Back to Journals » Clinical and Experimental Gastroenterology » Volume 11
Achalasia and esophageal cancer: risks and links
Authors Torres-Aguilera M, Remes Troche JM
Received 16 April 2018
Accepted for publication 29 June 2018
Published 6 September 2018 Volume 2018:11 Pages 309—316
DOI https://doi.org/10.2147/CEG.S141642
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Andreas M. Kaiser
Maura Torres-Aguilera,1 José María Remes Troche2
1Department of Pediatric Gastroenteritis, Hospital CMN “La Raza”, Mexico City, Mexico; 2Digestive Physiology and Motility Laboratory, Medical Biological Research Institute, Universidad Veracruzana, Veracruz, Mexico
Abstract: Esophageal cancer affects more than 4,50,000 persons worldwide, and its incidence has increased in recent years. It is the eighth most common cancer across the globe. The main histologic types are esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EA), and their associated risk factors are well known. Achalasia, an idiopathic esophageal disorder that conditions aperistalsis and the absence of lower esophageal sphincter relaxation, stands out among them. The prevalence of ESCC in subjects with esophageal achalasia is 26 in every 1,000 cases, whereas the prevalence of EA is 4 in every 1,000. Patients with achalasia have a 50 times higher risk of presenting with ESCC than the general population, and the disease manifests 20–25 years after achalasia symptom onset. Multiple mechanisms are related to the development of ESCC in achalasia, and they include bacterial overgrowth, food stasis, genetic alterations, and chronic inflammation. Regarding the risk of EA in achalasia patients, most cases are associated with Barrett’s esophagus, due to uncontrolled chronic acid reflux. Given that achalasia is a well-established factor for ESCC/EA, clinicians must be aware of said associations to enable the development of programs for the prevention and opportune detection of these cancers in patients with achalasia.
Keywords: esophageal cancer, achalasia, cancer, risk factors, gastroesophageal reflux
Introduction
Esophageal cancer affects more than 4,50,000 persons worldwide, and its incidence has increased in recent years.1 It is the eighth most common cancer across the globe.2 Nearly four out of five cases occur in nonindustrialized nations, with the highest rates in Asia and Africa.2 The National Cancer Institute has estimated 16,910 new cases of esophageal cancer and 15,910 deaths from the disease in 2016. In the majority of countries, the estimated range of 5-year survival in patients with esophageal cancer is from 15% to 25%.3 In most cases, the outcome is poor and the mortality rate is high.4
There are two main histologic types of esophageal cancer: esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EA). The incidence, ethnic pattern, and geographic distribution of the two pathologies are significantly heterogeneous.5 ESCC is the most frequent histologic subtype worldwide and more frequently presents in underdeveloped countries, whereas EA is the histologic subtype in up to 50% of the cases of esophageal cancer in Western countries, and its incidence has increased rapidly.2 The most widely accepted explanation for that phenomenon is the elevated prevalence of obesity, illustrated by the fact that the risk of EA is three times higher in obese subjects.1,5
Other less frequent (<5%) neoplasias can develop in the esophagus, such as esophageal sarcoma and small cell carcinoma in 1%–2% of cases. Melanomas, leiomyosarcomas, carcinoid tumors, and lymphomas are even rarer.1
As occurs in the majority of malignant neoplasias of the gastrointestinal tract, esophageal cancer is associated with well-known factors, but unlike other cancers, esophageal cancer screening is difficult and not very reliable, and late presentation is common in most cases.6
In addition, achalasia is a relatively rare condition with an annual incidence rate of 0.5–1.2 per 1,00,000 individuals.7 It is a motility disorder of the esophagus, and although its etiology has not been conclusively determined, there is increasing evidence that achalasia is the result of autoimmune mechanisms, based on its high association with other autoimmune disorders and with human leukocyte antigen abnormalities.8 The main pathophysiologic mechanism described is a decrease in the number of myenteric neurons, or their absence, causing aperistalsis and impaired relaxation of the lower esophageal sphincter. Most likely, the myenteric neurons disappear due to chronic ganglionitis.8 Clinically, patients develop dysphagia as a consequence of the loss of esophageal peristalsis and failure of the lower esophageal sphincter to relax, especially during swallowing.9 Achalasia is a relatively rare condition, but it has the complication risks of megaesophagus,10 aspiration pneumonia,11 and esophageal cancer, specifically ESCC.12,13
The present article is a review of the available evidence on the relationship between achalasia and ESCC/EA, taking into account the pathophysiologic mechanisms involved, the clinical evidence, and the possibility of disease detection and opportune treatment in patients presenting with those associated pathologies.
Risk factors for ESCC
As is the case of all malignant neoplasias, ESCC is the result of the interaction of numerous factors (Table 1) that provide the predisposing conditions for the esophageal mucosa to come into contact with carcinogens.2 The incidence of ESCC also increases with patient age and peaks in the seventh decade of life. ESCC frequency is three times higher in blacks than in whites, whereas adenocarcinomas are more common in white males.1
  | Table 1 Risk factors associated with esophageal squamous cell carcinoma and esophageal adenocarcinoma Note: aMore than three drinks per day. Abbreviation: SCC, squamous cell carcinoma. |
Most factors implicated in cancer of the esophagus, so far, appear to act directly on the esophagus, rather than behaving systemically. Smoking and chronic alcohol consumption are the greatest risk factors associated with ESCC, especially in combination. Research suggests that tobacco carcinogens, chiefly nitrosamines, come into contact with the esophageal mucosa through tobacco condensate ingestion.1,14 The number of cigarettes smoked per day and the length of time spent smoking are directly correlated with the risk of esophageal cancer.1,14 Hookah smoking, nass (a smokeless tobacco) use, and opium consumption are other factors related to ESCC.15 Interestingly, there is a higher risk of developing ESCC in patients who have presented with squamous cell cancer in other parts of the body (especially the head and neck),16,17 which is associated with the fact that the same risk factors of alcohol and tobacco are shared.16,17 The estimated excess risk of ESCC in patients with head and neck cancer expressed with a standardized incidence ratio is 21.16 Currently, the risk of developing a synchronous or metachronous ESCC is the highest in patients with hypopharyngeal and oropharyngeal cancers, followed by oral cavity, laryngeal, or nasopharyngeal cancers.18,19 Likewise, patients are at increased risk of metachronous esophageal cancer after endoscopic resection of ESCC. The risk of metachronous cancer is estimated at 4%–25% at 4 years of follow-up.20
Nutritional deficiencies, another factor related to ESCC, can develop through chronic alcohol use, as well as through poverty and lack of an adequate food supply. However, not everything can be explained from the dietary perspective. Chronic esophageal irritation also occurs when food is retained and decomposed by bacteria, releasing various chemical irritants. Frequent consumption of hot beverages also appears to increase the incidence of ESCC.21 Drinking green tea at high temperatures resulted in a six or seven times greater increase in the risk of ESCC in patients who were also smokers.22
Esophagitis due to caustic agents is another factor involved in the development of ESCC.16 The risk of presenting with ESCC in patients with esophagitis caused by corrosive agents has been described at 1,000–3,000 times higher than in the general population.23 ESCC can present within the first year of exposure to a corrosive agent or up to 40 years later.24,25 The mechanism associated with the development of ESCC due to caustic agents is related to the chronic inflammation they condition, as well as to the local damage they produce.
A genetic predisposition is also associated with the development of ESCC and is linked to specific genes involved in alcohol metabolism, such as those related to the alcohol dehydrogenase and the aldehyde dehydrogenase 2 enzymes.26 The cyclin D1 (CCND1) G870A polymorphism has also been associated with ESCC.27 In addition, tylosis, a disease with an autosomal dominant pattern of inheritance mapped to a region on chromosome 17q25, is related to the development of ESCC.16,28 Tylosis is characterized by a thickening of the palms of the hands and soles of the feet, and there is a 40%–95% increased risk of developing ESCC throughout the patient’s lifetime.16,28,29
The geographic variability in the incidence of esophageal cancer (more frequent in China, Africa, and the Middle East) strongly points to nutritional factors and is correlated with areas whose populations have a deficient nutritional status.1,2 Along with malnutrition itself, micronutrient deficiencies of zinc, molybdenum, magnesium, and iron in the soil are involved. The clearest relation is that of molybdenum deficiency. It enables the accumulation of nitrates and nitrites in plants, which in turn convert them into nitrosamines, the known esophageal carcinogens.30 Plummer–Vinson (or Paterson–Kelly) syndrome has been associated with carcinoma of the upper third of the esophagus and is related to both iron-deficiency anemia and vitamin B deficiencies.31 Human papillomavirus (HPV) and Epstein–Barr virus have also been associated with ESCC.32
Finally, the prevalence of esophageal cancer in achalasia is variable, but most cases in patients with achalasia correspond to ESSC. However, there have also been cases of EA associated with Barrett’s esophagus. These two associations and their mechanisms will be discussed in detail in the following sections.
Risk factors for EA
The main risk factor for EA is Barrett’s esophagus, a preneoplastic condition considered a chronic complication of gastroesophageal reflux disease. A linear increase in the incidence of EA has been described, which coincides with a greater incidence of Barrett’s esophagus. It is important to emphasize that the risk factors for Barrett’s esophagus are also considered risk factors “per se” for EA, such as smoking, chronic alcohol consumption, obesity, male sex, and white race.33 The increasing prevalence of obesity in the Western world is currently thought to be the main risk factor for EA. It has been postulated that obesity increases intraabdominal pressure, which serves as a chronic mechanism that induces gastroesophageal reflux through a specific mechanism, but other studies suggest that adipose tissue itself influences tumor development.34,35
Links
Achalasia as a risk factor for ESCC
Clinical evidence
The clinical manifestations of achalasia are characterized by progressive dysphagia, predominant nocturnal regurgitation, nondigested food aspiration, and weight loss. Nevertheless, in the early disease stages, characteristics can be similar to those of gastroesophageal reflux disease, including the typical retrosternal thoracic pain after eating and stomach acidity.36 Due to the nonspecific symptoms in the initial disease stage, the condition is often not diagnosed for years, resulting in late-stage disease characteristics and their associated complications of malnutrition, risk of pneumonia due to bronchoaspiration, and cancer of the esophagus.
Esophageal cancer is an infrequent complication of achalasia, and in several studies, it ranges from 0.4% to 9.2%.7,13,37–39 The relationship between achalasia and esophageal carcinoma was first reported in 1872.39 Since then, there have been several case reports and studies conducted on the theme, with limited but useful information.
There is considerable variation in the documented risk of ESCC in achalasia, and some authors have reported an increase in esophageal carcinoma up to 50 times higher than in a control population matched by age and sex.40,41 ESCC typically develops 10–15 years after achalasia diagnosis or 20–25 years after the onset of achalasia symptoms.42 Tumors usually arise in a widely dilated esophagus and, when detected, are large and at an advanced stage.
In a prospective study conducted in 1992 that evaluated the incidence of ESCC in subjects with achalasia who were treated with pneumatic balloon dilation, the risk of ESCC was estimated to be 33 times higher than in a control population.42 In that study, the mean time interval was 17 years from the onset of dysphagia to the diagnosis of cancer and was 5.7 years from the diagnosis of achalasia to the diagnosis of ESCC. In one of the better cohorts studied, Leeuwenburgh et al39 followed 448 patients for a mean 9.6 years (range: 0.1–32) and found that 3.3% of the patients developed ESCC, with an annual incidence of 0.34 (95% CI: 0.20–0.56). The relative hazard rate of esophageal cancer was 28.
In a recent meta-analysis of 40 studies (11,978 patients), Tustumi et al43 described an incidence of squamous cell carcinoma of 312.4 cases per 1,00,000 patient-years at risk. The prevalence of ESCC in subjects with esophageal achalasia was 26 cases in 1,000, with an increase in absolute risk of squamous cell carcinoma of 308.1 cases per 1,00,000 patients per year. Taking into account only cohorts from South American countries, where Chagas disease is endemic, the prevalence of carcinoma was 56 cases per 1,000 patients with achalasia. In the rest of the world, the prevalence of carcinoma was 26 cases per 1,000 patients with achalasia. The prevalence ratio was 3.35 (95% CI: 2.1–5.34; P<0.01). Other interesting data from that meta-analysis were that, contrary to what occurs in ESCC unrelated to achalasia, the majority of cases of achalasia-associated ESCC present in the lower third of the esophagus (42%), followed by the middle third of the esophagus (49%), and the upper third of the esophagus (17%; P=0.0013). Furthermore, the pooled data showed that the mean survival rate after cancer diagnosis was 12.7 months and only 4.54% of the patients survived longer than 5 years.43
Pathophysiology of ESCC in achalasia
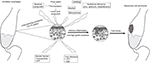
Multiple pathophysiologic mechanisms have been related to the development of ESCC in patients with achalasia (Figure 1). One hypothesis is that food stasis promotes lactic acid production and fermentation due to bacterial overgrowth, which stays in the distal portion of the esophagus, causing slow and continuous chronic inflammation, damaging the esophageal mucosa, and predisposing to dysplastic changes.10,37–42 In addition, a damaged esophageal mucosa is prone to be exposed to food carcinogens, such as nitrosamines, alcohol, and tobacco. In untreated patients with achalasia, 24-hour pH-study tracing shows episodes of slow elimination reflux or prolonged episodes of acid exposure with no acid elimination. The possible causes of the slow esophageal elimination of acid reflux could be secondary to an aperistaltic esophageal body or to the fermentation of retained food.44 Episodes of poor acid reflux clearance could also cause lesions.
With respect to histologic alterations and dysplasia markers, Chino et al45 conducted a study on six patients with achalasia and ESCC, carrying out histologic mapping of the esophageal samples. They reported marked hyperplastic changes in the stratified squamous epithelium and multiple foci of dysplastic changes. The ESCC was well differentiated, with low-grade atypia, closely associated with dysplastic foci. Immunohistochemical staining for p53, p21, p16, and the epidermal growth factor receptor suggested that the dysplastic epithelium was a borderline lesion between hyperplasia and carcinoma in situ. These findings imply that esophageal food stasis induces chronic hyperplasia that finally transforms into malignant epithelial cells of the esophagus, associated with the dysplasia-carcinoma sequence.45 Regarding p53, in addition to overexpression in ESCC with achalasia, there are also mutational changes of that tumor suppressor. On occasion, high-grade squamous dysplasia or superficially invasive squamous cell carcinoma is an incidental finding in achalasia patients.
Other genetic abnormalities described in ESCC associated with achalasia include mutations that could be associated with advanced megaesophagus due to Chagas disease. A silent mutation at codon 88 of exon 7 of the FHIT gene and a mutation involving exon 6 of the TP53 gene, as well as mutations in exons 5 and 7 of that gene, have been reported.46 Aneuploidies for chromosomes 7, 11, and 17 may possibly be associated with an increased risk of ESCC.47
Interestingly, idiopathic achalasia, like non-achalasia-related ESCC, is a disease that is associated with low socioeconomic levels and poverty. It is postulated that those situations predispose to malnutrition, vitamin deficiencies, and infections that could be related to achalasia (eg, HSV-1 and Epstein–Barr), as well as to ESCC (HPV).32
Achalasia as a risk factor for EA
Clinical evidence
As mentioned above, although most cases of achalasia-associated esophageal cancer are ESCC, there have been cases of adenocarcinoma associated with Barrett’s esophagus. The majority of those cases are due to the fact that once the achalasia is resolved, the exposure of the esophageal mucosa to acid is not properly controlled (through acid secretion inhibitors or even surgery), and the patients remain permanently exposed to acid, which induces Barrett’s esophagus.38
A Dutch study found that 8.4% of 331 patients with achalasia previously treated with pneumatic dilation developed Barrett’s esophagus over a period of up to 25 years.48 The annual incidence of Barrett’s esophagus was 1.00% (95% CI: 0.62–1.37), and a hazard ratio of 8.04 for developing Barrett’s esophagus was found if a hiatal hernia was present.
The effects of chronic acid exposure after esophagectomy have been described in patients with achalasia. The authors of a 10-year prospective study on 101 patients with advanced achalasia who underwent esophagectomy and cervical gastroplasty evaluated them histologically and endoscopically every 2 years and reported an incidence of esophagitis in the esophageal stump of 45.9% at 1 year, 71.9% at 5 years, and 70.0% at 10 years of follow-up.49 The appearance of ectopic columnar metaplasia and Barrett’s esophagus was 10.9% at 1–5 years, 29.5% at 5–10 years, and 57.5% at ≥10 years of follow-up. Cancer of the esophageal stump was detected in five patients: three with ESCC and two with EA.49
In their meta-analysis, Tustumi et al43 found that the incidence of EA in patients with achalasia was 21.3 cases per 1,00,000 patient-years at risk. The prevalence of EA in subjects with esophageal achalasia was 4 cases in 1,000, with an increase in the absolute risk of EA of 18.03 cases per 1,00,000 patients per year.
Even rarer, there are reports of achalasia as a symptom of EA. According to such clinical evidence, achalasia is a risk factor for EA.50
ESCC and EA prevention in achalasia
Once the “risks and links” between ESCC/EA and achalasia are established, it is logical to think of strategies to prevent the development of those neoplasias. Although a regular surveillance program for esophageal cancer as standard practice in patients with achalasia is controversial, clinicians who treat patients with achalasia should be keenly aware of the association to detect cancer in its early stages.16,51 However, current guideline recommendations are controversial. Some state that the data are insufficient to support routine endoscopic surveillance for patients with achalasia,52,53 but others suggest surveillance at 3-year intervals, if the disease has been present for more than 10–15 years.54 One of the arguments against surveillance in achalasia patients is the lack of studies regarding the cost-effectiveness of those programs, because the incidence of cancer is low.16,55 A large population-based study from Sweden showed that annual surveillance after the first year would require 406 endoscopic examinations in men and 2,220 in women to detect one case of ESCC.16,56
There are also other technical limitations that challenge the performance of appropriate screening of the achalasic esophagus, such as the fact that the entire esophagus is at risk.16,55 If we take into consideration that the mucosa is often covered with food debris and has a cobblestone appearance, a complete examination is almost impossible, and random biopsies might not be representative.55 In addition, the majority of surveillance and screening studies for ESCC in patients with achalasia have been performed with conventional white-light endoscopy, making it necessary to evaluate the usefulness of newer technologies, such as chromoendoscopy, narrow banding imaging, and confocal microscopy.
When combining the pros and cons, it seems useful to define high-risk patients and to develop a tailor-made surveillance program. For instance, if patients had other risk factors for ESCC besides achalasia, such as male sex, age >60 years, cigarette smoking, and alcohol use, perhaps advanced surveillance should be recommended earlier, compared with patients who do not have these risk factors.
Even though surgical treatment for achalasia has been successful, the risk of ESCC and EA can persist, raising the question of whether patients who have been operated on for achalasia should still be in a surveillance program. In a prospective follow-up study on 32 patients, Ota et al57 found that 6 patients (18%) developed esophageal cancer despite being in a surveillance program. An annual follow-up endoscopy was done, and the average duration of follow-up until cancer after surgery was 14.3 years (range: 5–40 years). Therefore, it is suggested that even though surgery for achalasia usually improves passage symptoms, esophageal cancer still arises in some cases, and the number of tumors developing after many years is not negligible. Accordingly, long-term endoscopic follow-up is needed for the detection of malignancy at an early stage. However, as mentioned above, there is no recommendation for when to begin such vigilance or how often and for how long it should be carried out.
Finally, several preventive strategies are under investigation to prevent ESCC (achalasia related or nonrelated) using agents such as nonsteroidal antiinflammatory drugs, selenium, alpha-difluoromethylornithine, and retinoids.1 Fruit and vegetable intake is considered to have a preventive role. Carotene, vitamin C, and vitamin E are protective elements, most likely in combination with each other and with other micronutrients.58
Conclusion
Achalasia, an idiopathic motor disorder of the esophagus that conditions aperistalsis and the absence of lower esophageal sphincter relaxation, is a risk factor for the development of ESCC/EA. Multiple mechanisms are related to the development of ESCC in achalasia, and they include bacterial overgrowth, food stasis, genetic alterations, and chronic inflammation. Regarding the risk of EA in achalasia patients, most cases are associated with Barrett’s esophagus, due to uncontrolled chronic acid reflux. The clinician must be aware of these associations so that programs for the prevention and opportune treatment of these neoplasias in patients with achalasia can be developed.
Disclosure
Dr José María Remes Troche is a member of the Advisory Board of Takeda Pharmaceuticals, Alfa-Wassermann, and Almirall. He is a speaker for Takeda, Asofarma, Alfa-Wassermann, Almirall, and Astra-Zeneca. The authors report no other conflicts of interest in this work.
References
Zhang Y. Epidemiology of esophageal cancer. World J Gastroenterol. 2013;19(34):5598–5606. | ||
Short MW, Burgers KG, Fry VT. Esophageal cancer. Am Fam Physician. 2017;95(1):22–28. | ||
Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. | ||
Liang H, Fan JH, Qiao YL. Epidemiology, etiology, and prevention of esophageal squamous cell carcinoma in China. Cancer Biol Med. 2017;14(1):33–41. | ||
Gupta B, Kumar N. Worldwide incidence, mortality and time trends for cancer of the oesophagus. Eur J Cancer Prev. 2017;26(2):107–118. | ||
Cowie A, Noble F, Underwood T. Strategies to improve outcomes in esophageal adenocarcinoma. Expert Rev Anticancer Ther. 2014;14(6):677–687. | ||
O’Neill OM, Johnston BT, Coleman HG. Achalasia: a review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol. 2013;19(35):5806–5812. | ||
Pressman A, Behar J. Etiology and pathogenesis of idiopathic achalasia. J Clin Gastroenterol. 2017;51(3):195–202. | ||
Salvador R, Voltarel G, Savarino E, et al. The natural history of achalasia: evidence of a continuum – “The evolutive pattern theory”. Dig Liver Dis. 2018;50(4):S702–S703. | ||
Eckardt AJ, Eckardt VF. Current clinical approach to achalasia. World J Gastroenterol. 2009;15(32):3969–3975. | ||
Akritidis N, Gousis C, Dimos G, Paparounas K, Fever PK. Fever, cough, and bilateral lung infiltrates. Achalasia associated with aspiration pneumonia. Chest. 2003;123(2):608–612. | ||
Ríos-Galvez S, Meixueiro-Daza A, Remes-Troche JM. Achalasia: a risk factor that must not be forgotten for esophageal squamous cell carcinoma. BMJ Case Rep. 2015;2015:bcr2014204418. | ||
Brücher BL, Stein HJ, Bartels H, Feussner H, Siewert JR. Achalasia and esophageal cancer: incidence, prevalence, and prognosis. World J Surg. 2001;25(6):745–749. | ||
Esophageal cancer: epidemiology, pathogenesis and prevention. Nat Clin Pract Gastroenterol Hepatol. 2008;5(9):517–526. | ||
Mao WM, Zheng WH, Ling ZQ. Epidemiologic risk factors for esophageal cancer development. Asian Pac J Cancer Prev. 2011;12(10):2461–2466. | ||
Chaber-Ciopinska A, Kiprian D, Kawecki A, Kaminski MF. Surveillance of patients at high-risk of squamous cell esophageal cancer. Best Pract Res Clin Gastroenterol. 2016;30(6):893–900. | ||
Ohashi S, Miyamoto S, Kikuchi O, Goto T, Amanuma Y, Muto M. Recent advances from basic and clinical studies of esophageal squamous cell carcinoma. Gastroenterology. 2015;149(7):1700–1715. | ||
Morris LG, Sikora AG, Patel SG, Hayes RB, Ganly I. Second primary cancers after an index head and neck cancer: subsite-specific trends in the era of human papillomavirus-associated oropharyngeal cancer. J Clin Oncol. 2011;29(6):739–746. | ||
Wang WL, Lee CT, Lee YC, et al. Risk factors for developing synchronous esophageal neoplasia in patients with head and neck cancer. Head Neck. 2011;33(1):77–81. | ||
Katada C, Yokoyama T, Yano T, et al. Alcohol consumption and multiple dysplastic lesions increase risk of squamous cell carcinoma in the esophagus, head, and neck. Gastroenterology. 2016;151(5):860–869. | ||
Lin J, Zeng R, Cao W, Luo R, Chen J, Lin Y. Hot beverage and food intake and esophageal cancer in southern China. Asian Pac J Cancer Prev. 2011;12(9):2189–2192. | ||
Chen Z, Chen Q, Xia H, Lin J. Green tea drinking habits and esophageal cancer in southern China: a case-control study. Asian Pac J Cancer Prev. 2011;12(1):229–233. | ||
Appelqvist P, Salmo M. Lye corrosion carcinoma of the esophagus: a review of 63 cases. Cancer. 1980;45(10):2655–2658. | ||
Kay M, Wyllie R. Caustic ingestions in children. Curr Opin Pediatr. 2009;21(5):651–654. | ||
Jain R, Gupta S, Pasricha N, Faujdar M, Sharma M, Mishra P. ESCC with metastasis in the young age of caustic ingestion of shortest duration. J Gastrointest Cancer. 2010;41(2):93–95. | ||
Wu C, Kraft P, Zhai K, et al. Genome-wide association analyses of esophageal squamous cell carcinoma in Chinese identify multiple susceptibility loci and gene-environment interactions. Nat Genet. 2012;44(10):1090–1097. | ||
Zhuo W, Zhang L, Wang Y, Zhu B, Chen Z. Cyclin D1 G870A polymorphism is a risk factor for esophageal cancer among Asians. Cancer Invest. 2012;30(9):630–636. | ||
Ellis A, Risk JM, Maruthappu T, Kelsell DP. Tylosis with oesophageal cancer: diagnosis, management and molecular mechanisms. Orphanet J Rare Dis. 2015;10:126. | ||
Varela AB, Blanco Rodríguez MM, Boullosa PE, Silva JG. Tylosis A with squamous cell carcinoma of the oesophagus in a Spanish family. Eur J Gastroenterol Hepatol. 2011;23(3):286–288. | ||
Rasool S, A Ganai B, Syed Sameer A, Masood A. Esophageal cancer: associated factors with special reference to the Kashmir Valley. Tumori. 2012;98(2):191–203. | ||
Aday U, Gündeş E, Ali Çetin D, Çiyiltepe H, Başak K, Duman M. Long-term evolution of squamous-cell cancer in Plummer-Vinson syndrome. Prz Gastroenterol. 2017;12(3):226–228. | ||
Al-Haddad S, El-Zimaity H, Hafezi-Bakhtiari S, et al. Infection and esophageal cancer. Ann N Y Acad Sci. 2014;1325:187–196. | ||
Thrift AP. Determination of risk for Barrett’s esophagus and esophageal adenocarcinoma. Curr Opin Gastroenterol. 2016;32(4):319–324. | ||
Duggan C, Onstad L, Hardikar S, Blount PL, Reid BJ, Vaughan TL. Association between markers of obesity and progression from Barrett’s esophagus to esophageal adenocarcinoma. Clin Gastroenterol Hepatol. 2013;11(8):934–943. | ||
Nieman KM, Romero IL, van Houten B, Lengyel E. Adipose tissue and adipocytes support tumorigenesis and metastasis. Biochim Biophys Acta. 2013;1831(10):1533–1541. | ||
Francis DL, Katzka DA. Achalasia: update on the disease and its treatment. Gastroenterology. 2010;139(2):369–374. | ||
Ribeiro U, Posner MC, Safatle-Ribeiro AV, Reynolds JC. Risk factors for squamous cell carcinoma of the oesophagus. Br J Surg. 1996;83(9):1174–1185. | ||
Zendehdel K, Nyrén O, Edberg A, Ye W. Risk of esophageal adenocarcinoma in achalasia patients, a retrospective cohort study in Sweden. Am J Gastroenterol. 2011;106(1):57–61. | ||
Leeuwenburgh I, Scholten P, Alderliesten J, et al. Long-term esophageal cancer risk in patients with primary achalasia: a prospective study. Am J Gastroenterol. 2010;105(10):2144–2149. | ||
Dunaway PM, Wong RK. Risk and surveillance intervals for squamous cell carcinoma in achalasia. Gastrointest Endosc Clin N Am. 2001;11(2):425–434. | ||
Streitz JM, Ellis FH, Gibb SP, Heatley GM. Achalasia and squamous cell carcinoma of the esophagus: analysis of 241 patients. Ann Thorac Surg. 1995;59(6):1604–1609. | ||
Meijssen MA, Tilanus HW, van Blankenstein M, Hop WC, Ong GL. Achalasia complicated by oesophageal squamous cell carcinoma: a prospective study in 195 patients. Gut. 1992;33(2):155–158. | ||
Tustumi F, Bernardo WM, da Rocha JRM, et al. Esophageal achalasia: a risk factor for carcinoma. A systematic review and meta-analysis. Dis Esophagus. 2017;30(10):1–8. | ||
Crookes PF, Corkill S, Demeester TR. Gastroesophageal reflux in achalasia. When is reflux really reflux? Dig Dis Sci. 1997;42(7):1354–1361. | ||
Chino O, Kijima H, Shimada H, et al. Clinicopathological studies of esophageal carcinoma in achalasia: analyses of carcinogenesis using histological and immunohistochemical procedures. Anticancer Res. 2000;20(5C):3717–3722. | ||
Safatle-Ribeiro AV, Ribeiro U, Sakai P, et al. Integrated p53 histopathologic/genetic analysis of premalignant lesions of the esophagus. Cancer Detect Prev. 2000;24(1):13–23. | ||
Manoel-Caetano FS, Borim AA, Caetano A, Cury PM, Silva AE. Cytogenetic alterations in chagasic achalasia compared to esophageal carcinoma. Cancer Genet Cytogenet. 2004;149(1):17–22. | ||
Leeuwenburgh I, Scholten P, Caljé TJ, et al. Barrett’s esophagus and esophageal adenocarcinoma are common after treatment for achalasia. Dig Dis Sci. 2013;58(1):244–252. | ||
da Rocha JR, Ribeiro U, Sallum RA, Szachnowicz S, Cecconello I. Barrett’s esophagus (BE) and carcinoma in the esophageal stump (ES) after esophagectomy with gastric pull-up in achalasia patients: a study based on 10 years follow-up. Ann Surg Oncol. 2008;15(10):2903–2909. | ||
Segal J, Lagundoye A, Carter M. Achalasia leading to diagnosis of adenocarcinoma of the oesophagus. BMJ Case Rep. 2017;2017:bcr-2017-219386. | ||
Ravi K, Geno DM, Katzka DA. Esophageal cancer screening in achalasia: is there a consensus? Dis Esophagus. 2015;28(3):299–304. | ||
ASGE Standards of Practice Committee, Evans JA, Early DS, et al. The role of endoscopy in Barrett’s esophagus and other premalignant conditions of the esophagus. Gastrointest Endosc. 2012;76(6):1087–1094. | ||
Vaezi MF, Pandolfino JE, Vela MF. ACG clinical guideline: diagnosis and management of achalasia. Am J Gastroenterol. 2013;108(8):1238–1249; quiz 1250. | ||
Eckardt AJ, Eckardt VF. Editorial: cancer surveillance in achalasia: better late than never? Am J Gastroenterol. 2010;105(10):2150–2152. | ||
Boeckxstaens GE, Zaninotto G, Richter JE. Achalasia. Lancet. 2014;383(9911):83–93. | ||
Sandler RS, Nyrén O, Ekbom A, Eisen GM, Yuen J, Josefsson S. The risk of esophageal cancer in patients with achalasia. A population-based study. JAMA. 1995;274(17):1359–1362. | ||
Ota M, Narumiya K, Kudo K, et al. Incidence of esophageal carcinomas after surgery for achalasia: usefulness of long-term and periodic follow-up. Am J Case Rep. 2016;17:845–849. | ||
Heath EI, Limburg PJ, Hawk ET, Forastiere AA. Adenocarcinoma of the esophagus: risk factors and prevention. Oncology. 2000;14(4):507–514; discussion 518–520, 522–523. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.