Back to Journals » Infection and Drug Resistance » Volume 13
ACEIs and ARBs and Their Correlation with COVID-19: A Review
Authors Yehualashet AS , Belachew TF
Received 28 May 2020
Accepted for publication 7 August 2020
Published 16 September 2020 Volume 2020:13 Pages 3217—3224
DOI https://doi.org/10.2147/IDR.S264882
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Awgichew Shewasinad Yehualashet,1 Teshome Fentik Belachew2
1Pharmacology and Toxicology Unit, Department of Pharmacy, College of Health Sciences, Debre Berhan University, Debre Berhan, Ethiopia; 2Department of Pharmacy, Debre Birhan Health Science College, Debre Birhan, Ethiopia
Correspondence: Awgichew Shewasinad Yehualashet Pharmacology and Toxicology Unit, Department of Pharmacy
College of Health Sciences, Debre Berhan University, PO BOX. 445, Debre Berhan, Ethiopia
Tel +251 935450290
Email [email protected]
Abstract: Although some animal studies suggested that the use of ACEIs/ARBs could contribute for the prevention and treatment of the effects of the COVID-19 infection, there are also contradictory scenarios indicating that their use may exacerbate the deleterious conditions of the infection. As a result of the paradoxical issue of using ACEIs/ARBs during COVID-19, it is still an area requiring extended investigation to prove. Additionally, a trial evidence of their efficacy and the possible benefit risk analysis of these conventional drugs during COVID-19 in connection with other comorbidities like hypertension, heart failure, and renal disease associated with diabetes should also be addressed.
Keywords: coronavirus, COVID-19, ACEIs, ARBs
Introduction
Coronaviruses (CoVs) of the family Coronaviridae and subfamily Corovirinae are positive-sense, single-stranded RNA viruses that affect a wide range of hots with diseases ranging from common cold to severe/fatal illnesses. The novel virus was initially termed as 2019-nCoV which later changed to “SARS-CoV-2” by the Coronavirus Study Group (CSG) of International Committee on Taxonomy of Viruses (ICTV) because of the higher resemblance of the virus with severe acute respiratory syndrome coronavirus (SARS-CoV).1 The continued coronavirus infection that was emerged in China has rapidly spread to each corner and led a declaration to be considered as a global health emergency by the World Health Organization (WHO). Although there are many efforts for the provision of suitable therapeutic approaches, there are no vaccines or direct-acting antiviral delivers to those infected with the virus.2 Right after the first reported case of death on the first week of January 2020 was confirmed as a result of the 2019 novel coronavirus (2019-nCoV; later renamed severe acute respiratory syndrome coronavirus 2 [SARSCoV-2]), the end of this month it was declared as an outbreak by WHO and subsequently named as coronavirus disease (COVID-19). Globally, as of 5 August 2020, there have been 18,318,928 confirmed cases of COVID-19, including 695,043 deaths, reported to WHO.3,4
Findings revealed that the genome sequence identity of SARS CoV 2 and bat CoV is about 96.2%, and based on virus genome sequencing results and evolutionary analysis, bat has been blamed as natural host of the virus SARSCoV-2 to transmit from bats via unknown intermediate hosts to infect humans. It was identified that SARS-CoV-2 uses angiotensin-converting enzyme 2 (ACE2), the same receptor as SARS-CoV to infect humans.5,6 Direct contact with intermediate host animals or consumption of wild animals was suspected to be the main route of SARS-CoV-2 transmission and the sources and mode of transmission is still open for further investigation.6
Virus–host interactions affect viral entry and replication. SARS-CoV-2 is an enveloped positive single-stranded RNA (ssRNA) coronavirus. About two-thirds of viral RNA, mainly located in the first open reading frame (ORF 1a/b), encode 16 non-structure proteins (NSPs). The remaining part of the virus genome encodes four essential structural proteins, including spike (S) glycoprotein, small envelope (E) protein, matrix (M) protein, and nucleocapsid (N) protein, and also several accessory proteins. The S glycoprotein of SARS-CoV-2 binds to host cell receptors, ACE2 which is assumed to be a critical step for the entry of the virus. However, the possible molecules facilitated membrane invaginations for SARS-CoV-2 endocytosis are still unclear and other virus proteins may contribute to pathogenesis.6
Unproven and Supportive Therapies of COVID-19
To date, the 2019 novel coronavirus (2019-nCoV) outbreak is being a major concern of the scientific community to treat with potential medications. Becuase there is lack of effective and scientifically proven antiviral therapy against COVID-19, the current treatment approaches mainly focused on the symptomatic and respiratory support based up on the Diagnosis and Treatment of Pneumonia Caused by COVID-19.7 Oxygen therapy is almost applied to all patients with the COVID-19 and WHO also recommended extracorporeal membrane oxygenation (ECMO) to patients with refractory hypoxemia. To certain critical case immunoglobulin G and convalescent plasma are used to save the life of the patients as per their condition.3,8 According to literature searched on web, few drugs other than vaccines are enumerated as unproven optional and adjuvant drugs for COVID-19 and other coronaviruses. These include antivirals (opinavir/ritonavir, remdesivir), chloroquine/hydroxychloroquine, and interferon, tocilizumab, corticosteroids, antibiotic therapy, NSAIDs/Ibuprofen, and some others.9
Previous studies reported that Chloroquine and Hydroxychloroquine possess a broad spectrum of antiviral effects on a variety of viruses as diverse as human immunodeficiency virus (HIV), Marburg virus, Zika virus, dengue virus, Ebola virus, and SARS-CoV-1, etc. In addition, Chloroquine and Hydroxychloroquine can regulate immune system by affecting cell signaling and production of pro-inflammatory cytokines.10–13 Chloroquine and Hydroxychloroquine have been shown by several studies to reduce the SARS-CoV-2 viral load and shorten the duration of viremia. Although the immunomodulatory effect of these drugs also plays a role in the treatment of COVID-19, there is still a huge need for further investigation. For coronaviruses, the potential therapeutic benefits of Chloroquine were notably reported for SARS-CoV-1. In vitro, Chloroquine can prevent SARS-CoV-1 from infecting the glycosylation of a virus cell surface receptor, ACE2.7,13 Remdesivir has been reported to treat the first US case of COVID-19 successfully. Results obtained from the recently conducted in vitro study against COVID-19 are promising since the drugs remdesivir and chloroquine were found to be highly effective in controlling the infection. Even though in vitro studies showed that remdesivir and chloroquine are highly effective in the control of 2019-nCoV infection, these compounds have been used in human patients with a safety track record and shown to be effective against various ailments.14,15
Pathogenesis of SARS-CoV-2
The pathophysiology and virulence mechanisms of SARS-CoV-2 have a linkage with the function of the nsps (non-structural proteins) and structural proteins. NSP is able to block the host innate immune response. Among the structural elements of CoVs, there are the spike glycoproteins which have two subunits; one subunit, S1, binds to the receptors on the cell surface; the other subunit, S2, fuses with the cell membrane. For many CoVs, S is cleaved at the boundary between the S1 and S2 subunits, which remain non-covalently bound in the prefusion conformation.16,17 The S1 subunit comprises the receptor-binding domain(s) and contributes to stabilization of the prefusion state of the membrane-anchored S2 subunit that contains the fusion machinery. For all CoVs, S is further cleaved by host proteases at the so-called S20 site located immediately upstream of the fusion peptide.16,18 This cleavage proposed to activate the protein for membrane fusion via extensive irreversible conformational changes.19
The entry of coronavirus into susceptible cells is something which is complicated and requires the great effort in understanding the action of receptor-binding and proteolytic processing of the S protein to promote virus-cell fusion. SARS-CoV and several SARS-related coronaviruses (SARSr-CoV) interact directly with ACE2 via SB to enter to the target. Studies showed that ACE2 is highly expressed in the mouth and tongue, facilitating viral entry in the host. In human lungs, ACE2 is expressed in lower lungs on type I and II alveolar epithelial cells. After infection, SARS-CoV-2 entry starts with the attachment of the spike glycoprotein expressed on the viral envelope to ACE2 on the alveolar surface. The attachment of SARSCoV-2 to ACE2 modulates the clathrin-dependent endocytosis of the SARS-CoV-2 and ACE2 complex, inducing fusion at the cell membrane. Once enter the cells, SARS-CoV-2 exploits the endogenous transcriptional machinery of alveolar cells to replicate it and spreads through the entire lung.20 ACE2 could assist SARS-CoV-2 S-mediated entry into cells, thereby establishing it as a functional receptor for this newly emerged coronavirus, COVID-19. The SARS-CoV2 SB engages human ACE2 (hACE2) with higher affinity than to SARS-CoV SB.21,22
Both ACE-1 and ACE-2 cleave angiotensin peptides in that ACE-1 cleaves angiotensin I and generating angiotensin (Ang) II, which causes vasoconstriction, broncho-constriction, increases vascular permeability, inflammation, and fibrosis and enhance the development of acute respiratory disease syndrome (ARDS) and lung failure in patients infected with SARS-CoV-2. The effect of ACE1 signaling is by the GPCRs; AT1 and AT2 receptors. ACE1-generated AT1 receptors function as the key mediator of Ang II actions and the opposing actions of ACE2-derived peptides. ACE2 is a carboxypeptidase (zinc metalloprotease), which is responsible for Ang II degradation to Ang (1–7).23 The conversion of Ang II to Ang (1–7) by the enzyme ACE2 produces effects that oppose the action of Ang II mediated by AT1. The SARS-CoV-2 virus infects alveolar pneumocytes by binding to ACE2 (as in Figure 1), leading to a decrease in Ang II conversion to ACE2-derived peptides, for example, a reduction in Ang (1–7) and its actions that counteract effects of Ang II. Higher imbalance between the action of ACE1 and ACE2 aggravates pathology, making it more likely that the immune response will be overcome. Blunting the ACE-1–Ang II–AT1R enhances the action of ACE-1–Ang II–AT2R, the ACE-2–Ang (1-7)-AT2R or the ACE-2–Ang (1–7) which likely protects from ARDS triggered by infectious pathogens, including coronaviruses.24,25
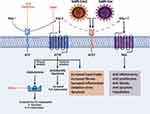 |
Figure 1 ACEIs/ARB in RAAS pathway and infectious mechanism of ACE2 receptors for SARS-COV-2. (Reprinted from J Am Coll Cardiol, 75(24), Brojakowska A, Narula J, Shimonay R, Bander J. Clinical implications of SARS-COV2 interaction withrenin angiotensin system. 3085–3095. Copyright (2020), with permission from Elsevier).23 |
ACE2 is expressed at apical plasma membranes of epithelial cells, including those of respiratory origin, the primary location of SARS-CoV infection.26 During the infection of SARS-CoV, the cell surface expression of ACE2 down-regulated through internalization of the receptor–ligand complex or activation of TACE-mediated ectodomain shedding of soluble ACE2. After attachment of viral spike proteins with ACE2, the amount of cell surface-expressed ACE2 is reduced. ACE2 receptor down-regulation provokes a worsening effect of lung failure.27,28 In addition to the alveolar cells in the lungs, ACE2 is expressed in other organs, including the kidney, the heart, and the gut. Whether robust ACE2 expression in these organs affects SARS-CoV-2 infectivity remains ill-defined. Acute kidney injury (AKI), cardiac damage, and abdominal pain are the most common co-morbidities of COVID-19.20
ACEIs and ARBs Use During COVID-19
Angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) have a different mechanism in the pathogenesis of COVID-19. As depicted in Figure 1 ACEIs in clinical use do not directly affect ACE2 activity23 and the intestinal messenger RNA level of ACE2 increased with ACEI but not observed with ARBs. SARS-CoV-2 down-regulates ACE2 expression that decreases protective effects of ACE2 on different organs.29 ACEIs increased the entrance more than twice the entrance with ARBs. This cannot definitely conclude risks or benefits of these therapies with patients confounding variables like age, hypertension, and impact of yet-unidentified comorbidities on outcome with the COVID-19 pandemic.30,31 In the other way, ACEIs or ARBs could prevent COVID-19 viral entry by stabilizing ACE2–AT1R interaction and preventing viral protein ACE2 interaction and internalization. The interaction between viral protein and ACE2 decreased in the presence of stabilized ACE2-AT1R complexes.31
ACEIs, the first drugs targeting the RAAS (Renin Angiotensin Aldosterone System), are used for the treatment of a wide range of indications related to hypertension, cardiovascular disease, and renal disease for over 30 years. ACEIs and ARBs not only lower blood pressure but also may possess unique cardioprotective properties.32 They improve endothelial function and regress both left ventricular hypertrophy and arterial mass. They also reduce rates of death, myocardial infarction, stroke, cardiac arrest, and revascularization procedures.33 ACEIs have been shown to protect against oxidative stress and prevent glycosylation of proteins, which may confer cardiovascular benefit. ACEI or ARB also used for conditions associated with insulin resistance, such as metabolic syndrome, hypertension, impaired fasting glucose, family history of diabetes, obesity, congestive heart failure, or other risks for the development of type 2 diabetes.33–35 The renin-angiotensin system (RAS) is a homeostatic regulator signaling pathway participated in the control of vascular function. The regulation of blood pressure, natriuresis, and blood volume recruits this signaling pathway. This system also has a local role in the regulation of regional blood flow and controlling our body’s responses to a range of stimuli.36
Following a rapid increase in the cases of COVID-19, different observational studies are undertaken to identify the possible risk factors for infection and related poor outcomes. With that Yang et al, 17% diabetes cases were reported among 52 critically ill patients with COVID-1937 and 16.2% DM and 23.7% hypertension cases were identified from 1099 patients by Guan et al.38 Similarly, out of 140 hospitalized patients, it was reported by Yang et al that 12% DM cases and 30% HTN cases were observed in critically ill patients with COVID-19.37
The large percentage of patients with hypertension undertaking ACEIs or ARBs was a remarkable characteristic among those presenting severe COVID-19 manifestations. These are classes of anti-hypertensive massively used as first-line therapy due to their additional kidney and cardiologic protections that occur regardless of their use for blood pressure control.39 ACE2 is established as a functional receptor for the entry of SARS-CoV-2 S- into cells. Considering the pathophysiologic mechanisms and possible drug targets the scientific community is intensively striving to develop therapeutics for the current pandemic COVID-19. Therefore, the driving force of this review is to discuss the correlation between ACEIs/ARBs with COVID-19 considering the existing scientific evidence.
ACE2 is a type 1 integral membrane glycoprotein which is expressed and identified to be active in most tissues. The highest expression of ACE2 is observed in the kidney, the endothelium, the lungs, and in the heart.36 The probable rational proposed for the possible relation between the use of ACEIs/ARBs, and progression to ARDS in COVID-19 is the increased availability of ACE-2 attached to surface in the lung endothelium, an inherent effect of these two classes, leading to enhanced coupling of SARS-CoV2 to ACE-2 and its consequent cell entry. Indeed, the receptor-binding domain (RBD) of the SARS-CoV2 has a stronger interaction with ACE-2, compared to other viruses from the same family, and any increase of ACE-2 expression may potentially amplify the virus capacity to entry the cells.40,41 In the case of ACEI’s, the reduced angiotensin II levels increase the proportion of ACE-2 uncoupled to this molecule, which would act transforming angiotensin II into angiotensin 1–7. The overcompensation of the ACE-2 action of transforming angiotensin I into angiotensin 1–9, which may be enhanced under the use of ACEI’s, since angiotensin I is increased, does not fully compensate the lack of coupling of the attached ACE-2. Distinctly, ARBs increase angiotensin II availability since this class blocks its coupling with AT2 receptor, leading to compensatory up-regulation of ACE-2 in the membrane. Studies differentiating the risk of ARDS and related pulmonary complications between ACEI’s and ARBs lack, precluding from a comparative analysis of the clinical outcomes between these two classes.40,42
While expression and availability of attached ACE-2 is directly correlated with COVID-19 severity, the free circulating form of ACE-2 may inactivate SARS-CoV2, and preclude from its entry in the pulmonary endothelium, and has been proposed that recombinant human soluble ACE-2 could act as a potential molecule to protect from the development of severe manifestations, ARDS, and death in COVID-19. Hence, a speculative ratio between attached ACE-2 availability and expression, and freely circulating ACE-2 could predict the lung pathogenicity of COVID-19.43,44
Controversial Issues of ACE2 Modulation in the Outcome of COVID-19
According to Yang et al (2020), higher prevalence of hypertension was observed in patients with severe COVID-19; however, a concluding remark has not been made in that the severity of the infection has a link with hypertension. Similarly, no data were reported with respect to the use of ACEIs and ARBs for the infection. Guo et al (2020), in evaluating cardiovascular implications of COVID-19 infection, the use of ACEIs/ARBs did not show any association with mortality.37,45 But previous animal studies showed that ACEIs and ARB increase ACE2 activity. Based on prior animal studies, it was suggested that proposed ACEIs and ARBs can enhance ACE2 activity and thereby increase infectivity of COVID-19 virus. Other studies in mice and humans showed in contrary to the above animal studies. ACE2 mRNA expression in rat heart cells treated with an ACEI 7 and ACE2 activity in the presence of either ACEI or ARBs in humans, in both scenarios the level of ACE2 does not show any change.46
As per Fang et al (2020), it was hypothesized that the use of ACE2 receptor increasing drugs is at higher risk for severe COVID-19 infection. ACEI initially inhibits ACE leading to decreased angiotensin I levels, causing a possible negative feedback loop that ultimately up-regulates more ACE2 receptor to be able to interact with the decreased angiotensin I substrate available. This ACE2 receptor up-regulation results in increased binding sites for SARS-CoV-2, leading to preferential COVID-19 infection. This is particularly observed in patients with diabetes and/or hypertension since they are usually taking ACEIs/ARB. It was, therefore, suggested that patients with cardiac diseases, hypertension, or diabetes, who are treated with ACE2-increasing drugs, are at higher risk for severe COVID-19 infection and, therefore, should be monitored for ACE2-modulating medications, such as ACEIs/ARBs.47
On the contrary, some studies indicated that ACEIs/ARB use may be beneficial in COVID-19 infection prevention. Because of a proposed mechanism that ACEI inhibition of ACE may stimulate a negative feedback (given the lack of angiotensin II, up-regulating ACE2 receptors and decreasing overall inflammation).48 In severe lung injury animal models, preclinical studies have showed that ACE2 is significantly downregulated and it has been shown that the inhibition of the angiotensin type 1 receptor by ARB like losartan reduces severe acute lung injury in mice administered with the spike glycoprotein of SARS-CoV.49,50 The above complementary approach reflects that ACE2 is protective in lung injury during infection of coronavirus. Based on this, there are ongoing trials studying the effect of Losartan (an ARB) in patients with COVID-19 in outpatient and inpatient settings.51,52
In order to determine the influence of the RAS system and active treatments on SARS-CoV-2 infection and on the development of COVID-19 disease, provided the fact that in many cardiovascular diseases increased levels of ACE2 play a protective role, so that its down-regulation by SARSCoV- 2 would be highly detrimental per se in this critical subset of patients due to an increase in angiotensin II,53 not only case series but also autoptic exams should investigate the expression and activation of RAS components in different organs during COVID-19 infection in patients treated or not with an ACEI/ARB, also investigating ACE2 polymorphisms which could impact the affinity for the spike protein of SARSCoV-2. Knock-in or knock-out models for RAS molecules could also be useful to understand how COVID-19 might develop interactions with the RAS. Moreover, randomized controlled trials should be initiated to show whether the modulation of RAS inhibition (starting, stopping, or continuing) would lead to better or worse outcomes in COVID-19. Lastly, experimental studies might show possible effective RAS-related therapies. In this regard, there are three active, but not yet recruiting trials [https://clinicaltrials.gov/ct2/show/NTC04332666, https://clinicaltrials.gov/ct2/show/NCT04312009, https://clinicaltrials.gov/ct2/show/NCT04322786] investigating the role of angiotensin (1–7) infusion, ACEIs, and losartan in patients affected by COVID-19. One randomized trial [https://clinicaltrials.gov/ct2/show/NCT04330300] is already recruiting to assess whether a shift to a non-ARB/ACEI regimen would be beneficial or detrimental for hypertensive patients affected by COVID-19.
Based on the present scientific evidence, patients who are taking ACEIs/ARBs during continual therapy of chronic diseases have generally been advised to continue taking their medicines. The Council on Hypertension of the European Society of Cardiology has reflected that there is insufficient data available to support the deleterious effect of ACEIs/ARBs in the current pandemic COVID-19. Because lack of evidence does not support that the medication’s benefit can potentially outweigh the risk, individualized treatment decision should be made taking into account of the patients hemodynamic status and clinical presentations.54
Conclusion and Future Perspectives
Depending on the current evidence available in the web, it is difficult to confirm the co-relation of ACEIs/ARB and their use during COVID-19 either to be beneficial or harmful. Therefore, much more research is highly in need to better elaborate the correlation of the RAAS with SARS-CoV2 infection. It is also very challenging to suggest on the consequences of using ACEIs/ARBs in patients infected with COVID-19 because of the complexity of the RAS system and lack of evidence in human to support the hypothesis.54 So that whether the benefits of ACE-1 inhibitors or ARBs during an episode of infection with SARS-CoV-2 outweigh the potential risk or not, require further investigation.
Although some animal studies suggested that their use could have value in preventing and treating the effects of the COVID-19, there are also contradictory scenarios suggesting that the use of ACEIs/ARBs may exacerbate the deleterious conditions of the infection. As a result of the paradoxical issue of using ACEIs/ARBs during COVID-19, it is still an area requiring investigation to prove. Additionally, a trial evidence of their efficacy and the possible benefit-risk analysis of these conventional drugs during COVID-19 in connection with other comorbidities like hypertension, heart failure, and renal disease associated with diabetes mellitus should also be addressed.
Author Contributions
Both authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Dhama K, Patel SK, Pathak M, et al. An update on SARS-COV-2/COVID-19 with particular reference on its clinical pathology, pathogenesis, immunopathology and mitigation strategies–a review. Travel Med Infect Dis. 2020.
2. Wang Z, Chen X, Lu Y, Chen F, Zhang W. Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined Chinese and Western medicine treatment. Biosci Trends. 2020;14(1):64–68. doi:10.5582/bst.2020.01030
3. Organization WHO. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: interim guidance; January 28, 2020.
4. Available from: https://covid19.who.int/?gclid=CjwKCAjwsan5BRAOEiwALzomX9QXX9YInbXvWdchwpisFFagRGptECzLWx6ZeUZ_vEqUsxwbfWzkhoC5F4QAvD_BwE.
5. Yin Y, Wunderink RG. MERS, SARS and other coronaviruses as causes of pneumonia. Respirology. 2018;23(2):130–137.
6. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi:10.1038/s41586-020-2012-7
7. Gao J, Tian Z, Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14(1):72–73. doi:10.5582/bst.2020.01047
8. Chen L, Xiong J, Bao L, Shi Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect Dis. 2020;20(4):398–400. doi:10.1016/S1473-3099(20)30141-9
9. Available from: Recommendation_Unproven_Therapies_COVID-19.pdf.
10. Akpovwa H. Chloroquine could be used for the treatment of filoviral infections and other viral infections that emerge or emerged from viruses requiring an acidic pH for infectivity. Cell Biochem Funct. 2016;34(4):191–196. doi:10.1002/cbf.3182
11. Lajoie J, Mwangi L, Fowke KR. Preventing HIV infection without targeting the virus: how reducing HIV target cells at the genital tract is a new approach to HIV prevention. AIDS Res Ther. 2017;14(1):46. doi:10.1186/s12981-017-0166-7
12. Kumar A, Liang B, Aarthy M, et al. Hydroxychloroquine inhibits zika virus NS2B-NS3 protease. ACS Omega. 2018;3(12):18132–18141. doi:10.1021/acsomega.8b01002
13. Zhang W, Zhao Y, Zhang F, et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the Perspectives of clinical immunologists from China. Clin Immunol. 2020;214:108393. doi:10.1016/j.clim.2020.108393
14. Agostini ML, Andres EL, Sims AC, et al. Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. mBio. 2018;9(2). doi:10.1128/mBio.00221-18.
15. Holshue ML, DeBolt C, Lindquist S, et al. First Case of 2019 Novel Coronavirus in the United States. N Engl J Med. 2020;382(10):929–936. doi:10.1056/NEJMoa2001191
16. Tortorici MA, Veesler D. Structural insights into coronavirus entry. Adv Virus Res. 2019;105:93–116.
17. Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020;109:102433. doi:10.1016/j.jaut.2020.102433
18. Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181(2):281–292.e6. doi:10.1016/j.cell.2020.02.058
19. Lau YL, Peiris JS. Pathogenesis of severe acute respiratory syndrome. Curr Opin Immunol. 2005;17(4):404–410. doi:10.1016/j.coi.2005.05.009
20. Perico L, Benigni A, Remuzzi G. Should Covid-19 concern nephrologists? Why and to what extent? The emerging impasse of angiotensin blockade. Nephron. 2020;1–9.
21. Crowley SD, Rudemiller NP. Immunologic effects of the renin-angiotensin system. J Am Soc Nephrol. 2017;28(5):1350–1361. doi:10.1681/ASN.2016101066
22. Heurich A, Hofmann-Winkler H, Gierer S, Liepold T, Jahn O, Pöhlmann S. TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J Virol. 2014;88(2):1293–1307. doi:10.1128/JVI.02202-13
23. Brojakowska A, Narula J, Shimony R, Bander J. Clinical implications of SARS-Cov2 interaction with renin angiotensin system. J Am Coll Cardiol. 2020;75(24):3085–3095. doi:10.1016/j.jacc.2020.04.028
24. Rossi GP, Sanga V, Barton M. Potential harmful effects of discontinuing ACE-inhibitors and ARBs in COVID-19 patients. Elife. 2020;9:e57278. doi:10.7554/eLife.57278
25. Simões e Silva A, Silveira K, Ferreira A, Teixeira M. ACE2, angiotensin‐(1‐7) and M as receptor axis in inflammation and fibrosis. Br J Pharmacol. 2013;169(3):477–492. doi:10.1111/bph.12159
26. Meng J, Xiao G, Zhang J, et al. Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg Microbes Infect. 2020;9(1):757–760. doi:10.1080/22221751.2020.1746200
27. Li F. Receptor recognition mechanisms of coronaviruses: a decade of structural studies. J Virol. 2015;89(4):1954–1964. doi:10.1128/JVI.02615-14
28. Fehr AR, Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Molecular Biol. 2015;1282:1–23.
29. Wysocki J, Lores E, Ye M, Soler MJ, Batlle D. Kidney and Lung ACE2 expression after an ACE inhibitor or an Ang II receptor blocker: implications for COVID-19. bioRxiv. 2020.
30. Guo J, Huang Z, Lin L, Lv J. Coronavirus disease 2019 (COVID‐19) and cardiovascular disease: a viewpoint on the potential influence of angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers on onset and severity of severe acute respiratory syndrome coronavirus 2 infection. J Am Heart Assoc. 2020;9(7):e016219.
31. Leclézio A, Robinson J, Banerjee I. SARS-CoV-2: ACE inhibitors, disastrous or desirable? J Biomed Sci. 2020;7(1):40–46. doi:10.3126/jbs.v7i1.29852
32. Maione A, Navaneethan SD, Graziano G, et al. Angiotensin-converting enzyme inhibitors, angiotensin receptor blockers and combined therapy in patients with micro- and macroalbuminuria and other cardiovascular risk factors: a systematic review of randomized controlled trials. Nephrol Dial Transplant. 2011;26(9):2827–2847. doi:10.1093/ndt/gfq792
33. Barnes M, Heywood AE, Mahimbo A, Rahman B, Newall AT, Macintyre CR. Acute myocardial infarction and influenza: a meta-analysis of case-control studies. Heart. 2015;101(21):1738–1747. doi:10.1136/heartjnl-2015-307691
34. Abuissa H, Jones PG, Marso SP, O’Keefe JH
35. Arendse LB, Danser AHJ, Poglitsch M, et al. Novel Therapeutic Approaches Targeting the Renin-Angiotensin System and Associated Peptides in Hypertension and Heart Failure. Pharmacol Rev. 2019;71(4):539–570. doi:10.1124/pr.118.017129
36. Tikellis C, Thomas M. Angiotensin-converting enzyme 2 (ACE2) is a key modulator of the renin angiotensin system in health and disease. Int J Pept. 2012;2012.
37. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi:10.1016/S2213-2600(20)30079-5
38. Guan W-J, Ni Z-Y, Hu Y, et al. Clinical characteristics of 2019 novel coronavirus infection in China. MedRxiv. 2020.
39. Formoso G, Lombardi M. Is drug choice by general practitioners influenced by exposure to specialists? Record-linkage study in Italy. J Health Serv Res Policy. 2016;21(1):24–28. doi:10.1177/1355819615599799
40. Chen Y, Guo Y, Pan Y, Zhao ZJ. Structure analysis of the receptor binding of 2019-nCoV. Biochem Biophys Res Commun. 2020.
41. Batlle D, Wysocki J, Satchell K. Soluble angiotensin-converting enzyme 2: a potential approach for coronavirus infection therapy? Clin Sci. 2020;134(5):543–545. doi:10.1042/CS20200163
42. Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e8. doi:10.1016/j.cell.2020.02.052
43. Lewis T. Smoking or vaping may increase the risk of severe coronavirus infection. Sci Am. 2020;17.
44. Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi:10.1016/S0140-6736(20)30251-8
45. Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5(7):811. doi:10.1001/jamacardio.2020.1017
46. Zou Z, Yan Y, Shu Y, et al. Angiotensin-converting enzyme 2 protects from lethal avian influenza A H5N1 infections. Nat Commun. 2014;5(1):1–7. doi:10.1038/ncomms4594
47. Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8(4):e21. doi:10.1016/S2213-2600(20)30116-8
48. Li XC, Zhang J, Zhuo JL. The vasoprotective axes of the renin-angiotensin system: physiological relevance and therapeutic implications in cardiovascular, hypertensive and kidney diseases. Pharmacol Res. 2017;125:21–38. doi:10.1016/j.phrs.2017.06.005
49. Kuba K, Imai Y, Rao S, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nat Med. 2005;11(8):875–879. doi:10.1038/nm1267
50. Gu H, Xie Z, Li T, et al. Angiotensin-converting enzyme 2 inhibits lung injury induced by respiratory syncytial virus. Sci Rep. 2016;6:19840. doi:10.1038/srep19840
51. Henery C, Zaizafoun M,Stock E, Ghamande S, Arroliga AC, White HD, editors.Impact of angiotensin-converting enzyme inhibitors and statins on viral pneumonia. Baylor University Medical Center Proceedings. Taylor & Francis; 2018;31(4):419–423.
52. Rabindranath K, Supershad S, Talreja NDDH, et al. A consensus statement on the use of angiotensin receptor blockers and angiotensin converting enzyme inhibitors in relation to COVID-19 (Corona Virus Disease 2019). N Z Med J. 2020;133(1512):85–87.
53. Battistoni A, Volpe M. Might renin–angiotensin system blockers play a role in the COVID-19 pandemic? Eur Heart J Cardiovasc Pharmacother. 2020;6(4):248–251. doi:10.1093/ehjcvp/pvaa030
54. Aronson JK, Ferner RE. Drugs and the renin-angiotensin system in COVID-19. BMJ. 2020;m1313. doi:10.1136/bmj.m1313
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
