Back to Journals » Patient Preference and Adherence » Volume 17
Adaptation and Implementation of Pictorial Conversation Aids for Early-Stage Breast Cancer Surgery and Reconstruction: A Quality Improvement Study
Authors Durand MA, Bannier M, Aim MA, Mancini J
Received 16 June 2023
Accepted for publication 23 September 2023
Published 4 October 2023 Volume 2023:17 Pages 2463—2474
DOI https://doi.org/10.2147/PPA.S421695
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Johnny Chen
Marie-Anne Durand,1– 3 Marie Bannier,4 Marie-Anastasie Aim,5,6 Julien Mancini7
1CERPOP, Université de Toulouse, Inserm, Université Toulouse III Paul Sabatier, Toulouse, France; 2The Dartmouth Institute for Health Policy & Clinical Practice, Dartmouth College Lebanon, Lebanon, NH, USA; 3Unisanté, Centre universitaire de médecine générale et santé publique, Lausanne, Switzerland; 4Institut Paoli-Calmettes, Marseille, France; 5AP-HM, Délégation à la Recherche Clinique et à l’Innovation, Marseille, France; 6Aix-Marseille Univ, LPS, Aix-en-Provence, France; 7Aix-Marseille Univ, APHM, INSERM, IRD, ISSPAM, SESSTIM, “Cancer, Biomedicine & Society” Group, Equipe Labellisée LIGUE, Hop Timone, Marseille, France
Correspondence: Julien Mancini, SESSTIM (Aix-Marseille Univ, Inserm, IRD) “Cancer, Biomedicine & Society” group, Institut Paoli-Calmettes, 232 Bd Ste Marguerite, BP 156, Marseille Cedex 9, Marseille, 13273, France, Tel +33 491 22 35 01, Email [email protected]
Purpose: After a diagnosis of early-stage breast cancer, women of lower socioeconomic position (SEP) report worse outcomes than women of higher SEP. A pictorial conversation aid was shown to improve decision outcomes in controlled contexts. No such intervention existed in France. In Phase 1, our aim was to adapt, for use in France, two pictorial conversation aids for breast cancer surgery and reconstruction. In Phase 2, our aim was to implement them in a regional cancer center serving a diverse population.
Patients and Methods: In phase 1, we used iterative qualitative methods to adapt the conversation aids with a convenience sample of patients and health professionals. In phase 2, we tested their implementation using PDSA cycles with volunteer surgeons.
Results: In phase 1, we interviewed 10 health professionals and 5 patients to reach thematic data saturation. They found the conversation aids usable and very acceptable (especially patients) and suggested small changes to further simplify the layout and content (including a glossary). In phase 2, three surgeons started the first PDSA cycle, for 4 weeks. Only one additional surgeon agreed to take part in the second cycle. The third cycle was cancelled since no new surgeon agreed to take part. Time was a barrier for 2 out of 4 surgeons, potentially explaining the difficulty recruiting for the third cycle. The evaluation was otherwise positive. The surgeons found the conversation aids very useful during their consultations and all intended to continue using them in the future.
Conclusion: It was possible to adapt, for use in France, pictorial conversation aids proven to be effective elsewhere. While the adapted conversation aids were deemed usable by health professionals and very acceptable to patients, their implementation using PDSA cycles proved slow.
Plain Language Summary: After a diagnosis of early-stage breast cancer, women of lower health literacy and socioeconomic position report worse outcomes than women of higher health literacy and socioeconomic position. Pictorial conversation aids were shown to improve decision outcomes in controlled contexts. No such intervention existed in France. Our aims were to adapt, for use in France, two lower health literacy pictorial conversation aids for breast cancer surgery and reconstruction, and then to implement them in a regional cancer center. Health professionals and patients found the conversation aids usable and very acceptable (especially patients) and suggested changes. Regarding implementation, the evaluation was also positive but time was a barrier for surgeons, and it was difficult to recruit many volunteers to use such conversation aids during their consultations.
Keywords: shared decision-making, decision support techniques, breast cancer disparities, lower socioeconomic position
Introduction
Breast cancer is the most commonly diagnosed malignancy and second leading cause of death in women.1,2 Therapeutic advances make it possible to provide satisfactory patient care and increase life expectancy.3–5 Unfortunately, major inequalities persist between patients from disadvantaged backgrounds and those from higher socio-economic positions (SEP).1,6–10 This is a public health problem that affects both France and the United States.11–13 After a diagnosis of early-stage breast cancer, extensive evidence suggests that women of lower SEP have significantly poorer communication with their clinicians, lower knowledge of breast cancer surgery options, and worse cancer-related and patient-centered health outcomes compared to women of higher SEP.6–10,13–16 They also tend to receive breast cancer care that is inferior to that offered to women of higher SEP and deviates from established clinical guidelines.14,16 Specific efforts must be made to help all women make informed decisions congruent with their wishes, regardless of their SEP and health literacy level.
Although breast conserving surgery is the recommended treatment for early-stage breast cancer (stages I to IIIA), research confirms equivalent survival between mastectomy and breast conserving surgery with adjuvant radiation therapy.17–20 Both options are offered routinely yet have distinct harms and benefits, which are valued differently by each patient.21 In this context, patient preferences play an essential role in decision-making and warrant patient participation in medical decision-making. While shared decision-making (SDM) is recommended, only 44–51% of women with early-stage breast cancer across socioeconomic strata achieve the degree of participation that they desire.6,15,22–25 In France, more than 25% of breast cancer survivors would have wished higher involvement in treatment decision-making, especially low-literate women.26 However, there is a growing interest in promoting SDM at the national level.27
Patient decision aids provide evidence-based information about the harms and benefits of reasonable healthcare options to promote SDM and help individuals deliberate about their preferences.28 One systematic review suggested that women who had used a patient decision aid were 25% more likely to choose breast-conserving surgery over mastectomy.29 Further, research has shown that although decision aids improve outcomes in controlled settings (with literate audiences), their use in routine care remains rare because of resistance to implementation.30 Clinicians argue that consultation time is limited and that complex pre-encounter decision aids are not designed for use in face-to-face encounters and disrupt workflows.30–36
Shorter, simpler interventions designed for use in clinic visits, also called conversation aids, have received less attention than complex pre-encounter interventions37 despite evidence of benefits38–40 and possible integration into routine care and electronic health records.40 A pictorial conversation aid for early-stage breast cancer surgery evaluated in a comparative effectiveness trial in the United States41 resulted in higher knowledge, improved decision process, lower decision regret and higher self-reported and observed SDM compared to usual care. It had more impact on knowledge and quality of life among disadvantaged patients.42
In France, pictorial conversation aids have never been used or implemented in routine breast cancer care. The planned research was aimed at exploring the challenges of developing bespoke material and ensuring that it is adopted using Plan Do Study Act (PDSA) cycles, a quality improvement method that consists of testing the acceptability of an intervention in clinical settings by introducing the tool progressively, and studying the impact of the intervention on teams, the clinical environment and patients.42 Our aims were to 1) adapt pictorial conversation aids for breast cancer surgery and reconstruction for use in France (phase 1) and 2) implement the conversation aids in a regional comprehensive cancer center using PDSA cycles (phase 2).
Materials and Methods
All qualitative methods and results are reported using the COnsolidated criteria for REporting Qualitative (COREQ) research checklist (see Supplementary Table 1).
Overview
In phase 1, two paper-based pictorial conversation aids for early-stage breast cancer were translated and adapted from validated English versions, and tested for usability, acceptability and feasibility with a convenience sample of patients and health professionals at a regional comprehensive cancer center in France. In phase 2, we tested the implementation of these two conversation aids using PDSA cycles with volunteer surgeons and their patients.
This project was reviewed and approved by the local ethics committee (GSPC: ‘Groupe de Sélection des Projets Cliniques’) of the participating cancer center on 6 November 2018.
Design and Procedure
In phase 1, we translated each conversation aid from English to French using an adapted TRAPD (Translation, Review, Adjudication, Pretesting and Documentation) protocol. Each conversation aid was independently translated by two certified translators from a US-based translation agency. The two French translations were then reviewed by a bilingual researcher (Marie-Anne Durand) to compare the original English version and the two French translations, make corrections to one of the versions (deemed superior) or create a third version from the two translations.
In parallel, we developed semi-structured interview guides using cognitive debrief and think aloud techniques (see Supplementary Figures 1 and 2). The interviewer had experience successfully using those techniques before.43,44 We targeted a 30-minute interview duration and based the content of the interview guide on previously tested interview guides developed in English with our patient partners in the US. We piloted the interview guide with one French team member, and made necessary revisions before starting data collection. Revisions included making sure the interview questions flowed smoothly and reducing repetition.
Once the French translations were deemed acceptable to the bilingual reviewer, we conducted semi-structured interviews. In total, and depending on thematic data saturation, we planned to conduct up to 20 interviews (up to 10 with patients and up to 10 with healthcare professionals). The interviewer was the principal investigator (Marie-Anne Durand), a female health services researcher with a PhD, trained in qualitative data collection and analysis methods, with 15 years of experience conducting qualitative and mixed-methods research, including in oncology. The interviewees had not met the interviewer before. Information about the study was provided when recruiting volunteer participants using an information sheet. Consent was obtained verbally before starting the interview. Written consent was waived and approved by the local ethics committee (GSPC: ‘Groupe de Sélection des Projets Cliniques’, Institut Paoli-Calmettes, Marseilles). This project was conducted within the French reference methodology standards MR-004. Projects that are labelled MR-004 and that are conducted in one center only can use verbal consent (also referred to as verbal non-opposition in the MR-004 methodology) instead of written consent. As stated in the MR-004 methodology, if the study is conducted in one center only (which was the case here), it is not necessary to document verbal consent. Given the quality improvement context of this project, we did not record the interviews but took detailed notes instead. The interviewer is a French native speaker and was therefore comfortable using abridged writing and taking detailed notes while progressing with the interview guides. The interviewer also took 30 minutes on average after each interview to read the notes and add additional thoughts.
In this phase, the researcher also gave a 30-minute presentation to health professionals in the breast cancer care unit including a very brief introductory training session about SDM and conversation aids. This did not include role-playing.45
In phase 2, we used PDSA cycles. PDSA cycles are an iterative problem-solving model used to improve a process and carry out change. Using PDSA cycles in quality improvement offers the opportunity to test out changes on a small scale before larger scale implementation. Changes were made during each cycle to facilitate the integration of the intervention in the next cycle. A short (8 questions) anonymous online survey was also sent to each participating surgeon after each PDSA cycle (see Supplementary Figure 3).
Setting
In phase 1, the conversation aids for breast cancer (Supplementary Figures 4 and 5) were adapted with patients, clinicians and other healthcare professionals from an urban cancer center.
In phase 2, to begin the PDSA cycles, we recruited volunteer surgeons working in the participating cancer center to use the conversation aids in their clinical practice over a 4-month period in total.
Participants
In phase 1, we recruited a convenience sample of 6 to 10 healthcare professionals and 6 to 10 patients from the cancer center to adapt and test the translations of conversation aids for breast cancer. For the patient sample, an effort was made to recruit a diverse sample of participants.
We included all health professionals working in the breast cancer unit of the participating regional comprehensive cancer center. There were no exclusion criteria.
We also included a convenience sample of women who had previously had breast cancer, had completed treatments and were able to read simple textual content in French on their own or assisted by a close relative or caregiver.
These women were selected by a participating surgeon and approached by the researcher over the telephone. The researcher introduced the study over the telephone with a script-based information sheet. If the potential participant agreed to participate, the researcher arranged a face-to-face interview at the cancer center or a phone-based interview according to the participant’s stated preference.
In phase 2, we recruited a convenience sample of volunteer surgeons from the participating cancer center. This included surgeons who had already participated in phase 1. Their patients with early-stage breast cancer considering surgical treatment or breast reconstruction were automatically engaged in PDSA cycles.
Interventions
Both conversation aids (see Figure 1) have been developed and tested in English-speaking countries:42 1) Decision of mastectomy or lumpectomy + radiotherapy (see Supplementary Figure 4); 2) decision to undertake reconstruction for patients who have opted for mastectomy, and if so, immediate or delayed, and flap or prosthesis (see Supplementary Figure 5).
 |
Figure 1 Example of conversation aid developed and validated in English. |
Both breast cancer conversation aids incorporated images and plain language to promote their use in the general population, regardless of the patient’s health literacy level.
Outcome Measures
In phase 1, we measured the usability, acceptability and feasibility of the two conversation aids. We adapted each tool in order to optimize their use in routine clinical settings.
In phase 2, we measured:
- The number of PDSA cycles;
- The size of each cycle (number of surgeons involved and number of conversation aids used);
- The number of changes made to the tools and the nature of each change;
- The length of each cycle.
We also asked participating surgeons to complete a short online survey about their experience of using the conversation aid in a PDSA cycle (see Supplementary Figure 3).
Analyses
In phase 1, we undertook a two-step thematic analysis derived from descriptive phenomenology. Notes taken during interviews were analyzed in relation to the following three themes: usability, acceptability and feasibility of each tool. One researcher coded the data. Given the small number of interviews, data analysis was done manually.
In phase 2, we collected and analyzed the PDSA data in a descriptive manner.
Data collected with the online survey were also analyzed descriptively.
Patient and Public Involvement
Patient and public involvement was central to the development and validation of the English version of the conversation aids. In France, involvement was sought through a convenience sample of women who had experienced breast cancer, and who provided, in addition to the work undertaken in phases 1 and 2, their opinions on the project and on the adaptation of the pictorial conversation aids.
Results
Phase 1
Characteristics of Participants and Interviews
We interviewed 10 health professionals and 5 patients and reached thematic data saturation for both target groups. We approached 8 patients in total, and 5 accepted to participate. We approached 12 health professionals in total, and 10 agreed to participate. No reasons for declining participation were provided. Given the quality-improvement nature of the project and associated ethical approval, we were not authorized to collect any sociodemographic information about the participants. Interviews conducted with health professionals were spontaneous, occurring between consultations or surgical procedures, the researcher was thus unable to accurately time their duration. Two out of five patient participants attended the interview with their husbands. Interviews with patients ranged from 10 to 46 minutes and lasted 26 minutes on average. Three interviews were conducted face-to-face and two over the phone.
Usability, Acceptability and Feasibility of the Conversation Aids from the Health Professionals’ Perspective
Healthcare professionals were positive and receptive to the SDM approach and use of pictorial conversation aids. Several mentioned regularly drawing pictures for patients during their consultations. This type of interventions therefore appeared to fit well within their routine clinical practice and usual consultation style, by providing illustrations combined with evidence-based content. They all indicated being willing to use this type of patient-centered intervention during a consultation with eligible patients. However, a minority voiced concerns about the extra time spent using this type of intervention and promoting SDM. They worried that using the conversation aid would create additional questions not normally raised in the consultation.
They suggested changes to the textual content and clinical terms used to better fit the French clinical context, as well as changes to specific images (eg, removing the thumbs up in the reconstruction conversation aid and changing the chemotherapy image which was deemed depressing). Changes to the format were also suggested (eg, larger images and more white spaces). Several also suggested changes to the risk of recurrence provided and recovery information (different duration of hospital stay in France) and removing the question about cost, less relevant in France. A few health professionals (2/10) suggested reordering the frequently asked questions. Since most patients (see below) liked the initial order, we chose not to change it. Health professionals also indicated liking the colors used in each conversation aid. Interestingly, several health professionals talked about the importance of framing content positively, avoiding to present information that could worry their patients and were concerned about presenting outcome probabilities. Several surgeons felt that the SDM approach was more suitable in the context of breast reconstruction, where clinical equipoise was not debatable. For breast cancer surgery, some surgeons did not identify true clinical equipoise and considered breast conserving surgery the first option to discuss for early cancer. They explained that mastectomy could be discussed, but at the patient’s request. Several health professionals felt that pictograms might be difficult to understand and suggested bar graphs instead.
Usability, Acceptability and Feasibility of the Conversation Aids from the Health Professionals’ Perspective
All five patients interviewed were also very receptive to the use of a pictorial conversation aid used during the consultation. They particularly liked the use of images and simplified textual content but asked for additional simplifications and layout improvements. The majority of patients interviewed (n=4/5) did not support the use of pictograms and preferred the use of bar graphs instead, mirroring health professionals’ suggestions (see Figures 2 and 3). They suggested (n=3/5) introducing a glossary to explain complex medical terms with simpler language, which was added at the bottom of each page (see Figures 2 and 3). They liked the colors used (n=5/5). Most liked the order of the frequently asked questions (n=4/5). To improve the layout and facilitate comprehension at a time of heightened emotional distress, they suggested spacing content out a little more, to show two pictures per page only and adding space for questions underneath each frequently asked question. Two illustrations were also modified in response to their comments (see Figure 2). Examples of the changes made are shown in Figures 2 and 3; fully adapted and original documents are provided in Supplementary Figures 4 and 5.
 |
Figure 2 Example of changes made to breast surgery conversation aid after phase 1. |
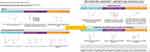 |
Figure 3 Example of changes made to reconstruction conversation aid after phase 1. |
Phase 2
We had initially planned to conduct a minimum of three PDSA cycles. Each cycle lasted 4 weeks. We were only able to conduct two cycles in total. No new surgeon answered repeated invitation emails for the third cycle.
PDSA Cycle 1
For the first cycle, we approached 4 surgeons, 1 declined due to relocation, and three accepted to use the conversation aids in their routine clinical practice for 4 weeks. We asked them to choose how many printed pictorial conversation aids they wanted to use during the 4-week cycle. Each surgeon took 4 or 5 copies of each conversation aid, that is a total of 8 or 10 copies per surgeon. After two weeks, each surgeon received a short email asking them how the cycle had gone so far. All three surgeons replied to the email and indicated that it was going well, that the conversation aids were an interesting tool for their consultation, and that they had each used at least 3 copies. One surgeon mentioned that it was occasionally difficult to integrate the conversation aid in the workflow. The surgeon mentioned recurrent time pressure, with frequent long delays in the consultation schedule (often over 2 hours). The surgeon thus reported occasionally skipping the use of the conversation aid, fearing that it would take too much time.
When the 4-week cycle ended, each surgeon completed the short 8-question online survey.
One out of three surgeons reported no major difficulties using the conversation aids. Two surgeons, however, indicated, in the free text box, that using the conversation aid for breast cancer surgery generated more emails and occasionally, an additional consultation. One surgeon explicitly expressed that time was a challenge. This surgeon suggested giving the conversation aid to eligible patients before the consultation to minimize disruptions to the clinic workflow. All three surgeons rated the conversation aids as very useful (mean score of 8.7 out of 10, with 0 being not useful at all and 10 being extremely useful). Two out of three surgeons felt that the approach (introducing the conversation aid in the consultation) was received positively or extremely positively by patients. The third surgeon indicated that since they had not formally collected patient-reported data, it was difficult to accurately answer. However, the overall impression was positive. They did not suggest changes to the content but recommended a longitudinal evaluation of the impact of the conversation aid. Two out of three surgeons indicate not needing additional information or training on the use of the conversation aids. One surgeon felt that a training video explaining how to use the pictorial conversation aid would be helpful. All three surgeons intended to continue using the conversation aids in the future (mean 8.7 out of 10, with 0 being absolutely no intention of using it in the future and 10 being absolutely intend to use it in the future).
PDSA Cycle 2
For the second cycle, we approached 4 additional surgeons. Only one additional surgeon agreed to participate. The other surgeons did not answer our two email invitations. The fourth surgeon, newly recruited, chose to take 8 copies of the conversation aids. The other three surgeons continued to use the conversation aids and requested additional copies. No new check-in email was sent to the surgeons from cycle 1, since they had previously used the conversation aids and had positively replied to the online survey. The fourth surgeon did not answer the check-in email.
When the 4-week cycle ended, the fourth surgeon completed the short 8-question online survey. The surgeon reported no major difficulty using the conversation aid. They typically used the conversation aids as instructed. They introduced the tool during the consultation, used it with the patient and encouraged the patient to take it home. The surgeon scored 7 out of 10 to indicate the usefulness of the conversation aid. The surgeon indicated that the conversation aid was positively received by the patients but would have liked more information and training about the conversation aid. The surgeon scored 9 out of 10 to indicate his/her intention to continue using the conversation aid in the future. No change was suggested.
Discussion
In phase 1, the translation and adaptation of validated conversation aids with a convenience sample of health professionals and patients appeared feasible and successful at maximizing their usability and acceptability in the French oncology context. Both groups, and particularly patients, found the translated conversation aids very acceptable, and welcomed the approach. They suggested several changes to further simplify the layout and content. Surgeons also suggested changes to the clinical content to fit the French context. In phase 2, a total of four surgeons took part in two PDSA cycles that lasted 4 weeks each. Despite repeated invitations, no additional surgeon accepted to take part in a third cycle. Feedback suggested that time was a barrier for 2 out of 4 surgeons, potentially explaining the difficulty recruiting other surgeons. The evaluation was otherwise very positive. The surgeons found the conversation aids very useful during the consultations and intended to continue using them in the future.
This study highlighted the importance of local and clinical adaptation of an intervention validated elsewhere. Mere translation was not sufficient. The initial content, although carefully translated, evidence-based and validated in a large randomized controlled trial for the breast cancer surgery conversation aid,42 needed contextual adaptation. This mirrors our experience in other countries where contextual adaptation was an essential step and could sometimes take several months and multiple iterative interview cycles, until the intervention was deemed fully acceptable.46,47 A sizeable proportion of health professionals interviewed in France expressed repeated concerns about sharing evidence-based facts and outcome probabilities with patients that could worry and distress them. It is worth noting, although the sample size was small, that none of the patients interviewed expressed those concerns. This tendency to select or withdraw evidence-based information considered too complex or potentially worrying and avoid offering a treatment choice could be understood in the context of information sharing and protective paternalism in France.48,49 The patient information law has been introduced fairly recently in France (2002) and seems relatively nuanced. Article 35 stipulates that the doctor has a duty to inform their patients on their health status, procedures and treatments offered. However, it also specifies that if a person asks not to be informed, information should be withdrawn. The French patient has therefore less autonomy than the full determination granted to an American patient since the information law passed in 1960 in the USA.49 These differences may explain some of our findings. Further, while a brief introductory training was offered to health professionals, more in-depth training about SDM and conversation aids could have facilitated conversation aid use and implementation. Potential next steps have included the development of an online training module that could be rolled out to health professionals more easily and systematically.
Time was a frequently cited concern and likely barrier to using and implementing pictorial conversation aids in routine care. This concern has been extensively documented in the literature and is the most frequently reported barrier to SDM.30,35,50,51 There is, however, no reliable evidence that promoting shared decision-making and using conversation aids takes more time than a standard consultation.28,50 On the contrary, a recent systematic review and meta-analysis of 23 randomized controlled trials of conversation aids demonstrated that using those interventions in controlled contexts did not increase visit durations.38 However, surgeons argue that these trials have been conducted in different countries and clinical contexts and may be irrelevant in naturalistic French settings. Further research in the French context, using a hybrid design where both effectiveness and implementation outcomes are measured (including time) seems warranted. Further, Pieterse et al argue that time will remain a barrier to SDM as long as this approach is considered a nice-to-have extra for which additional time may need to be freed.50 This also rings true in France, illustrated by the phase 2 findings.
The strengths of this study were the careful, stepwise translation process adapted from TRAPD and the iterative data collection procedure with key stakeholders in a regional comprehensive cancer center. The limitations are related to the quality improvement nature of the project, which precluded the collection of sociodemographic information. The small sample size and small number of PDSA cycles is another limitation. We must also consider the impact of desirability bias for the surgeons who took part in the PDSA cycles and completed the online survey. Although the survey was anonymous, the sample size was very small and each surgeon could have been identified by the researcher based on the information provided. This may have influenced the surgeons’ responses (usefulness of the conversation aids and intention to use them in the future).
Conclusion
It was possible to adapt pictorial conversation aids proven to be effective elsewhere, for use in France. While the adapted conversation aids were deemed usable and acceptable by health professionals, patients seemed particularly receptive to this approach and welcomed the integration of a simple pictorial conversation aid in their clinic visit. Implementing the conversation aids using PDSA cycles proved slow, despite positive feedback provided by all participating surgeons. It was difficult to identify new surgeons willing to integrate the conversation aids in their routine clinical practice. Time was a frequently mentioned barrier to integration in both phases. Further research is needed to evaluate the impact of the conversation aids on time (duration of the consultation) in controlled contexts, longitudinally and explore ways to minimize the impact on busy clinic workflows.
Abbreviations
PDSA, Plan Do Study Act; SDM, shared decision-making; SEP, socioeconomic position; TRAPD, Translation, Review, Adjudication, Pretesting and Documentation.
Ethics
This project was reviewed and approved by the local ethics committee (GSPC: “Groupe de Sélection des Projets Cliniques”, Institut Paoli-Calmettes, Marseille) of the participating cancer center on 6 November 2018.
Acknowledgments
We would like to acknowledge the patients and health professionals who have made this study possible. Funding for this project was provided by the Institut Paoli Calmettes Cancer Center and the Institute for Advanced Studies (IMéRA) of Aix-Marseille University.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Marie-Anne Durand has contributed to the development of Option Grid patient decision aids (from which Picture Option Grid is derived). EBSCO Information Services sells subscription access to Option Grid patient decision aids. She receives consulting income from EBSCO Health, and royalties. The authors report no other conflicts of interest in this work.
References
1. Donepudi MS, Kondapalli K, Amos SJ, Venkanteshan P. Breast cancer statistics and markers. J Cancer Res Ther. 2014;10(3):506–511. doi:10.4103/0973-1482.137927
2. American Cancer Society. What are the key statistics about breast cancer?; 2015. Available from: http://www.cancer.org/cancer/breastcancer/detailedguide/breast-cancer-key-statistics.
3. Cameron D, Piccart-Gebhart MJ, Gelber RD, et al. 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet. 2017;389(10075):1195–1205. doi:10.1016/S0140-6736(16)32616-2
4. Lowery AJ, Kell MR, Glynn RW, Kerin MJ, Sweeney KJ. Locoregional recurrence after breast cancer surgery: a systematic review by receptor phenotype. Breast Cancer Res Treat. 2012;133(3):831–841. doi:10.1007/s10549-011-1891-6
5. Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366(9503):2087–2106 doi:10.1016/S0140-6736(05)67887-7.
6. Chen JY, Diamant AL, Thind A, Maly RC. Determinants of breast cancer knowledge among newly diagnosed, low-income, medically underserved women with breast cancer. Cancer. 2008;112(5):1153–1161. doi:10.1002/cncr.23262
7. Mac Bride MB, Neal L, Dilaveri CA, et al. Factors associated with surgical decision making in women with early-stage breast cancer: a literature review. J Womens Health. 2013;22(3):236–242. doi:10.1089/jwh.2012.3969
8. McVea KLSP, Minier WC, Palensky JEJ. Low-income women with early-stage breast cancer: physician and patient decision-making styles. Psycho-Oncology. 2001;10:137–146. doi:10.1002/pon.503
9. Siminoff LA, Graham GC, Gordon NH. Cancer communication patterns and the influence of patient characteristics: disparities in information-giving and affective behaviors. Patient Educ Couns. 2006;62(3):355–360. doi:10.1016/j.pec.2006.06.011
10. Wheeler SB, Reeder-Hayes KE, Carey LA. Disparities in breast cancer treatment and outcomes: biological, social, and health system determinants and opportunities for research. Oncologist. 2013;18(9):986–993. doi:10.1634/theoncologist.2013-0243
11. Delaporte I. Face au cancer du sein, les inégalités se creusent. l’Humanité; 2015.
12. Observatoire societal des cancers, Ligue Contre le Cancer. 4eme rapport de l’observatoire societal des cancers; 2014. Available from: https://www.ligue-cancer.net/sites/default/files/docs/observatoire_societal_des_cancers_rapport_2014.pdf.
13. Polacek GN, Ramos MC, Ferrer RL. Breast cancer disparities and decision-making among U.S. women. Patient Educ Couns. 2007;65(2):158–165. doi:10.1016/j.pec.2006.06.003
14. Hurd TC, James T, Foster JM. Factors that affect breast cancer treatment: underserved and minority populations. Surg Oncol Clin N Am. 2005;14(1):119–130. doi:10.1016/j.soc.2004.08.001
15. Hawley ST, Lantz PM, Janz NK, et al. Factors associated with patient involvement in surgical treatment decision making for breast cancer. Patient Educ Couns. 2007;65(3):387–395. doi:10.1016/j.pec.2006.09.010
16. Richardson LC. Treatment of breast cancer in medically underserved women: a review. Breast J. 2004;10(1):2–5. doi:10.1111/j.1524-4741.2004.09511.x
17. Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347(16):1233–1241. doi:10.1056/NEJMoa022152
18. Jatoi I, Proschan MA. Randomized trials of breast-conserving therapy versus mastectomy for primary breast cancer: a pooled analysis of updated results. Am J Clin Oncol. 2005;28(3):289–294. doi:10.1097/01.coc.0000156922.58631.d7
19. Morris AD, Morris RD, Wilson JF, et al. Breast-conserving therapy vs mastectomy in early-stage breast cancer: a meta-analysis of 10-year survival. Cancer J Sci Am. 1997;3(1):6–12.
20. Poggi MM, Danforth DN, Sciuto LC, et al. Eighteen-year results in the treatment of early breast carcinoma with mastectomy versus breast conservation therapy: the national cancer institute randomized trial. Cancer. 2003;98(4):697–702. doi:10.1002/cncr.11580
21. National Cancer Institute. Breat cancer treatment; 2016. Available from: http://www.cancer.gov/types/breast/patient/breast-treatment-pdq#link/_229_toc.
22. Degner LF, Kristjanson LJ, Bowman D, et al. Information needs and decisional preferences in women with breast cancer. JAMA. 1997;277(18):1485–1492. doi:10.1001/jama.1997.03540420081039
23. Keating NL, Guadagnoli E, Landrum MB, Borbas C, Weeks JC. Treatment decision making in early-stage breast cancer: should surgeons match patients’ desired level of involvement? J Clin Oncol. 2002;20(6):1473–1479. doi:10.1200/JCO.2002.20.6.1473
24. Fagerlin A, Lakhani I, Lantz PM, et al. An informed decision? Breast cancer patients and their knowledge about treatment. Patient Educ Couns. 2006;64(1–3):303–312. doi:10.1016/j.pec.2006.03.010
25. Lee CN, Chang Y, Adimorah N, et al. Decision making about surgery for early-stage breast cancer. J Am Coll Surg. 2012;214(1):1–10. doi:10.1016/j.jamcollsurg.2011.09.017
26. Moumjid N, Carretier J, Marsico G, Blot F, Durif-Bruckert C, Chauvin F. Moving towards shared decision making in the physician-patient encounter in France: state of the art and future prospects. Z Evid Fortbild Qual Gesundhwes. 2017;123–124:41–45. doi:10.1016/j.zefq.2017.05.017
27. Moumjid N. Prise de décision partagée en France: point et perspectives synthétiques en 2019. Rev Med Suisse. 2019;15(669):1998.
28. Stacey D, Legare F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4:CD001431. doi:10.1002/14651858.CD001431.pub5
29. Waljee JF, Rogers MA, Alderman AK. Decision aids and breast cancer: do they influence choice for surgery and knowledge of treatment options? J Clin Oncol. 2007;25(9):1067–1073. doi:10.1200/JCO.2006.08.5472
30. Elwyn G, Scholl I, Tietbohl C, et al. “Many miles to go…”: a systematic review of the implementation of patient decision support interventions into routine clinical practice. BMC Med Inform Decis Mak. 2013;13(2):S14. doi:10.1186/1472-6947-13-S2-S14
31. Gravel KLF, Graham ID, Graham ID. Barriers and facilitators to implementing shared decision-making in clinical practice: a systematic review of health professionals’ perceptions. Implement Sci. 2006;1(16). doi:10.1186/1748-5908-1-16
32. Holmes-Rovner MVD, Draus C, Draus C, Nabozny-Valerio B, Keiser S, Keiser S. Implementing shared decision-making in routine practice: barriers and opportunities. Health Expect. 2000;3(3):182–191. doi:10.1046/j.1369-6513.2000.00093.x
33. Silvia KA, Ozanne EM, Sepucha KR. Implementing breast cancer decision aids in community sites: barriers and resources. Health Expect. 2008;11(1):46–53. doi:10.1111/j.1369-7625.2007.00477.x
34. Silvia KA, Sepucha KR. Decision aids in routine practice: lessons from the breast cancer initiative. Health Expect. 2006;9(3):255–264. doi:10.1111/j.1369-7625.2006.00393.x
35. Legare F, Ratte S, Gravel K, Graham ID. Barriers and facilitators to implementing shared decision-making in clinical practice: update of a systematic review of health professionals’ perceptions. Patient Educ Couns. 2008;73(3):526–535. doi:10.1016/j.pec.2008.07.018
36. Legare F, Ratte S, Stacey D, et al.Interventions for improving the adoption of shared decision making by healthcare professionals. Cochrane Database Syst Rev. 2010;(5):Cd006732. doi:10.1002/14651858.CD006732.pub2
37. Wyatt KD, Branda ME, Anderson RT, et al. Peering into the black box: a meta-analysis of how clinicians use decision aids during clinical encounters. Implement Sci. 2014;9:26. doi:10.1186/1748-5908-9-26
38. Scalia P, Durand MA, Berkowitz JL, et al. The impact and utility of encounter patient decision aids: systematic review, meta-analysis and narrative synthesis. Patient Educ Couns. 2019;102(5):817–841. doi:10.1016/j.pec.2018.12.020
39. Scalia P, Elwyn G, Durand MA. “Provoking conversations”: case studies of organizations where option grid decision aids have become ‘normalized’. BMC Med Inform Decis Mak. 2017;17(1):124. doi:10.1186/s12911-017-0517-2
40. Inselman J, Branda M, Castaneda-Guarderas A, et al. Uptake and documentation of the use of an encounter decision aid in usual practice: a retrospective analysis of the use of the statin/aspirin choice decision aid. Med Decis Making. 2015;36:557–561. doi:10.1177/0272989X15618175
41. Durand MA, Yen RW, O’Malley AJ, et al. What matters most: protocol for a randomized controlled trial of breast cancer surgery encounter decision aids across socioeconomic strata. BMC Public Health. 2018;18(1):241. doi:10.1186/s12889-018-5109-2
42. Durand MA, Yen RW, O’Malley AJ, et al. What matters most: randomized controlled trial of breast cancer surgery conversation aids across socioeconomic strata. Cancer. 2021;127(3):422–436. doi:10.1002/cncr.33248
43. Durand MA, Alam S, Grande SW, Elwyn G. “Much clearer with pictures”: using community-based participatory research to design and test a picture option grid for underserved patients with breast cancer. BMJ Open. 2016;6(2):e010008. doi:10.1136/bmjopen-2015-010008
44. Durand MA, Song J, Yen RW, et al. Adapting the breast cancer surgery decision quality instrument for lower socioeconomic status: improving readability, acceptability, and relevance. MDM Policy Pract. 2018;3(2):2381468318811839. doi:10.1177/2381468318811839
45. Nikendei C, Kraus B, Schrauth M, et al. Integration of role-playing into technical skills training: a randomized controlled trial. Med Teach. 2009;29(9–10):956–960.
46. Scalia P, Elwyn G, Barr P, et al. Exploring the use of Option Grid patient decision aids in a sample of clinics in Poland. Z Evid Fortbild Qual Gesundhwes. 2018;134:1–8. doi:10.1016/j.zefq.2018.04.002
47. Hahlweg P, Witzel I, Muller V, Elwyn G, Durand MA, Scholl I. Adaptation and qualitative evaluation of encounter decision aids in breast cancer care. Arch Gynecol Obstet. 2019;299(4):1141–1149. doi:10.1007/s00404-018-5035-7
48. Hwang DY, Bernat JL. Neurologists and end-of-life decision-making: the role of “protective paternalism”. Neurol Clin Pract. 2015;5(1):6–8. doi:10.1212/CPJ.0000000000000096
49. Raoul JL, Maraninchi D. L’autonomie de décision du patient: du concept à la pratique cancérologique. Bull Cancer. 2017;104:695–700. doi:10.1016/j.bulcan.2017.06.002
50. Pieterse AH, Stiggelbout AM, Montori VM. Shared decision making and the importance of time. JAMA. 2019;322(1):25–26. doi:10.1001/jama.2019.3785
51. Joseph-Williams N, Elwyn G, Edwards A. Knowledge is not power for patients: a systematic review and thematic synthesis of patient-reported barriers and facilitators to shared decision making. Patient Educ Couns. 2014;94(3):291–309. doi:10.1016/j.pec.2013.10.031
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
