Back to Journals » Neuropsychiatric Disease and Treatment » Volume 16
Abnormal Anhedonia as a Potential Endophenotype in Obsessive–Compulsive Disorder
Authors Xu C, Chen J, Cui Z, Wen R , Han H, Jin L, Wan G, Wei Z, Peng Z
Received 17 June 2020
Accepted for publication 2 November 2020
Published 8 December 2020 Volume 2020:16 Pages 3001—3010
DOI https://doi.org/10.2147/NDT.S268148
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Yuping Ning
Chuanyong Xu,1,* Jierong Chen,2,* Zitian Cui,1 Rongzhen Wen,1 Hongying Han,3 Lili Jin,1 Guobin Wan,2 Zhen Wei,2 Ziwen Peng1
1Center for Studies of Psychological Application, School of Psychology, South China Normal University, Guangzhou, People’s Republic of China; 2Department of Child Psychiatry and Rehabilitation, Affiliated Shenzhen Maternity & Child Healthcare Hospital, Southern Medical University, Shenzhen, People’s Republic of China; 3Department of Psychiatry, The Third Affiliated Hospital, Sun Yat-Sen University, Guangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ziwen Peng
School of Psychology, South China Normal University, No. 55, West of Zhongshan Avenue, Guangzhou 510631, People’s Republic of China
Tel/Fax +86 20-85210369
Email [email protected]
Zhen Wei
Department of Child Psychiatry and Rehabilitation, Affiliated Shenzhen Maternity & Child Healthcare Hospital, Southern Medical University, No. 3012 Fuqiang Road, Futian District, Shenzhen, 518048, People’s Republic of China
Email [email protected]
Background: Obsessive–compulsive disorder (OCD) is often accompanied by cognitive, particularly executive function, impairments. Recently, anhedonia has emerged as an apparently important symptom of OCD reflecting altered emotion regulation. These two aspects are often comorbid in OCD. However, little is known about whether anhedonia may be a trait marker for OCD.
Methods: To verify the role of executive function and evaluate the role of anhedonia in OCD and its relationship with OCD symptoms, we recruited 60 OCD patients, 30 unaffected first-degree relatives (FDRs), and 60 healthy controls (HCs). Participants completed psychometric testing to assess depression, anxiety, and anhedonia symptoms, as well as two cognitive tests to assess executive function, namely the Wisconsin Card Sorting Test (WCST) and the Stroop Color-Word Test (SCWT).
Results: Compared to HCs, OCD patients and FDRs had significantly lower anticipatory and consummatory pleasure scores. The severity of anticipatory anhedonia correlated positively with obsessive–compulsive symptoms (r = 0.253, p = 0.009), even after controlling for depression and anxiety symptoms. Compared to HCs, OCD patients and FDRs made more errors and achieved fewer categories in the WCST. For all three SWCT components, OCD patients and FDRs took more time to name colors than HCs, but the three groups had similar numbers of errors.
Conclusion: This family-based study showed dampened pleasure together with cognitive dysfunction in OCD patients. The similar consummatory pleasure findings between OCD and FDR groups suggest anhedonia may be considered as a candidate OCD endophenotype.
Keywords: anhedonia, obsessive–compulsive disorder, executive function, endophenotype
Background
Anhedonia is defined as an inability to experience pleasure or a reduced ability to feel happiness.1,2 Numerous investigations have characterized anhedonia and depressed mood as the most important diagnostic criteria for major depression.3–5 Anhedonia is also the most common negative symptom of schizophrenia6,7 and may also be seen in patients with Parkinson's disease, drug dependence, post-traumatic stress disorder, and bipolar disorder.8–11 Recently reported findings have suggested that obsessive–compulsive disorder (OCD) may also be associated with anhedonia symptoms.12,13 Abramovitch et al14 reported a significantly higher prevalence of anhedonia among individuals with OCD, compared with the general population, and also found that OCD symptoms correlated directly with anhedonia severity. Observations of anhedonia in obsessive–compulsive personality disorder15 are suggestive of a transdiagnostic dimension.16 However, there has been little research into anhedonia in the context of OCD, and whether the symptom of anhedonia is a trait or phenotype characteristic.
Cognitive flexibility, an ability to flexibly and quickly adapting to a changing environment.17 The flexibility and emotion regulation ability as two aspects of cognition, or named “hot” and “cold” cognition,18 are the two subdomains of executive functions.19 The anhedonia and deficits of cognitive flexibility are often comorbid in depression disorder. Recently, some researchers find OCD is characterized by repetitive thoughts and behaviors, not only reflecting deficits in the subdomain of cognitive flexibility in executive function but also the emotion regulation subdomain.19–21 On one hand, OCD patients exhibit impaired emotion regulation.22,23 When OCD patients repeat behaviors over and over, they may be experiencing anxiety and depression symptoms, which might lead to infrequent experiences of pleasure or anhedonia at the moment.24 On the other hand, OCD has been associated with deficits in cognitive flexibility,25,26 which is important for adjusting one’s thoughts and behaviors to a changeable environment or rewards. Some researchers have observed the characteristic of inflexible mind in OCD patients27 and this inflexibility of mind appears to be driven by perfectionistic tendencies.28 It may be difficult for these patients to terminate an ongoing behavior even when the goal of the behavior has been devalued.29 They do not attain consummatory pleasure readily from repeated behaviors.30 Thus, OCD patients may have deficits in both cognitive flexibility and the ability to experience pleasure. Anhedonia may be closely related to cognitive dysfunction. In particular, these two are influenced by the abnormal metabolism of the ventrolateral prefrontal cortex in major depressive disorder.19 However, there have been no studies focusing on anhedonia and cognitive flexibility in the context of OCD.
The results of case–control OCD studies may be influenced by patient characteristics as well as medication effects.31 It is difficult to identify whether some symptoms or deficits may be trait markers in patients. Furthermore, although OCD is a highly hereditary neuropsychiatric disorder,32,33 a specific OCD-causative gene has not been found. These issues have led researchers to pursue family-based research.
Gottesman and Shields et al34,35 proposed the concept of the “endophenotype” as an intrinsic manifestation that can be found only through biochemical testing or microscopic examination.34 It represents an intermediary between disease phenotype and genotype but is more closely related to genetics than environment. The criteria for a presentation to be deemed an endophenotype are coexisting with a disease or disorder, heritability, state independence, and co-segregation with illness within a family, with a higher rate among family members than in the general population. Because endophenotypes are useful in complex mechanism studies of psychiatric disorders, family-based studies are increasingly being used to elucidate psychiatric disorders. To our knowledge, although recent studies have recognized anhedonia as an appropriate endophenotype of depression,36,37 there is no pedigree research on anhedonia symptoms in OCD. Therefore, assessing and discussing anhedonia in unaffected first-degree relatives (FDRs) is meaningful for early detection and diagnosis of OCD.
Previous studies have described anhedonia as a clinical manifestation and typical symptom of OCD.12 We are interested in anhedonia symptoms experienced by OCD patients and their unaffected FDRs, as well as whether these patients and FDRs with anhedonia also have deficits in cognitive flexibility. We designed a family-based study to investigate these issues. We hypothesized that: (1) compared to healthy controls (HCs), OCD and FDR groups would have lower Temporal Experience of Pleasure Scale (TEPS) subscale scores; (2) anhedonia severity would correlate with Yale-Brown Obsessive–Compulsive Scale (Y-BOCS) total scores in the OCD group; and (3) compared to HCs, OCD and FDR groups would perform worse on the Wisconsin Card Sorting Test (WCST) and Stroop Color-Word Test (SCWT), cognitive tests of executive function.
Methods
Participants
OCD patients and their relatives were recruited in and out of our psychiatric institution in Guangzhou, China. HCs were recruited via advertisements targeted at college students and workers. The study enrolled 150 subjects, including 60 OCD patients, 30 unaffected FDRs, and 60 HCs, within the age range of 18–50 years old. All OCD patients were diagnosed using the Mini-International Neuropsychiatric Interview, version 7.0 for the DSM-V and were diagnosed with OCD by two psychiatrists at the psychiatric institution in Guangzhou. The patients had no history of any neurological disorder, Tourette’s syndrome, head injury, serious medical conditions, or a history of drug or alcohol addiction. Treatment details of the OCD patients can be found in Table S1. The FDR group, which consisted of parents and siblings of the OCD patients, was screened to exclude any history of neurological disorders. The HC subjects did not use psychotropic medications and were without a known family history of OCD.
To make full use of the subjects’ data, the entire group of 60 OCD patients and 60 HCs were included in the preliminary analysis (Table 1). However, some of these OCD patients did not have FDRs in this study. To conduct a 1:1:1 matched family-based study, we selected 30 subjects from the OCD and HC groups randomly for the latter analyses. One-way analysis of variance (ANOVA) or chi-square (χ2) testing showed that years of education, IQ, and gender were similar among all three groups, and that FDRs were older on average than members of the other two groups and also included more married individuals (Table 2).
 |
Table 1 Demographic Characteristics of Individuals with OCD and HC in Preliminary Analysis |
 |
Table 2 Demographic Characteristics for OCD, FDR and HC Groups in Latter Analysis |
All subjects gave written informed consent after receiving a detailed introduction to the study. The study was approved by the Institutional Research and Ethics Committee of Guangzhou Huiai Hospital. All procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Assessment of Clinical Symptoms
The Y-BOCS38 is used to measure OCD symptom severity and consensually acknowledged as the gold standard for rating obsessive–compulsive symptomatology.39 In our study, we used the 10-item Chinese version of the Y-BOCS. Scores for each item ranged from 0 (no symptoms) to 4 (extreme symptoms). The instrument has been shown to be appropriate for clinical and non-clinical samples, with good reliability and validity.40–42
The TEPS43 was designed to capture the anticipatory and consummatory facets of pleasure. Consummatory pleasure is defined as a sense of joy at the moment or the pleasure of having one's desires met, such as the pleasure of eating. Anticipatory pleasure is considered to be the pursuit of joy, related to motivation and goal-directed behavior, such as a hungry man watching a sumptuous lunch being prepared for him. The Chinese version of the TEPS used in this study is comprised of 20 items, including 2 items not in the original scale.44 Each item is answered on a 6-point Likert scale ranging from “1” (very false for me) to “6” (very true for me), with lower total scores reflecting more severe anhedonia.
The Beck Depression Inventory45 is a 21-item self-report instrument designed to assess depression severity. The Chinese version of the Back Depression Inventory used in this study has good internal consistency and sensitivity.46,47 Each item is rated from “0” (not present) to “3” (severe), with a total score ranging from 0 to 63.
The State-Trait Anxiety Inventory48 consists of two subscales that measure state anxiety and trait anxiety, respectively. The state anxiety subscale (S-AI) is designed to assess in-the-moment feelings of fear, tension, nervousness, and worry. The trait anxiety subscale (T-AI) is used to measure how one feels generally. The Chinese version of the State-Trait Anxiety Inventory used in this study has been shown to have acceptable psychometric properties and high internal consistency.49
Assessments of Cognitive Function
The WCST50 is a neuropsychological test of set-shifting (ie, the ability to display flexibility in the face of changing environmental contingencies). Four cards (one red triangle, two green stars, three yellow crosses, and four blue circles) are presented on the top of the screen as stimuli. A response card that varies in the shape (triangle, star, cross, or circle), color (red, green, yellow, or blue), and number of shapes (one to four) shown appears on the bottom of the screen during each trial. The participant pairs the response card with stimulus cards, according to criteria of similitude. After every pairing, the computer provides feedback about whether the choice was correct. The number of stages completed and total errors were recorded.
The SCWT51 evaluates cognitive flexibility. It includes color, color-word, irrelevant color-word tasks. For the color task, there are 24 dots (6 rows × 4 columns) printed on a card and colored in green, blue, yellow, or red. In the color-word task, four Chinese color characters (green, blue, yellow, red) are printed in inconsistent colors (eg, the word “red” printed in green font). Every color character is printed in the other colors and presented in a table (6 rows × 4 columns) shown twice to participants. In the irrelevant color-word task, 24 Chinese characters irrelevant to color (eg, “ask”, “go”, “live”) are displayed in each of the four colors (eg, the word “go” printed in red) in a table (6 rows × 4 columns), with each color presented 6 times. Participants were required to identify stimulus color from left to right and top to bottom as fast and accurately as possible. The number of errors and time required to name the colors in the entire card were recorded by the experimenter.
Statistical Analysis
In the preliminary analysis, independent t-tests were conducted to compare clinical task performance and neuropsychological scores between the OCD and HC groups. Then, Pearson correlation analyses were performed to assess whether the severity of anhedonia relates to the total Y-BOCS score. In the latter analysis, ANOVAs were performed to identify differences among the OCD, FDR, and HC groups in the TEPS and neuropsychological tasks. Statistically significant results were further analyzed by post-hoc inter-group least significant difference (LSD) testing.
Results
The 60 OCD patients and 60 HC subjects in the preliminary analysis were matched for age, years of education, IQ, gender, and marital status (see Table 1). OCD patients scored higher than HCs on the Y-BOCS, BDI, and S-AI/T-AI (t-tests, p < 0.01). The OCD group had significantly lower TEPS-consummatory pleasure (CON) scores than HCs, reflecting greater consummatory anhedonia severity in OCD, but only a near-significant trend toward a difference in TEPS-anticipatory pleasure (ANT) scores. In terms of neuropsychological cognitive testing, OCD patients performed significantly worse than HCs on the WCST and SCWT. The OCD group had higher total error scores and lower stage numbers than the HC group on the WCST. Similarly, the OCD group took more time than the HC group to name colors during the color, color-word, and irrelevant color-word components of the SCWT. There was no significant difference between the groups in the number of errors made in any of these three components of the SCWT (Table 3).
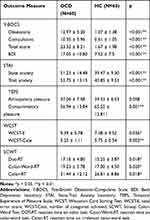 |
Table 3 Clinical Characteristics of OCD Patients and Healthy Comparison Subjects in Preliminary Analysis |
Pearson correlation analysis examining the potential association of Y-BOCS total scores with TEPS subscale scores in the OCD sample indicated that only anticipatory anhedonia scores correlated with Y-BOCS total scores (r = 0.253, p = 0.009; Figure 1). Anticipatory anhedonia scores correlated with Y-BOCS total scores, regardless of depression and anxiety symptom severity (r = 0.248, p = 0.011). The procedure in our study to control the influence of depression on obsessive–compulsive symptoms is the same as a previous study, which just regressed out the total scores of BDI.30
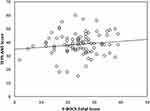 |
Figure 1 Correlation between severity of anticipatory anhedonia (TEPS-ANT scores) and OCD symptom severity (Y-BOCS scores). A linear regression graph is presented. |
The demographic characteristics of the OCD, FDR, and HC groups (N = 30/group) in the latter analyses were similar among the three groups in terms of years of education, IQ, and gender, but there were more married persons among FDRs than in the other two groups, owing to FDRs being older than members of the other two groups (Table 2). ANOVAs indicated that TEPS subscale scores differed significantly among the groups (TEPS-ANT, F2, 87 = 3.706, p = 0.029; TEPS-CON, F2, 87 = 4.487, p = 0.014). Post-hoc analyses showed that, compared to HCs, OCD patients and unaffected FDRs had significantly lower levels of pleasure (Table 4 and Figure 2). Post-hoc analyses also revealed that OCD patients and FDRs had greater numbers of WCST errors and completed fewer categories than HCs. In the SCWT, OCD patients took more time to identify colors than FDRs and HCs (post hoc p < 0.05), but the three groups had similar numbers of errors on the color, color-word, and irrelevant color-word tasks. FDRs did not differ significantly from OCD patients in terms of TEPS, WCST, or SCWT scores (Table 4).
 |
Table 4 Clinical Characteristics of OCD Patients, Unaffected First-Degree Relatives and Healthy Comparison Subjects in Latter Analysis |
Discussion
Our preliminary analysis showed that OCD patients had more consummatory anhedonia, as indicated by TEPS-CON scores, than HCs, but a similar level of anticipatory anhedonia, as indicated by TEPS-ANT scores, consistent with the findings of a previous report.30 These findings suggest that people with OCD may have a lower pleasure level when performing activities in real time. Reduced consummatory pleasure could be related to an ego-dystonic state of mind52 and perfectionism28 in OCD. A previous study also showed that OCD patients had less enjoyable sex.53 Although consummatory pleasure is not attained from repeated behaviors, OCD patients lack the ability to control these behaviors and they still expect the behaviors to produce pleasure and to results in some relief from anxiety.54 A normal anticipatory pleasure capacity with a disruption in consummatory pleasure may enable the reinforcement of rigid behavior patterns. Thus, we speculate that restricted, repetitive patterns of behavior may alleviate anxiety, through negative reinforcement, leading to anticipatory pleasure.
We found that more severe obsessive–compulsive symptoms (Y-BOCS scores) were associated with a lack of anticipatory pleasure (TEPS-ANT scores) in OCD patients (Figure 1). This association may provide further evidence of ongoing reinforcement of repetitive patterns in OCD, such that more repeated behaviors yield more anticipatory pleasure. This association remained after controlling for depression and anxiety. Similarly, Abramovitch et al12 found that depressive severity was not sufficient to explain the presence of anhedonia in OCD. Depression is characterized by putamen hyperactivity, while OCD is characterized by hypoactivity in the orbitofrontal-striatal loop during reward processing,55–57 suggesting that the neuromechanism responsible for anhedonia in OCD may differ from those in depression.
In our latter analysis, the FDR group showed higher anhedonia severity (lower TEPS-ANT and TEPS-CON scores) than HCs, without a striking difference from patients with OCD. Simultaneously, there was no significant difference in Y-BOCS total scores between the FDR and HC groups. Moreover, there was no clear relationship between Y-BOCS scores and TEPS-ANT and TEPS-CON anhedonia scores in the FDR group, and no significant relationship between Y-BOCS and TEPS-CON scores in the OCD group. Statistically speaking, anhedonia, especially consummatory anhedonia was stated independent, as would be needed for it to be an objective and easily measurable endophenotypic marker for OCD. Previously, anhedonia was confirmed to be an endophenotype of depression, schizophrenia, and other diagnoses.58,59 Our TEPS results differed in part from a previous study showing significant differences in TEPS-CON but not TEPS-ANT scores between OCD patients and HCs.30 Although we did not find a significant difference in TEPS-ANT scores between our OCD and HC groups in our preliminary analysis, a difference emerged in our later analysis. It may be that TEPS-ANT scores are unstable in OCD patients, perhaps being sensitive to participant selection in relation to factors such as age, obsessive–compulsive symptoms, or medicine status. To some extent, we may have to infer that consummatory, but not anticipatory, anhedonia may be state independent. Our results suggest that OCD patients may have a typical ability to feel anticipatory pleasure, with a specific consummatory pleasure deficit. The neural bases of anhedonia-related reward systems involve both emotional and motivational pathways.43 The emotional pathway related to consummatory pleasure has been reported to be disrupted in OCD patients.60,61 Gillan et al62 found that OCD patients showed a deficit in goal-directed control in an outcome devaluation test; they did not stop goal-directed behaviors despite the target goal being devalued. Analogously, OCD patients may be hard to stop the use of objects which have no capacity to produce consummatory pleasure. Recent neuroimaging evidence points to reduced functional connectivity between the caudate nucleus, orbitofrontal cortex, and anterior cingulate cortex in OCD patients. These regions have been found to affect goal-directed behavior in animals.63
We applied the WCST and SCWT to test whether OCD patients and FDRs had deficits in domains of executive function, such as cognitive flexibility and interference control.64 OCD patients and their relatives showed poorer performance than HCs on the WCST and SCWT, consistent with previous findings65 suggesting that alterations in executive function may be considered as an endophenotype for OCD. Moreover, Chamberlain et al32 found that deficits in cognitive flexibility and motor inhibition may be appropriate markers for OCD. Zhang et al66 have similarly found that mental flexibility and response inhibition may represent cognitive endophenotypes of early-onset OCD. On a neuropsychological level, alterations in the frontal lobe and anterior cingulate cortex67 have been found in OCD patients during the performance of the WCST68 and SCWT.51,69 And the deficits in cognitive flexibility may be related to the abnormal ability to achieve pleasure. Franke et al70 found that more severe anhedonia was related to worse performance on the WCST and that these deficits were associated with frontal lobe alterations even in healthy siblings of schizophrenics. A previous brain-imaging study57 reported blunted reward-associated right medial orbitofrontal cortex responsiveness as well as decreased activities in the dorsolateral and anterior prefrontal cortices in OCD patients performing affective switching in a reversal-learning task. Light et al19 found a relationship between trait emotionality and executive function in major depressive disorder that was mediated by the ventrolateral prefrontal cortex. These frontal brain region abnormalities may disrupt the ability to get pleasure from rewards in addition to impairing cognitive function.19,71–73 If so, OCD patients with anhedonia may also have executive function (eg cognitive flexibility) deficits. This hypothesis could be tested in functional neuroimaging studies.
Limitations
This study had several limitations. First, the generalizability of the study is limited by its small sample size. Further research should enroll larger samples to probe the validity of our conclusions. Secondly, with regard to unaffected FDR, we did not have a specific limit to parents or siblings of OCD patients. Age and genotype factors may also have influenced the results. Thirdly, we did not divide our OCD patients according to OCD symptoms. OCD with checking compulsions may be related to brain regions that are important for motor and attentional functions, whereas OCD with contamination fears may be more associated with dysfunctional emotion processing circuits.74 Figee et al60 have shown that OCD with contamination fears exhibit reduced nucleus accumbens activity during reward anticipation. Further research is necessary to elucidate anhedonia among different subtypes of OCD patients.
Conclusion
The present family-based study showed an independent role of anhedonia, especially consummatory anhedonia, in OCD beyond depression. Our findings support the qualification of anhedonia as an endophenotype of OCD. Additionally, our exploratory analysis suggested that OCD patients may have deficits in cognitive flexibility in addition to deficits in experiencing pleasure.
Abbreviations
OCD, obsessive–compulsive disorder; WCST, Wisconsin Card Sorting Test; SCWT, Stroop Color-Word Test; FDR, first-degree relatives; HC, healthy control subjects; PTSD, post-traumatic stress disorder; IGT, Iowa Gambling Task; NAc, nucleus accumbens; OFC, orbitofrontal cortex; fMRI, Functional magnetic resonance imaging; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; IQ, intelligence quotient; ANOVA, analysis of variance; TEPS, Temporal Experience of Pleasure Scale; C-BDI, Chinese version of the Beck Depression Inventory; S-AI, state anxiety questionnaire; T-AI, trait anxiety questionnaire; LSD, least significant difference; TEPS-CON, Temporal Experience of Pleasure Scale–consummatory pleasure scores; TEPS-ANT, Temporal Experience of Pleasure Scale–anticipatory pleasure scale; ACC, anterior cingulated cortex; DLPFC, decreased dorsolateral prefrontal cortex.
Data Sharing Statement
All related data are present in the manuscript.
Ethics Approval and Consent to Participate
This study was approved by the Human Research Ethics Committee for Non-Clinical Faculties of the School of Psychology, South China Normal University (reference number: 114). All participants were provided with an information sheet that provided details about the study, including its purpose, safeguards for their anonymity and use of data. Informed written consent was obtained from all subjects who participated in the current study.
Consent for Publication
Not applicable.
Acknowledgments
The authors would like to thank all the participants who took part, provided support and contributed to this piece of research.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed on the journal to which the article will be submitted; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This study was partly supported by the National Natural Science Foundation of China (31871113), the project of Shenzhen science and technology innovation committee (JCYJ20160427192001852, JCYJ20150729104249783) and Sanming Project of Medicine in Shenzhen (SZSM201512009) and Guangdong Key Project in the development of new tools for diagnosis and treatment of autism (2018B030335001).
Disclosure
The authors report no conflicts of interest for this work.
References
1. American P. Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition, Text Revised) (DSM-IV-TR). American Psychiatric Association; 1994.
2. Dichter GS. Anhedonia in unipolar major depressive disorder: a review. Open Psychiatr J. 2010;4(1):1–9. doi:10.2174/1874354401004010001
3. Keedwell PA, Andrew C, Williams SCR, Brammer MJ, Phillips ML. The neural correlates of anhedonia in major depressive disorder. Biol Psychiatry. 2005;58(11):843–853. doi:10.1016/j.biopsych.2005.05.019
4. Pelizza L, Ferrari A. Anhedonia in schizophrenia and major depression: state or trait? Ann Gen Psychiatry. 2009;8(1):22. doi:10.1186/1744-859X-8-22
5. Gabbay V, Johnson AR, Alonso CM, Evans LK, Babb JS, Klein RG. Anhedonia, but not irritability, is associated with illness severity outcomes in adolescent major depression. J Child Adolesc Psychopharmacol. 2015;25(3):194–200. doi:10.1089/cap.2014.0105
6. Gard DE, Kring AM, Gard MG, Horan WP, Green MF. Anhedonia in schizophrenia: distinctions between anticipatory and consummatory pleasure. Schizophr Res. 2007;93(1–3):253–260. doi:10.1016/j.schres.2007.03.008
7. Strauss GP, Gold JM. A new perspective on anhedonia in schizophrenia. Am J Psychiatry. 2012;169(4):364–373. doi:10.1176/appi.ajp.2011.11030447
8. Isella V. Physical anhedonia in parkinson’s disease. J Neurol Neurosurg Psychiatry. 2003;74(9):1308–1311. doi:10.1136/jnnp.74.9.1308
9. Leentjens AFG, Dujardin K, Marsh L, et al. Apathy and anhedonia rating scales in parkinson’s disease: critique and recommendations. Mov Disord. 2008;23(14):2004–2014. doi:10.1002/mds.22229
10. Pizzagalli DA, Goetz E, Ostacher M, Iosifescu DV, Perlis RH. Euthymic patients with bipolar disorder show decreased reward learning in a probabilistic reward task. Biol Psychiatry. 2008;64(2):162–168. doi:10.1016/j.biopsych.2007.12.001
11. Nawijn L, van Zuiden M, Frijling JL, Koch SBJ, Veltman DJ, Olff M. Reward functioning in PTSD: a systematic review exploring the mechanisms underlying anhedonia. Neurosci Biobehav Rev. 2015;51:189–204. doi:10.1016/j.neubiorev.2015.01.019
12. Abramovitch A, Pizzagalli DA, Reuman L, Wilhelm S. Anhedonia in obsessive-compulsive disorder: beyond comorbid depression. Psychiatry Res. 2014;216(2):223–229. doi:10.1016/j.psychres.2014.02.002
13. Xia J, Fan J, Du H, et al. Abnormal spontaneous neural activity in the medial prefrontal cortex and right superior temporal gyrus correlates with anhedonia severity in obsessive-compulsive disorder. J Affect Disord. 2019;259:47–55. doi:10.1016/j.jad.2019.08.019
14. Abramovitch A, Pizzagalli DA., Reuman L, Wilhelm S. Anhedonia in obsessive-compulsive disorder: beyond comorbid depression. Psychiatry Res. 2014;216(2):223–29. doi:10.1016/j.psychres.2014.02.002
15. Der-Avakian A, Markou A. The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci. 2012;35(1):68–77. doi:10.1016/j.tins.2011.11.005
16. Spano MC, Lorusso M, Pettorruso M, et al. Anhedonia across borders: transdiagnostic relevance of reward dysfunction for noninvasive brain stimulation endophenotypes. CNS Neurosci Ther. 2019;25(11):1229–1236. doi:10.1111/cns.13230
17. Diamond A. Executive functions. Annu Rev Psychol. 2013;64(1):135–168. doi:10.1146/annurev-psych-113011-143750
18. Roiser JP, Sahakian BJ. Hot and cold cognition in depression. CNS Spectr. 2013;18(3):139–149. doi:10.1017/S1092852913000072
19. Light SN, Bieliauskas LA, Zubieta J-K. “Top-down” mu-opioid system function in humans: mu-opioid receptors in ventrolateral prefrontal cortex mediate the relationship between hedonic tone and executive function in major depressive disorder. J Neuropsychiatry Clin Neurosci. 2017;29(4):357–364. doi:10.1176/appi.neuropsych.16090171
20. Shin NY, Lee TY, Kim E, Kwon JS. Cognitive functioning in obsessive-compulsive disorder: a meta-analysis. Psychol Med. 2014;44(6):1121–1130. doi:10.1017/S0033291713001803
21. Calkins AW, Berman NC, Wilhelm S. Recent advances in research on cognition and emotion in OCD: a review. Curr Psychiatry Rep. 2013;15(5):357. doi:10.1007/s11920-013-0357-4
22. Whitehead MR, Suveg C. Difficulties in emotion regulation differentiate depressive and obsessive–compulsive symptoms and their co-occurrence. Anxiety Stress Coping. 2016;29(5):507–518. doi:10.1080/10615806.2015.1088523
23. Robinson LJ, Freeston MH. Emotion and internal experience in obsessive compulsive disorder: reviewing the role of alexithymia, anxiety sensitivity and distress tolerance. Clin Psychol Rev. 2014;34(3):256–271. doi:10.1016/j.cpr.2014.03.003
24. Ferreira GM, Yücel M, Dawson A, Lorenzetti V, Fontenelle LF. Investigating the role of anticipatory reward and habit strength in obsessive-compulsive disorder. CNS Spectr. 2017;22(3):295–304. doi:10.1017/S1092852916000535
25. Chamberlain S. Motor inhibition and cognitive flexibility in obsessive-compulsive disorder and trichotillomania. Am J Psychiatry. 2006;163(7):1282. doi:10.1176/appi.ajp.163.7.1282
26. Vaghi MM, Vértes PE, Kitzbichler MG, et al. Specific frontostriatal circuits for impaired cognitive flexibility and goal-directed planning in obsessive-compulsive disorder: evidence from resting-state functional connectivity. Biol Psychiatry. 2017;81(8):708–717. doi:10.1016/j.biopsych.2016.08.009
27. Zetsche U, Rief W, Westermann S, Exner C. Cognitive deficits are a matter of emotional context: inflexible strategy use mediates context-specific learning impairments in OCD. Cogn Emot. 2015;29(2):360–371. doi:10.1080/02699931.2014.911144
28. Bouchard C, Rhéaume J, Ladouceur R. Responsibility and perfectionism in OCD: an experimental study. Behav Res Ther. 1999;37(3):239–248. doi:10.1016/S0005-7967(98)00141-7
29. Gillan CM, Robbins TW. Goal-directed learning and obsessive–compulsive disorder. Philos Trans R Soc B Biol Sci. 2014;369(1655):20130475. doi:10.1098/rstb.2013.0475
30. Li S, Zhang Y, Fan J, et al. Patients with obsessive-compulsive disorder exhibit deficits in consummatory but not anticipatory pleasure. Front Psychol. 2019;10. doi:10.3389/fpsyg.2019.01196
31. de SRB CL, Rodrigues L, Vivan ADS, Kristensen CH. Heterogeneity of Obsessive-Compulsive Disorder (OCD): a selective review of the literature. Context Clínicos. 2010;3(2):132–140. doi:10.4013/ctc.2010.32.07
32. Chamberlain SR, Fineberg NA, Menzies LA, et al. Impaired cognitive flexibility and motor inhibition in unaffected first-degree relatives of patients with obsessive-compulsive disorder. Am J Psychiatry. 2007;164(2):335–338. doi:10.1176/ajp.2007.164.2.335
33. Pauls DL, Alsobrook JP, Goodman W, Rasmussen S, Leckman JF. A family study of obsessive-compulsive disorder. Am J Psychiatry. 1995;152(1):76–84. doi:10.1176/ajp.152.1.76
34. Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160(4):636–645. doi:10.1176/appi.ajp.160.4.636
35. Gottesman II, Shields J. Genetic theorizing and schizophrenia. Br J Psychiatry. 1973;122(566):15–30. doi:10.1192/bjp.122.1.15
36. McCabe C. Neural correlates of anhedonia as a trait marker for depression. In: Anhedonia A, editors. Comprehensive Handbook Volume II: Neuropsychiatric and Physical Disorders. Netherlands: Springer;2014:159–174. doi:10.1007/978-94-017-8610-2_6
37. Wen-Hua L, Raymond C, Min-Er H. Endophenotype of depression: anhedonia and its measurements. Adv Psychol Ence. 2010;2:10.
38. Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown obsessive compulsive scale. II. Validity. Arch Gen Psychiatry. 1989;46(11):1012–1016. doi:10.1001/archpsyc.1989.01810110054008
39. Moritz S, Meier B, Kloss M, et al. Dimensional structure of the Yale–Brown Obsessive-Compulsive Scale (Y-BOCS). Psychiatry Res. 2002;109(2):193–199. doi:10.1016/S0165-1781(02)00012-4
40. Zhang Y, Men F, C Y, Gan X, Guo W. Reliability and validity of revised the Yale-Brown obsessive compulsive scale. China Acad J Electron Publ House. 1996;010(005):205–207.
41. Lee EB, Zhang CC, Gong H, et al. Psychometric evaluation of the Mandarin Chinese version of the Yale-Brown obsessive-compulsive scale–self report. J Obsessive Compuls Relat Disord. 2018;19:29–33. doi:10.1016/j.jocrd.2018.07.006
42. Xu Y, Zhang H. The reliability and validity of the Chinese version of Yale-Brown obsessive-compulsive scale. Shanghai Arch Psychiatry. 2006;18(6):321–323.
43. Gard DE, Gard MG, Kring AM, John OP. Anticipatory and consummatory components of the experience of pleasure: A scale development study. J Res Pers. 2006;40(6):1086–1102. doi:10.1016/j.jrp.2005.11.001
44. Chan RCK, Shi Y, Lai M, Wang Y, Wang Y, Kring AM. The Temporal Experience of Pleasure Scale (TEPS): exploration and confirmation of factor structure in a healthy Chinese sample. Heaton RK, ed. PLoS One. 2012;7(4):e35352. doi:10.1371/journal.pone.0035352
45. Beck AT. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4(6):561. doi:10.1001/archpsyc.1961.01710120031004
46. Shek DTL. Reliability and factorial structure of the chinese version of the beck depression inventory. J Clin Psychol. 1990;46(1):35–43. doi:10.1002/1097-4679(199001)46:1<35::AID-JCLP2270460106>3.0.CO;2-W
47. Chan DW. The beck depression inventory: what difference does the Chinese version make? Psychol Assess. 1991;3(4):616–622. doi:10.1037/1040-3590.3.4.616
48. Spielberger CD. Manual for the State-Trait Anxiety Inventory (STAI: Form Y). Palo Alto (CA): Mind Garden; 1983.
49. Shek DTL. Reliability and factorial structure of the chinese version of the state-trait anxiety inventory. J Psychopathol Behav Assess. 1988;10(4):303–317. doi:10.1007/BF00960624
50. Heaton RK, Staff P. Wisconsin card sorting test: computer version 2. Psychol Assess Res. 1993;4:1–4.
51. Kulaif T, Valle LER. Alternative to the Stroop color-word test for illiterate individuals. Clin Neuropsychol. 2008;22(1):73–83. doi:10.1080/13854040601186964
52. Vaghi MM, Luyckx F, Sule A, Fineberg NA, Robbins TW, De Martino B. Compulsivity reveals a novel dissociation between action and confidence. Neuron. 2017;96(2):348–354.e4. doi:10.1016/j.neuron.2017.09.006
53. Vulink N, Denys D, Bus L, Westenberg H. Sexual pleasure in women with obsessive-compulsive disorder? J Affect Disord. 2006;91(1):19–25. doi:10.1016/j.jad.2005.12.006
54. Rachman S. Obsessions, responsibility and guilt. Behav Res Ther. 1993;31(2):149–154. doi:10.1016/0005-7967(93)90066-4
55. Price JL, Drevets WC. Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn Sci. 2012;16(1):61–71. doi:10.1016/j.tics.2011.12.011
56. Harrison BJ, Soriano-Mas C, Pujol J, et al. Altered corticostriatal functional connectivity in obsessive-compulsive disorder. Arch Gen Psychiatry. 2009;66(11):1189. doi:10.1001/archgenpsychiatry.2009.152
57. Remijnse PL, Nielen MMA, van Balkom AJLM, et al. Differential frontal–striatal and paralimbic activity during reversal learning in major depressive disorder and obsessive–compulsive disorder. Psychol Med. 2009;39(9):1503–1518. doi:10.1017/S0033291708005072
58. Schürhoff F, Szöke A, Bellivier F, et al. Anhedonia in schizophrenia: a distinct familial subtype? Schizophr Res. 2003;61(1):59–66. doi:10.1016/S0920-9964(02)00237-2
59. Vrieze E, Claes S. Anhedonia and increased stress sensitivity: two promising endophenotypes for major depression. Curr Psychiatry Rev. 2009;5(3):143–152. doi:10.2174/157340009788971083
60. Figee M, Vink M, de Geus F, et al. Dysfunctional reward circuitry in obsessive-compulsive disorder. Biol Psychiatry. 2011;69(9):867–874. doi:10.1016/j.biopsych.2010.12.003
61. Oikonomidis L, Santangelo AM, Shiba Y, Clarke FH, Robbins TW, Roberts AC. A dimensional approach to modeling symptoms of neuropsychiatric disorders in the marmoset monkey. Dev Neurobiol. 2017;77(3):328–353. doi:10.1002/dneu.22446
62. Gillan CM, Papmeyer M, Morein-Zamir S, et al. Disruption in the balance between goal-directed behavior and habit learning in obsessive-compulsive disorder. Am J Psychiatry. 2011;168(7):718–726. doi:10.1176/appi.ajp.2011.10071062
63. Lee SW, Shimojo S, O’Doherty J. Neural computations underlying arbitration between model-based and model-free learning. Neuron. 2014;81(3):687–699. doi:10.1016/j.neuron.2013.11.028
64. Yun J-Y, Jang JH, Jung WH, et al. Executive dysfunction in obsessive-compulsive disorder and anterior cingulate-based resting state functional connectivity. Psychiatry Investig. 2017;14(3):333. doi:10.4306/pi.2017.14.3.333
65. Cavedini P, Zorzi C, Piccinni M, Cavallini MC, Bellodi L. Executive dysfunctions in obsessive-compulsive patients and unaffected relatives: searching for a new intermediate phenotype. Biol Psychiatry. 2010;67(12):1178–1184. doi:10.1016/j.biopsych.2010.02.012
66. Zhang J, Yang X, Yang Q. Neuropsychological dysfunction in adults with early-onset obsessive-compulsive disorder: the search for a cognitive endophenotype. Rev Bras Psiquiatr. 2015;37(2):126–132. doi:10.1590/1516-4446-2014-1518
67. Funahashi S. Neuronal mechanisms of executive control by the prefrontal cortex. Neurosci Res. 2001;39(2):147–165. doi:10.1016/S0168-0102(00)00224-8
68. Jones CRG. Wisconsin Card Sorting Test (WCST). New York: Springer; 2013.
69. Gruber SA, Rogowska J, Yurgelun-Todd DA. Decreased activation of the anterior cingulate in bipolar patients: an fMRI study. J Affect Disord. 2004;82(2):191–201. doi:10.1016/j.jad.2003.10.010
70. Franke P, Maier W, Hardt J, Hain C. Cognitive functioning and anhedonia in subjects at risk for schizophrenia. Schizophr Res. 1993;10(1):77–84. doi:10.1016/0920-9964(93)90079-X
71. Waltz JA, Kasanova Z, Ross TJ, et al. The roles of reward, default, and executive control networks in set-shifting impairments in Schizophrenia. Lu L, ed. PLoS One. 2013;8(2):e57257. doi:10.1371/journal.pone.0057257
72. Dichter GS, Bellion C, Casp M, Belger A. Impaired modulation of attention and emotion in Schizophrenia. Schizophr Bull. 2010;36(3):595–606. doi:10.1093/schbul/sbn118
73. Stinson EJ, Krakoff J, Gluck ME. Depressive symptoms and poorer performance on the Stroop task are associated with weight gain. Physiol Behav. 2018;186:25–30. doi:10.1016/j.physbeh.2018.01.005
74. Mataix-Cols D, Wooderson S, Lawrence N, Brammer MJ, Speckens A, Phillips ML. Distinct neural correlates of washing, checking, and hoarding symptom dimensions in obsessive-compulsive disorder. Arch Gen Psychiatry. 2004;61(6):564. doi:10.1001/archpsyc.61.6.564
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

