Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Adalimumab for Sintilimab-Induced Toxic Epidermal Necrolysis in a Patient with Metastatic Gastric Malignancy: A Case Report and Literature Review
Received 24 December 2022
Accepted for publication 4 February 2023
Published 20 February 2023 Volume 2023:16 Pages 457—461
DOI https://doi.org/10.2147/CCID.S401286
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Li Zhang, Zhongxiao Wu
Department of Dermatology, Ningbo No 6 Hospital, Ningbo, 315040, People’s Republic of China
Correspondence: Zhongxiao Wu, Email [email protected]
Abstract: Sintilimab is a recombinant fully human anti-programmed cell death protein 1 (PD-1) monoclonal antibody that blocks the interaction of PD-1 with its ligand. It was approved to use in patients with gastric malignancy. Toxic epidermal necrolysis (TEN) is a rare, life-threatening cutaneous drug reaction. Here, we report a 70-year-old female patient with gastric malignancy who developed severe TEN 10 days after initiation of sintilimab. The patient did not respond to the systemic corticosteroids and intravenous immunoglobulin therapies but improved after the subcutaneous injection of adalimumab (40 mg) that is a monoclonal antibody directed against antitumor necrosis factor-α. Her rashes rapidly resolved within 24 hr. By the seventh day, the bullae had scabbed and most skin lesions had subsided. The patient showed no sign of organ dysfunction. This is the first reported case of immune checkpoint inhibitor-induced TEN successfully treated with adalimumab.
Keywords: adverse events, gastric malignancy, sintilimab, programmed death-1 inhibitor, toxic epidermal necrolysis, antitumor necrosis factor-α
Introduction
With an increasing use of immune checkpoint inhibitors (ICIs) in cancer patients, reports have emerged on the cutaneous adverse events after the ICIs treatments,1 while the toxic epidermal necrolysis (TEN) has been less studied. TEN is a severe drug-induced adverse reaction that mainly manifests as the systemic epidermal necrosis. Its incidence is low but carries a high fatality rate. The treatment outcomes by high-dose corticosteroids or gamma-globulins on TEN caused by ICIs were unsatisfactory.2
Here, we report a 70-year-old patient who developed TEN following the initiation of sintilimab (Tyvyt®) treatment for gastric malignancy. Her symptoms completely resolved after the treatment with HUMIRA® (AbbVie Inc. U.S.A) that is a monoclonal antibody directed against antitumor necrosis factor-α (anti-TNF-α) and also referred as adalimumab. The patient was treated in an outpatient setting and did not require hospitalization.
Case Report
A 70-year-old female was diagnosed with advanced gastric malignancy. Her primary treatments included oxaliplatin (150 mg intravenous infusion) and tiggio (120 mg oral administration). At the 3-month follow-up visit, the disease had metastasized to the lung and liver. The patient agreed to the adjuvant systemic biologic treatment. She was given her first cycle of combination therapy with intravenous sintilimab (0.2 g), together with oxaliplatin (150 mg intravenous infusion) and tiggio (120 mg oral administration). Ten days after receiving the first cycle treatment, the patient developed a widespread erythematous maculopapular rash covering the majority of the skin areas of her chest, back, and arms. After receiving methylprednisolone (80 mg, intravenously) daily for three days, the patient's general condition deteriorated with persistent eruptions of new rashes, which were more extensive and severe. In addition, the rashes started to blister and burst, with subsequent skin denudation. The patient then visited our outpatient clinic. She denied a history of food and drug allergies or a family history of inherited disorders.
At our outpatient clinic, her physical examination revealed systemically widespread rashes in black with slight iron gray color, which were partially fused and contained multiple flaccid blisters in various sizes. There were also epidermolysis, positive Nikolsky’s sign, partial epidermal detachment with a bright red, scalded erosion surface, and significant palpation tenderness covering more than 40% of the skin. According to her medical history and rashes, the clinical diagnosis of TEN was considered for her, however the patient refused treatment and hospitalization.
After the presentation to our clinic, the patient immediately received intravenous immunoglobulin (IVIG) 400mg/kg/d for three consecutive days. However, the rashes did not improve. The blisters rapidly grew to fuse into bullae. In the meantime, some bullae ruptured with erosions. The skin rashes developed sheet‑like epidermal detachment and epidermal necrosis involving nearly 70% of the body surface area (Figure 1). The patient had a body temperature of 38.5°C. The SCORTEN was 5, suggesting a >90% mortality risk. Then, subcutaneous injection of adalimumab (40 mg) was administered. The rashes resolved within 24 h. There was no new epidermal detachment. No new bullae appeared 48 h after the adalimumab treatment. Physical examination showed negative Nikolsky’s sign and a body temperature of 37°C. By the 10th day, the existing bullae had scabbed and most skin lesions had subsided. The desquamation had occurred in part of the primary rashes (Figure 2). Adalimumab (40 mg) was provided once again. At the follow-up visit 45 days later, the majority of the skin lesions had disappeared (Figure 3). Currently, the patient is being followed up in the clinic for continuous treatments and monitoring. There is no sign of rash recurrence.
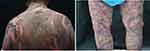 |
Figure 1 The 3rd day after intravenous immunoglobulin. |
 |
Figure 2 The 7th day after subcutaneous injection of adalimumab, AbbVie Inc. USA. |
 |
Figure 3 The 35th day after subcutaneous injection of adalimumab, AbbVie Inc. USA. |
Discussion
ICIs include cytotoxic T lymphocyte-associated antigen-4 (CTLA-4: monoclonal antibody ipilimumab), programmed cell death protein 1 (PD-1: monoclonal antibody nivolumab, pembrolizumab), and programmed cell death ligand 1 (PD-L1: monoclonal antibody atezolizumab, durvalumab). Ir-CAEs related to ICI therapy that may cause adverse events in the cutaneous and extracutaneous systems. Despite the high rates of cutaneous irAEs associated with ICI therapy, severe and life-threatening skin reactions, such as TEN, were reported in approximately 2% to 3% of patients treated with a single-agent ICI.3,4
In previous studies on TEN cases after the treatment by ICIs, patients were commonly treated by the high-dose corticosteroids or IVIG but with poor outcomes. We summarize these previously reported cases of ICI-induced TEN in Table 1. All of these patients with TEN had long-term hospitalizations or even intensive care unit admissions. Most of them still had a poor response after treatments with corticosteroids or gamma globulin.
 |
Table 1 Previously Reported Cases of Immune Checkpoint Inhibitor-Induced Toxic Epidermal Necrolysis (TEN) |
In the present study, the patient experienced the disease progression after the use of high-dose systemic corticosteroids and IVIG treatment. Administration of the TNF inhibitor adalimumab resulted in the rapid resolution of the blisters. The patient showed no sign of organ dysfunction. The whole treatment was performed in the outpatient setting without hospitalization.
Pathogenesis of TEN is thought to be a cell-mediated cytotoxic reaction against keratinocytes, leading to massive apoptosis.13 Several studies have demonstrated that certain medications could directly bind to the major histocompatibility complex I and the T-cell receptor, which resulted in the clonal expansion of a population of drug-specific cytotoxic T cells to kill keratinocytes.14,15 This process could occur directly and indirectly through the recruitment of other cells, such as natural killer cells (releasing cytokines), Fas ligand, perforin/granzyme, TNF-α, and TNF-related apoptosis inducing ligand including granulysin, which has a key role in the pathogenesis of TEN.13,16 Histopathology typically suggests keratinocyte necrosis, ranging from partial to full-thickness necrosis of the epidermis. With the known mechanism of the action of sintilimab,17 we consider that sintilimab might lead to the activation and proliferation of the CD8+ T cells and downstream pathways, which resulted in massive keratinocyte apoptosis, manifesting as TEN in our patient. Adalimumab, a TNF inhibitor with a high affinity to TNF-α receptor that is used for severe drug rashes, may target and deactivate TNF-α by suppressing its binding with the receptor to finally block the development of drug rashes. Serum inflammatory profiles in TEN patients were characterized by massive upregulation of type 1 immune response and proinflammatory markers;18 anti-tumor necrosis factor can suppress the inflammation formation cascade effectively, thus blocking the development of drug rashes.19
In this case, the patient refused hospitalization for various reasons. However, we attempted to explain to the patient the risk of the disease and the necessity for hospitalization. Nonetheless, the patient requested outpatient treatment. To respect the wishes of patient, we allowed them to return to the clinic every day and to provide feedback on the disease status at any time through the Internet. This case provides a useful reference on how patients can receive home care and provide timely feedback remotely, using Internet applications. By facilitating the ability of patients to provide feedback to the clinic daily, we can achieve similar results to hospitalization.
Conclusion
TEN is a dermatologic emergency that causes significant morbidity and mortality. In this case, we used IVIG plus adalimumab to treat TEN caused by the antitumor drug sintilimab, and achieved satisfactory results, including faster skin healing, a reduced period of treatment, and the ability to perform outpatient treatment. To our knowledge, this was the first reported case of the complete resolution of irAEs after the adalimumab therapy. If this treatment regimen can be validated by other studies, this can provide a novel management strategy in patients with similar clinical situations.
Data Sharing Statement
The authors confirm that the data supporting the findings of this study are available within the article.
Ethics Approval and Informed Consent
Informed consent for publication of the case details and associated images was obtained from the patient, and all procedures were performed in accordance with the Helsinki Declaration. Institutional approval was not required to publish the case details.
Consent Statement
Informed consent was obtained from the patient to publish his clinical information and relevant images.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Geisler AN, Phillips GS, Barrios DM, et al. Immune checkpoint inhibitor-related dermatologic adverse events. J Am Acad Dermatol. 2020;83:1255–1268. doi:10.1016/j.jaad.2020.03.132
2. Si X, He C, Zhang L, et al. Management of immune checkpoint inhibitor-related dermatologic adverse events. Thorac Cancer. 2020;11:488–492. doi:10.1111/1759-7714.13275
3. Nadelmann ER, Yeh JE, Chen ST. Management of cutaneous immune-related adverse events in patients with cancer treated with immune checkpoint inhibitors: a systematic review. JAMA Oncol. 2022;8:130–138. doi:10.1001/jamaoncol.2021.4318
4. Koh HK, Fook-Chong SMC, Lee HY. Improvement of mortality prognostication in patients with epidermal necrolysis the role of novel inflammatory markers and proposed revision of SCORTEN (Re-SCORTEN). JAMA Dermatol. 2022;158:160–166. doi:10.1001/jamadermatol.2021.5119
5. Vivar KL, Deschaine M, Messina J, et al. Epidermal programmed cell death-ligand 1 expression in TEN associated with nivolumab therapy. J Cutan Pathol. 2017;44:381–384. doi:10.1111/cup.12876
6. Griffin LL, Cove-Smith L, Alachkar H, et al. Toxic epidermal necrolysis (TEN) associated with the use of nivolumab (PD-1 inhibitor) for lymphoma. JAAD Case Rep. 2018;4:229–231. doi:10.1016/j.jdcr.2017.09.028
7. Logan IT, Zaman S, Hussein L, Perrett CM. Combination therapy of ipilimumab and nivolumab-associated toxic epidermal necrolysis (TEN) in a patient with metastatic melanoma: a case report and literature review. J Immunother. 2020;43:89–92. doi:10.1097/CJI.0000000000000302
8. Gopee NH, Oliphant TJ, Hampton PJ. Toxic epidermal necrolysis occurring with immune checkpoint inhibitors. Dermatol Online. 2020;26(8):42.
9. Kim MC, Khan HN. Nivolumab-induced toxic epidermal necrolysis: rare but fatal complication of immune checkpoint inhibitor therapy. Cureus. 2021;13:e15017–e15017. doi:10.7759/cureus.15017
10. Kian W, Zemel M, Elobra F, et al. Intravenous immunoglobulin efficacy on pembrolizumab induced severe toxic epidermal necrolysis. Anticancer Drugs. 2022;33:E738–E740. doi:10.1097/CAD.0000000000001162
11. Gallo Marin B, Oliva R, Kahn B, et al. Pembrolizumab-induced toxic epidermal necrolysis in a patient with metastatic esophageal adenocarcinoma. R I Med J. 2022;105:34–36.
12. Zhao Y, Cao Y, Wang X, et al. Treatment of PD-1 inhibitor-associated toxic epidermal necrolysis: a case report and brief review. Onco Targets Ther. 2022;5(15):345–351. doi:10.2147/OTT.S353743
13. Kuijper EC, French LE, Tensen CP, Vermeer MH, Bouwes Bavinck JN. Clinical and pathogenic aspects of the severe cutaneous adverse reaction epidermal necrolysis (EN). J Eur Acad Dermatol Venereol. 2020;34:1957–1971. doi:10.1111/jdv.16339
14. Charlton OA, Harris V, Phan K, et al. Toxic epidermal necrolysis and Steven-Johnson syndrome: a comprehensive review. Adv Wound Care. 2020;9:426–439. doi:10.1089/wound.2019.0977
15. Posadas SJ, Padial A, Torres MJ, et al. Delayed reactions to drugs show levels of perforin, granzyme B, and Fas-L to be related to disease severity. J Allergy Clin Immunol. 2002;109:155–161. doi:10.1067/mai.2002.120563
16. Kinoshita M, Ogawa Y, Hama N, et al. Neutrophils initiate and exacerbate Stevens-Johnson syndrome and toxic epidermal necrolysis. Sci Transl Med. 2021;13. doi:10.1126/scitranslmed.aax2398
17. Wang S, Yuan P, Mao B, et al. Genomic features and tumor immune microenvironment alteration in NSCLC treated with neoadjuvant PD-1 blockade. NPJ Precis Oncol. 2022;6. doi:10.1038/s41698-021-00244-6
18. Schmidt V, Lalevée S, Traidl S, et al. Intravenous immunoglobulins, cyclosporine, and best supportive care in epidermal necrolysis: diverse effects on systemic inflammation. Allergy. 2022. doi:10.1111/all.15608
19. Ataseven A, Temiz SA, Eren G, et al. Comparison of anti-TNF and IL-inhibitors treatments in patients with psoriasis in terms of response to routine laboratory parameter dynamics. J Dermatol Treat. 2022;33(2):1091–1096. doi:10.1080/09546634.2020.1801975
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
