Back to Journals » Infection and Drug Resistance » Volume 16
Acute Colonic Perforation with Septic Shock Secondary to Disseminated Histoplasmosis in an Autologous Bone Marrow Transplant Recipient
Authors Bhatti H , Batbileg E, De S, Friedman G, Antony S
Received 7 January 2023
Accepted for publication 9 May 2023
Published 16 May 2023 Volume 2023:16 Pages 3029—3034
DOI https://doi.org/10.2147/IDR.S402228
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Harsimrandeep Bhatti, Enkhbileg Batbileg, Srijisnu De, Glen Friedman, Suresh Antony
Department of Internal Medicine, Las Palmas Del Sol Medical Center, El Paso, TX, USA
Correspondence: Harsimrandeep Bhatti, Department of Internal Medicine, Las Palmas Del Sol Medical Center, 10175 Gateway W. Suite 100, El Paso, TX, 79925, USA, Tel +1 915-539-4346, Email [email protected]
Abstract: Histoplasma capsulatum is an opportunistic pathogen which can lead to a wide variety of clinical presentations in the immunocompromised host. Post-transplant histoplasmosis in hematopoietic cell transplant recipients is exceedingly rare, with an incidence of < 1%. We present a case of acute caecal perforation resulting from disseminated histoplasmosis in a patient who had undergone autologous bone marrow transplant for plasma cell dyscrasia. This is a 71-year-old patient who initially presented due to progressive weakness associated with shortness of breath.
Keywords: infectious disease, bone marrow transplant, pancytopenia, disseminated histoplasmosis, colonic perforation, surgery
Introduction
Histoplasma capsulatum is a dimorphic fungus endemic to the Ohio, Missouri, and Mississippi River valleys in the United States. Infection in immunocompetent individuals is most commonly asymptomatic; however, immunocompromised patients, including those with hematologic malignancies, may experience severe invasive disease. Multiple organ systems can become involved with disseminated disease and prognosis is poor despite maximal intervention.1 Disseminated histoplasmosis is rarely encountered in nonendemic areas, especially in patients who have previously undergone autologous bone marrow transplant.2 This case highlights the importance of maintaining disseminated histoplasmosis in the differential in patients who have previously undergone autologous bone marrow transplant and have features consistent with invasive fungal disease.
Case Report
This patient is a 71-year-old male with a past medical history of hypothyroidism, multiple myeloma status post-autologous bone marrow transplant 4 years prior, who initially presented with a 2-week history of progressive generalized weakness associated with shortness of breath. Review of systems was pertinent for anorexia, fatigue, weight loss, and shortness of breath. The patient denied any fevers, chills, or cough. He also denied any similar symptoms in the past and denied any aggravating/alleviating factors. Vital signs on initial evaluation were notable for a low-grade fever of 100.2°F, a heart rate of 116 beats per minute, oxygen saturation of 94%, and a respiratory rate of 22. Physical exam was remarkable for cachexia, generalized weakness, and moderate respiratory distress.
Of note, the patient had completed all maintenance therapy by time of presentation, although it was unclear as to when maintenance therapy was terminated based on the patient’s provided history and lack of availability of prior medical records. Autologous hematopoietic stem cell transplant had taken placed 4 years prior to presentation. At that time induction therapy had been completed but the exact course was unavailable to us. No complications were reported during or immediately after HCT and the patient had been doing well. His ECOG score was 0 as the patient had returned to complete independent activity after HCT and completion of maintenance therapy. Additional details regarding his diagnosis and treatment were unfortunately unavailable to us during this patient’s hospital course.
Diagnosis/Treatment
Initial laboratory evaluation revealed: WBC 1.67 k/mm3, hemoglobin 6.8 g/dL, and platelets 18 k/mm3. Baseline blood counts were unavailable for our review; however, as per his history, the patient had attained remission post-bone marrow transplant and was not currently taking any maintenance therapy by this time. Liver function tests and electrolytes were unremarkable. Chest X-ray on admission was largely unremarkable, revealing minimal subsegmental atelectatic changes at the right lung base but no focal areas of acute airspace disease. Given the patient’s history of malignancy and high clinical suspicion for pulmonary embolus (PE), a CT angiogram of the chest/thorax was obtained, which was negative for PE but did reveal a small cavitary lesion in the left lower lobe (Figure 1). The patient was subsequently initiated on cefepime 1gm IV q6h and micafungin 150mg IV q24h due to suspected disseminated fungal infection. On hospital day 3, the patient acutely decompensated and went into septic shock, with physical exam findings consistent with an acute abdomen. A stat CT of the abdomen was obtained and revealed free intra-abdominal air concerning for bowel perforation. The patient was subsequently taken for emergent exploratory laparotomy with right hemicolectomy for perforated cecum. Post-operative pathology evaluation revealed diffuse Histoplasma capsulatum involving the cecum (Figure 2). Bone marrow aspiration was performed and also revealed diffuse Histoplasma capsulatum. The patient was transitioned to lipophilic amphotericin B as per IDSA and American Society of Transplantation guidelines.
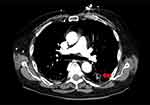 |
Figure 1 CT chest demonstrating small cavitary lesion in the left lower lobe (arrow). |
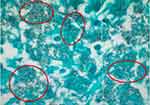 |
Figure 2 Cecum biopsy showing diffuse Histoplasma capsulatum (GMS stain). |
Follow-Up and Outcomes
Unfortunately, this patient’s post-operative course was complicated by the persistence of septic shock and metabolic encephalopathy. He continued to remain on ventilator support, requiring intermittent pressors for management of his septic shock despite appropriate interventions. The patient’s family elected to transition to hospice care given the patient’s poor clinical status and lack of improvement. The patient ultimately expired secondary to multi-organ failure in the setting of persistent shock.
Discussion
Histoplasma capsulatum infection, the most prevalent endemic mycosis in the US, is mainly transmitted via inhalation. Regions surrounding the Ohio and Mississippi River valleys are well-known hot spots.3 The highest rate of 6.1 cases per 100,000 population was recorded in the Midwestern US.3 Given the rarity of cases, incidence amongst immunocompromised patients, including those with a history of solid organ/bone marrow transplants and blood disorders, seems to be underestimated as they are often overlooked and misdiagnosed; currently the incidence in these populations is estimated at 0.02% annually.4
Invasive fungal infections are a significant cause of mortality in immunocompromised patients. Patients with plasma cell disorders, lymphoproliferative disorders, and myelodysplastic syndrome pursue hematopoietic stem-cell transplants (HCT) post-chemotherapy or as adjuvant therapy for prolonged remission and improved survival.5 Although HCT is pivotal in certain cases for transplant-eligible multiple myeloma patients, the associated and anticipated complications are equally important to consider. These patients are most sensitive to endemic fungi infection between weeks 5 and 18 post-transplantation.5 The overall incidence of invasive fungal infection in patients with hematopoietic stem-cell transplants is around 4%.5
Previous studies have reported that HCT recipients are at the highest risk for candida species infection during the pre-engraftment period and for aspergillosis species post-engraftment.5–7 Current guidelines recommend antifungal prophylaxis in all HCT recipients with fluconazole, although newer studies recommend voriconazole and posaconazole due to their increased activity against Aspergillus.7,8 This empiric and prophylactic antifungal treatment is now being challenged as it could be contributing to an underestimation of other invasive fungal infections.
Disseminated histoplasmosis is an aggressive, rapidly progressive granulomatous disease; 70–90% of cases with gastrointestinal involvement arise due to hematogenous seeding.3,9 The terminal ileum and proximal colon are the most common sites of focal plaques and ulcers.9,10 Clinical features include melena, colonic perforation, hematochezia, colonic mass/strictures leading to obstruction.11 Extrapulmonary manifestation of the disease has the potential to mimic other more common granulomatous diseases, such as IBD, malignancy, or other intestinal diseases.6,7,9,10,12 Diagnosis is rarely made from GI tract involvement alone, as in this case, as disseminated histoplasmosis typically has a multi-system presentation (Table 1).
 |
Table 1 Cases of Disseminated Histoplasmosis in Current Literature |
According to the IDSA, the current guideline for the treatment of severe disseminated histoplasmosis is lipophilic amphotericin B 3–5mg/kg/day for 1–2 weeks followed by Itraconazole 200mg BID for 12 months. In the case of intolerability of the first line azole, fluconazole can be considered for at least 12 months. Newer agents, such as Posaconazole, are considered as rescue treatment in cases with poor response.11,13 In this case, our patient was treated with amphotericin B deoxycholate.
Conclusion
We present a case of disseminated histoplasmosis in a patient with a history of multiple myeloma status post-HCT. We detailed the aggressive nature of disease progression, as well as clinical and pathology findings in his case. Furthermore, we highlight the medical and surgical management of disseminated histoplasmosis and its complications in accordance with IDSA and American Society of Transplantation guidelines. Although Histoplasmosis capsulatum is documented as endemic to the Ohio, Missouri, and Mississippi River valleys in the US, we emphasize the importance in considering disseminated histoplasmosis as a differential in patients with a history of HCT and immunocompromise regardless of geographical presentation in order to allow for swift recognition and early intervention. One area of future research may consider evaluating changes in endemicity of Histoplasmosis capsulatum given that more and more cases are being identified in “non-endemic” areas. IDSA and American Society for Transplantation guideline-directed medical therapy can reduce mortality and morbidity if initiated early.
Abbreviations
WBC, white blood cell; PE, pulmonary embolus; CT, computerized tomography; IDSA, Infectious Disease Society of America; HCT, hematopoietic cell transplant.
Data Sharing Statement
The data supporting the findings of this study can be obtained from the corresponding author according to reasonable request, and the corresponding author can be directly contacted for further inquiry.
Declarations
We declare the work presented is original, has been written in a collaborative manner by all authors, has been reviewed and approved by all authors, and has not been previously disseminated. This research was supported (in whole or in part) by HCA Healthcare and/or an HCA Healthcare-affiliated entity. The views expressed in this publication represent those of the authors and do not necessarily represent the official views of HCA Healthcare or any of its affiliated entities.
Informed Consent
Informed consent to publish the case details and any accompanying images was provided by the patient. Institutional approval was required via Centralized Algorithms for Research Rules on IRB Exemption (C.A.R.R.I.E) in order to publish case details.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Furcolow ML, Doto IL, Tosh FE, Lynch HJ. Course and prognosis of untreated Histoplasmosis: a United States public health service cooperative mycoses study. JAMA. 1961;177(5):292–296. doi:10.1001/jama.1961.03040310010003
2. Sy A, Chanson D, BeranoTeh J, et al. Late-occurring infections in a contemporary cohort of hematopoietic cell transplantation survivors. Cancer Med. 2021;10(9):2956–2966. doi:10.1002/cam4.3896
3. Histoplasmosis Statistics. Centers for Disease Control and Prevention; 2020. Available from: https://www.cdc.gov/fungal/diseases/histoplasmosis/statistics.html#one.
4. Xiong X-F, Fan -L-L, Kang M, Wei J, Cheng D-Y. Disseminated histoplasmosis: a rare clinical phenotype with difficult diagnosis. Respirol Case Rep. 2017;5(3):e00220. doi:10.1002/rcr2.220
5. Rahi MS, Jindal V, Pednekar P, et al. Fungal infections in hematopoietic stem-cell transplant patients: a review of epidemiology, diagnosis, and management. Ther Adv Infect Dis. 2021;8:204993612110390. doi:10.1177/20499361211039050
6. Goodwin JR, Robert A, Shapiro JL, et al. Disseminated Histoplasmosis: clinical and pathologic correlations. Medicine. 1980;59(1):1–33. doi:10.1097/00005792-198001000-00001
7. Bowden R, Ljungman P, Snydman D. Endemic mycoses after hematopoietic stem cell or solid organ transplantation. In: Transplant Infections. Section VI. Fungal Infections. Chapter 38. Springer; 2010.
8. Kauffman CA, Freifeld AG, Andes DR, et al. Endemic fungal infections in solid organ and hematopoietic cell transplant recipients enrolled in the Transplant‐Associated Infection Surveillance Network (TRANSNET). Transpl Infect Dis. n.d.;16(2):213–224. doi:10.1111/tid.12186
9. Kauffman CA, Miceli MH. Endemic mycoses after hematopoietic stem cell or solid organ transplantation. In: Ljungman P, Snydman D, Boeckh M, editors. Transplant Infections. Cham: Springer; 2016. doi:10.1007/978-3-319-28797-3_41
10. Mahajan VK, Raina RK, Singh S, et al. Case report: Histoplasmosis in Himachal Pradesh (India): an emerging endemic focus. Am J Trop Med Hyg. 2017;97(6):1749–1756. doi:10.4269/ajtmh.17-0432
11. Restrepo A, Tobón A, Clark B, et al. Salvage treatment of histoplasmosis with posaconazole. J Infect. 2007;54(4):319–327. doi:10.1016/j.jinf.2006.05.006
12. Kahi CJ, Wheat LJ, Allen SD, et al. Gastrointestinal Histoplasmosis. Am J Gastroenterol. 2005;100(1):220–231. doi:10.1111/j.1572-0241.2005.40823.x
13. Yang B, Lu L, Li D, et al. Colonic involvement in disseminated histoplasmosis of an immunocompetent adult: case report and literature review. BMC Infect Dis. 2013;13(143). doi:10.1186/1471-2334-13-143
14. Agrawal V, Brinda BJ, Farag SS. Histoplasma capsulatum infection in an allogeneic hematopoietic stem cell transplant patient receiving voriconazole prophylaxis. Case Rep Hematol. 2020;2020:8124137. PMID: 32099699; PMCID: PMC7039042. doi:10.1155/2020/8124137
15. Natarajan M, Swierzbinski MJ, Maxwell S, et al. Pulmonary Histoplasma infection after allogeneic hematopoietic stem cell transplantation: case report and review of the literature. Open Forum Infect Dis. 2017;4(2):ofx041. PMID: 28470019; PMCID: PMC5407209. doi:10.1093/ofid/ofx041
16. Haydoura S, Wallentine J, Lopansri B, Ford CD, Saad D, Burke JP. Disseminated histoplasmosis in allogeneic bone marrow transplant: a diagnosis not to be missed. Transpl Infect Dis. 2014;16(5):822–826. doi:10.1111/tid.12261
17. Grover S, Midha N, Gupta M, Sharma U, Talib V. Imaging spectrum in disseminated histoplasmosis: case report and brief review. Australas Radiol. 2005;49(2):175–178. doi:10.1111/j.1440-1673.2005.01369.x
18. Simon CT. Unexpected disseminated Histoplasmosis detected by bone marrow biopsy in a solid organ transplant patient. Clin Case Rep. 2017. https://onlinelibrary.wiley.com/doi/10.1002/ccr3.1282.
19. Shaikh MS, Memon AM. Disseminated histoplasmosis in an immuno-competent young male: role of bone marrow examination in rapid diagnosis. Diagn Cytopathol. 2018;46:273–276. doi:10.1002/dc.23834
20. Kumari M, Udayakumar M, Kaushal M, Madaan GB. Unusual presentation of disseminated histoplasmosis in an immunocompetent patient. Diagn Cytopathol. 2017;45:848–850. doi:10.1002/dc.23742
21. Kutkut I, Vater L, Goldman M, et al. Thrombocytopenia and disseminated Histoplasmosis in immunocompetent adults. Clin Case Rep. 2017;5(12):1954. doi:10.1002/ccr3.1182
22. Angius AG, Viviani MA, Muratori S, Cusini M, Brignolo L, Alessi E. Disseminated histoplasmosis presenting with cutaneous lesions in a patient with acquired immunodeficiency syndrome. J Eur Acad Dermatol Venereol. 1998;10(2):182–185. doi:10.1111/j.1468-3083.1998.tb00724.x
23. Mohan M, Fogel B, Eluvathingal T, Schinke C, Kothari A. Gastrointestinal histoplasmosis in a patient after autologous stem cell transplant for multiple myeloma. Transpl Infect Dis. 2016;18(6):939–941. doi:10.1111/tid.12619
24. Fewings JD, Lander H, Anderson KF, Henning FR, Radden BG, Jeanes BJ. Disseminated Histoplasmosis. Australas Ann Med. 1970;19(2):151–158. doi:10.1111/imj.1970.19.2.151
25. Angsutararux T, Chongtrakool P, Sukpanichnant S, Wongwaipijarn K, Wangchinda W. Disseminated histoplasmosis in a kidney transplant patient. Transpl Infect Dis. 2021;23(1):e13405. doi:10.1111/tid.13405
26. Lo MM, Mo JQ, Dixon BP, Czech KA. Disseminated Histoplasmosis associated with hemophagocytic lymphohistiocytosis in kidney transplant recipients. Am J Transplant. 2010;10(3):687–691. doi:10.1111/j.1600-6143.2009.02969.x
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
