Back to Journals » Infection and Drug Resistance » Volume 15
Additional Usefulness of Bronchoscopy in Patients with Initial Microbiologically Negative Pulmonary Tuberculosis: A Retrospective Analysis of a Korean Nationwide Prospective Cohort Study
Authors Oh JY , Lee SS , Kim HW, Min J , Ko Y , Koo HK , Jeong YJ , Kang HH, Kang JY, Kim JS, Park JS, Kwon Y, Yang J, Han J, Jang YJ, Lee MK, Jegal Y, Kim YC, Kim YS
Received 31 December 2021
Accepted for publication 26 February 2022
Published 12 March 2022 Volume 2022:15 Pages 1029—1037
DOI https://doi.org/10.2147/IDR.S354962
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Jee Youn Oh,1 Sung-Soon Lee,2 Hyung Woo Kim,3 Jinsoo Min,4 Yousang Ko,5 Hyeon-Kyoung Koo,2 Yun-Jeong Jeong,6 Hyeon Hui Kang,7 Ji Young Kang,8 Ju Sang Kim,3 Jae Seuk Park,9 Yunhyung Kwon,10 Jiyeon Yang,10 Jiyeon Han,10 You Jin Jang,10 Min Ki Lee,11 Yangjin Jegal,12 Young-Chul Kim,13 Yun Seong Kim14
1Division of Pulmonary, Allergy, and Critical Care Medicine, Department of Internal Medicine, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Republic of Korea; 2Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Inje University Ilsan Paik Hospital, Inje University College of Medicine, Goyang, Republic of Korea; 3Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Incheon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Republic of Korea; 4Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Daejeon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Republic of Korea; 5Division of Pulmonary, Allergy and Critical Care Medicine, Department of Internal Medicine, Kangdong Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Republic of Korea; 6Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Dongguk University Ilsan Hospital, Goyang, Republic of Korea; 7Division of Pulmonary, Critical Care and Sleep Medicine, Department of Internal Medicine, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan, Republic of Korea; 8Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Republic of Korea; 9Division of Pulmonary Medicine, Department of Internal Medicine, Dankook University College of Medicine, Cheonan, Republic of Korea; 10Division of Tuberculosis Prevention and Control, Korea Disease Control and Prevention Agency, Cheongju, Republic of Korea; 11Department of Internal Medicine, Pusan National University Hospital, Pusan National University School of Medicine, Pusan, Republic of Korea; 12Division of Pulmonary, Critical Care and Sleep Medicine, Department of Internal Medicine, Ulsan University Hospital, Ulsan University College of Medicine, Ulsan, Republic of Korea; 13Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Chonnam National University Hwasun Hospital, Chonnam National University Medical School, Hwasun, Republic of Korea; 14Department of Internal Medicine, Pusan National University Yangsan Hospital, Pusan National University School of Medicine, Yangsan, Republic of Korea
Correspondence: Sung-Soon Lee, Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Inje University Ilsan Paik Hospital, Inje University College of Medicine, Goyang, Republic of Korea, Tel +82-10-7173-6575, Fax +82-2-2626-1166, Email [email protected]
Purpose: Bronchoscopy is widely used for microbiological diagnosis of patients with minimal sputum production. However, the usefulness of bronchoscopy in patient groups who benefit from subsequent microbiological confirmation has not been established.
Patients and Methods: We retrospectively analyzed Korean tuberculosis (TB) cohort data from September 2018 to October 2019 to evaluate the usefulness of bronchoscopy in patients with microbiologically negative pulmonary TB (based on initial sputum polymerase chain reaction and culture results). The primary outcome was the proportion of microbiological diagnoses made after bronchoscopy. Secondary outcomes were the predictors of microbiological confirmation and the percentage of additional resistance detection after bronchoscopy.
Results: A total of 5194 patients were diagnosed with pulmonary TB, 937 of whom were microbiologically negative for pulmonary TB based on the initial sputum findings. Of these, 319 patients underwent bronchoscopy, and further microbiological confirmation was achieved in 157 (49.1%) patients. The predictors of microbiological confirmation after bronchoscopy were age > 65 years, female sex, and low body mass index (BMI). The rate of additional resistance detection was 10.5% (multidrug resistant/rifampin-resistant 3.8%; isoniazid-resistant 5.7%).
Conclusion: Bronchoscopy can be used for the detection of resistant pathogens. Bronchoscopy should be considered for microbiologically negative pulmonary TB in women aged > 65 years and with low BMI for subsequent microbiological confirmation.
Keywords: tuberculosis, pulmonary, bronchoscopy, cohort studies
Introduction
Tuberculosis (TB) is one of the most common infectious diseases and remains a major global health problem.1–3 According to the Global Tuberculosis Report 2020, an estimated 10 million individuals were diagnosed with TB, and 1.2 million TB-related deaths were reported in 2019.1,4 The disease burden in South Korea is intermediate, with a TB incidence of 51.5 cases per 100,000 population in 2018.5 Proper diagnosis is crucial for curing the disease and preventing TB transmission, and microbiological confirmation of TB from respiratory secretions is a key factor for diagnosis.6
However, this remains a challenge as up to 70% of patients with TB are smear negative and 40% are culture-negative in South Korea.7 The diagnosis of TB is challenging for physicians when a patient shows multiple negative sputum results, even if there is a high suspicion of active disease.8,9 Therefore, the choice remains whether to proceed with empiric treatment for pulmonary TB, wait for the TB culture with possible further delay, or perform an invasive test such as bronchoscopy to confirm the diagnosis.10 This issue is on the rise because many patients with suspected TB visit clinics without any symptoms or screening chest images.11,12
Bronchoscopy has recently been widely used to aid microbiological diagnosis in patients with little sputum production.13,14 The existing literature on the diagnostic yields of bronchoscopy in the diagnosis of TB varies from 30% to 80%.15–20 However, the usefulness of bronchoscopy and the rate of subsequent microbiological confirmation have not yet been established. Moreover, it is unknown if the patient groups will benefit further from the diagnosis after bronchoscopy. To maximize the benefit of bronchoscopy while minimizing costs and complications, it is necessary to identify parameters to predict the yield of microbiological diagnosis for diagnosing sputum-negative pulmonary TB. Thus, we aimed to evaluate the effectiveness of microbiological diagnoses after bronchoscopy, predictors of microbiological confirmation, and percentage of additional resistance detection after bronchoscopy.
Patients and Methods
Retrospective Analysis of the Prospective Cohort Data
We constructed a nationwide multicenter prospective observational cohort database called the “Korean TB cohort database.” This cohort study was conducted in September 2018 to evaluate the characteristics of Korean patients with TB and to improve their management. Data were systematically collected from patients with TB who visited hospitals under the national public-private mix (PPM) TB control project and were notified of the national TB surveillance system. All notified patients with TB were followed up at regular intervals during anti-TB treatment, as recommended by the Korean TB guidelines. For this database, every patient with TB notified from the first to the tenth day each month was consecutively enrolled across the country. Data of participants during this period were collected by TB specialist nurses using the prespecified questionnaire and case report form, and were entered into Microsoft Access (Redmond, WA, USA). The regional data manager then organized the data gathered from local hospitals every month and sent them to the central data manager every quarter. To improve and maintain data quality, regional and central data managers conducted audits and identified missing and erroneous data. We retrieved and retrospectively analyzed data from the Korea TB cohort database from September 2018 to October 2019.
Study Setting and Participants
Every hospital under the PPM project in Korea participated in this study. The Republic of Korea, a country with an intermediate TB burden, has a high incidence of TB among other high-income countries. The national PPM TB control project was initiated in 2009 and expanded nationwide in 2011.7 There are more than 210 TB specialist nurses at 127 PPM hospitals and 236 public health officials at 254 public health centers across the country. Approximately 70.7% of the newly notified patients with TB in Korea were treated at PPM hospitals in 2018. The main inclusion criterion for this study was the presence of pulmonary TB. TB patients were diagnosed based on clinical, radiological, microbiological, and pathological data, as judged by physicians based on the Korean TB guidelines, as follows: 1) if a patient has positive polymerase chain reaction (PCR) results, TB is considered, and 2) if a patient has negative PCR results, and pulmonary TB is clinically and radiologically suspected but the patient does not respond to antibiotics, smear-negative pulmonary TB is considered. After clinical treatment of pulmonary TB and confirmation of culture positivity, we can diagnose the patient with culture-positive pulmonary TB. After a trial of TB medication and clinical and radiological improvement but negative TB culture results, we can make a final diagnosis of culture-negative pulmonary TB. We can additionally perform chest computed tomography, bronchoscopy, and biopsy, and if the results of these tests show active TB, smear-negative pulmonary TB can be diagnosed. Drug-resistant TB was defined as pulmonary TB with resistance to anti-TB medications. Multidrug-resistant TB is caused by an organism that is resistant to at least isoniazid and rifampin, the two most potent TB drugs. Isoniazid-resistant TB refers to resistance to isoniazid and susceptibility to rifampin. Rifampin-resistant TB refers to resistance to rifampin and susceptibility to isoniazid.
Patients with TB and extrapulmonary involvement were excluded from the study. We also excluded patients without initial sputum, those with positive initial sputum PCR or TB culture, and those who did not undergo bronchoscopy for outcome analysis.
Independent Variables
Demographic and clinical data were collected based on in-depth interviews with TB specialist nurses at PPM-participating hospitals. Baseline characteristics such as age, sex, smoking history and amount, body mass index (BMI), comorbidities, initial presenting symptoms, prior TB history, sites of TB involvement, and laboratory findings were collected. The sputum study date and results, bronchoscopic washing date, results of bronchoaspiration, initial symptoms, and chest imaging were also collected. For the sputum study, expectorated sputum was collected, and bronchoscopic washing was performed on the bronchoscopic specimens. Data were coded as bivariate variables, except for microbiological test results, which were coded as categorical variables.
Primary and Secondary Outcomes
We aimed to evaluate the usefulness of bronchoscopy in patients with microbiologically negative pulmonary TB (based on initial sputum PCR and culture results). The primary outcome was the proportion of microbiological diagnoses made after bronchoscopy. Secondary outcomes were the predictors of microbiological confirmation and the percentage of additional resistance detection after bronchoscopy.
Statistical Analysis
Clinical data are presented as the mean and standard deviation and were compared using the t-test for continuous variables. For categorical variables, data are presented as percentages and numbers and were compared using Pearson’s χ2 test or Fisher’s exact test. For the predictors, multivariate logistic regression analysis was performed by adjusting for significant factors (P < 0.05) in the univariate model. Statistical significance was defined as p < 0.05. All statistical analyses were performed using SPSS Statistics for Windows (version 20.0; IBM Corp., Armonk, NY, USA).
Results
Proportion of Microbiological Diagnoses After Bronchoscopy in Patients with Microbiologically Negative Pulmonary TB
A total of 5194 patients were diagnosed with pulmonary TB. Among them, we excluded patients without initial sputum acid-fast bacilli, TB PCR, and TB culture (n=1054), and those with missing sputum or bronchoscopic washing results (n=68). A total of 3135 (77.0%) patients were microbiologically positive and 937 (23.0%) were microbiologically negative based on the initial sputum findings. Of the microbiologically negative patients, 319 (34.0%) underwent bronchoscopy, and further microbiological confirmation was achieved in 157 (49.1%) patients (Figure 1). A total of 105 patients had additional TB culture-positive results, with the results of 52 of them confirmed only by PCR.
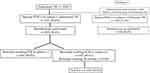 |
Figure 1 Flowchart of the analysis. *Initial sputum study: smear, PCR, and culture were performed. Abbreviations: PCR, polymerase chain reaction; TB, tuberculosis. |
Characteristics of Microbiologically Negative Pulmonary TB According to Microbiological Confirmation After Bronchoscopy
When we compared the baseline characteristics of patients with or without additional microbiological confirmation after bronchoscopy (Table 1), those with additional microbiological confirmation (n=157) were older, leaner, and had a lower percentage of male patients than those without additional microbiological confirmation (n=162). Mean age was 56.9±20.8 years and 56.1±17.3 years in patients with and without microbiological confirmation, respectively. Smoking status, past TB history, comorbidities, initial symptoms, and chest radiographic results did not differ significantly between the two groups.
Predictors of Microbiological Confirmation After Bronchoscopy
The predictors of microbiological confirmation after bronchoscopy were age >65 years (odds ratio [OR] 1.94, 95% confidence interval [CI], 1.232–3.077; p=0.004), female sex (male sex OR, 0.614, 95% CI, 0.387–0.974; p=0.038), and low BMI (BMI <18.5; OR, 3.237; 95% CI, 1.720–6.292; p<0.001). Smoking history, past TB history, comorbidities, initial symptoms, and chest radiographic results did not affect the results of bronchoscopic washing. On multivariate analysis after adjusting for age, sex, BMI, symptoms, and the presence of cavitation on chest radiographs, age (age ≥65 years, OR 1.751, 95% CI 1.093–2.816; p=0.020), sex (male OR 0.609, 95% CI, 0.378–0.982), and BMI (BMI <18.5, OR 3.007, 95% CI 1.534–5.894) were still significant predictors of microbiological positivity after bronchoscopy (Table 2).
Percentage of Additional Resistance Detection After Bronchoscopy
The rate of additional resistance detection among additional culture-positive patients after bronchoscopy (n=105) was 10.5% (multidrug-resistant/rifampin-resistant 3.8%; isoniazid-resistant 5.7%) (Table 3).
 |
Table 3 Additional Resistance Detection After Bronchoscopy |
Discussion
In our study, bronchoscopy proved to be an effective tool for the diagnosis of sputum-negative pulmonary TB, achieving microbiological confirmation in 49.1% of the patients, with 10.5% of patients being found to have resistance to anti-TB medication. We also found that the benefit was maximized, particularly for those aged >65 years, female patients, and those with low BMI.
There is a need to identify the optimal methods for diagnosing pulmonary TB in bacteriologically negative patients.21 Kwak et al reported that in more than half of the patients with smear-negative pulmonary TB, the diagnosis was delayed, which led to a poor prognosis.22 Further mycobacterial culture could provide additional information on drug sensitivity for a more accurate treatment of drug-resistant TB.23
Previous studies have shown that bronchial aspirates increase the culture rate of patients with suspected TB with negative sputum smear results.20,24,25 However, the yields of bronchoscopic washing samples varied as reported previously.16 Park et al reported that 68% of paucibacillary pulmonary TB showed positive results on mycobacterial culture after bronchoscopy.19 Schoch et al reported that bronchoscopy, performed in 87 of 92 sputum smear-negative cases, yielded four additional smear-positive and six culture-positive cases.20 Ahmad et al reported that bronchoalveolar lavage detected 61/190 (32.1%) pulmonary TB cases in a subset of patients with negative sputum results, and suspected pulmonary TB.21 The difference in the additive yields of bronchoscopy might depend on the characteristics of the patients and/or TB.
Considering the different yields of bronchoscopic washing results according to patient characteristics and the potential adverse effects of bronchoscopy,26 there should be evidence of patient groups who would benefit from bronchoscopy. Our study has several strengths that differ from those of previous studies that dealt with the indicators for the prediction of TB positivity detected using bronchoscopy. First, our study included patients within the prevalent age range of TB patients compared with previous studies that included younger patients. A previous study showed that bronchoscopy was useful in obtaining culture confirmation of pulmonary TB in approximately 10% of patients with negative results.27 However, these patients were predominantly young men (median age, 37 years). In another previous single-center study investigating factors associated with microbiological positivity after bronchoscopy, patients were also too young (mean age, 37.4 years) to represent the common diagnosis of pulmonary TB.28
Second, this was a multicenter prospective study, and various variables, including BMI, other epidemiologic factors, and drug sensitivity results, were collected from a nationwide quality-controlled database. A previous study conducted in this regard was a single-center study and did not evaluate other factors such as BMI or further drug sensitivity results.29 However, we observed additional roles of BMI, age, and sex as predictive factors. Patient factors other than TB (symptoms or imaging findings) were more affected in our study. Chest imaging findings with cavity or bilateral infiltration did not affect the results. This might be because such severe cases with a subsequent high bacterial burden could be ruled out in the current study because patients with this condition tested positive for mycobacteria in sputum specimens. Older lean female patients benefit after bronchoscopy because they are more fragile and may not be able to expectorate well.29 Furthermore, the drug sensitivity test (with approximately 10% of patients with additional microbiological confirmation observed to have resistance) enabled suitable treatment and prevention of treatment failure in these patients.
Our study had some limitations. Although the cohort design was prospective, we retrospectively analyzed yearly data. Therefore, a selection bias could not be ruled out. This national cohort included only patients with TB; therefore, those who were excluded from the diagnosis of TB by bronchoscopy were excluded from this cohort. One benefit of performing bronchoscopy is the differential diagnosis of pulmonary TB from non-TB mycobacterial diseases or lung malignancies. As patients classified as having other diseases were excluded, the important role of bronchoscopy in ruling out other important pulmonary diseases might have been underestimated. Nevertheless, we found 50% more microbiological confirmation of TB diagnosis in our study. Another limitation is that the decision to perform bronchoscopy was not randomly distributed. Patients who underwent bronchoscopy might have been the ones who physicians judged to have an additive yield on bronchoscopy. However, we overcame this limitation by multivariate adjustment, including age, sex, BMI, symptoms, and presence of cavitation on chest radiographs, which are the main characteristics that physicians focus on for the yield of sputum studies. Moreover, bronchoscopy yields differ depending on the technical expertise of the operators performing the procedures. However, this is the same in the real world, and more than 200 different pulmonary specialists from 127 PPM hospitals performed bronchoscopy; therefore, some physician technical errors might not have influenced the results in comparison with the study performed at a single-center hospital.28
Although this is the first retrospective analysis of a prospective multicenter cohort database that dealt with the predictors of microbiological yield after bronchoscopy for TB diagnosis, further prospective randomized studies including those across countries are needed to confirm our findings.
Conclusion
Bronchoscopy can be used for the detection of resistant pathogens. Bronchoscopy should be considered for microbiologically negative pulmonary TB in women aged >65 years and with low BMI for subsequent microbiological confirmation in countries with intermediate TB burden.
Abbreviations
BMI, body mass index; PCR, polymerase chain reaction; PPM, public-private mix; TB, tuberculosis.
Acknowledgments
The abstract of this paper was presented at the Korean Academy of Tuberculosis and Respiratory Diseases International Conference 2020 as a poster presentation with interim findings. The poster’s abstract was published in “Poster Abstracts” in the Korean Academy of Tuberculosis and Respiratory Diseases. The Korea Disease Control and Prevention Agency has the authority to hold and analyze surveillance data for public health and research purposes. The Korea Disease Control and Prevention Agency approved the use of the data and provided data without personal identification information. The study was conducted in accordance with the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of Inje University Ilsan Paik Hospital No. The ISPAIK 2021–08–012 waived the need for informed consent because no patients were at risk. The Korea Centers for Disease Control and Prevention (KCDC) has the authority to hold and analyze surveillance data for public health and research purposes.
Funding
This study was supported by the National Health Promotion Fund funded by the Korea Disease Control and Prevention Agency, Republic of Korea. This work was supported by a Korea University Guro Hospital grant (No. O1905151).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Harding E. WHO global progress report on tuberculosis elimination. Lancet Respir Med. 2020;8(1):19. doi:10.1016/S2213-2600(19)30418-7
2. Menzies NA, Bellerose M, Testa C, et al. Impact of effective global tuberculosis control on health and economic outcomes in the United States. Am J Respir Crit Care Med. 2020;202(11):1567–1575. doi:10.1164/rccm.202003-0526OC
3. Koegelenberg CFN, Schoch OD, Lange C. Tuberculosis: the past, the present and the future. Respiration. 2021;100(7):553–556. doi:10.1159/000516509
4. He Y, Wu YH, Han C, Gong HZ, Wang MS. Bronchial brushing Xpert improves the diagnostic efficiency of sputum Xpert in patients with pulmonary tuberculosis. Ther Adv Infect Dis. 2021;8:20499361211020174. doi:10.1177/20499361211020174
5. Son E, Jeon D. Current situation of tuberculosis and National Strategic Plan for tuberculosis Control in Korea. J Korean Med Assoc. 2021;64(4):316–323. doi:10.5124/jkma.2021.64.4.316
6. Datta S, Evans CA. The uncertainty of tuberculosis diagnosis. Lancet Infect Dis. 2020;20(9):1002–1004. doi:10.1016/S1473-3099(20)30400-X
7. Min J, Kim HW, Ko Y, et al. Tuberculosis surveillance and monitoring under the national public-private mix tuberculosis control project in South Korea 2016–2017. Tuberc Respir Dis. 2020;83(3):218–227. doi:10.4046/trd.2020.0016
8. Soto A, Solari L, Agapito J, et al. Development of a clinical scoring system for the diagnosis of smear-negative pulmonary tuberculosis. Braz J Infect Dis. 2008;12(2):128–132. doi:10.1590/S1413-86702008000200006
9. Soto A, Solari L, Gotuzzo E, Acinelli R, Vargas D, Van der Stuyft P. Performance of an algorithm based on WHO recommendations for the diagnosis of smear-negative pulmonary tuberculosis in patients without HIV infection. Trop Med Int Health. 2011;16(4):424–430. doi:10.1111/j.1365-3156.2010.02715.x
10. Theron G, Peter J, Dowdy D, Langley I, Squire SB, Dheda K. Do high rates of empirical treatment undermine the potential effect of new diagnostic tests for tuberculosis in high-burden settings? Lancet Infect Dis. 2014;14(6):527–532. doi:10.1016/S1473-3099(13)70360-8
11. Wong EB. It is time to focus on asymptomatic tuberculosis. Clin Infect Dis. 2021;72(12):e1044–e1046. doi:10.1093/cid/ciaa1827
12. Yoon C, Dowdy DW, Esmail H, MacPherson P, Schumacher SG. Screening for tuberculosis: time to move beyond symptoms. Lancet Respir Med. 2019;7(3):202–204. doi:10.1016/S2213-2600(19)30039-6
13. Kilaru SC, Chenimilla NP, Syed U, et al. Role of Xpert MTB/RIF in bronchoalveolar lavage fluid of sputum-scarce, suspected pulmonary TB patients. J Clin Tuberc Other Mycobact Dis. 2019;14:7–11. doi:10.1016/j.jctube.2018.11.003
14. To KW, Kam KM, Chan DPC, et al. Utility of GeneXpert in analysis of bronchoalveolar lavage samples from patients with suspected tuberculosis in an intermediate-burden setting. J Infect. 2018;77(4):296–301. doi:10.1016/j.jinf.2018.06.011
15. Yoo H, Song JU, Koh WJ, et al. Additional role of second washing specimen obtained during single bronchoscopy session in diagnosis of pulmonary tuberculosis. BMC Infect Dis. 2013;13(1):404. doi:10.1186/1471-2334-13-404
16. Luo W, Lin Y, Li Z, Wang W, Shi Y. Comparison of sputum induction and bronchoscopy in diagnosis of sputum smear-negative pulmonary tuberculosis: a systemic review and meta-analysis. BMC Pulm Med. 2020;20(1):146. doi:10.1186/s12890-020-01192-w
17. Fakey Khan DF, Suleman M, Baijnath P, et al. Multiple microbiologic tests for tuberculosis improve diagnostic yield of bronchoscopy in medically complex patients. AAS Open Res. 2019;2:25. doi:10.12688/aasopenres.12980.1
18. Prasad R, Singh A. Role of bronchoscopy in diagnosis of smear-negative pulmonary tuberculosis. Egypt J Bronchol. 2019;13:1–5.
19. Park JH, Jo KW, Shim TS, Kim SH. Diagnostic yield of post-bronchoscopy sputum for diagnosing pauci-bacillary pulmonary tuberculosis. Ann Med. 2021;53(1):576–580. doi:10.1080/07853890.2021.1908587
20. Schoch OD, Rieder P, Tueller C, et al. Diagnostic yield of sputum, induced sputum, and bronchoscopy after radiologic tuberculosis screening. Am J Respir Crit Care Med. 2007;175(1):80–86. doi:10.1164/rccm.200608-1092OC
21. Ahmad M, Ibrahim WH, Al Sarafandi SA, et al. Diagnostic value of bronchoalveolar lavage in the subset of patients with negative sputum/smear and mycobacterial culture and a suspicion of pulmonary tuberculosis. Int J Infect Dis. 2019;82:96–101. doi:10.1016/j.ijid.2019.03.021
22. Kwak SH, Choi JS, Lee EH, et al. Characteristics and risk factors associated with missed diagnosis in patients with smear-negative pulmonary tuberculosis. Korean J Intern Med. 2021;36(Suppl 1):S151–S159. doi:10.3904/kjim.2019.435
23. Gandhi NR, Nunn P, Dheda K, et al. Multidrug-resistant and extensively drug-resistant tuberculosis: a threat to global control of tuberculosis. Lancet. 2010;375(9728):1830–1843. doi:10.1016/S0140-6736(10)60410-2
24. Mondoni M, Repossi A, Carlucci P, Centanni S, Sotgiu G. Bronchoscopic techniques in the management of patients with tuberculosis. Int J Infect Dis. 2017;64:27–37. doi:10.1016/j.ijid.2017.08.008
25. Jamil SM, Oren E, Garrison GW, et al. Diagnosis of tuberculosis in adults and children. Ann Am Thorac Soc. 2017;14(2):275–278. doi:10.1513/AnnalsATS.201608-636CME
26. Facciolongo N, Patelli M, Gasparini S, et al. Incidence of complications in bronchoscopy. Multicentre prospective study of 20,986 bronchoscopies. Monaldi Arch Chest Dis. 2009;71(1):8–14. doi:10.4081/monaldi.2009.370
27. Iyer VN, Joshi AY, Boyce TG, et al. Bronchoscopy in suspected pulmonary TB with negative induced-sputum smear and MTD (®) Gen-probe testing Gen. Respir Med. 2011;105(7):1084–1090. doi:10.1016/j.rmed.2011.03.003
28. Liu X, Hou XF, Gao L, et al. Indicators for prediction of mycobacterium tuberculosis positivity detected with bronchoalveolar lavage fluid. Infect Dis Poverty. 2018;7(1):22. doi:10.1186/s40249-018-0403-x
29. Okada R, Kawasaki Y. Relationship between cough peak flow and onset of pneumonia in residents of geriatric health services facilities. Nihon Ronen Igakkai Zasshi. 2020;57(3):267–272. doi:10.3143/geriatrics.57.267
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.