Back to Journals » Infection and Drug Resistance » Volume 14
Acid-Fast Bacilli Positivity Rate and Associated Factors among Leprosy Suspected Cases attending Selected Health Facilities located in West Arsi Zone, Oromia, Ethiopia
Authors Bekala D, Reda DY, Ali MM
Received 13 September 2021
Accepted for publication 20 October 2021
Published 3 November 2021 Volume 2021:14 Pages 4581—4589
DOI https://doi.org/10.2147/IDR.S339102
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Degefa Bekala,1 Dawit Yihdego Reda,2 Musa Mohammed Ali2
1Bulchana Health Center, Oromia, Ethiopia; 2School of Medical Laboratory, College of Medicine and Health Sciences, Hawassa University, Hawassa, Ethiopia
Correspondence: Musa Mohammed Ali Email [email protected]
Introduction: Leprosy is one of the neglected tropical diseases that affect skin and peripheral nervous system often results in severe, lifelong disabilities and deformities. Even though multidrug therapy was in place for more than 30 years to treat and prevent leprosy worldwide including Ethiopia, its epidemiology is not well studied in the West Arsi zone.
Objective: The aim of this study was to determine the prevalence of acid-fast bacilli (AFB) positivity rate and associated factors among leprosy suspected cases.
Methods: A health facility-based cross-sectional study was conducted among 422 leprosy suspected cases from August 2020 to December 2020. To detect AFB, skin slit specimens were collected and examined using the Ziehl-Neelsen staining technique. Socio-demographic and clinical data were collected using a structured questionnaire. Data were analyzed using Statistical Package for Social Sciences (SPSS) version 24. Logistic regression was employed to determine predictors of AFB positivity rate.
Results: Acid-fast bacilli were detected among 46 leprosy suspected cases which gives a prevalence of 10.9% with 95% CI (8.2‒15.6). Suspected leprosy cases with multibacillary type were 4 times more likely to be AFB positive (p=0.021) than their counterparts. Study participants who had contact with known leprosy cases were 2 times more likely to be AFB positive (p = 0.032) and those with no formal education were 2 times more likely to be AFB positive (p = 0.03). Participants who had close contact with leprosy patients for ≥ 3 years were 8 times more likely to be AFB positive (p = 0.02).
Conclusion: This study revealed a high prevalence of AFB positivity rate in the era of multidrug therapy. Types of leprosy, close contact with known leprosy cases, educational status, and duration of closer contact with leprosy cases were significantly associated with AFB positivity rate.
Keywords: acid-fast bacilli, Ethiopia, Mycobacterium leprae, prevalence, West Arsi zone, Ziehl-Neelsen staining
Introduction
Leprosy is a neglected tropical disease affecting the skin and peripheral nervous system often results in severe, lifelong disabilities, and deformities. Moreover, it has a negative impact on the socioeconomic status of the patient and the patient’s relatives.1,2 Until 2008, leprosy was thought to be caused by Mycobacterium leprae. Another species of Mycobacterium associated with Leprosy, M. lepromatosis, was reported by Han et al3,4 The transmission of Leprosy is not well known. The clinical observation indicated that living in close contact with leprosy patients as the main risk factor for Leprosy. Transmission of Leprosy occurs mainly through infectious aerosols and skin-to-skin contact.1,5
In 1981, World Health Organization (WHO) developed a multidrug therapy (MDT) for leprosy which was revised later in 1998.6 If MDT is not initiated as early as possible, permanent damage, neural damage, and permanent physical disability may not be averted.6,7 Multidrug therapy was effective and has played a critical role in the reduction of the leprosy burden, by reducing human-to-human transmission.8
Prevalence of Leprosy was deceased in all WHO regions; however, antimicrobial resistant (AMR) M. leprae was increased from 34,358 in 2018 to 35,231 in 2019. Globally, 202,185 new cases were detected representing a global decrease of 6506 cases.9 There was a constant and gradual reduction in the number of new leprosy cases in the last 10 years. Brazil, India, and Indonesia reported >10,000 new cases, while 13 other countries including Ethiopia reported 1000–10,000 new cases. In 2019, about 3201 new cases of leprosy were detected in Ethiopia.9
Even though the prevalence of leprosy has significantly decreased and most highly endemic countries have reached eradication level which is defined as a registered prevalence rate of < 1 case/10,000 population, it continues to be a global health concern.10 In 2010, Ethiopia was one of the 17 nations reporting 1000 or more incidence per year. Ethiopia was the second uppermost nation in Sub-Saharan Africa in leprosy prevalence.11 Greater than 76% of the leprosy cases in Ethiopia were reported from Oromia and Amhara regional states in 2012 (unpublished data: Federal Ministry of Health (FmoH); presentation at the National Leprosy Workshop at AHRI; 2012). Furthermore, there are pocket areas that report a high number of cases yearly.
In Ethiopia, the prevalence of leprosy has sharply declined from 19.8 per 10,000 populations in 1983 to 0.5 per 10,000 populations in 2012 following the introduction of MDT.1 Registered prevalence is a useful proxy indicator of the disease burden as it reflects the number of active leprosy cases diagnosed with the disease and receiving treatment with MDT. The aim of this study was to determine the prevalence of Acid-Fast bacilli (AFB) positivity rate and associated factors among Leprosy suspected cases attending selected health facilities.
Materials and Methods
Study Design and Area
A cross-sectional study was conducted in selected health facilities located in West Arsi zone, Oromia Regional State, Ethiopia from August 2020‒December 2020 (Figure 1). The Zone has 15 (13 rural, 2 towns) woredas with an altitude ranging from 2850 to 3000 meters above sea level. The zonal temperature ranges from 14°C to 27°C that divided the zone into three clusters: Lowland (14.9%), Midland (39.6%), and Highland area (45.5%). According to the Zonal report of 2019, the zone has an estimated population of 2,695,099 out of which 1,544,879 are females. There are 417 public health facilities of which 5 are hospitals, 88 are health centers, 324 are health posts and 203 are private medium and higher clinics including one non-governmental organization hospital and two private hospitals. Leprosy diagnosis and treatment services are given in all government health institutions and some selected private hospitals and clinics.
In the present study, the following 8 health facilities were selected: Shashamane Comprehensive Specialized hospital (SCSH), Gambo hospital (GH), Kokosa hospital (KH), Kofale health center (KHC), Kore health center (KoHC), Adaba health center (AHC), Nensebo health center (NHC), and Gedeb hasasa health center (GHHC). Out of eight health facilities selected, seven were located in a high land area whereas only one (SCSH) was located in a midland area (Figure 1).
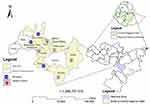 |
Figure 1 Study sites include three hospitals and five health centers in the West Arsi zone. |
Study Population
For this study, all Leprosy suspected cases that reside in the West Arsi zone during the study period were considered as source population whereas all Leprosy suspected cases who visited one of the selected Government health facilities found in West Arsi zone during the study period and fulfilled inclusion criteria were considered as a study population.
Eligibility Criteria
Inclusion criteria: Any person who was suspected of leprosy, visited one of the participating health facilities, never took the anti-leprosy drug before, did not know their leprosy status at the time of recruitment, and who were voluntary to participate in the study were included in the study.
Exclusion criteria: Leprosy patients who revisited one of the participating health facilities for the treatment and patients who were referred from other health facilities to continue the treatment were excluded from the study.
Study Variables
Dependent variable: AFB positivity rate.
Independent variables: Age, sex, residence, occupational status, educational status, monthly income, family size, number of rooms in a house, types of Leprosy, history of a family member with leprosy, close contact with Leprosy patients, duration of contact with leprosy patients, and clinical characteristics of study participants.
Sample Size Determination
A single proportion population formula was used to calculate the sample size by considering 50% prevalence, 5% margin of error, 95% confidence interval, and 10% for non-response rate. Accordingly, the total sample size was 422. To recruit participants, a convenient sampling technique was used.
Data Collection
After written informed consent and assent (wherever it is applicable) was obtained, socio-demographic characteristics such as age, sex, marital status, educational status, place of residence, monthly income, and clinical characteristics which include the type of Leprosy, presence of hypopigmented skin, types of lesion, lesion with pain, numbness on hand, eyelid exhaustion, difficulty in closing eyelids, presence of nodules on face and ears, and history of a family member with leprosy were collected using a structured questionnaire by face-to-face interview.
Skin slit specimens were collected from suspected cases by attending laboratory personnel. The samples were collected from both ear lobes, elbow of both arms, and thighs with visible skin lesions or areas with altered sensation. A 5 mm punch biopsy was taken from the most erythematous, contagious, expanding area that was a blood-free part of the skin.12 The skin slit was collected aseptically using a sterile blade for each patient. The smears were stained by the Ziehl-Neelsen method for the detection of AFB.
Quality Control
A questionnaire, which was originally prepared in English, was translated to Afaan Oromo (widely spoken in the area) and translated back to English to check the similarity. To assess the appropriateness of the questionnaire, a pre-test was conducted on 5% of study participants 2 weeks before the actual data collection period at Halaba primary hospital. The standard operating procedure was followed during sample collection, sample processing, Ziehl-Neelsen staining, and recording of the data.
Data Analysis
For data entry and analysis Statistical Package for Social Sciences version 24 was used. Descriptive statistics were used to present summarized data. Logistic regression was employed to determine the association between dependent and independent variables. Factors with a p-value less than 0.25 in bivariate analysis were further analyzed using multivariate analysis. A p<0.05 was considered statistically significant.
Operational Definition
Leprosy suspected cases: A dark-skinned person with patches on skin, loss or decrease of sensation in the skin patch, numbness of feet or hand, and swelling or lumps in the face or earlobes.
Leprosy confirmed cases: A person with at least one of the three cardinal signs: (i) definite loss of sensation in a pale or reddish skin patch, (ii) thickened or enlarged peripheral nerve which lost sensation and or weakness of the muscles, or (iii) positive for AFB in a slit skin smear.13
Leprosy contacts: Individuals who live in the same house and share kitchen and social area. Persons those who are indicated by the index case and have other types of associations, which includes blood relatives, neighbors, friends, and colleagues.14
Multibacillary: Is a case of leprosy with greater than five skin lesions, or with nerve involvement, or with bacilli in a slit-skin smear, irrespective of the number of skin lesions.
Paucibacillary: Is a case of leprosy with 1 to 5 skin lesions, without demonstrated presence of bacilli in a skin smear.
Results
Socio-Demographic Characteristics
In this study, 422 leprosy suspected cases were included with 100% response rate. Most participants were from SCSH (n = 110, 26.1%) followed by KH (n = 90, 21.3%), GH (n = 77, 18.3%), KoHC (n = 36, 8.5%), GHHC (n = 31, 7.4%), KHC (n = 29, 6.9%), AHC (n = 29, 6.9%), and NHC (n = 20, 4.7%). The mean age of participants with standard deviation was 34.9 ±15.4 years (Range: 7‒74 years). Most participants were in the age category of 46‒74 years. Place of residence for 381 (90.3%) was rural. One hundred fifty-four (36.5%) of the study participants were farmers whereas 94 (22.3%) were housewives. Participants with no formal education accounted for 46.2% whereas 26.5% and 1.7% of participants completed grade 1‒4 and 9‒12 respectively (Table 1).
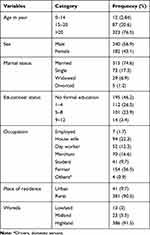 |
Table 1 Socio-Demographic Characteristics of Leprosy Suspected Cases Attending Selected Health Facilities Located in West Arsi Zone, August 2020‒December 2020 (N = 422) |
Clinical Characteristics of Participants
Of 422 Leprosy suspected cases included in the present study, 97.9% live with their family, 70.6% have a family size greater than 4. Clinically, 73% of participants had hypopigmented skin; most of the lesions were papule type. About 18% of participants had a family member with a history of leprosy and 40.3% had contact with known leprosy cases (Table 2).
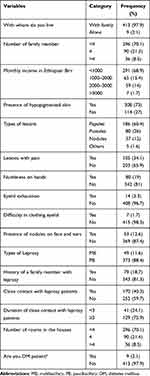 |
Table 2 Clinical Characteristics of Leprosy Suspected Cases Attending Selected Health Facilities Located in West Arsi Zone, August 2020‒December 2020 (N = 422) |
Prevalence of AFB Positivity Rate
Of the 422 Leprosy suspected cases, 46 (10.9%) were AFB positive with 95% CI (8.2‒15.6). Acid-Fast bacilli detection rates according to the sites of specimen collection were as follows: ear lobes 4/37 (10.8%), arms 9/68 (13.2), and thighs 33/317 (10.4%). A high AFB positivity rate (72%) was found from thighs (Figure 2). Acid-Fast bacilli detection rates across the study sites were as follows: SCSH 10/110 (9.1%), GH 8/77 (10.4%), KH 11/90 (12.2%), KHC 3/29 (10.5%), KoHC 3/36 (8.3%), GHHC 3/31 (9.7%), NHC 5/20 (25%), and AHC 5/29 (17.2%) (Figure 3). Regarding AFB distribution in age groups 1/99 (1%), 4/100 (4%), 13/104 (12.5%), and 28/119 (23.5%) participants in age group 7‒20, 21‒34, 35‒45, 46‒74 were positive for AFB respectively.
Factors Associated with AFB Positivity Rate
Nearly all independent variables analyzed by bivariate analysis had a p-value less than 0.25. Factors such as types of Leprosy, close contact with known Leprosy cases, educational status, number of rooms in a house, and duration of close contact with Leprosy cases were significantly associated with AFB positivity rate (p < 0.05). Study participants with multibacillary (MB) type leprosy were about 4 times more likely to be AFB-positive (p = 0.021). Study participants with a history of close contact with known Leprosy cases were about 2 times more likely to be AFB-positive (p = 0.032) whereas those with no formal education were about 2 times more likely to be positive for AFB (p = 0.03). Participants who had close contact with leprosy patients for ≥3 years were about 8 times more likely to be positive for AFB (p = 0.02) and those who lived in a house with room number ≥ 4 were also about 8 times more likely to be AFB positive (p = 0.04) (Table 3).
Discussion
Even though Leprosy cases were declined after the implementation of MDT in Ethiopia, still there are some cases reported from some parts of Ethiopia.1,15 In this study, the overall AFB positivity rate among Leprosy suspected cases was 10.9%, which was high compared to the report from different regions of the world16 and a recent report from the Eastern part of Ethiopia.17 Before initiation of MDT, the prevalence of Leprosy in Ethiopia was 19.8% which was higher than our finding; however, in 2012 it was dropped below 0.5%.1 The current study showed the increment of Leprosy cases as compared to overall leprosy cases reported in 2012.1 Besides, WHO in 2019 reported a global prevalence of 22.9 per million population9 and a prevalence of 0.21 per 10,000 population from Africa which is lower than our findings.
A relatively recent study conducted at Gambo hospital, Ethiopia, reported a high prevalence of Leprosy among children and adolescents (31.7%), and low prevalence was found among adults (11.7%).7 Both findings from Gambo hospital were higher than the current study. Moreover, our finding was relatively high compared to the prevalence of Leprosy reported from Indonesia (~7.5%)16 and Colombia (7%).18 On the other hand, India reported a higher burden of leprosy cases (55.5%) than the current study.16
The difference in prevalence might be due to the variation of M. leprae epidemiology in the general population, study design, study period, attitude, and traditional practices. Most importantly, wide use of MDT might be the main reason for variation observed. In countries where MDT is effectively applied, the number of new cases of Leprosy is expected to decline over time. According to this study, there are a significant number of leprosy cases in the West Arsi zone which need attention from concerned bodies.
In this study, we assessed some factors that could predispose to AFB positivity rate. Among factors analyzed, the type of leprosy was associated with the AFB positivity rate. The frequency of AFB was high among Leprosy suspected cases with multibacillary type Leprosy. It is well known that in multibacillary type Leprosy the immune system is polarized to a humoral immune response, which is incapable in limiting multiplication of M. leprae.2 As a result, Leprosy suspected cases with a multibacillary type of Leprosy tend to be positive for AFB as compared to their counterparts.
Study participants who had a history of close contact with known Leprosy cases were at increased risk of being AFB-positive (p = 0.032). One of the transmission ways of M. leprae is close contact especially long-term contact increases the acquisition of M. leprae multiple folds. In this study, participants who had close contact with known leprosy patients for more than three years had a high chance to be positive for AFB (p = 0.02). This indicates living with leprosy patients for a long time without prophylaxis could predispose them to M. leprae infection.
It seems education is a key for the prevention of Leprosy transmission in the community, especially among family members. In the current study, participants with no formal education were more likely to be positive for AFB (p = 0.03) unlike those who had some form of formal education. This is because educated individuals might have knowledge on how the disease is transmitted and apply their knowledge in preventing the transmission of the disease.
Limitation of the Study
As the sensitivity of Ziehl-Neelsen staining is low it may not show the true prevalence of AFB positivity rate. Since we used a convenient sampling technique, selection bias might have not been avoided. Study participants may not remember all questions asked as a result recall bias might have been introduced.
Conclusions
The finding of this study indicated a high prevalence of AFB positivity rate among Leprosy suspected cases in selected health facilities found in West Arsi zone, Oromia, Ethiopia. Types of Leprosy, close contact with known Leprosy cases, educational status, and duration of closer contact with Leprosy cases were significantly associated with AFB positivity rate.
Abbreviations
MDT, Multidrug therapy; WHO, World Health Organization; AFB, Acid-Fast bacilli; SCSH, Shashamane comprehensive specialized hospital; GH, Gambo hospital; KH, Kokosa hospital; GHHC, Gedeb Hasasa health center; KHC, Kofale health center; AHC, Adaba health center; KoHC, Kore health center; NHC, Nensebo health center.
Data Sharing Statement
All relevant data are available within the paper.
Ethical Approval and Consent to Participate
This study was ethically cleared by the IRB of the College of Medicine and Health Sciences, Hawassa University with the letter Ref number IRB/228/12. Permission letter was obtained from the Oromia Regional Health Bureau (BEFO/HITFU/1-16/195) and from the West Arsi Zone Health office (EFI 362/2012). Permission was also obtained from all health facilities selected for the present study. All study participants and parents or legal guardians were informed about the study and written informed consent was obtained from all participants. Informed consent was also obtained from parents or legal guardians of participants under age 18 years before initiation of the study. All methods were carried out in accordance with the Declaration of Helsinki.
Acknowledgments
We would like to thank all selected health facilities located in the West Arsi Zone for their cooperation during data collection. We thank Mr Getachew Meka for drawing the map. We would also like to thank all study participants for their willingness to participate in the study.
Funding
No funding was received for this study.
Disclosure
The authors have declared no conflicts of interest for this work.
References
1. Sori EA. Review on the burden of leprosy in Ethiopia. J Trop Dis. 2019;7(297):2.
2. Khadge S, Banu S, Bobosha K, et al. Longitudinal immune profiles in type 1 leprosy reactions in Bangladesh, Brazil, Ethiopia and Nepal. BMC Infect Dis. 2015;15(1):1–2. doi:10.1186/s12879-015-1128-0
3. Han XY, Seo YH, Sizer KC, et al. A new Mycobacterium species causing diffuse lepromatous leprosy. Am J Clin Pathol. 2008;130(6):856–864. doi:10.1309/AJCPP72FJZZRRVMM
4. Han XY, Mistry NA, Thompson EJ, Tang HL, Khanna K, Zhang L. Draft genome sequence of new leprosy agent Mycobacterium lepromatosis. Genome Announc. 2015;3(3):e00513–15. doi:10.1128/genomeA.00513-15
5. Ploemacher T, Faber WR, Menke H, Rutten V, Pieters T. Reservoirs and transmission routes of leprosy; A systematic review. PLoS Negl Trop Dis. 2020;14(4):e0008276.
6. Lazo-Porras M, Prutsky GJ, Barrionuevo P, et al. World Health Organization (WHO) antibiotic regimen against other regimens for the treatment of leprosy: a systematic review and meta-analysis. BMC Infect Dis. 2020c;20(1):1–4. doi:10.1186/s12879-019-4665-0
7. Ramos JM, Ortiz-Martínez S, Lemma D, et al. Epidemiological and clinical characteristics of children and adolescents with leprosy admitted over 16 years at a rural hospital in Ethiopia: a retrospective analysis. J Trop Pediatr. 2018;64(3):195–201. doi:10.1093/tropej/fmx048
8. Rodrigues LC, Lockwood DN. Leprosy now: epidemiology, progress, challenges, and research gaps. Lancet Infect Dis. 2011;11(6):464–470. doi:10.1016/S1473-3099(11)70006-8
9. World Health Organization (WHO). Weekly Epidemiology Record. No 36. 2020;95:417–440. Available from: https://reliefweb.int/report/world/weekly-epidemiological-record-wer-4-september-2020-vol-95-no-36-pp-417-440-enfr. Accessed October 28, 2021.
10. Mondiale de la Santé O; World Health Organization. Global leprosy update, 2018: moving towards a leprosy-free world – Situation de la lèpre dans le monde, 2018: parvenir à un monde exempt de lèpre. Wkly Epidemiol Rec. 2019;94(35/36):389–411. Available from: https://apps.who.int/iris/handle/10665/326776. Accessed October 28, 2021.
11. World Health Organization (WHO). Global leprosy situation, beginning of 2008. Wkly Epidemiol Rec. 2008;83(33):293–300. Available from: https://www.who.int/publications-detail-redirect/who-wer8333. Accessed October 28, 2021.
12. Reibel F, Cambau E, Aubry A. Update on the epidemiology, diagnosis, and treatment of leprosy. Med Mal Infect. 2015;45(9):383–393. doi:10.1016/j.medmal.2015.09.002
13. World Health Organization. WHO expert committee on leprosy: eighth report. World Health Organization; 2012. Available from: https://apps.who.int/iris/handle/10665/75151. Accessed October 28, 2021.
14. Dos Santos DS, Duppre NC, Sales AM, Nery JA, Sarno EN, Hacker MA. Kinship and leprosy in the contacts of leprosy patients: cohort at the Souza Araujo outpatient clinic, Rio de Janeiro, RJ, 1987–2010. J Trop Med. 2013;2013:1–8. doi:10.1155/2013/596316
15. Abdela SG, Diro E, Zewdu FT, et al. Delayed diagnosis and ongoing transmission of leprosy in the post-elimination era in Boru Meda hospital, Ethiopia. J Infect Dev Ctries. 2020;14(06.1):10S–5S. doi:10.3855/jidc.11706
16. World Health Organization. Global health observatory data repository; 2017. Available from: https://apps.who.int/gho/data/node.main. Accessed October 28, 2021.
17. Urgesa K, Bobosha K, Seyoum B, et al. Evidence for hidden leprosy in a high leprosy-endemic setting, Eastern Ethiopia: the application of active case-finding and contact screening. PLoS Negl Trop Dis. 2021;15(9):e0009640. doi:10.1371/journal.pntd.0009640
18. Cardona-Castro N. Leprosy in Colombia. Curr Trop Med Rep. 2018;5(2):85–90. doi:10.1007/s40475-018-0145-7
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.