Back to Journals » International Medical Case Reports Journal » Volume 14
Adenocarcinoma in a Recurrent Retrorectal Cyst: A Case Report
Authors Ujaimi R
Received 14 December 2020
Accepted for publication 11 March 2021
Published 7 April 2021 Volume 2021:14 Pages 223—228
DOI https://doi.org/10.2147/IMCRJ.S294090
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ronald Prineas
Reem Ujaimi
Department of Radiology, Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia
Correspondence: Reem Ujaimi
King Abdulaziz University, Jeddah, Saudi Arabia
Tel +966 555608014
Email [email protected]
Abstract: I present a case of an adenocarcinoma in a retrorectal cyst in a 63-year-old female with a prior history of a congenital cyst excised as a newborn. The patient had a resection with positive margins, followed by chemotherapy and radiotherapy at progression. Her disease did not respond to chemotherapy or radiotherapy and she died with systemic manifestations related to her disease 28 months after diagnosis.
Keywords: adenocarcinoma, recurrent, retrorectal cyst, tailgut cyst
Background
Retrorectal cysts, also known as tailgut cysts or retrorectal cystic hamartomas, are developmental cysts that arise from the residue of the tailgut of the intestine during the embryonic period,1,2 and are located in the retrorectal space.3 The retrorectal space is found behind the rectum, in front of the sacrum, with the ureters, iliac blood vessels, sacral nerves, and lateral stalks of the rectum serving as its lateral walls, which makes both the diagnosis and surgical approaches used to address these lesions difficult.4 These tumors are extremely rare, with hospital admissions data estimating their incidence at 1 in 40,000 to 60,000 admissions.5 They have a female predominance, as they are five times more common in females than in males.6,7 These cysts present at any age, but are more common between the ages of 30 and 60 years.6 They are usually asymptomatic and might be identified as an incidental finding on examination, but they may present as a mass in the perianal area,8 and may be associated with changes in bowel movements or perineal pain.9 Affected patients may also present with varied symptoms that can mimic other proctological disorders.10 In the literature, cases presenting with lower back pain have been reported.11 Tailgut cysts are usually benign, but can undergo malignant transformation.12 Seven percent (7%) of those cysts undergo malignant transformation, with adenocarcinoma being one of the most common histological variants.12,13
Diagnosis is usually performed using computed tomography (CT), magnetic resonance imaging (MRI), biochemical analysis, and histological findings following excision.1 Radiological tests are essential in differentiating between benign and malignant lesions; however, a definitive diagnosis is supported by surgical and histological findings.12 Neither fine-needle aspirate nor ultrasound-guided biopsy is recommended, as these procedures may lead to complications that include the formation of fistulae and may lead to the potential spread of malignant cells, if present.9 Elevated tumor markers, including carcinoembryonic antigen (CEA), at the time of diagnosis are useful in assessing the possibility of malignant transformation.2
Complete resection with negative margins is usually the treatment of choice.4 Complete excision usually leads to a favorable prognosis. Excision also prevents recurrent drainage.1,9,10 Where there is malignant transformation, resection and chemotherapy are usually recommended. Other supplemental therapy includes adjuvant radiotherapy.12 We present a case of a retrorectal cyst in a 63-year-old female with a prior cyst excised at birth. The deceased patient’s son provided his informed consent to write and publish this case report. Institutional ethical approval is not required for publication of case reports at our institution.
Case Presentation
This is a 63-year-old female who was referred to the radiotherapy clinic at our tertiary hospital in December 2016, for consideration of adjuvant radiotherapy for a resected retrorectal adenocarcinoma with a positive margin.
The patient provided a remote history of a congenital cyst that had been surgically removed when she was a newborn. Her past surgical history was also significant for a hysterectomy and unilateral oophorectomy, which were performed to address a fibroid and benign ovarian cyst more than 17 years prior to this presentation. Her past medical history was significant for diabetes, high blood pressure, high cholesterol and acid reflux. Her current presentation was that of a recent, progressive gluteal swelling associated with chronic lower back pain, which has progressed in the few months prior to presentation. Upon presentation, a cystic mass was felt in the gluteal area and needle aspiration was attempted. Over the following couple of weeks, the cystic fluid reaccumulated and she apparently developed multiple abscesses and fistulas.
The patient underwent surgical resection of the cystic mass through a posterior approach and recovered well from her surgery. Her back pain had completely resolved. At her first visit to our tertiary center, the only finding in her exam was that of thickening in the gluteal area scar.
Laboratory Investigations
Her initial blood work revealed normal blood counts, and normal liver and renal profiles. CEA and CA 19–9 levels were not obtained at her initial presentation. The initial fluid aspirate was negative for malignancy. The final pathology of the resected mass was positive for mucinous adenocarcinoma arising within a cystic hamartoma. A review of the slides at another private tertiary hospital revealed a mucinous adenocarcinoma; primary ovarian or cervical primaries were to be excluded.
A review of the slides was performed at our hospital. The microscopic description was that of an invasive mucinous adenocarcinoma, as evident throughout the provided tissue samples. The mucin had extravasated to the surrounding tissue and elicited a histiocytic reaction (Figure 1). The tumor was seen at the margin and reached the skin’s surface with ulceration. Features associated with teratoma, epidermoid, duplication, or tailgut cyst were not seen.
Immunohistochemistry was positive for CK7 (diffuse, strong cytoplasmic), CEA (diffuse, moderately strong, cytoplasmic), and CDX2 (focally strong, nuclear), and negative for 4CK 20, P 16, GCDFP, ER, and p63.
Radiological Investigations
At presentation, an MRI scan of the pelvis was obtained and revealed a large, multiloculated cyst in the ischiorectal fossa, with a clear fat plane between the cyst and the rectum. No other abnormality was noted on MRI. The uterus and ovaries could not be visualized on MRI. Unfortunately, we could not retrieve the first MRI image from the other hospital.
Staging CT scan of the chest, abdomen, and pelvis, as well as a postoperative MRI of the pelvis, were obtained after referral to our hospital. The CT of the chest was unremarkable for any metastatic disease. The CT of the abdomen revealed an ill-defined, solid mass at the right adnexa measuring 3.2 cm that could represent ovarian metastases or a metastatic lymph node; an inguinal lymph node measuring 1.6 cm was also noted on the left side (Figure 2). As a result, an ultrasound of the pelvis was obtained and revealed a solid mass arising from the right adnexa, but no further characterization was possible. An MRI scan of the pelvis revealed fluid collection with high signal intensity and no enhancement on postcontrast images at the presacral region, likely representing a hematoma at the surgical area. The surgical bed demonstrated diffuse, soft-tissue edema and muscular edema, primarily involving the gluteus muscle associated with enhancement. There was circumferential wall thickening involving the rectum. The right ovary was well visualized and appeared unremarkable. There was a left inguinal lymph node measuring 1.4 cm, most likely representing a metastatic lymph node.
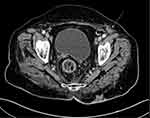 |
Figure 2 Axial cut of a computed tomography scan of the pelvis showing a left inguinal lymph node (white arrow). |
Since the question arose of whether a gynecological or gastrointestinal primary mass was present, the patient was referred to a gastroenterologist and a gynecologist. The patient underwent a colonoscopy, which did not reveal any perianal, rectal, or colonic masses. As the MRI did not reveal any abnormality in the ovary and given that the patient was examined by a gynecologist who did not find any abnormalities, a gynecological malignancy had also been ruled out.
Management and Clinical Course
The patient was discussed at the tumor board. Given the remote history of the cyst and the absence of a clear primary gastrointestinal mass, the potential diagnosis of an adenocarcinoma arising in a recurrent cyst was thought to be likely, with the main differential being an occult adenocarcinoma of the rectum. The decision was made to proceed with chemotherapy. The patient received five cycles of XELOX chemotherapy (capecitabine and oxaliplatin), starting 6 weeks after her surgery.
Once the patient completed her chemotherapy course, a repeat staging work-up was obtained. The CT of the abdomen and pelvis revealed heterogeneous soft tissue thickening of the rectum, and clinical examination and proctoscopy/biopsy to exclude recurrent disease in the rectum was recommended. A mild reduction in the size of the metastatic left inguinal lymph node was noted. The chest CT scan did not reveal any mediastinal or pulmonary metastases. The patient was again discussed at the tumor board and the recommendation was to have her assessed by a surgical oncologist, and to repeat the colonoscopy to exclude rectal or colonic disease. The repeat colonoscopy did not reveal any masses, and a few polyps were removed and were benign. The patient was seen by the surgical oncologist and thickening at the sacrum in proximity to the previous resection was again noted, as was the inguinal lymph node. Fine-needle aspiration of the inguinal lymph node was obtained and was positive for metastatic adenocarcinoma. A CT-guided biopsy of the thickened sacral area was also obtained and revealed adenocarcinoma (Figure 3).
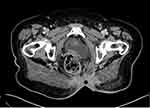 |
Figure 3 Axial cut of a computed tomography scan of the pelvis at recurrence showing thickened sacral area (Black dot). |
Four months after the completion of chemotherapy, a repeat MRI scan was done. It showed a large, speculated, intense, enhancing, soft-tissue mass at the surgical bed. It measured 9 cm×5.3 cm (Figure 4). The tumor was inseparable from the coccyx and was associated with multiple enlarged mesorectal lymph nodes measuring up to 5 mm. There was a large, pelvic lymph node on the left-side wall. Multiple foci of cystic components were also identified adjacent to the tumor. The rectum was unremarkable. In conclusion, there was significant Interval progression at the surgical bed of the tumor, in keeping with recurrence. The patient was again discussed at the tumor board, and the surgical oncologist advised that she had unresectable disease. The patient with then referred to the radiation oncology service for palliative radiotherapy, as she started complaining of pain at this time.
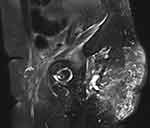 |
Figure 4 Speculated, intense, enhancing, soft-tissue mass at the surgical bed on MRI (sagittal, T1 with contrast). |
In October 2017 (10 months after diagnosis), the patient completed a long course of high-dose palliative radiation therapy. She received a dose of 45 Gy in 28 fractions to the entire pelvis and inguinal area, with concomitant 58.8 Gy delivered to the sacral mass and the enlarged inguinal lymph node (Figure 5).
 |
Figure 5 Radiotherapy dose distribution with high dose areas in green. |
In January 2018, the patient repeated the MRI scan, and no significant interval change was noted. She continued to suffer from moderate to severe pain and was referred to the pain clinic, as her pain was not responding to regular narcotics. A superior hypogastric nerve block was administered to her.
A repeat MRI in March 2018 revealed stable disease in the pelvis; however, now in the field of view of this MRI scan were new bone lesions at the level of the lumbar spine. In April 2018, a repeat CT scan of the abdomen and pelvis, as well as a CT scan of the chest revealed no distant metastatic disease apart from the bone metastasis. At this point, the patient’s clinical presentation included non-healing ulcers in the sacral area with skin nodules. Her CEA measured 21.9 and her carcinoembryonic antigen (CA)19.9 measured 4767. She was re challenged with maintenance capecitabine. Figure 6 reflects the change in her CEA level over the clinical course.
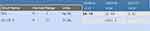 |
Figure 6 Changes in levels of CEA and CA 19–9 over the clinical course. |
The patient was last seen at our hospital in December 2018. At that time, she demonstrated major clinical progression and had a poor performance status. Her chemotherapy was stopped, as she had refused further treatment apart from pain and symptom management.
The patient was last seen in our hospital in December 2018. According to her family, she died on March 26, 2019 (28 months after her diagnosis). She had suffered from severe anemia and went into a coma secondary to a major stroke, potentially having suffered from disseminated intravascular coagulation.
Discussion
I present the case of an elderly female patient with longstanding back pain and a <1-year history of gluteal swelling that was initially aspirated. Later, the cyst was excised, but with positive margins, and adjuvant chemotherapy was provided. Unfortunately, the patient’s condition advanced and she received radiotherapy and chemotherapy, as her disease was not resectable She succumbed to death due to the systemic effects associated with her disease a few months after completing second-line chemotherapy.
The challenges of diagnosing a retrorectal cyst include its nonspecific symptoms. Although our case had a short history of gluteal swelling, her 10–15-year history of back pain was relieved following excision of the cyst. Since symptom relief usually follows surgical excision,14 it is possible that the cyst had been there prior to the gluteal swelling. Our case had a cyst that was excised at birth, but in the absence of definitive information about the congenital cyst, it is challenging to know whether this is a recurrent cyst. It is possible that our patient experienced cyst recurrence, as recurrence has been reported up to 58 years following excision.15
Conflicting information exists about the use of fine-needle aspirates to manage retrorectal cysts.4 For this reason, CT or MRI imaging is recommended for their diagnosis. Aspiration and biopsy, which are the most accurate diagnostic methods, can only be used after all differential diagnoses have been carefully considered, or when managing cysts where the preoperative finding of malignant degeneration may affect management.2 Furthermore, where aspiration has been performed and the cysts has been deemed operable, it is important to resect the biopsy tract during surgery.10 Cyst aspiration in this patient possibly led to the formation of fistulas and abscesses, and potentially resulted in the spread of malignant cells.
Although retrorectal cysts usually have a good prognosis, this is dependent on timely diagnosis and appropriate management. As our patient had multiple aspirations of what was thought to be a benign cyst and given that the excision was not complete (as based on the positive margin and questionable residual noted on images), this may or may not have affected her outcome, as she presented with lymph node metastasis soon after. Due to the rarity of her disease, it is not clear which adjuvant treatment is optimal and should be instituted; as such, multidisciplinary team management of the disease is of paramount importance. The treatment of choice for retrorectal cysts with adenocarcinomatous transformation is surgery followed often times by chemotherapy,12 which our patient received. This can also be combined with radiotherapy, if needed, to sterilize the site of origin of the tumor.16 Because of the rarity of the disease, no evidence is available to dictate the use of adjuvant chemotherapy or radiotherapy, however one modality or the combination is usually offered similar to the management of rectal cancer.17
In conclusion, tailgut cysts should be considered as a potential diagnosis for any presacral cysts that are present at any age ranging from childbirth to the 8th decade of life.18 An exhaustive workup is essential to establish the diagnosis, exclude the involvement of other pelvic organs, and initiate appropriate, individualized patient management. This requires a high index of suspicion and timely referral of patients to tertiary centers. Additionally, future research should focus on identifying feasible schedules and the investigation requirements for the follow up of asymptomatic patients following the excision of tailgut cysts.
Acknowledgments
The author acknowledges Dr Salwa Baksh, MD, FRCPC, Dr Nora Trabulsi, MD, FRCSC, and Dr Marwan Al-Hajeili, MD.
Disclosure
The author reports no conflicts of interest for this work.
References
1. Wang M, Liu G, Mu Y, He H, Wang S, Li J. Tailgut cyst with adenocarcinoma transition: a rare case report. Medicine (Baltimore). 2020;99(27):e20941. doi:10.1097/MD.0000000000020941
2. Zhao XR, Gao C, Zhang Y, Yu YH. The malignant transformation of retrorectal cystic hamartomas with blood irregular antibodies positive: a case report. Medicine (Baltimore). 2015;94(49):e2253. doi:10.1097/MD.0000000000002253
3. de Castro Gouveia G, Okada LY, Paes BP, Moura TM, da Conceição Júnior AH, Pinheiro RN. Tailgut cyst: from differential diagnosis to surgical resection-case report and literature review. J Surg Case Rep. 2020;2020(7):rjaa205. doi:10.1093/jscr/rjaa205
4. Chand P, Bhatnagar S, Kumar A, Rani N, Rare A. Presentation of lower back swelling as tailgut cyst. Niger J Surg. 2016;22(2):134–137. doi:10.4103/1117-6806.189023
5. Jao SW, Beart RW
6. Haydar M, Griepentrog K. Tailgut cyst: a case report and literature review. Int J Surg Case Rep. 2015;10:166–168. doi:10.1016/j.ijscr.2015.03.031
7. Sloan M, Fantus RJ, Paner GP, Faris S. Perineal dermoid cyst in a young male. Urol Case Rep. 2020;33:101358. doi:10.1016/j.eucr.2020.101358
8. Al-Shoura R, Malaekah H, Al Bassam W. Giant retrorectal epidermoid cyst masquerading as a perianal swelling. Case Rep Surg. 2020;2020:5750382. doi:10.1155/2020/5750382
9. Yalav O, Topal U, Eray İC, Deveci MA, Gencel E, Rencuzogullari A. Retrorectal tumor: a single-center 10-years’ experience. Ann Surg Treat Res. 2020;99(2):110–117. doi:10.4174/astr.2020.99.2.110
10. Martins P, Canotilho R, Peyroteo M, Afonso M, Moreira A, de Sousa A. Tailgut cyst adenocarcinoma. Autops Case Rep. 2019;10(1):e2019115. doi:10.4322/acr.2019.115
11. Joyce EA, Kavanagh DO, Winter DC. A rare cause of low back pain: report of a tailgut cyst. Case Rep Med. 2012;2012:623142. doi:10.1155/2012/623142
12. Liang F, Li J, Yu K, Zhang K, Liu T, Li J. Tailgut cysts with malignant transformation: features, diagnosis, and treatment. Med Sci Monit. 2020;26:e919803. doi:10.12659/MSM.919803
13. Almeida Costa NA, Rio G. Adenocarcinoma within a tailgut cyst. BMJ Case Rep. 2018;bcr2018226107. doi:10.1136/bcr-2018-226107
14. Au E, Anderson O, Morgan B, Alarcon L, George M. Tailgut cysts: report of two cases. Int J Colorectal Dis. 2008;24(3):345–350. doi:10.1007/s00384-008-0598-6
15. Kearney D, Valente M. Excision of a recurrent retrorectal tailgut cyst after 58 years. BMJ Case Rep. 2019;12(5):e230286. doi:10.1136/bcr-2019-230286
16. Chhabra S, Wise S, Maloney-Patel N, Rezac C, Poplin E. Adenocarcinoma associated with tail gut cyst. J Gastrointest Oncol. 2013;4(1):97–100. doi:10.3978/j.issn.2078-6891.2012.043
17. Kaistha S, Gangavatiker R, Harsoda R, Kinra P. A case of adenocarcinoma in a tail gut cyst and review of literature. Med J Armed Forces India. 2018;74(4):390–393. doi:10.1016/j.mjafi.2017.06.002
18. Alhasani AA, Shamaon RS. Tailgut cyst: a case report in a female neonate. MOJ Surg. 2015;2(2):44–45. doi:10.15406/mojs.2015.02.00016
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

