience Communications, Springer Healthcare; this assistance was funded by Bayer Pharma AG.
Disclosure
Jörg Elliesen and Dietmar Trummer are employees of Bayer Pharma AG. The authors report no other conflicts of interest in this work.
References
Association of Reproductive Health Professionals [webpage on the Internet]. What You Need to Know: Menstrual Suppression. 2008. Available form: http://www.arhp.org/uploadDocs/menstruationfactsheet.pdf. Accessed October 27, 2015. | ||
Guilbert E, Boroditsky R, Black A, et al; Society of Obstetricians and Gynaecologists of Canada. Canadian consensus guideline on continuous and extended hormonal contraception, 2007. J Obstet Gynaecol Can. 2007;29(7 suppl 2):S1–S32. | ||
Anderson FD, Hait H. A multicenter, randomized study of an extended cycle oral contraceptive. Contraception. 2003;68(2):89–96. | ||
Sulak PJ, Kuehl TJ, Coffee A, Willis S. Prospective analysis of occurrence and management of breakthrough bleeding during an extended oral contraceptive regimen. Am J Obstet Gynecol. 2006;195(4):935–941. | ||
Edelman A, Micks E, Gallo MF, Jensen JT, Grimes DA. Continuous or extended cycle vs. cyclic use of combined hormonal contraceptives for contraception. Cochrane Database Syst Rev. 2014;7:CD004695. | ||
Aubeny E, Buhler M, Colau JC, Vicaut E, Zadikian M, Childs M. Oral contraception: patterns of non-compliance. The Coraliance study. Eur J Contracept Reprod Health Care. 2002;7(3):155–161. | ||
Klipping C, Duijkers I, Fortier MP, Marr J, Trummer D, Elliesen J. Long-term tolerability of ethinylestradiol 20 μg/drospirenone 3 mg in a flexible extended regimen: results from a randomised, controlled, multicentre study. J Fam Plann Reprod Health Care. 2012;38(2):84–93. | ||
Klipping C, Duijkers I, Fortier MP, Marr J, Trummer D, Elliesen J. Contraceptive efficacy and tolerability of ethinylestradiol 20 μg/drospirenone 3 mg in a flexible extended regimen: an open-label, multicentre, randomised, controlled study. J Fam Plann Reprod Health Care. 2012;38(2):73–83. | ||
Strowitzki T, Kirsch B, Elliesen J. Efficacy of ethinylestradiol 20 μg/drospirenone 3 mg in a flexible extended regimen in women with moderate-to-severe primary dysmenorrhoea: an open-label, multicentre, randomised, controlled study. J Fam Plann Reprod Health Care. 2012;38(2):94–101. | ||
Wiegratz I, Elliesen J, Paoletti AM, Walzer A, Kirsch B. Adherence with ethinylestradiol 20 μg/drospirenone 3 mg in a flexible extended regimen supported by the use of a digital tablet dispenser with or without acoustic alarm: an open-label, randomized, multicenter study. Int J Womens Health. 2015;7:19–29. | ||
Lete I, Doval JL, Perez-Campos E, et al. Self-described impact of noncompliance among users of a combined hormonal contraceptive method. Contraception. 2008;77(4):276–282. | ||
Martinez-Astorquiza-Ortiz de Zarate T, Diaz-Martin T, Martinez-Astorquiza-Corral T; MIA Study Investigators. Evaluation of factors associated with noncompliance in users of combined hormonal contraceptive methods: a cross-sectional study: results from the MIA study. BMC Womens Health. 2013;13:38. | ||
Singh S, Sedgh G, Hussain R. Unintended pregnancy: worldwide levels, trends, and outcomes. Stud Fam Plann. 2010;41(4):241–250. | ||
Rosenberg MJ, Waugh MS, Meehan TE. Use and misuse of oral contraceptives: risk indicators for poor pill taking and discontinuation. Contraception. 1995;51(5):283–288. | ||
Elliesen J, Kirsch B, Paoletti A, Walzer A, Wiegratz I. Open-label, randomized, multicentre study assessing compliance with ethinylestradiol 20 μg/drospirenone 3 mg in a flexible extended regimen supported by a tablet dispenser. Proceedings of the 10th European Society of Gynaecology Congress. Brussels, Belgium; 2013. | ||
Dinger J, Minh TD, Buttmann N, Bardenheuer K. Effectiveness of oral contraceptive pills in a large U.S. cohort comparing progestogen and regimen. Obstet Gynecol. 2011;117(1):33–40. | ||
Potter L, Oakley D, de Leon-Wong E, Canamar R. Measuring compliance among oral contraceptive users. Fam Plann Perspect. 1996;28(4):154–158. | ||
Chin-Quee D, Wong E, Cuthbertson C. Evaluating information on oral contraceptive use: a randomized controlled trial to assess missed pill instructions. Hum Reprod. 2006;21(12):3137–3145. | ||
Zapata LB, Steenland MW, Brahmi D, Marchbanks PA, Curtis KM. Patient understanding of oral contraceptive pill instructions related to missed pills: a systematic review. Contraception. 2013;87(5):674–684. | ||
Fox MC, Creinin MD, Murthy AS, Harwood B, Reid LM. Feasibility study of the use of a daily electronic mail reminder to improve oral contraceptive compliance. Contraception. 2003;68(5):365–371. | ||
Lachowsky M, Levy-Toledano R. Improving compliance in oral contraception: ‘the reminder card’. Eur J Contracept Reprod Health Care. 2002;7(4):210–215. | ||
Hou MY, Hurwitz S, Kavanagh E, Fortin J, Goldberg AB. Using daily text-message reminders to improve adherence with oral contraceptives: a randomized controlled trial. Obstet Gynecol. 2010;116(3):633–640. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.