Back to Journals » Patient Preference and Adherence » Volume 16
Accepting Immunotherapy After Multiline Treatment Failure: An Exploration of the Anxiety and Depression in Patients with Advanced Cancer Experience
Authors Xie Q, Sun C, Fei Z, Yang X
Received 25 October 2021
Accepted for publication 20 December 2021
Published 4 January 2022 Volume 2022:16 Pages 1—9
DOI https://doi.org/10.2147/PPA.S346171
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Naifeng Liu
Qingqing Xie,1 Caixia Sun,2 Zhenghua Fei,1 Xujing Yang1
1Department of Radiation and Medical Oncology, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, 325000, People’s Republic of China; 2Department of Nursing, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, 325000, People’s Republic of China
Correspondence: Xujing Yang
Department of Radiation and Medical Oncology, The First Affiliated Hospital of Wenzhou Medical University, No. 2 Fuxue Lane, Wenzhou, 325000, People’s Republic of China
Email [email protected]
Background: Tumor immunotherapy is a promising therapeutic strategy for patients with advanced cancers, and some tumors have profound and durable tumor regression. However, immunotherapy is still in the clinical trial stage with elusive long-term effects and complications as a new strategy. It is unclear whether patients possess an accurate understanding of the clinical benefits associated with these agents.
Objective: To investigate the anxiety and depression of patients with advanced cancer who received immunotherapy using programmed death-1 or programmed death-ligand 1 after multiline treatment failure, explore the influencing factors, and provide a reference for clinical medical staff and psychological support for patients.
Methods: The Hospital Anxiety and Depression Scale was used to calculate the anxiety and depression scores before and after 1, 2, and 3 courses of treatment, respectively. The patients with anxiety and depression were counted. Purposive sampling was used to conduct face-to-face semi-structured interviews with 21 patients to find out the reasons. The obtained data were analyzed and collated using Colaizzi’s phenomenological method.
Results: One hundred and twenty-six patients with advanced cancers were included in the study. Before and after 1, 2 and 3 courses of treatment, 18.26%, 23.0%, 50% and 54% of patients suffered from anxiety and depression, respectively. The proportion of patients with anxiety and depression during immunotherapy kept increasing, mainly due to therapeutic efficacy below expectation, lack of timely information after treatment, lack of awareness of treatment and drugs, and lack of family and social support.
Conclusion: Patients with advanced tumors after multiline treatment failure are susceptible to anxiety and depression during immunotherapy. It is necessary to test the emotional state of patients in time and carry out early intervention. Nursing staffs and medical staffs should adopt personalized measures to meet the psychological needs of patients.
Keywords: anxiety, depression, cancer, immunotherapy, quality of life
Introduction
As a significant public health problem worldwide, malignant tumors have seriously threatened human health and increased the economic burdens of patients with cancers, their families, and society.1 In China, patients who die of malignant tumors account for 23.91% of all death cases.1 The incidence and mortality rates of malignant tumors have continued to increase in the past decades, and the annual medical expenses have exceeded 35 billion Dollars.2 Therefore, the treatment of advanced malignant tumors is challenging and urgent worldwide.3
In recent years, immunotherapy, especially immune checkpoint inhibitor-related therapy represented by programmed death-1 (PD-1)/programmed death-ligand 1 (PD-L1), has been successfully employed to treat melanoma,4 non-small cell lung cancer,5 kidney cancer,6 bladder cancer,7 and other advanced tumors.8 There are two main mechanisms for immunotherapy. PD-1 binds PD-L1 on the surface of tumor cells to inhibit the immune killing effect of the human body, and tumors undergo immune escape via the PD-1/PD-L1 signaling pathway.9 PD-1/PD-L1 antibody blocks this signal pathway and enhances the sustained killing effect of cytotoxic T lymphocytes on tumor cells without transforming their essence, which can even deplete the cells. The effector cells restore the immune killing function, finally boosting the tumor-killing effect.10
Patients with cancer are prone to anxiety and depression, with an average incidence rate of 24%.11,12 The causes of the anxiety and depression in patients with advanced cancer are many, including uncontrolled symptoms, effects of the disease itself, treatment, and loss of independence.13,14 As a new strategy, immunotherapy is still in the clinical trial stage with elusive long-term effects and complications.15,16 The psychological status of patients receiving this treatment is different from that of ordinary ones.17 Thereby motivated, we explored factors related to exacerbate anxiety and depression in these patients, aiming to provide valuable evidence for clinical diagnosis, treatment, and nursing.
Methods
Samples
A consecutive series of 126 patients attending a range of outpatient clinics in The First Affiliated Hospital of Wenzhou Medical University (Wenzhou, China), who received immunotherapy after multiline treatment failure, were recruited between January and December 2019. Inclusion criteria:
1) Patients who received immunotherapy after multiline treatment failure; 2) those without speech/communication impairment or mental retardation, and with the ability to complete the scale (the patients could fill out the general demographics information form by themselves); 3) self-care of medication costs, without participating in other clinical trials. More than two medical staff members (one of them was a psychologist) confirmed all the reports, conclusions, and interview transcripts through patient communication.
Survey Contents and Methods
The study purpose and procedures were carefully explained to all participants by three trained study staff, and any questions were answered. Face-to-face semi-structured interviews were conducted for objective sampling by purpose sampling methodology. The sample size was determined according to the data saturation principle, ie, no critical or new topics appeared.18
Anxiety and depression were assessed using the Hospital Anxiety And Depression Scale (HADS).19 HADS is a self-administered rating scale with 14 items divided into two subscales (measuring anxiety and depression respectively), each containing seven items. Each item was rated on a four-point scale from 0 to 3 points, with a maximum subscale of 21 and an overall distress score of 0–42 points, and a higher score indicates more severe distress. A score of ≥8 corresponds to anxiety or depression. Patients with both scores of ≥8 points were included in an anxiety and depression group.20,21
Survey procedure: 1) the patient was informed by the first author and asked whether she was willing to participate. 2) the medical staff recorded the baseline clinical data of included patients, including name, gender, age, past medical history, current medical history, occupation, family members, and economic income. 3) Before the interview, we call the patient to make an appointment. At the time of the phone call, we described the study again and asked the patient whether they were willing to participate in the study. In addition to this, the patient is interviewed or postponed according to their physical and mental conditions. 4) at the beginning of the interview, the patients were explained the purpose of this study, the principle of confidentiality, and the need for full recording. The participants completed the HADS questionnaire for the first time and signed an informed consent form. 5) The interview was conducted in a classroom of our department, which was equipped with a small round table and a sofa. The environment was quiet and comfortable Only the patient or accompanied by the patient’s close family was mentally well and stable The interview time was controlled within 40~60 min. During the interview, the medical staff kept the theme, listened carefully, and asked questions in time. The flow chart of the study can be seen in Figure 1. 6) After completes one, two, and three courses treatment with immunotherapy, patients filled HADS questionnaire for the second, third, and fourth time respectively.
 |
Figure 1 A study flowchart. Abbreviation: HADS, Hospital Anxiety and Depression Scale. |
Questions asked about interviewing patients mainly include: 1) You have undergone surgery or chemotherapy, radiotherapy, targeted therapy, and now choose P-1. Can you tell me your treatment experience and story? 2) Do you have any difficulties and stress in receiving immunotherapy? 3) Are you confident in immunotherapy? 4) Have you heard of this medicine before? How make you understand it? 5) Have you actively reported adverse reactions during immunotherapy? 6) Do you think your family gives you incredible support?
Analysis Information
Colaizzi’s22 method of analysis for phenomenological studies was utilized to elucidate the central themes and distill the essence of the participants’ experience. 1) Investigators independently reviewed the verbatim transcripts several times to immerse themselves in the data; 2) Investigators underlined significant statements and formulated a meaning separately; 3) Investigators reviewed and agreed upon the significant statements and formulated meanings; 4) Investigators grouped the common themes into clusters; 5) Each cluster was carefully examined and gradually distilled into four emergent themes; 6) a detailed and no omission description was written; 7) Member checking of detailed description completed for final validation of findings.
Results
Characteristics of Samples
All the 126 sent out questionnaires were valid (recovery: 100%). These data are displayed in Table 1. The largest group, by educational level, (63/126, 50.00%) had below junior high school, followed by Junior high school (34/126, 26.98%). When classified according to occupation, most patients (65/126, 51.59%) had retired. There were no significant in sex, age, classification of cancer, educational level, occupation, number of medication, or know their condition between patients with and without anxiety and depression (p > 0.05). There were significant in the percentage of know their condition (42.86% vs 21.90%; P=0.045), heard of immunotherapy (61.90% vs 33.33%; P=0.014), and mean (S.D.) HADS score (10.67±2.12 vs 2.40±1.50, <0.001) between patients with and without anxiety and depression.
 |
Table 1 Clinical Characteristics of Respondents Received Immunotherapy After Multiline Treatment Failure |
After 1 and 2 courses of treatment, all 126 questionnaires were valid (recovery: 100%). Before the third course of treatment, nine patients stopped immunotherapy, so 117 questionnaires were distributed after three courses (recovery: 100%). The survey results of HADS are shown in Table 2, and the clinical characteristics of 21 patients included in the sample study are listed in Table 3. With the degree of treatment with immunotherapy, there increased significantly in score of HADS and the proportion of patients with anxiety and depression (P<0.001, P=9.7333e-12; respectively). From the aforementioned results, it was concluded that the depression and anxiety increased significantly as immunotherapy progressed. Next, we looked for a cause for increased depression and anxiety.
 |
Table 2 Survey Results of HADS |
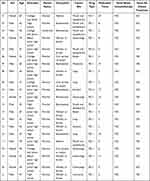 |
Table 3 Clinical Characteristics of Patients |
Topic 1: Low Expectations for Immunotherapy
Slow and Unobvious Effects of Immunotherapy
Patient A: “PD-1 is the once-in-a-century savior for us. It is unfortunate to have such a disease.
Fortunately, now, I have the chance to cure it (smiling). I have received 24 courses of treatment, but a CT scan showed that the mass did not become larger or smaller. The doctor said that it was effective, but how come? The mass is not controlled, and I do not know how long I will undergo the treatment.” Patient S:
PD-1 was bought by one of my family members in the United States. He told me that it had been on the market for a long time in the United States as the most advanced antitumor drug currently. I have received 11 courses of treatment, but the effect is not obvious, and the mass remains.
Patient G:
I have received ten courses of treatment. Look at my thigh (groin). It’s getting worse. Useless and lies, I’m hopeless. (clenching the fist and knocking on the table).
Patient D:
The doctor said that tumor cells continuously metastasized, and it was difficult to be controlled, so I received PD-L1 therapy. I have received eight courses, but I do not know whether it is effective. I feel hopeless. They said that chemotherapy and targeted therapy might be effective, but also in vain (tears in eyes). I think I would not waste money on this drug if my son did not ask our relatives to buy it in Hong Kong.
Patient A and Patient S expressed their euphoria for the new treatment. However, tumor size did not change much after several courses of treatment, which dramatically reduced the patient’s expectations for immunotherapy treatment, and they even felt hopeless. Patient G and Patient D directly talked about the condition, and as the severity worsens, they developed complaints. These four patients experienced 8–24 courses of immunotherapy, and all talked about the slow therapeutic effect.
Patients Felt Poor Health Status
Patients with malignant tumors usually have long-term suffering. After multiline treatment such as surgery, radiotherapy, chemotherapy, and targeted therapy, their physical fitness is weakened, accompanied by poor self-cognitive health. The medical staff asked if the patients wanted to know what they were doing. Patient M:
I do not want to know. It is impossible to cure this disease. I have taken this drug for five courses, but I feel worse every day. I will die before the Spring Festival. It is a waste of money.
Patient U: “I have fecal incontinence and fatigue. The disease will kill me finally, and no drug is effective.”
Topic 2: Lack of Information Needs About Immunotherapy
Patient L:
I have heard that immunotherapy is the most effective new method for cancers, but I do not know if it is effective for this disease. After many courses of chemotherapy, the index levels did not drop. The doctor asked me to take PD-1, so I tried. There is no better way for me now.
Patient H:
I have had lung cancer for two years, and also received surgery and chemotherapy. Cancer cells were found in bones this year. During the last treatment in Shanghai, I heard that the patient hospitalized next to me had the same disease and received several courses of PD-1 treatment, and the cancer was well controlled. I asked the doctor if I could try this drug. The doctor said that I could try if economic conditions permitted.
Patient R:
I’m eager to know the effective rate of this drug for patients like me. I can’t take it blindly. At least the doctor should detect one index every time after treatment to prove that it is effective.
Although some patients heard the clinical effects of individual patients after receiving PD-1 treatment, none of the patients asked the researchers about the current status of immunotherapy in the world. Moreover, no participants actively consulted the researchers to confirm the mechanism of action and potential side effects.
Topic 3: Unclear Understanding of Side Effects of Immunotherapy and Insufficient Clinical Experience
Inadequate Clinical Experience of Medical Staff
At present, studies on immunotherapy are still far from mature, especially in the aspect of nursing, which has not been included in daily training hitherto. Patient B: “After two courses of treatment, I feel stabbing pain in both lower extremities and fatigue sometimes. The nurses do not know why.” Patient J:
The drug has not been used for a long time, so doctors hardly received training about it at school. Our family members even know more about the drug than many doctors. It is difficult for doctors to explain immunotherapy in detail. They also get confused at problems, let alone answer questions from patients.
Unclear Understanding of Side Effects of Immunotherapy
The safety management of immunotherapy is of great significance. Patients must be informed of possible adverse reactions before immunotherapy, and doctors should evaluate and screen- related risk factors. Patients are worried about the severe adverse reactions caused by PD-1 and the frustration of encountering withdrawal. Patient C:
Before the first course of treatment, I checked the information about the possible adverse reactions of PD-1 on the Internet. One relative, also a doctor, also helped me search for literature, so I was very worried about the drug. When I saw some erythema and felt itchy, I reported to the doctor immediately. The doctor confirmed that it was only a mosquito bite.
Patient H:
During the first course of treatment, the nurse on duty failed to inject PD-1 three times due to the poor conditions of blood vessels, so the head nurse performed PICC for me. After I took off my tops, she found large areas of erythema on the chest, arms and back, and asked me if I felt itchy and why I didn’t report to the doctor. I think I can tolerate the pain of chemotherapy before, not to mention such side effects. If the doctor knows, he will withdraw the therapy. Then I can do nothing but wait to die.
Topic 4: Lack of Adequate Social and Family Support
Social Insurance Does Not Include Immunotherapy
Some anticancer drugs have been included in social insurance in China, but PD-1 remains excluded, thus rendering the drug unaffordable. Patient G:
I have taken the drug for six months. Re-examinations showed that the neck mass did not enlarge anymore, and I do not feel pain now. The doctor says that the tumor in the oral cavity has also been controlled, so PD-1 is effective. However, it is too expensive and not included in social insurance. My family has run out of money on this disease in the past few years, so I can do nothing but stop taking the drug. (choking)
Patient E:
I have heard that PD-1 works well with lung cancer. I have received four courses of treatment, but my family can’t bear the expenses any longer. I raised some money through Shuidichou App the year before last, but this drug is so expensive and can’t get reimbursed, so I ask the doctor if there are other cheaper drugs.
It can be concluded that patients expect PD-1 or PD-L1 to be included in social insurance as soon as possible. “National Basic Medical Insurance, Industrial Injury Insurance and Maternity Insurance Drug Catalog” issued by the Chinese People’s Government does not PD-1 or PD-L1. (http://www.nhsa.gov.cn/art/2019/8/20/art_37_1666.html).
Family Members Disagree and Cannot Provide Adequate Support
Patients with malignant tumors obtain emotional support mostly from their spouses, children, or parents, and these emotional supports also affect the patients’ thinking and behavior. Patient H:
I’m taking K (PD-1), and my son tells me to receive two courses first. If it works, I will continue the treatment even at the cost of selling our house. If not, I will try other drugs. I will follow his suggestions.
Patient E:
I noticed recently that my spouse often whispered to my father, sometimes in a loud voice. I’m sure they lack money again. So please persuade my father to stop buying this drug. I do not feel better. I just wish all my family members stay well together.
In this study, three families had poor economic conditions, and they had a family meeting and decided to be aided by relatives and the Red Cross Society of China.
Immunotherapy Drugs are Expensive, and Some Local Hospitals Do Not Buy Them
PD-1 is expensive due to patent cost, market monopoly, and tariff on imported anticancer drugs. Hospitals in some areas of China do not have the drug, and patients cannot receive immunotherapy. Patient G:
PD-1 is very effective for melanoma, which has been reported in the United States previously. The doctor said that the surgery was successful, but I needed to receive PD-1 for one year for radical treatment. As long as it can cure the disease, my family members will always support me. However, it is often difficult to buy this drug in China, so I can only ask others to buy it abroad. If I do not get the drug in time, the treatment will be delayed.
Patient K: “Why PD-1 is not popular yet? I did not get this drug this time, so the doctor asked me to try a domestic one, but I wonder if its efficacy is the same.”
Discussion
Depression and anxiety are common in patients with advanced cancer.23,24 The incidence of anxiety and depression in some young, advanced or individual types of cancer cases even exceeds 50%.23 Patients with advanced tumors who receive immunotherapy after multiline treatment failure are different from other cancer cases. For example, the patients have long-term suffering, significant threats to life, high early treatment costs, low self-healing expectations, and low economic affordability. As a result, they are more vulnerable to emotional stress. After multiline treatment failure, the incidence rate of anxiety and depression was 54% after three courses of immunotherapy. The possible reasons for inducing or increasing anxiety and depression in patients with advanced cancer after multiline treatment failure are as follows.
- Immunotherapy is a suitable option for patients with refractory cancer after multiline treatment failure due to its good tolerance and mild side effects. However, the effect of immunotherapy is slow and non-immediate. After several treatment failures, patients took immunotherapy as the only hope and were eager to achieve a curative effect. Once the immunotherapy effect deviated from the expected effect, patients were prone to despair, increasing anxiety and depression.
- Young and educated patients are overly concerned about the effectiveness of immunotherapy. After long waiting and treatment processes, their anxiety and depression are aggravated. It is reported in the literature that acceptance and Support-seeking coping styles were used most frequently for young adults with cancer. After controlling for sadness and depression, patients who seek support but fail to get enough support may develop higher levels of anxiety.25 This study has found that patients believe more in the introduction of medical specialists and the practical experience of cured cases. Some patients search literature through the Internet or relatives (doctors) to get the latest advances in immunotherapy. Therefore, medical staff should describe the current global situation of immunotherapy patiently in plain language. Well-educated patients should be provided as many ways of information inquiry as possible for their convenience. If the patient’s close relatives are well-educated, it should also provide as many ways of information inquiry as possible to facilitate them. It is also necessary to regularly give lectures about immunotherapy and supply patients with multiple information sources.
- Immune-related adverse events are still largely unclear.26 If severe adverse reactions occur in clinical practice, doctors have to recommend discontinuation and symptomatic treatment; the lengthy treatment process of patients with malignant tumors is bound to induce tremendous economic and mental stresses. As the treatment proceeded, the expenditure is not directly proportional to the therapeutic effect, thereby exacerbating the anxiety and depression of patients.
Regardless, this study has limitations. Since the interviewees are from Wenzhou, Zhejiang Province, and surrounding cities and counties, the results may differ from those of other regions in terms of economic basis and medical environment. Besides, the sample size is not large enough. Patients with different cancer types might have the different possible underlying factors that activate anxiety and depression. In this study, survey was based on cancer metastasis or no-metastases only, whereas no classification was based on cancer types. The interview results may differ if cases are selected from other general hospitals or regions, and the sample size is further increased. We also need to acknowledge the fact that Colaizzi’s method limitations existed in our study. There may be differences in the dictation of the patient and truth. On the other hand, the doctor’s understanding of the patient’s statement may be biased. In addition, we did not collect the anxiety and depression of patients before and after each treatment after diagnosed cancer and are unable to present that the difference between the anxiety and depression of patients received immunotherapy and chemoradiotherapy or other therapeutic approaches in this paper. The analyses mainly were qualitative rather than quantitative, which is a limitation of the study.
Conclusion
In summary, patients with advanced tumors after multiline treatment failure are susceptible to anxiety and depression during immunotherapy. It is necessary to test the emotional state of patients in time and carry out early intervention.
Ethical Considerations
This study was approved by the Regional Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University (Code: 2018-52). All procedures performed in the study were carried out in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained from each patient before treatment.
Acknowledgment
The authors most honestly appreciate the patients for their participation in this study and all colleagues in the radiation and medical oncology department of the first affiliated hospital of Wenzhou medical university for their assistance in the present study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi:10.3322/caac.21442
2. Zheng RS, Sun KX, Zhang SW, et al. [Report of cancer epidemiology in China, 2015]. Zhonghua Zhong Liu Za Zhi. 2019;41(1):19–28. Chinese. doi:10.3760/cma.j.issn.0253-3766.2019.01.005
3. Yang P, Shen B. Minimally invasive and precise treatment of malignant tumor. Chin J Clin Oncol. 2016;11:452–456.
4. Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–330. doi:10.1056/NEJMoa1412082
5. Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. doi:10.1056/NEJMoa1507643
6. Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab for metastatic renal cell carcinoma: results of a randomized Phase II trial. J Clin Oncol. 2015;33(13):1430–1437. doi:10.1200/JCO.2014.59.0703
7. Inman BA, Longo TA, Ramalingam S, Harrison MR. Atezolizumab: a PD-L1-blocking antibody for bladder cancer. Clin Cancer Res. 2017;23(8):1886–1890. doi:10.1158/1078-0432.CCR-16-1417
8. Li HZ, Zhang YS, Zheng GY. Commentary on and expectation of tumor immunotherapy. Med J Peking Union Med Coll Hosp. 2018;9(4):289–294.
9. Kotsakis A, Georgoulias V. Avelumab, an anti-PD-L1 monoclonal antibody, shows activity in various tumour types. Lancet Oncol. 2017;18(5):556–557. doi:10.1016/S1470-2045(17)30227-9
10. Jiao Q, Ren Y, Ariston Gabrie AN, et al. Advances of immune checkpoints in colorectal cancer treatment. Biomed Pharmacother. 2020;123:109745. doi:10.1016/j.biopha.2019.109745
11. Shankar A, Dracham C, Ghoshal S, Grover S. Prevalence of depression and anxiety disorder in cancer patients: an institutional experience. Indian J Cancer. 2016;53(3):432–434. doi:10.4103/0019-509X.200651
12. Amirifard N, Payandeh M, Aeinfar M, Sadeghi M, Sadeghi E, Ghafarpor S. A survey on the relationship between emotional intelligence and level of depression and anxiety among women with breast cancer. Int J Hematol Oncol Stem Cell Res. 2017;11(1):54–57.
13. Chen X, Wei Q, Jing R, Fan Y. Effects of music therapy on cancer-related fatigue, anxiety, and depression in patients with digestive tumors: a protocol for systematic review and meta-analysis. Medicine. 2021;100(22):e25681. doi:10.1097/md.0000000000025681
14. Meng X, Wang X, Dong Z. Impact of non-pharmacological interventions on quality of life, anxiety, and depression scores in patients with colorectal cancer: a systematic review and meta-analysis of randomized controlled trials. Support Care Cancer. 2021;29(10):5635–5652. doi:10.1007/s00520-021-06185-x
15. Tøndell A, Subbannayya Y, Wahl SGF, et al. Analysis of intra-tumoral macrophages and T cells in Non-Small Cell Lung Cancer (NSCLC) indicates a role for immune checkpoint and CD200-CD200R interactions. Cancers. 2021;13(8):1788. doi:10.3390/cancers13081788
16. Kagabu M, Nagasawa T, Sato C, et al. Immunotherapy for uterine cervical cancer using checkpoint inhibitors: future directions. Int J Mol Sci. 2020;21(7):2335. doi:10.3390/ijms21072335
17. Bergerot CD, Bergerot PG, Philip EJ, et al. Perception of cure among patients with metastatic genitourinary cancer initiating immunotherapy. J Immunother Cancer. 2019;7(1):71. doi:10.1186/s40425-019-0557-5
18. Cuthbert CA, Moules N. The application of qualitative research findings to oncology nursing practice. Oncol Nurs Forum. 2014;41(6):683–685. doi:10.1188/14.ONF.683-685
19. Thomas BC, Devi N, Sarita GP, et al. Reliability and validity of the Malayalam hospital anxiety and depression scale (HADS) in cancer patients. Indian J Med Res. 2005;122(5):395–399.
20. Mykletun A, Stordal E, Dahl AA. Hospital Anxiety and Depression (HAD) scale: factor structure, item analyses and internal consistency in a large population. Br J Psychiatry. 2001;179:540–544. doi:10.1192/bjp.179.6.540
21. Smith AB, Selby PJ, Velikova G, et al. Factor analysis of the hospital anxiety and depression scale from a large cancer population. Psychol Psychother. 2002;75(Pt 2):165–176. doi:10.1348/147608302169625
22. Colaizzi P. Psychological research as a phenomenologist views it. In: Existential Phenomenological Alternatives for Psychology. Oxford University Press; 1979.
23. Khue PM, Thom VT, Minh DQ, Quang LM, Hoa NL. Depression and anxiety as key factors associated with quality of life among lung cancer patients in Hai Phong, Vietnam. Front Psychiatry. 2019;10:352. doi:10.3389/fpsyt.2019.00352
24. Jewett BE, Miller MN, Ligon LA, Carter Z, Mohammad I, Ordway GA. Rapid and temporary improvement of depression and anxiety observed following niraparib administration: a case report. BMC Psychiatry. 2020;20(1):171. doi:10.1186/s12888-020-02590-4
25. Trevino KM, Maciejewski PK, Fasciano K, et al. Coping and psychological distress in young adults with advanced cancer. J Support Oncol. 2012;10(3):124–130. doi:10.1016/j.suponc.2011.08.005
26. Myers G. Immune-related adverse events of immune checkpoint inhibitors: a brief review. Curr Oncol. 2018;25(5):342–347. doi:10.3747/co.25.4235
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
