Back to Journals » OncoTargets and Therapy » Volume 12
Aberrant expression of PAFAH1B3 associates with poor prognosis and affects proliferation and aggressiveness in hypopharyngeal squamous cell carcinoma
Authors Xu J, Zang Y, Cao S, Lei D, Pan X
Received 28 November 2018
Accepted for publication 21 February 2019
Published 11 April 2019 Volume 2019:12 Pages 2799—2808
DOI https://doi.org/10.2147/OTT.S196324
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr William C. Cho
Jianing Xu,1,2 Yuanwei Zang,3 Shengda Cao,1,2 Dapeng Lei,1,2 Xinliang Pan1,2
1Department of Otorhinolaryngology, Qilu Hospital, Shandong University, Jinan, Shandong 250012, People’s Republic of China; 2NHC Key Laboratory of Otorhinolaryngology, Shandong University, Jinan, Shandong 250012, People’s Republic of China; 3Department of Urology, Qilu Hospital, Shandong University, Jinan, Shandong 250012, China
Background: Hypopharyngeal squamous cell carcinoma (HSCC) is among the most lethal tumors encountered in the head and neck, and currently lacks satisfactory therapeutic targets. Platelet activating factor acetylhydrolase 1B3 (PAFAH1B3), a cancer-relevant metabolic driver, is reported to play a critical role in controlling tumorigenesis and aggressiveness in several types of cancers. However, the role of PAFAH1B3 in HSCC progression has not yet been identified.
Methods: The expression pattern of PAFAH1B3 was examined using immunohistochemistry in 83 HSCC tumor tissues and 44 paired adjacent non-tumor samples. Univariate and multivariate analyses were conducted to explore its association with prognosis of HSCC. In vitro loss-of-function assays were performed to explore the impact of PAFAH1B3 knockdown on the biological phenotype of the human HSCC cell line, ie, FaDu cells.
Results: PAFAH1B3 was overly expressed in the HSCC tumor tissues compared with the adjacent non-tumor samples. Moreover, high expression of PAFAH1B3 was positively correlated with cervical lymph node metastasis. PAFAH1B3 overexpression was associated with poor outcome in HSCC, but it was not an independent prognostic indicator. Furthermore, in vitro loss-of function experiments demonstrated that PAFAH1B3 knockdown suppressed cell proliferation by inducing apoptosis and disrupting cell cycle process, and the migratory and invasive capacities were also attenuated in the absence of PAFAH1B3.
Conclusion: This study for the first time demonstrated the clinical value and the role of PAFAH1B3 in the biological function of HSCC. This work suggested that PAFAH1B3 might serve as a potential therapeutic target for HSCC patients.
Keywords: hypopharyngeal squamous cell carcinoma, platelet activating factor acetylhydrolase 1B3, prognosis, cell proliferation, migration, invasion
Introduction
Head and neck squamous cell carcinoma (HNSCC), one of the most prevalent malignances worldwide, refers to a large heterogeneous group of cancers arising from oral cavity, oropharynx, hypopharynx, and larynx.1,2 Hypopharyngeal squamous cell carcinoma (HSCC) is one of the most lethal tumors encountered in HNSCC, and overall survival for HSCC is poor with a 5-year survival rate of 30%.3 By virtue of its inconspicuous location, more than 70% of the HSCC patients manifest at an advanced stage (stage III or IV) at the time of diagnosis,4 commonly after spread to the lymph nodes in the neck. The presence of metastasis in the cervical lymph nodes is considered the most important prognostic factor for HSCC: ipsilateral cervical nodal metastasis in 60%–80% of patients and contralateral occult nodal metastasis in up to 40% of cases.5 Local recurrence also negatively impacts the outcome of HSCC patients, with the reported locoregional recurrence rate up to 54% in advanced cases.6 Indeed, the overall survival rate has remained relatively unchanged over the last few decades,7 although improvements in functional outcomes, attributable to multi-modality therapeutic strategies, are observed. Therefore, there is a robust need for the identification of the novel therapeutic targets, with the aim of achieving a more favorable clinical outcome for HSCC patients.
Head and neck cancer cells, like other tumor cells, possess fundamentally dysregulated metabolism, including changes in metabolites related to energetics, lipid metabolism, inflammation, markers of oxidative stress, and xenobiotics.8 Of note, lipid metabolic abnormalities in head and neck cancer cells have received less concern but are increasingly being recognized for the past few years, such as acetyl-CoA carboxylase (ACC),9 fatty acid synthase (FASN),10 stearoy-CoA desaturase (SCD),11 lipid phosphate phosphatase 1 (LPP1),12 and faconi anemia pathway–dependent lipid metabolism.13 For instance, FASN, a well-established HNSCC metabolic oncoprotein,10,14,15 is one of the most attractive targets in cancer chemotherapy,16 as its inhibitors can kill cancer cells directly or sensitize tumor cells to other therapies such as 5-fluorouracil (5-FU).17 Additionally, 5-FU, another known antimetabolite, is widely used in HNSCC treatment to increase the therapeutic efficacy of cisplatin.18 Moreover, rewiring of lipid metabolisms, including ACC, FASN, and SCD, also plays an important role in cancer metastasis.19 Hence, the more the exploration of the lipid metabolic molecules in head and neck cancer, the better we might exploit the novel targets for therapeutic intervention in HNSCC, including HSCC.
Platelet-activating factor (PAF), as one of the most potent lipid mediators, plays a critical role in oncogenic transformation,20 apoptosis,21 metastasis,22 and angiogenesis in cancers.23 The activity of PAF is regulated by deacetylation, which is catalyzed by PAF acetylhydrolase (PAFAH).24 Platelet-activating factor acetylhydrolase 1B3 (PAFAH1B3) is the one of the catalytic subunits of PAFAH. PAFAH1B3 is reported to be among the 50 most commonly upregulated metabolic enzymes across >1,000 primary human tumors across 19 types of cancers,25 and is dysregulated broadly across many types of cancers.26 PAFAH1B3 can maintain tumor cell aggressiveness via regulating tumor-suppressing lipids.26,27 In addition, PAFAH1B3 participates in multiple signaling pathways, including PAF signaling pathways,28 Wnt pathways,29 and reelin pathways.30,31 Furthermore, PAFAH1B3 is identified as a target for combination therapy with tyrosine kinase inhibitors (TKIs) in BCR-ABL1+ BCP-ALL.32 Despite the critical role that PAFAH1B3 plays in tumor progression and malignancy, it remains obscure as to what is the clinical value of PAFAH1B3 in HSCC and whether it makes a difference to the biological phenotype of hypopharyngeal cancer cells.
Here, we first explored the expression patterns of PAFAH1B3 in HSCC tumor tissues and adjacent non-tumor samples, and determined the correlation of PAFAH1B3 expression with overall survival and clinicopathological parameters based on the immunohistochemical results. Next, in vitro loss-of-function assays were performed to explore impacts of PAFAH1B3 on biological phenotype of the human HSCC FaDu cell line. Overall, we suggest that PAFAH1B3 potentially serves as a promising therapeutic target for HSCC patients.
Materials and methods
Patients and specimens
Eighty-three formalin-fixed, paraffin-embedded HSCC specimens, of which 44 had paired adjacent non-tumor samples, obtained from patients who underwent surgical resection from June 2011 to June 2013 in Qilu Hospital, Jinan, Shandong, China, were subjected to immunohistochemical examination. Patients who underwent neoadjuvant chemotherapy or radiotherapy were excluded. All the patients underwent postoperative radiotherapy. Retrospective clinicopathological data of these patients were also obtained, including age, sex, smoking status, alcohol consumption, clinical stage, T stage, N stage, and differentiation. All these clinicopathological parameters are listed in Table 1. Overall survival was defined as the time from the date of surgery to the date of death. The study was conducted in accordance with the Declaration of Helsinki. All patients provided written consent for the use of their specimens and disease information for future investigation according to the ethics committee of Qilu Hospital of Shandong University.
  | Table 1 Clinical characteristics of the study patients with HSCC (n=83) |
Immunohistochemistry (IHC)
IHC was used to detect PAFAH1B3 expression in HSCC tumor tissues and adjacent non-tumor samples. In brief, paraffin-embedded tissues of HSCC patients were serially sectioned as 5 μm slices, deparaffinized in xylene, and rehydrated through a series of graded alcohols. A citrate buffer (10 mM, pH 6.0) was used to retrieve antigens at 95°C. Endogenous peroxidase activity was quenched by 3% (v/v) hydrogen peroxide for 15 minutes and nonspecific binding was blocked by BSA (5% w/v) for 30 minutes. Then, the sections were incubated with PAFAH1B3 antibody (26564–1-AP, 1:100 dilution; Proteintech, Wuhan, China) overnight at 4°C. Detection was performed with a peroxidase-conjugated secondary antibody at room temperature for 30 minutes and a DAB substrate kit was used to perform chromogenic reaction. The sections were counterstained with hematoxylin. These sections were then dehydrated by graded alcohols and covered with coverslip.
Staining intensity and percentage of PAFAH1B3-positive tumor cells were assessed. Staining intensity was evaluated by the following criteria: 0, negative; 1, low; 2, medium; and 3, high. The proportion of positive-staining cells was semi-quantitatively estimated and graded from 0 to 3: 0, 0% stained; 1, 1%–25% stained; 2, 26%–50% stained; and 3, 51%–100% stained. Final scores were calculated by multiplying the intensity scores by the proportion scores. According to the final scores, samples were divided into four grades: 0, absent (−); 1–2, weak (+); 3–5, moderate (++); and 6–9, strong (+++). Samples with moderate and strong scores were considered to show high expression of PAFAH1B3.
Cell culture and reagents
Human HSCC cell line FaDu was obtained from the ATCC (Manassas, VA, USA) and cultured in minimum essential medium (MEM; HyClone Laboratories, Logan, UT, USA) with 10% (v/v) FBS (Thermo Fisher Scientific, Waltham, MA, USA) at 37°C in a humidified incubator with 5% CO2 (v/v).
PAFAH1B3 small interfering RNA transfection
PAFAH1B3 expression was knocked down with small interfering RNAs (siRNAs) with the following sequences (GenePharma, Shanghai, China). The sequence of PAFAH1B3-specific siRNA1 was 5′-CCU CUG CAU GCA CUU AAC UTT-3′. The sequence of PAFAH1B3-specific siRNA2 was 5′-GCA AAG AUA AGG AAC CCG ATT-3′. The sequence of negative control was 5′-UUC UCC GAA CGU GUC ACG UTT-3′. These siRNAs were transfected into FaDu cells at a concentration of 100 nM with the aid of Lipofectamine 3000 (Thermo Fisher Scientific) following manufacturer’s instructions.
Western blotting
For Western blotting, 3.5×105 FaDu cells were seeded in six-well plates and harvested 72 hours after transfection. The total amount of protein was extracted using RIPA lysis buffer (Beyotime, Shanghai, China) and phenylmethanesulfonyl fluoride (Beyotime). Protein concentrations were measured utilizing the BCA Protein Quantitative Kit (DBI Bioscience, Ludwigshafen, Germany). Equal amounts of proteins were subjected to Western blotting. Proteins were separated on 10% (w/v) SDS-PAGE gels and transferred to polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA, USA). After blocking with 5% (w/v) skimmed milk, membranes were incubated with PAFAH1B3 antibody (ab166906, 1:1,000 dilution; Abcam, Cambridge, UK) and anti-cleaved PARP (ab32064, 1:1,000 dilution; Abcam).
Cell proliferation
FaDu cells (5×103 cells/well) were seeded in 96-well plates and incubated for 0, 24, 48, 72, and 96 hours after transfection. At each time point, 10 μL of Cell Counting Kit-8 (CCK-8) reagent (Bestbio, Shanghai, China) was added to each well and ODs at 450 nm were measured 2 hours after CCK-8 reagent was added. Subsequently, the cell numbers were calculated at the indicated time points and the proliferation curves were drawn accordingly.
Apoptosis and cell cycle assay
After 48 hours of transfection, cells were collected and subjected to flow cytometry. Cell apoptosis was quantified using Annexin V-fluorescein isothiocyanate apoptosis detection kit I (BD Biosciences, San Jose, CA, USA) and analyzed using FCS Express software (version 3; Denovo Software, Glendale, CA, USA). For evaluation of cell cycle status, the cell cycle kit (Bestbio) was used for staining, and then Modifit LT 4.1 software (Verity Software House, Topsham, ME, USA) was used for analysis.
Wound healing assay
FaDu cells were seeded at a concentration of 5×105 cells/well in six-well plates and transfected with PAFAH1B3 or control siRNAs for 48 hours. Vertical scratches on the cell monolayers were created using sterile p200 pipette tips. Then the detached cells were removed by washing with PBS, and the serum-free MEM was added to each well. The percentage of wound closure was quantified by dividing the wound width that had healed at 24 hours by the initial width at 0 hours.
Transwell migration and invasion assays
The transwell plates (8 μm diameter pores; Corning, Corning, NY, USA) were used for migration and invasion assays. To evaluate the migratory capacity of FaDu cells, cell suspensions (1.2×105 cells/200 μL, FBS-free medium) were transferred to the upper chambers and 600 μL of complete medium was added to the lower chambers. After 60 hours, cells were fixed using 100% methanol and stained with 0.1% (w/v) crystal violet. The upper faces of the filters were wiped with the cotton swabs. Images of five 200× fields were captured from each membrane, and the number of migrating cells was counted and averaged. For the invasion assays, the upper faces of the membranes were coated with Matrigel (60 μL, 1:6 dilution; BD Biosciences) at 37°C for 1 hour. The subsequent steps were the same as described earlier.
Statistical analysis
Statistical analyses were performed using the SPSS version 17.0 (IBM Corporation, Armonk, NY, USA) and GraphPad Prism version 5.0 (GraphPad Software, La Jolla, CA, USA). The PAFAH1B3 expression levels measured by IHC and the correlation with clinicopathological parameters were analyzed using the chi-squared or Fisher’s exact test. Kaplan–Meier analysis followed by log-rank test was conducted to analyze the differences in overall survival between the patient subgroups showing different PAFAH1B3 expression levels. Differences between the experimental groups and negative control were assessed by the Student’s t-test. Data were presented as the mean ± SD. P<0.05 was considered statistically significant.
Results
PAFAH1B3 is overly expressed in HSCC tumor tissues
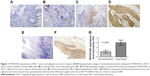
The expression levels of PAFAH1B3 in HSCC tumor tissues (n=83) and adjacent non-tumor samples (n=44) were examined by IHC. PAFAH1B3 was mainly observed in the cytoplasm of HSCC cells. PAFAH1B3 was overly expressed in 62.7% (52/83, Figure 1A–D) of the HSCC tumor specimens, which was markedly higher than adjacent non-tumor samples (22.7%, 10/44, Figure 1E and F) (P<0.0001, Table 2). Moreover, we statistically compared PAFAH1B3 staining in the 44 paired tumor and non-tumor specimens, which confirmed that PAFAH1B3 was significantly overexpressed in HSCC tumor tissues (Figure 1G). These findings suggest that PAFAH1B3 is overly expressed in HSCC.
High expression of PAFAH1B3 was associated with poor prognosis, lymph node metastasis, and advanced clinical stage in HSCC
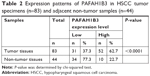
The clinical value of PAFAH1B3 expression was analyzed in a total of 83 HSCC patients. The associations of PAFAH1B3 with clinicopathological parameters are shown in Table 3. Notably, high expression of PAFAH1B3 was significantly correlated with advanced clinical stage and cervical lymph node metastasis (P=0.032 and 0.010, respectively, Table 3). Moreover, the overall survival time of HSCC patients with low PAFAH1B3 expression was significantly longer than those with high PAFAH1B3 level (P=0.022, Figure 2).
Univariate analysis identified four prognostic indicators: lymph node metastasis, T stage, clinical stage, and PAFAH1B3 expression level. Other clinicopathological parameters such as sex, age, and differentiation were not statistically significant prognostic indicators for HSCC. However, multivariate analysis showed that only clinical stage was an independent prognostic factor (P=0.006, Table 4).
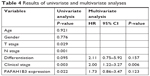  | Table 4 Results of univariate and multivariate analyses |
PAFAH1B3 depletion suppressed cell proliferation, promoted cell apoptosis, and affected cell cycle process in HSCC FaDu cells
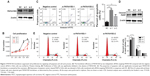
Loss-of-function assays were conducted to explore the biological function of PAFAH1B3 in HSCC FaDu cells. PAFAH1B3 expression was significantly knocked down in FaDu cells transfected with PAFAH1B3-specific siRNA1 and siRNA2 compared with the negative control (NC) siRNA (Figure 3A). Cell proliferation was significantly suppressed in FaDu cells treated with PAFAH1B3 siRNAs in comparison with the NC group (siRNA1 vs NC: P=0.0001; siRNA2 vs NC: P=0.0046, Figure 3B). As PAFAH1B3 depletion inhibited cell proliferation, whether cell apoptosis and cell cycle were involved was explored subsequently by flow cytometry. PAFAH1B3 silencing significantly induced early-phase cell apoptosis than in NC group (siRNA1 vs NC: P=0.0002; siRNA2 vs NC: P=0.0061, Figure 3C). Furthermore, the expression of c-PARP, a marker of apoptosis, was also increased in FaDu cells transfected with PAFAH1B3 siRNAs (Figure 3D). In cell cycle analysis, the proportion of cells in G1-phase was significantly increased (siRNA1 vs NC: P=0.0106; siRNA2 vs NC: P=0.0192), and the percentage of cells in S-phase was markedly reduced (siRNA1 vs NC: P=0.0196; siRNA2 vs NC: P=0.0376) upon PAFAH1B3 knockdown (Figure 3E).
PAFAH1B3 knockdown significantly inhibited cell migration and invasion potential of FaDu cells
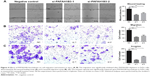
The wound healing assay and transwell assays were conducted to explore the role of PAFAH1B3 in HSCC aggressiveness. The wound healing assay showed that percentage of wound closure in PAFAH1B3 siRNAs transfected FaDu cells was significantly lower than in NC (siRNA1 vs NC: P=0.0012; siRNA2 vs NC: P=0.0039, Figure 4A), indicating that migratory capacity of FaDu cells was significantly impaired upon PAFAH1B3 knockdown. Likewise, the transwell assay for migration also showed that the number of cells traversing the membrane was significantly decreased after PAFAH1B3 depletion (siRNA1 vs NC: P=0.0005; siRNA2 vs NC: P=0.0017, Figure 4B). Similar with the results of the migration assays, the cell number on the bottom of the membranes in PAFAH1B3 siRNAs groups was also significantly reduced (siRNA1 vs NC: P=0.0002; siRNA2 vs NC: P=0.0013, Figure 4C).
Discussion
HSCC has one of the worst prognoses among all the squamous cell carcinomas of the head and neck, and hence there is an urgent need to identify novel therapeutic targets. Recent studies are focused on discovering new molecular markers associated with dysregulated metabolisms in cancers including HNSCC.33,34 PAFAH1B3, one of the most common upregulated metabolic enzymes in cancers, is identified as a critical driver of breast cancer pathogenicity.27 Furthermore, the pharmacological blockade of PAFAH1B3 can impair cancer pathogenicity in multiple types of cancer cells, including melanoma, breast, ovarian, and prostate cancers.26 However, the role of PAFAH1B3 in HSCC has not yet been clarified.
In this study, we showed that PAFAH1B3 was overly expressed in the HSCC tumor tissues compared with the adjacent non-tumor samples. Moreover, PAFAH1B3 overexpression was significantly associated with cervical lymph node metastasis and reduced overall survival in HSCC patients. PAFAH1B3 was selectively overexpressed in HSCC tumor tissues, making it a potentially good target for cancer therapy. Meanwhile, the tumor-associated PAFAH1B3’s features may be used in the diagnosis and prognosis of HSCC. Likewise, PAFAH1B3 was significantly heightened in breast cancers compared with the normal mammary tissues, and its upregulation was significantly associated with reduced recurrence-free survival in breast cancer patients overall, in lymph-node-positive tumors and in grade 1–3 cases.27 This evidence also supports the cancer-related protein role of PAFAH1B3 in hypopharyngeal cancers.
Furthermore, in vitro loss-of-function assays demonstrated that depletion of PAFAH1B3 suppressed cell proliferation by inducing cell apoptosis and compromising cell cycle process in FaDu cells. Previously, RNAi-mediated knockdown and pharmacological blockade of PAFAH1B3 caused unique changes in lipid metabolism, including elevated levels of tumor-suppressing lipids with anti-tumorigenic or proapoptotic effects. These lipids include ceramide, phosphatidylcholine, sphingomyelin, and phosphatidylserine.26,27 In addition, PAF can elevate the expression of antiapoptotic factors in melanoma cell line via NF-κB activation.21 As a potent deacetylase for the signaling lipid PAF, PAFAH1B3 might exert its antiapoptotic function through controlling PAF signaling pathways. These findings support our results that PAFAH1B3 knockdown can promote cell apoptosis in FaDu cells, and further investigation is needed for identification of the underlying mechanisms. Furthermore, our data showed that cell accumulation at G1-phase was increased and cell proportion at S-phase was decreased upon PAFAH1B3 knockdown. It is reported that PAFAH1B3 can modulate the Wnt pathway29 which is connected with the cell cycle regulation;35 cell cycle regulation is an important factor for radio-sensitivity of tumor cells, and radiation is one of the basic therapeutic strategies for HSCC. Therefore, our findings encourage future studies to pay close attention to the role of PAFAH1B3 in cell cycle.
Like the Warburg effect, alteration of lipid metabolism is another nearly ubiquitous change in tumor cells.36 Emerging evidence demonstrates that lipid metabolisms play a vital role in tumor metastasis including HNSCC.9,14,19 Mulvihill et al suggested that PAFAH1B3 knockdown ameliorated cell migration and invasion in breast cancer cells.27 Similar to the previous study, our results showed that the migratory and invasive capacities of FaDu cells were significantly impaired in the absence of PAFAH1B3. These findings supported the fact that PAFAH1B3 expression was positively correlated with cervical lymph node metastasis of HSCC. PAFAH1B3 can modulate the reelin pathway by binding specifically to the reelin receptor (very-low-density lipoprotein receptor), and then compete for receptor occupancy.30 Reduced expression of reelin is observed in many tumors,37 and knockdown of reelin, as well as of its receptors in a pancreatic cancer cell line, promotes cell migration and colony formation.38 As mentioned earlier, PAFAH1B3 serves as a deacetylase in PAF signaling pathways, and PAF can enhance MMP-2 production in vascular smooth muscle cells via the β-arrestin2-dependent activation of ERK signaling pathways.39 Considering that emergence of lymph node metastasis in the neck is the most mortal aspect of HSCC, understanding the underlying mechanisms might provide new strategy to ameliorate metastasis.
Projecting forward, we would like to discuss the future therapeutic prospect of PAFAH1B3 in HSCC. Specific inhibitor of PAFAH1B3 does exist, which can impair cancer cell survival in neuroblastoma, prostate cancer, and breast cancer cells.26,40 Moreover, the combination of PAFAH1B3 inhibition with TKI therapy is suggested to possibly this be efficacious in BCR-ABL1+ BCP-ALL.32 Collectively, these evidence strengthens the potential of PAFAH1B3 as anticancer target once further investigation has been conducted to explore the efficiency of PAFAH1B3 inhibitors in HSCC.
Nevertheless, there are several limitations in our study. First, the in vitro assays were only performed in FaDu cells, which is the only HSCC cell line available in China for now. Second, this being a single-center study, the clinical value of PAFAH1B3 was investigated in a relatively small patient sample. Thus, large multicenter studies including large cohorts of HSCC patients are warranted to study the prognosis of HSCC. Finally, the underlying mechanism of PAFAH1B3 in HSCC has not been clarified in this study. Therefore, we will validate these findings and expand our investigation by including additional HSCC cell lines in the future.
Conclusion
Overall, our study suggests that PAFAH1B3 is overly expressed in HSCC tumor tissues, and that its overexpression is correlated with poor prognosis and cervical lymph node metastasis and advanced clinical stage. Furthermore, loss-of-function experiments demonstrate that PAFAH1B3 knockdown suppresses cell proliferation and aggressiveness in FaDu cells. Therefore, inhibiting PAFAH1B3 might serve as a novel therapeutic strategy for patients with HSCC.
Ethics approval and informed consent
This study protocol was approved by the Ethics Committee of Qilu Hospital of Shandong University. Informed consent was obtained from all individual participants included in the study.
Data availability
The data used during the current study are available from the corresponding author on reasonable request.
Acknowledgments
This work was supported by the Taishan Scholars Program (No tshw20130950), Shandong Province, Department of Science & Technology of Shandong Province (Nos ZR2013HM107, ZR2014HM005, 2015GSF118014, 2015GSF118030, and 2017GSF18166), and Science Foundation of Qilu Hospital of Shandong University and the Fundamental Research Funds of Shandong University (No 2014QLKY05).
Disclosure
The authors report no conflicts of interest in this work.
References
Safdari Y, Khalili M, Farajnia S, Asgharzadeh M, Yazdani Y, Sadeghi M. Recent advances in head and neck squamous cell carcinoma–a review. Clin Biochem. 2014;47(13–14):1195–1202. doi:10.1016/j.clinbiochem.2014.05.066 | ||
Stransky N, Egloff AM, Tward AD, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333(6046):1157–1160. doi:10.1126/science.1208130 | ||
Pracy P, Loughran S, Good J, Parmar S, Goranova R. Hypopharyngeal cancer: United Kingdom national multidisciplinary guidelines. J Laryngol Otol. 2016;130(S2):S104–S110. doi:10.1017/S0022215116000529 | ||
Takes RP, Strojan P, Silver CE, et al. Current trends in initial management of hypopharyngeal cancer: the declining use of open surgery. Head Neck. 2012;34(2):270–281. doi:10.1002/hed.21613 | ||
Buckley JG, MacLennan K. Cervical node metastases in laryngeal and hypopharyngeal cancer: a prospective analysis of prevalence and distribution. Head Neck. 2000;22(4):380–385. | ||
Ang KK, Trotti A, Brown BW, et al. Randomized trial addressing risk features and time factors of surgery plus radiotherapy in advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001;51(3):571–578. | ||
Kim JW, Kim MS, Kim SH, et al. Definitive chemoradiotherapy versus surgery followed by adjuvant radiotherapy in resectable stage III/IV hypopharyngeal cancer. Cancer Res Treat. 2016;48(1):45–53. doi:10.4143/crt.2014.340 | ||
Kamarajan P, Rajendiran TM, Kinchen J, Bermudez M, Danciu T, Kapila YL. Head and neck squamous cell carcinoma metabolism draws on glutaminolysis, and stemness is specifically regulated by glutaminolysis via aldehyde dehydrogenase. J Proteome Res. 2017;16(3):1315–1326. doi:10.1021/acs.jproteome.6b00936 | ||
Su YW, Lin YH, Pai MH, et al. Association between phosphorylated AMP-activated protein kinase and acetyl-CoA carboxylase expression and outcome in patients with squamous cell carcinoma of the head and neck. PLoS One. 2014;9(4):e96183. doi:10.1371/journal.pone.0096183 | ||
Silva SD, Agostini M, Nishimoto IN, et al. Expression of fatty acid synthase, ErbB2 and Ki-67 in head and neck squamous cell carcinoma. A clinicopathological study. Oral Oncol. 2004;40(7):688–696. doi:10.1016/j.oraloncology.2004.01.004 | ||
Nanjappa V, Renuse S, Sathe GJ, et al. Chronic exposure to chewing tobacco selects for overexpression of stearoyl-CoA desaturase in normal oral keratinocytes. Cancer Biol Ther. 2015;16(11):1593–1603. doi:10.1080/15384047.2015.1078022 | ||
Vishwakarma S, Agarwal R, Goel SK, et al. Altered expression of sphingosine-1-phosphate metabolizing enzymes in oral cancer correlate with clinicopathological attributes. Cancer Invest. 2017;35(2):139–141. doi:10.1080/07357907.2016.1272695 | ||
Zhao X, Brusadelli MG, Sauter S, et al. Lipidomic profiling links the fanconi anemia pathway to glycosphingolipid metabolism in head and neck cancer cells. Clin Cancer Res. 2018;24(11):2700–2709. doi:10.1158/1078-0432.CCR-17-3686 | ||
Agostini M, Almeida LY, Bastos DC, et al. The fatty acid synthase inhibitor orlistat reduces the growth and metastasis of orthotopic tongue oral squamous cell carcinomas. Mol Cancer Ther. 2014;13(3):585–595. doi:10.1158/1535-7163.MCT-12-1136 | ||
Silva SD, Cunha IW, Younes RN, Soares FA, Kowalski LP, Graner E. ErbB receptors and fatty acid synthase expression in aggressive head and neck squamous cell carcinomas. Oral Dis. 2010;16(8):774–780. doi:10.1111/j.1601-0825.2010.01687.x | ||
Tennant DA, Duran RV, Gottlieb E. Targeting metabolic transformation for cancer therapy. Nat Rev Cancer. 2010;10(4):267–277. doi:10.1038/nrc2817 | ||
Vazquez-Martin A, Ropero S, Brunet J, Colomer R, Menendez JA. Inhibition of Fatty Acid Synthase (FASN) synergistically enhances the efficacy of 5-fluorouracil in breast carcinoma cells. Oncol Rep. 2007;18(4):973–980. | ||
Adelstein DJ, Moon J, Hanna E, et al. Docetaxel, cisplatin, and fluorouracil induction chemotherapy followed by accelerated fractionation/concomitant boost radiation and concurrent cisplatin in patients with advanced squamous cell head and neck cancer: a Southwest oncology group phase II trial (S0216). Head Neck. 2010;32(2):221–228. doi:10.1002/hed.21179 | ||
Luo X, Cheng C, Tan Z, et al. Emerging roles of lipid metabolism in cancer metastasis. Mol Cancer. 2017;16(1):76. doi:10.1186/s12943-017-0646-3 | ||
Kume K, Shimizu T. Platelet-activating factor (PAF) induces growth stimulation, inhibition, and suppression of oncogenic transformation in NRK cells overexpressing the PAF receptor. J Biol Chem. 1997;272(36):22898–22904. | ||
Heon Seo K, Ko HM, Kim HA, et al. Platelet-activating factor induces up-regulation of antiapoptotic factors in a melanoma cell line through nuclear factor-kappaB activation. Cancer Res. 2006;66(9):4681–4686. doi:10.1158/0008-5472.CAN-05-3186 | ||
Melnikova VO, Mourad-Zeidan AA, Lev DC, Bar-Eli M. Platelet-activating factor mediates MMP-2 expression and activation via phosphorylation of cAMP-response element-binding protein and contributes to melanoma metastasis. J Biol Chem. 2006;281(5):2911–2922. doi:10.1074/jbc.M508683200 | ||
Denizot Y, Descottes B, Truffinet V, Valleix D, Labrousse F, Mathonnet M. Platelet-activating factor and liver metastasis of colorectal cancer. Int J Cancer. 2005;113(3):503–505. doi:10.1002/ijc.20585 | ||
Satoh K, Imaizumi T, Kawamura Y, et al. Platelet-activating factor (PAF) stimulates the production of PAF acetylhydrolase by the human hepatoma cell line, HepG2. J Clin Invest. 1991;87(2):476–481. doi:10.1172/JCI115020 | ||
Nilsson R, Jain M, Madhusudhan N, et al. Metabolic enzyme expression highlights a key role for MTHFD2 and the mitochondrial folate pathway in cancer. Nat Commun. 2014;5:3128. doi:10.1038/ncomms5972 | ||
Kohnz RA, Mulvihill MM, Chang JW, et al. Activity-based protein profiling of oncogene-driven changes in metabolism reveals broad dysregulation of PAFAH1B2 and 1B3 in cancer. ACS Chem Biol. 2015;10(7):1624–1630. doi:10.1021/acschembio.5b00053 | ||
Mulvihill MM, Benjamin DI, Ji X, et al. Metabolic profiling reveals PAFAH1B3 as a critical driver of breast cancer pathogenicity. Chem Biol. 2014;21(7):831–840. doi:10.1016/j.chembiol.2014.05.008 | ||
Manya H, Aoki J, Kato H, et al. Biochemical characterization of various catalytic complexes of the brain platelet-activating factor acetylhydrolase. J Biol Chem. 1999;274(45):31827–31832. | ||
Livnat I, Finkelshtein D, Ghosh I, Arai H, Reiner O. PAF-AH catalytic subunits modulate the Wnt pathway in developing GABAergic neurons. Front Cell Neurosci. 2010;4:19. doi:10.3389/fncel.2010.00019 | ||
Zhang G, Assadi AH, McNeil RS, et al. The Pafah1b complex interacts with the reelin receptor VLDLR. PLoS One. 2007;2(2):e252. doi:10.1371/journal.pone.0000252 | ||
Zhang G, Assadi AH, Roceri M, Clark GD, D’Arcangelo G. Differential interaction of the Pafah1b alpha subunits with the Reelin transducer Dab1. Brain Res. 2009;1267:1–8. doi:10.1016/j.brainres.2009.02.059 | ||
Fiedler ERC, Bhutkar A, Lawler E, Besada R, Hemann MT. In vivo RNAi screening identifies Pafah1b3 as a target for combination therapy with TKIs in BCR-ABL1(+) BCP-ALL. Blood Adv. 2018;2(11):1229–1242. doi:10.1182/bloodadvances.2017015610 | ||
Sandulache VC, Ow TJ, Pickering CR, et al. Glucose, not glutamine, is the dominant energy source required for proliferation and survival of head and neck squamous carcinoma cells. Cancer. 2011;117(13):2926–2938. doi:10.1002/cncr.25868 | ||
Vander Heiden MG. Targeting cancer metabolism: a therapeutic window opens. Nat Rev Drug Discov. 2011;10(9):671–684. doi:10.1038/nrd3504 | ||
Bryja V, Cervenka I, Cajanek L. The connections of Wnt pathway components with cell cycle and centrosome: side effects or a hidden logic? Crit Rev Biochem Mol Biol. 2017;52(6):614–637. doi:10.1080/10409238.2017.1350135 | ||
Zhang F, Du G. Dysregulated lipid metabolism in cancer. World J Biol Chem. 2012;3(8):167–174. doi:10.4331/wjbc.v3.i8.167 | ||
Bock HH, May P. Canonical and non-canonical reelin signaling. Front Cell Neurosci. 2016;10:166. doi:10.3389/fncel.2016.00018 | ||
Sato N, Fukushima N, Chang R, Matsubayashi H, Goggins M. Differential and epigenetic gene expression profiling identifies frequent disruption of the RELN pathway in pancreatic cancers. Gastroenterology. 2006;130(2):548–565. doi:10.1053/j.gastro.2005.11.008 | ||
Kim YH, Lee SJ, Seo KW, et al. PAF enhances MMP-2 production in rat aortic VSMCs via a beta-arrestin2-dependent ERK signaling pathway. J Lipid Res. 2013;54(10):2678–2686. doi:10.1194/jlr.M037176 | ||
Chang JW, Zuhl AM, Speers AE, et al. Selective inhibitor of platelet-activating factor acetylhydrolases 1b2 and 1b3 that impairs cancer cell survival. ACS Chem Biol. 2015;10(4):925–932. doi:10.1021/cb500893q |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.