Back to Journals » Journal of Multidisciplinary Healthcare » Volume 12
Abdominal Compartment Syndrome: Improving Outcomes With A Multidisciplinary Approach – A Narrative Review
Authors Padar M, Reintam Blaser A , Talving P, Lipping E, Starkopf J
Received 12 September 2019
Accepted for publication 24 October 2019
Published 19 December 2019 Volume 2019:12 Pages 1061—1074
DOI https://doi.org/10.2147/JMDH.S205608
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Martin Padar,1,2 Annika Reintam Blaser,2,3 Peep Talving,4,5 Edgar Lipping,6 Joel Starkopf1,2
1Department of Anaesthesiology and Intensive Care, Tartu University Hospital, Tartu, Estonia; 2Department of Anaesthesiology and Intensive Care, University of Tartu, Tartu, Estonia; 3Department of Intensive Care, Lucerne Cantonal Hospital, Lucerne, Switzerland; 4Department of Surgery, University of Tartu, Tartu, Estonia; 5Management Board, North Estonia Medical Centre, Tallinn, Estonia; 6Department of Surgery, Division of Acute Care Surgery, North Estonia Medical Centre, Tallinn, Estonia
Correspondence: Annika Reintam Blaser
Department Of Intensive Care, Lucerne Cantonal Hospital, Spitalstrasse, Lucerne 6000, Switzerland
Tel +41 79 514 21 21
Email [email protected]
Abstract: Abdominal compartment syndrome (ACS) refers to a severe increase in intra-abdominal pressure associated with single or multiorgan failure. ACS with specific pathophysiological processes and detrimental outcomes may occur in a variety of clinical conditions. Patients with ACS are predominantly managed in critical care settings, however, a wide range of multidisciplinary interventions are frequently required from medical, surgical, radiological and nursing specialties. The medical management, aiming to prevent the progression of intra-abdominal hypertension to ACS, is extensively reviewed. Timing and techniques of surgical decompression techniques, as well as management of open abdomen, are outlined. In summary, the current narrative review provides data on history, definitions, epidemiology and pathophysiology of the syndrome and highlights the importance of multidisciplinary approach in the management of ACS in adults.
Keywords: intra-abdominal hypertension, abdominal compartment syndrome, abdominal decompression, decompressive laparotomy, damage control surgery, open abdomen
Introduction
Abdominal compartment syndrome (ACS) has been increasingly recognized as a life-threatening syndrome, where success of treatment depends above all in timely application of appropriate management principles. This narrative review summarizes the contemporary evidence on epidemiology, pathophysiology and therapeutic interventions to provide comprehensive overview for multidisciplinary management of ACS in adult patients.
Methods
We performed literature searches using PubMed and Medline electronic databases using the following search terms: “intra-abdominal hypertension” OR “abdominal compartment syndrome” OR “intra-abdominal pressure”; “abdominal decompression” OR “decompressive laparotomy” OR “open abdomen” OR (“damage control surgery” AND “intra-abdominal hypertension”). Searches were limited to English language and studies in children were not searched (“NOT children”). Additional studies were identified via reference lists of identified papers and related articles feature in PubMed.
History
The evolution of abdominal compartment syndrome and its management have undergone a dramatic evolution during the recent century. In early 19th century, it was initially suggested that muscular compartment syndrome may occur in extremity musculofascial compartments as a result of elevated internal and/or external compartmental pressures. Similar pathophysiological mechanism was subsequently proposed for abdominal compartment resulting in abdominal organ hypoperfusion due to increased intra-abdominal pressure.1,2 Furthermore, it was noted that intra-abdominal pressure (IAP) measured via rectum increased during inspirations and was associated with an oliguria supporting the pressure-related hypothesis for abdominal compartment syndrome.3,4 Also, Emerson and co-authors observed that a significant increase in IAP resulted in cardiovascular collapse.5 Evacuation of peritoneal ascites was noted to improve cardiovascular function.5 Sir Heneage Ogilvie suggested the concept of abdominal decompression and introduced the management of open abdomen in severe war wounds during the 2nd World War.6
Likewise, it was observed that closure of abdominal wounds with increased tension resulted in tension-pneumoperitoneum and wound dehiscence resulting in detrimental outcomes.7 The development of laparoscopic surgery contributed significantly to observations of IAP-related cardiovascular and respiratory complications. Subsequently, Kron and co-authors proposed IAP as a criterion for reoperation and decompression following abdominal aortic aneurysm repair.8 It was also observed that abdominal decompression restored urine output thus intravesical pressure measurements became a common practice in surgical critical care.1,9 The clinical experience pertinent to ACS was consolidated and advanced by the inauguration of the World Society of Abdominal Compartment Syndrome (WSACS) in 2004. The WSACS, comprising international experts of critical care, trauma and general surgery, vascular surgery, anesthesiology and other specialties defined the concept of intra-abdominal hypertension (IAH) and ACS. The WSACS introduced evidence-based guidelines of IAH and ACS in 2006, with subsequent revision in 2013 resulting in significantly improved outcomes following abdominal compartment syndrome.10–12 Current international consensus definitions, gradings of IAH and classification of ACS are shown in Table 1.
 |
Table 1 Definitions |
Epidemiology And Outcome
The precise prevalence of IAH and ACS in hospitalized patients is not well known, because many studies have included only selected patients. A few recent studies including all consecutive ICU admissions describe the true prevalence in critically ill patients.14–16 According to these data, approximately every third ICU patient may suffer from mild to moderate IAH, while mortality is conversely related to the grade of IAH (Figure 1). Approximately two thirds of IAH patients present with elevated IAP already at the day of ICU admission, while one third develop this syndrome later.16
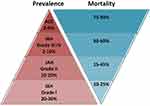 |
Figure 1 Estimated prevalence and mortality of different grades of IAH and of ACS. Note: Data from Iyer et al,13 Murphy et al,14 and Reintam Blaser et al.15Abbreviations: IAH, intra-abdominal hypertension; ACS, abdominal compartment syndrome. |
ACS is rare among ICU patients. Two single-center studies describe it in 2–3% of patients, while the only multicenter study found a prevalence of 6% in consecutive patients.14–16 Mortality is very high: 87.5% at ICU discharge (single-center study) or 68% at day 28 and 76% at day 90 (multicenter study).15,16 Accordingly, despite latest improvements in awareness, recognition and management,17 ACS still remains a deadly syndrome.
Pathophysiology Of IAH And ACS
Development Of IAH
IAP is determined by intra-abdominal volume and abdominal compliance (mainly anterolateral abdominal wall compliance).18 Increase in IAP may result either from increased intra-abdominal volume, decreased abdominal compliance or both.19 Intra-abdominal volume may increase from intra- or extraluminal gas or fluid, tissue oedema or solids such as fat, tumor or a pregnant uterus. Abdominal compliance cannot be readily measured at bedside but the concept is important to recognize. With normal compliance, a large change in volume produces a slight rise in IAP and only at higher volumes IAP starts to rise rapidly.18 With reduced compliance, a lower amount of added volume causes a sharp rise in IAP.18 Abdominal compliance may be reduced in severe burn injuries, massive fluid resuscitation with capillary leak, external constraints and previous abdominal surgery.18
IAH And Organ Dysfunctions/Failures
Elevated IAP is transmitted to the thoracic compartment through elevation of the diaphragm. Resulting direct pulmonary compression complicates mechanical ventilation, reflected by reduced lung compliance, functional residual capacity, tidal volumes and elevated airway pressures. Lung compression also leads to the development of atelectasis, reduced capillary blood flow and increased alveolar dead space. Oxygen uptake and carbon dioxide excretion are reduced and intrapulmonary shunt increases, together leading to hypoxaemia and hypercapnia.20,21 Compression of the heart by pressure transmitted through the diaphragm is associated with decreased cardiac filling and contractility. The inferior vena cava (IVC) is compressed at high IAP, impeding venous return and thereby reducing cardiac output. Concomitant hypovolaemia and application of high PEEP exacerbate these issues. Simultaneously, congestion develops in the venous system of the lower body, promoting oedema and venous thrombosis. Finally, vascular compression in pulmonary and abdominal vascular beds brings along increases in pulmonary and systemic vascular resistance (SVR), respectively.22,23 Clinically, worsening of shock state with increasing need of vasopressors, decline in cardiac output and development of lactic acidosis is observed. Renal perfusion is reduced due to renal arterial and venous compression. Oliguria is seen, often being the first sign of evolvement of IAH to ACS. Worsening perfusion causes ischaemia and acute renal failure develops.19,24 High IAP has severe effects on splanchnic perfusion in animal models.25 From other settings, it is known that hypoperfusion of to the gut may cause ischaemia, promote oedema and ileus, and in case of mucosal barrier failure, lead to sepsis, shock and multiple organ failure.26 Liver perfusion begins to deteriorate at IAP levels as low as 10 mmHg.25 In IAH, decreased lactate clearance and altered glucose metabolism can be expected.27 Increased intrathoracic pressure from elevated IAP is also transmitted to the upper vena caval system, impeding venous return from the brain. This may lead to increased intracranial pressure, having clinical relevance in patients with concomitant brain trauma or oedema.27,28
Risk Factors For ACS
Systematic review and meta-analysis of 14 studies, enrolling 2500 ICU patients from 2002 to 2013 revealed disease severity, metabolic derangement (hypothermia, anaemia, acidosis), hypotension/shock, and large volume crystalloid and packed red blood cell resuscitation as significant risk factors for ACS.29 Studied populations comprised surgical, trauma and pancreatitis patients.29 As none of the studies included in this meta-analysis enrolled all consecutive patients, it remained impossible to identify true risk factors.
In a recent multicenter study including 491 consecutive patients in 15 ICUs worldwide, body mass index, Acute Physiology and Chronic Health Evaluation II score ≥18, abdominal distension, absence of bowel sounds, and positive end-expiratory pressure ≥7 cmH2O were identified as independent risk factors predicting IAH at ICU admission.16 Daily positive fluid balance was independently associated with development of IAH beyond ICU admission day.16 No similar study identifying true risk factors for ACS has been conducted. In our view, the following conditions should warrant particularly high vigilance for ACS: abdominal trauma, in context of damage control surgery and massive transfusions in particular, ruptured abdominal aortic aneurysm (rAAA) repair, extensive abdominal surgery in peritonitis, and severe acute pancreatitis.29,13
Management Of ACS
The care of patients with abdominal compartment syndrome involves interventions across several medical specialties, nursing and allied health professionals. ACS may occur independent of primary diagnosis and medical specialty dealing with it. Establishing the optimal treatment requires merging the therapy concept of the primary disease, stabilization of the patient and management of ACS. For complex management of ACS (including treatments becoming necessary after decompression), other specialties next to intensivists and primary specialty of the particular patient need to be involved in the process. For example, in a patient with neutropenic colitis as a cause of ACS, pathophysiological mechanisms may involve bowel distension, as well as bowel oedema and ascites due to positive fluid balance in shock. Therefore, next to oncologists and intensivists, other specialists need to be involved to develop a plan for therapy, whereas early involvement and discussion with all specialists together should allow agreement on the optimal strategy considering all different aspects. Nephrologists may help regarding the optimal form of CRRT, surgeons to agree on criteria for the re-evaluation of decompression, radiologists to evaluate the signs of bowel ischemia and possibilities to drain any intra-abdominal fluid collections, etc.
ACS is rare, thus testing of treatment options in randomized controlled trials (RCT) is almost impossible. Most management suggestions are therefore based on observational studies, expert opinion or derived from pathophysiology. Decompressive laparotomy is obviously effective and must be performed if the patient presents already with overt ACS. However, in many cases development of ACS can either be delayed or even avoided with timely and meticulous medical care.
Vigilance And Prophylaxis
IAP must be measured to diagnose IAH and ACS. Clinical examination alone is not accurate in detecting elevated IAP.30,31 Also, management of IAH and ACS is based on serial measurements of IAP. Several important risk factors for the development of ACS are given above. IAP measurements should be initiated if any known risk factor for IAH or ACS is present.13
It is important to keep in mind that critically ill patients with extra-abdominal and nonsurgical pathologies may also be at risk of developing IAH and ACS. A multicenter prospective study indicated that only in 46.3% of the cases of IAH was of primary origin. IAH was equally frequent in medical and surgical patients.16
Nonsurgical Management
In our opinion, whereas mild IAH can often just be observed, nonsurgical management strategy should always be used if IAH is progressing towards ACS. Careful monitoring of dynamics of IAP together with general condition of the patient (eg, continuing fluid resuscitation, persisting shock) is essential for timing of aggressive medical treatment to avoid ACS. Even though surgical decompression is highly effective in reducing IAP and restoring organ functions, IAH persists in many patients with the possibility of recurrent ACS.32,33 IAP should be measured regularly, at least every 4 to 6 hrs, or more frequently if indicated.13 Comprehensive medical management should, therefore, continue also after surgical decompression. Options of nonsurgical management are reviewed below and summarized in Figure 2.
 |
Figure 2 Nonsurgical management of IAH and ACS.Note: Data from Kirkpatrick et al13 and Starkopf et al34Abbreviations: IAP, intra-abdominal pressure; IAH, intra-abdominal hypertension; ACS, abdominal compartment syndrome. |
Evacuation Of Intraluminal Contents
Intraluminal accumulation of fluid or gas is a major contributor to IAH. Common causes include bowel obstruction, bowel paralysis and acute colonic pseudo-obstruction (also Ogilvie syndrome). Other reasons may include insufflation of air with non-invasive or mask ventilation, but also intraluminal bleeding and accumulation of bowel contents. If applicable, nasogastric or rectal drainage should be used as a first step for treating mild to moderate IAH, being fairly simple and noninvasive.13 Prokinetics (metoclopramide, erythromycin) may be used to resolve gastroparesis.35 Neostigmine can be considered to evacuate the contents of colon in patients with Ogilvie syndrome, resulting in rapid reduction of intraluminal volume and IAP.13,36
Colonoscopic decompression is effective in resolving colonic volvulus and decompressing the bowel in Ogilvie syndrome.37 Repeated procedures are sometimes needed in the latter, with ultimate clinical success in 73–88% of patients.38
Patients in whom previously described methods are not applicable or fail are best managed by surgery in order to avoid ischaemic intestinal damage and perforation.37
Evacuation Of Intra-Abdominal Space-Occupying Lesions
Hemoperitoneum and ascites are common fluid collections causing IAH; however, retroperitoneal hematoma, free air and intra-abdominal abscesses may likewise be at fault. Such collections should be actively sought using ultrasound (US) and if necessary, CT scan.13 Percutaneous catheter drainage (PCD) is suggested to decrease IAP in patients with IAH/ACS.13,39 This can be done bedside by the intensivist or by interventional radiologist under US or CT guidance. Drainage of ascites may be highly effective in reducing IAP and avoiding ACS, as demonstrated in a single-center case-control study comparing PCD to surgical decompression.40 PCD potentially avoided 81% of surgical decompressions, while a successful procedure was associated with >1 L of drainage, or >9 mmHg decrease of IAP in the first 4 hrs.40 ACS developing after endovascular repair of rAAA was effectively managed by CT-guided drainage of retroperitoneal haematoma together with local tissue plasminogen activator administration.41
Improvement Of Abdominal Wall Compliance
Pain, shivering, agitation, accessory muscle use and ventilator dyssynchrony all increase abdominal wall muscle tone, an important determinant of abdominal wall compliance.36 Resolving these problems should increase abdominal wall compliance and help accommodate a given intra-abdominal volume at lower IAP; however, good data to back specific recommendations, especially on sedation and analgesia are lacking. Patient comfort and avoidance of excessive abdominal muscle contraction might be reasonable goals.
ACS as a complication of burn injury can be remedied by performing escharotomies, shown to reduce IAP significantly while improving ventilation, oxygenation and haemodynamics.42,43
IAP is correctly measured in a supine patient with no active abdominal muscle contractions with the transducer zeroed at the mid-axillary line.13 However, current practice of caring for ICU patients is different – keeping the head of bed (HOB) elevated to reduce the risk of ventilator-associated pneumonia. HOB elevation to 30 degrees from the supine position has been shown to increase IAP by 1.5–5.2 mmHg.44–47 This increase in IAP may become relevant in patients with impending ACS. A reverse Trendelenburg position, suggested in this situation by some authors,13,36 cannot be recommended as it can elevate IAP instead, demonstrated in a study of 10 ICU patients with normal baseline IAP.48 Caution should also be used with the prone position which may somewhat increase IAP.49,50 If proning is needed, it is advisable to suspend the abdomen.51
Provided adequate analgosedation is used and less aggressive means considered or already applied, neuromuscular blockade (NMB) can be used to decrease IAP in the short term.13 NMB reduces IAP effectively and quickly in patients with mild to moderate IAH.28,52
Optimization Of Fluid Administration
High volume fluid resuscitation is a known risk factor of ACS.29 Damage control resuscitation (DCR) refers to the practice of early blood product use in trauma patients aiming to restore blood volume and physiologic stability, addressing coagulopathy and avoiding massive crystalloid use.53 DCR is shown to reduce resuscitation volume and improve patient outcomes,54 and significantly reduces the incidence of ACS in trauma laparotomy patients.55 In patients with severe acute pancreatitis, controlled fluid expansion, compared to rapid infusion of fluids, reduced fluid requirements and was associated with lower incidence of ACS.56 In burn patients, the change in IAP in the course of fluid resuscitation was significantly greater in a crystalloid-only (+27 mmHg) vs combined crystalloid-colloid regimen (+11 mmHg), with more fluids given in the crystalloid group.57
After initial fluid resuscitation and attenuation of the acute phase, removal of excess fluid should be attempted as soon as possible, using diuretics or renal replacement therapy if needed, as these approaches have been shown to reduce IAP.58
Optimization Of Systemic And Regional Perfusion
In IAH, cardiac filling pressures are often elevated due to abdominothoracic transmission of pressure.27 Parameters often used to evaluate cardiac preload/right heart function may therefore be misleading. For example, high central venous pressure may be observed in a hypovolaemic patient without right heart insufficiency, whereas compressed IVC may be seen in ultrasound in a normo- or hypervolemic patient.
Abdominal perfusion pressure (APP) is calculated as mean arterial pressure minus IAP.13 It has been suggested that an optimal APP is 50–60 mmHg and maintaining it appears to be associated with improved outcomes.23,59 Current WSACS guidelines give no recommendation on the use of APP as a resuscitation endpoint.13
Mean arterial pressure may be elevated due to increased SVR even though cardiac output is low. Thus, hypoperfusion may occur despite acceptable APP. Initiating advanced haemodynamic monitoring is reasonable in patients with threatening ACS given the pathophysiological changes, however, evidence of better outcomes with improved monitoring in this patient population is lacking.
Testing fluid responsiveness through heart–lung interactions of mechanically ventilated patients using stroke volume variation (SVV) and pulse pressure variation (PPV) is problematic in the presence of high IAP.23 These values tend to increase owing to changes in aortic compliance and aortic transmural pressures, increases in intrathoracic pressures and concomitant disease states.23 Predetermined changes of SVV and PPV are also used in response to a fluid bolus to predict volume responsiveness. The passive leg raise test performs differently in IAH, because elevated IAP impairs blood return from lower limbs and mesenteric veins making the bolus effect smaller than without the presence of IAH.23
Transpulmonary thermodilution techniques are validated in patients with IAH/ACS and volumetric indices of preload have been shown as accurate.23 However, no exact values can be given for volumetric indices as resuscitation end-points in this setting and an individual approach is warranted.23
Surgical Management Of ACS
Surgical management of IAH with open abdomen (OA) has saved many lives during the recent decades. Main indications for OA therapy are listed in Table 2. According to the international registry of 649 patients from 2015 to 2017, the main indication for OA was peritonitis (51.2% of the cases). A total of 16.8% of causes were traumatic lesions, followed by hemorrhage and vascular emergencies (11.9%), bowel ischemia (8.2%), and pancreatitis (5.7%).60
 |
Table 2 Indications And Complications For Open Abdomen |
Decompressive laparotomy is recommended when medical treatment of ACS fails.13 A timely and complete midline laparotomy is undisputedly the most efficient method of reducing IAP. The procedure is usually performed in operating room but can be also carried out in ICU settings in selected cases.
Timing For Decompressive Laparotomy
High index of suspicion of IAH must prevail among patients suffering severe injuries, burns and emergency surgical diseases. Delaying decompressive laparotomy in ACS is associated with excessive morbidity and mortality rates up to 88%.61 Likewise, it is crucial to perform decompressive laparotomy early at manifestation of ACS. Decompressive laparotomy within 4 days from disease onset in severe acute pancreatitis associated with ACS, was shown to be related to improved outcomes.62 Likewise, in a prospective study, decompressive laparotomy performed early, at a median of 3 hrs after the diagnosis of ACS with median IAP of 22 mmHg, demonstrated improved outcomes.63
Surgical Techniques Of Decompression And Early Definitive Closure
Whenever a decompressive laparotomy is performed, a multidisciplinary treatment plan is set for early definitive closure including judicious fluid administration, utilization of vacuum-assisted wound therapy and early definitive closure of the fascia.64 Surgical abdominal decompression is a vertical full-thickness midline incision through all the layers of the abdominal wall from the xiphoid process to pubic symphysis. A bilateral subcostal transverse laparotomy has also been described but its role in routine treatment of ACS remains unclear.65 Subcutaneous anterior fasciotomy is a new promising technique which uses three small skin incisions to divide the linea alba, leaving the skin and the peritoneum intact. This method avoids OA and associated morbidity, but creates a ventral hernia requiring subsequent repair.66 Currently, more data are needed to recommend these two techniques over the classical midline laparotomy.
Temporary Abdominal Wall Closure In OA
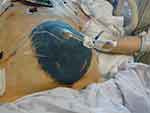
Classical surgical decompression results in OA, and carries subsequent risk for infections, fluid loss, enteroatmospheric fistulas, and incisional hernia. To avoid serious complications and facilitate early definitive or primary fascial closure, various temporary abdominal closure techniques have been deployed. The materials and techniques of temporary closure have undergone significant evolution. Previously applied temporary closure techniques with either “Bogota Bag”, sandwich vacuum dressing, Wittmann patch or mesh-mediated fascial traction-techniques have been currently replaced with use of vacuum-assisted negative pressure therapy at index operation.64 Accordingly, all recent guidelines suggest negative pressure wound therapy (NPWT) with continuous fascial traction as preferred technique for temporary closure both in trauma and non-trauma patients.67,68 This technique ensures appropriate evacuation of ascites rich in pro-inflammatory markers and contamination,69 improves nursing care, and prevents retraction of fascial edges, facilitating early definitive fascial closure (Figure 3).68,70 Temporary closure in OA without negative pressure can be currently considered only if NPWT is not available (low resource setting), resulting frequently in a delayed fascial closure and increased intestinal fistula rates.67,68
Definitive Fascial Closure
The ultimate goal of abdominal wall reconstruction following decompressive laparotomy is primary fascial closure, defined as abdominal fascia-to-fascia closure.71 This should be attempted within 4–7 days after the index operation and is the recommended strategy for both trauma and non-trauma patients.67,68,72 Primary closure is more likely possible in non-infectious indications of OA such as post-traumatic or non-trauma haemorrhage, reported to be achievable in 75–100% of patients.73 In abdominal sepsis, primary closure is possible only in half of the cases.74,75 Success rate is higher if NPWT is used for temporary closure.76
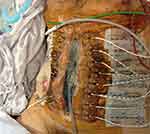
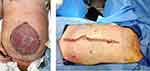
Delaying primary fascial closure beyond 7 days poses significant risks of increased complications.77 When primary tension-free fascial closure fails, different surgical reconstruction techniques may facilitate definitive closure. Both anterior or posterior component separation may facilitate a tension-free definitive closure.78 Likewise, prosthetic mesh-traction is recommended by many investigators to facilitate the approximation of fascial edges.79,80 In settings of contamination, a range of biologic meshes have been introduced with a benefit of fewer hernia rates.81,82 Placing the mesh in a sublay fashion is recommended as the technique results in low recurrence rates.83 Likewise, dynamic retraction techniques have been introduced, such as the ABRA (Dynamic Tissue Systems, Barrie, Ontario, Canada) system that uses elastomers through the full thickness of the abdominal wall to slowly pull the fascia together under variable tension (Figure 4). The very last resort of abdominal closure when all options of primary fascial closure fail is a split-thickness skin-graft on visceral surface with a planned delayed definitive closure (Figure 5).
Prophylactic Open Abdomen
Post-operative IAH may frequently develop following surgery for severe abdominal or pelvic trauma, intra-abdominal sepsis or rAAA. To prevent the development of ACS in these instances, the abdominal cavity is left open at index surgery with a meticulous plan for definitive closure in timely fashion when the condition of the patient approves.84
Damage Control Surgery In Abdominal Trauma
Patients in extremis following abdominal trauma are frequently managed per damage control surgery (DCS) principles with the creation of OA at index operation (Figure 3). Risk factors of ACS in these settings are bleeding requiring massive transfusion (>10 units of packed red blood cells), metabolic acidosis (pH <7.3), hypothermia (<35ºC), coagulopathy and operative time >90 mins.85 DCS strategies such as controlling critical haemorrhage and bowel contamination with abbreviated surgery have remarkably reduced mortality rates of major abdominal trauma patients.54
After the initial surgery, DCS patients are subjected to ICU care with correction of coagulopathy, acidosis and body temperature. At the subsequent surgery, definitive repair of the injuries together with primary fascial closure is attempted.
Severe Complicated Intra-Abdominal Sepsis
Patients with complicated intra-abdominal sepsis and critical derangements in whom definitive surgical repair is not appropriate are likewise subjected to damage control strategy with abbreviated surgery and temporary abdominal closure.86 OA with NPWT in these settings may control toxic ascites,69 drain and control any residual infection and prevent the development of ACS.87 The use of OA has been reported in numerous case series as a potentially beneficial alternative for non-trauma patients with severe abdominal sepsis but there are no contemporary RCTs to outline the best practice. A single RCT published in 2007 that randomized patients with severe secondary peritonitis to OA and closed abdomen groups found an increase in the risk of death for OA.88 Patients with OA were managed with a non-absorbable polypropylene mesh sutured to fascial edges without covering the viscera with a protective layer, thus placing the underlying bowel in danger, as this is known to be associated with adhesions, erosion and fistula formation.89,90 The technique used in the trial can be considered inadequate as significant improvements have been made recently in the management of OA such as the ABThera OA Negative Pressure Therapy System (KCI USA, San Antonio, USA) or ABRA, described above, which use NPWT and a dedicated layer of film to protect the viscera. A prospective international multicentre RCT comparing conventional-closed abdomen vs OA and NPWT after surgical source control for severe complicated intra-abdominal sepsis is currently enrolling patients.91
Patient With Open Abdomen In Intensive Care
OA creates a number of risks and challenges. Timing and techniques of decompression, re-exploration and primary closure, derangements in respiratory, cardiovascular, renal and gastrointestinal function, specific aspects of nursing, monitoring, fluid and nutritional management all demand multidisciplinary and complex approach.73,92
Plan For Surgical Management
A plan for re-exploration no later than 24–48 hrs after index operation has to be made between the multidisciplinary care team.67,68,72 Dynamics of IAP, persistence/resolution of shock, organ failures and coagulation disorders, ongoing infection, amount and characteristics of abdominal drainage output should be discussed daily in details between surgeons, intensivists and intensive care nurses to decide whether primary fascial closure should be attempted at the next re-exploration. The decision-making during re-exploration can be further assisted by anaesthesia team by continuing IAP measurements in the operating room together with monitoring of airway pressures and haemodynamic changes in relation to closure attempt. The determinants to maintain the abdomen open at re-exploration are the need for ongoing resuscitation (trauma, bleeding), infection source control and/or necessity for second look for ischaemic intestine.67 Concerns of development or persistence of ACS also define the need for maintenance of OA.
Dressings And Wound Care
OA therapy requires extensive nursing. Complete collection of peritoneal fluids is often difficult to achieve and may lead to serious skin compromise even despite frequent changes of dressings. Application of NPWT has significantly reduced the complication rate as well as nursing workload. Different commercial products for NPWT are available.64,70 The optimal negative pressure has been suggested to be −125 mmHg, with lower level (−70 mmHg) when active bleeding due to coagulopathy is suspected.67 Instillation of the abdomen with fluids may be considered together with NPWT as this may improve bowel loop moisture, prevent adhesions and improve abdominal closure rates.67 It has also been shown that OA with NPWT does not harm intestinal anastomoses unless they are located very superficially.64,67 Techniques of NPWT usually require dressing changes every 48 hrs, associated with significant use of operating room resources. Recently it has been demonstrated that bedside dressing change for OA in the ICU is feasible.93
Nutritional Support
Patients with OA are as a rule in a hyper-metabolic state, associated with significant fluid, protein and nitrogen losses.68,94,95 Nutritional support thus becomes especially important.96 Indirect calorimetry is recommended to estimate energy expenditure as complex formulas or body weight based calculations are largely unreliable.97 OA itself is not a contraindication for enteral nutrition (EN); therefore, starting EN within 48 hrs after ICU admission (early EN) is encouraged.35,96 Feasibility of EN depends on the presence of bowel injury. The largest study investigating EN in OA included 597 trauma patients over a period of 7 years.98 EN was associated with higher fascial closure rate and reduced mortality compared to no EN, but this beneficial effect was evident only in patients without bowel injury. Presence or formation of enterocutaneous or enteroathmosphaeric fistulas may, in particular, hamper enteral feeding. In case of high output fistulas attention should be paid on enteral delivery vs losses. Establishing feeding access distant to the fistula is important in this setting.99 If this cannot be achieved, EN should be discontinued.35 Overall, patients with OA seldom receive full caloric requirements by enteral route only. Therefore, supplemental parenteral nutrition should be initiated and gradually increased over days 3 to 7 in ICU.100 Vitamins and microelements should be prescribed as suggested by recent guidelines.97 Additional nitrogen losses may occur in patients with OA.95 Considering the complexity described above, involvement of a nutritional specialist in the multidisciplinary team caring patients with OA would be highly desirable. In a cohort study including 179 ICUs worldwide, presence of a critical care dietitian was associated with better compliance with nutrition guidelines, use of early enteral nutrition and providing at least 80% of target energy.101
Mobilization
OA therapy itself does not demand deep sedation and paralysis. The main indication for sedation/paralysis in these patients is threatening recurrent ACS. If ACS is effectively managed with therapeutic measures including OA, gradual awakening of the patient should be undertaken. Gradual application of passive movements at first, and gradual increasing of spontaneous physical activity thereafter can be decided individually. The optimal time for mobilization of patients with OA, however, is not known.68 Early mobilization, the practice of applying physical therapy within the first 2 to 5 days of critical illness,102 has been shown to improve mobility status, muscle strength and increase days alive and out of hospital;103 however, the role of physical therapists is not specifically clear in patients with OA. According to an expert consensus, out-of-bed exercises pose significant risk of adverse events in patients with OA while with in-bed exercises potential benefits should be weighed against potential risks.102 NPWT systems may allow active movements and mobilization more freely thanks to abdominal wall support.
Long-Term Outcome
OA therapy is associated with significant morbidity and mortality.74–76,104 Main complications are listed in Table 2. Long-term outcome is mainly affected by loss of abdominal wall integrity, formation of ventral hernias and loss of bowel function. Still, dedicated and complex management ensures acceptable long-term results. Seternes et al reviewed retrospectively 118 patients treated with OA mainly because of peritonitis and abdominal sepsis.75 Primary fascial closure was achieved in 84% of cases, and 68% of patients survived to hospital discharge. Similar mortality rate has been reported in other studies.74,104 A single-center study from Germany shows that long-term recovery after OA therapy is similar to that of entire ICU population with severity of illness being the only predictor of physical functioning.105
Summary
ACS is a rare syndrome with exceedingly high morbidity and mortality. Recognizing the predisposing risk factors of IAH in conjunction with vigilant IAP monitoring allows prompt detection of patients at risk of ACS. Timely application of IAH management principles is the key factor for improved outcomes. When conservative management fails, early decompressive laparotomy prevents ACS with detrimental outcomes. NPWT is the method of choice for temporary abdominal closure following index laparotomy and all efforts should be made for primary fascial closure within 4 to 7 days. The patient with OA requires dedicated intensive care management considering specific aspects of hemodynamic monitoring, wound care, nutrition and physiotherapy. With proper multidisciplinary approach, acceptable long-term results can be achieved.
Disclosure
Dr Annika Reintam Blaser reports personal fees from Nestlé, personal fees from Fresenius Kabi, grants from Fresenius Kabi, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Van Hee R. Historical highlights in concept and treatment of abdominal compartment syndrome. Acta Clin Belg. 2007;62(Suppl 1):9–15. doi:10.1179/acb.2007.62.s1.003
2. Weber E. Ueber die Anwendung des Wellenlehre auf die Lehre vom Kreislauf des Blutes und insbesondere auf die Pulslehre. Arch Anat Physio Wissensch Medizin. 1851;497–546.
3. Bert P. Leçons sur la physiologie comparée de la respiration. J Anat Physiol. 1870;5(Pt 1):191.
4. Wendt E. Ueber den Einfluss des intraabdominalen Druckes auf die Absonderungsgeschwindigkeit des Harnes. Arch Heilkunde. 1876;17:527–546.
5. Emerson H. Intra-abdominal pressures. Arch Intern Med. 1911;7:754–784. doi:10.1001/archinte.1911.00060060036002
6. Ogilvie WH. The late complications of abdominal war-wounds. Lancet. 1940;236(6105):253–257. doi:10.1016/S0140-6736(01)08769-4
7. Baggot MG. Abdominal blow-out: a concept. Curr Res Anesth Analg. 1951;30(5):295–299. doi:10.1213/00000539-195101000-00055
8. Kron IL, Harman PK, Nolan SP. The measurement of intra-abdominal pressure as a criterion for abdominal re-exploration. Ann Surg. 1984;199(1):28–30. doi:10.1097/00000658-198401000-00005
9. Smith JH, Merrell RC, Raffin TA. Reversal of postoperative anuria by decompressive celiotomy. Arch Intern Med. 1985;145(3):553–554. doi:10.1001/archinte.1985.00360030205035
10. Malbrain MLNG, Cheatham ML, Kirkpatrick A, et al. Results from the international conference of experts on intra-abdominal hypertension and abdominal compartment syndrome. I. Definitions. Intensive Care Med. 2006;32(11):1722–1732. doi:10.1007/s00134-006-0349-5
11. Cheatham ML, Malbrain MLNG, Kirkpatrick A, et al. Results from the International conference of experts on intra-abdominal hypertension and abdominal compartment syndrome. II. Recommendations. Intensive Care Med. 2007;33(6):951–962. doi:10.1007/s00134-007-0592-4
12. Kirkpatrick AW, De Waele JJ, De Laet I, et al. WSACS – the Abdominal Compartment Society. A Society dedicated to the study of the physiology and pathophysiology of the abdominal compartment and its interactions with all organ systems. Anaesthesiol Intensive Ther. 2015;47(3):191–194. doi:10.5603/AIT.a2015.0024
13. Kirkpatrick AW, Roberts DJ, De Waele J, et al. Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med. 2013;39(7):1190–1206. doi:10.1007/s00134-013-2906-z
14. Iyer D, Rastogi P, Åneman A, D’Amours S. Early screening to identify patients at risk of developing intra-abdominal hypertension and abdominal compartment syndrome: intra-abdominal hypertension: screening. Acta Anaesthesiol Scand. 2014;58(10):1267–1275. doi:10.1111/aas.12409
15. Murphy PB, Parry NG, Sela N, Leslie K, Vogt K, Ball I. Intra-abdominal hypertension is more common than previously thought: a prospective study in a mixed medical-surgical ICU. Crit Care Med. 2018;46(6):958–964. doi:10.1097/CCM.0000000000003122
16. Reintam Blaser A, Regli A, De Keulenaer B, et al. Incidence, risk factors, and outcomes of intra-abdominal hypertension in critically Ill patients – a Prospective Multicenter Study (IROI study). Crit Care Med. 2019;47(4):535–542. doi:10.1097/CCM.0000000000003623
17. Balogh ZJ, Lumsdaine W, Moore EE, Moore FA. Postinjury abdominal compartment syndrome: from recognition to prevention. Lancet. 2014;384(9952):1466–1475. doi:10.1016/S0140-6736(14)61689-5
18. Blaser AR, Björck M, De Keulenaer B, Regli A. Abdominal compliance: a bench-to-bedside review. J Trauma Acute Care Surg. 2015;78(5):1044–1053. doi:10.1097/TA.0000000000000616
19. Cheatham ML. Abdominal Compartment Syndrome: pathophysiology and definitions. Scand J Trauma Resusc Emerg Med. 2009;17(1):10. doi:10.1186/1757-7241-17-10
20. Ridings PC, Bloomfield GL, Blocher CR, Sugerman HJ. Cardiopulmonary effects of raised intra-abdominal pressure before and after intravascular volume expansion. J Trauma Inj Infect Crit Care. 1995;39(6):1071–1075. doi:10.1097/00005373-199512000-00010
21. Obeid F. Increases in intra-abdominal pressure affect pulmonary compliance. Arch Surg. 1995;130(5):544. doi:10.1001/archsurg.1995.01430050094016
22. Cheatham ML, Malbrain MLNG. Cardiovascular implications of abdominal compartment syndrome. Acta Clin Belg. 2007;62(sup1):98–112. doi:10.1179/acb.2007.62.s1.013
23. Malbrain MLNG, De Waele JJ, De Keulenaer BL. What every ICU clinician needs to know about the cardiovascular effects caused by abdominal hypertension. Anaesthesiol Intensive Ther. 2015;47(4):388–399. doi:10.5603/AIT.a2015.0028
24. Mohmand H, Goldfarb S. Renal dysfunction associated with intra-abdominal hypertension and the abdominal compartment syndrome: table 1. J Am Soc Nephrol. 2011;22(4):615–621. doi:10.1681/ASN.2010121222
25. Diebel LN, Wilson RF, Dulchavsky SA, Saxe J. Effect of increased intra-abdominal pressure on hepatic arterial, portal venous, and hepatic microcirculatory blood flow. J Trauma Inj Infect Crit Care. 1992;33(2):279–283. doi:10.1097/00005373-199208000-00019
26. Sertaridou E, Papaioannou V, Kolios G, Pneumatikos I. Gut failure in critical care: old school versus new school. Ann Gastroenterol. 2015;28(3):309–322.
27. Malbrain M, Waele JD. Intra-Abdominal Hypertension. Cambridge: Cambridge University Press;2013.
28. Deeren DH, Dits H, Malbrain MLNG. Correlation between intra-abdominal and intracranial pressure in nontraumatic brain injury. Intensive Care Med. 2005;31(11):1577–1581. doi:10.1007/s00134-005-2802-2
29. Holodinsky JK, Roberts DJ, Ball CG, et al. Risk factors for intra-abdominal hypertension and abdominal compartment syndrome among adult intensive care unit patients: a systematic review and meta-analysis. Crit Care. 2013;17(5):R249. doi:10.1186/cc13075
30. Kirkpatrick AW, Brenneman FD, McLean RF, Rapanos T, Boulanger BR. Is clinical examination an accurate indicator of raised intra-abdominal pressure in critically injured patients? Can J Surg J Can Chir. 2000;43(3):207–211.
31. Sugrue M, Bauman A, Jones F, et al. Clinical examination is an inaccurate predictor of intraabdominal pressure. World J Surg. 2002;26(12):1428–1431. doi:10.1007/s00268-002-6411-8
32. Balogh Z, McKinley BA, Holcomb JB, et al. Both primary and secondary abdominal compartment syndrome can be predicted early and are harbingers of multiple organ failure. J Trauma Inj Infect Crit Care. 2003;54(5):848–861. doi:10.1097/01.TA.0000070166.29649.F3
33. Zhou J, Xu Q, Pan K, Mao C, Jin C. Effect of increased intra-abdominal pressure and decompressive laparotomy on aerated lung volume distribution. J Zhejiang Univ Sci B. 2010;11(5):378–385. doi:10.1631/jzus.B0900270
34. Starkopf J, Reintam Blaser A, Malbrain MLNG, Moonen P-J, Oudemans-van Straaten H. Intra-abdominal hypertension and abdominal compartment syndrome (IAH/ACS).
35. Reintam Blaser A, Starkopf J; ESICM Working Group on Gastrointestinal Function, et al. Early enteral nutrition in critically ill patients: ESICM clinical practice guidelines. Intensive Care Med. 2017;43(3):380–398. doi:10.1007/s00134-016-4665-0
36. Cheatham ML. Nonoperative management of intraabdominal hypertension and abdominal compartment syndrome. World J Surg. 2009;33(6):1116–1122. doi:10.1007/s00268-009-0003-9
37. Vogel JD, Feingold DL, Stewart DB, et al. Clinical practice guidelines for colon volvulus and acute colonic pseudo-obstruction. Dis Colon Rectum. 2016;59(7):589–600. doi:10.1097/DCR.0000000000000602
38. Saunders MD. Acute colonic pseudo-obstruction. Best Pract Res Clin Gastroenterol. 2007;21(4):671–687. doi:10.1016/j.bpg.2007.03.001
39. Ouellet J-F, Leppaniemi A, Ball CG, Cheatham ML, D’Amours S, Kirkpatrick AW. Alternatives to formal abdominal decompression. Am Surg. 2011;77(Suppl 1):S51–5.
40. Cheatham ML, Safcsak K. Percutaneous catheter decompression in the treatment of elevated intraabdominal pressure. Chest. 2011;140(6):1428–1435. doi:10.1378/chest.10-2789
41. Hörer T, Skoog P, Pirouzram A, Larzon T. Tissue plasminogen activator-assisted hematoma evacuation to relieve abdominal compartment syndrome after endovascular repair of ruptured abdominal aortic aneurysm. J Endovasc Ther. 2012;19(2):144–148. doi:10.1583/11-3699.1
42. Tsoutsos D, Rodopoulou S, Keramidas E, Lagios M, Stamatopoulos K, Ioannovich J. Early escharotomy as a measure to reduce intraabdominal hypertension in full-thickness burns of the thoracic and abdominal area. World J Surg. 2003;27(12):1323–1328. doi:10.1007/s00268-003-6962-3
43. Oda J, Ueyama M, Yamashita K, et al. Effects of escharotomy as abdominal decompression on cardiopulmonary function and visceral perfusion in abdominal compartment syndrome with burn patients. J Trauma Inj Infect Crit Care. 2005;59(2):369–374. doi:10.1097/01.ta.0000174917.90514.4a
44. Cheatham ML, De Waele JJ, De Laet I, et al. The impact of body position on intra-abdominal pressure measurement: a multicenter analysis. Crit Care Med. 2009;37(7):2187–2190. doi:10.1097/CCM.0b013e3181a021fa
45. Vasquez DG, Berg-Copas GM, Wetta-Hall R. Influence of semi-recumbent position on intra-abdominal pressure as measured by bladder pressure. J Surg Res. 2007;139(2):280–285. doi:10.1016/j.jss.2006.10.023
46. McBeth PB, Zygun DA, Widder S, et al. Effect of patient positioning on intra-abdominal pressure monitoring. Am J Surg. 2007;193(5):644–647. doi:10.1016/j.amjsurg.2007.01.013
47. Chionh JJL, Wei BPC, Martin JA, Opdam HI. Determining normal values for intra-abdominal pressure. ANZ J Surg. 2006;76(12):1106–1109. doi:10.1111/j.1445-2197.2006.03849.x
48. Malbrain M, Van Mieghem N, Verbrugghe W, Daelemans R, Lins R. Effects of different body positions on intra-abdominal pressure and dynamic respiratory compliance. Crit Care. 2003;7(Suppl 2):P179. doi:10.1186/cc2068
49. Hering R, Wrigge H, Vorwerk R, et al. The effects of prone positioning on intraabdominal pressure and cardiovascular and renal function in patients with acute lung injury. Anesth Analg. 2001;92(5):1226–1231. doi:10.1097/00000539-200105000-00027
50. Hering R, Vorwerk R, Wrigge H, et al. Prone positioning, systemic hemodynamics, hepatic indocyanine green kinetics, and gastric intramucosal energy balance in patients with acute lung injury. Intensive Care Med. 2002;28(1):53–58. doi:10.1007/s00134-001-1166-5
51. Regli A, Pelosi P, Malbrain MLNG. Ventilation in patients with intra-abdominal hypertension: what every critical care physician needs to know. Ann Intensive Care. 2019;9(1):52. doi:10.1186/s13613-019-0522-y
52. De Laet I, Hoste E, Verholen E, De Waele JJ. The effect of neuromuscular blockers in patients with intra-abdominal hypertension. Intensive Care Med. 2007;33(10):1811–1814. doi:10.1007/s00134-007-0758-0
53. Ball C. Damage control resuscitation: history, theory and technique. Can J Surg. 2014;57(1):55–60. doi:10.1503/cjs.020312
54. Cotton BA, Reddy N, Hatch QM, et al. Damage control resuscitation is associated with a reduction in resuscitation volumes and improvement in survival in 390 damage control laparotomy patients. Ann Surg. 2011;254(4):598–605. doi:10.1097/SLA.0b013e318230089e
55. Joseph B, Zangbar B, Pandit V, et al. The conjoint effect of reduced crystalloid administration and decreased damage-control laparotomy use in the development of abdominal compartment syndrome. J Trauma Acute Care Surg. 2014;76(2):457–461. doi:10.1097/TA.0b013e3182a9ea44
56. Mao E, Tang Y, Fei J, et al. Fluid therapy for severe acute pancreatitis in acute response stage. Chin Med J (Engl). 2009;122(2):169–173.
57. O’Mara MS, Slater H, Goldfarb IW, Caushaj PF. A prospective, randomized evaluation of intra-abdominal pressures with crystalloid and colloid resuscitation in burn patients. J Trauma. 2005;58(5):1011–1018. doi:10.1097/01.ta.0000162732.39083.15
58. Regli A, De Keulenaer B, De Laet I, Roberts D, Dabrowski W, Malbrain MLNG. Fluid therapy and perfusional considerations during resuscitation in critically ill patients with intra-abdominal hypertension. Anaesthesiol Intensive Ther. 2015;47(1):45–53. doi:10.5603/AIT.a2014.0067
59. Horoz OO, Yildizdas D, Sari Y, Unal I, Ekinci F, Petmezci E. The relationship of abdominal perfusion pressure with mortality in critically ill pediatric patients. J Pediatr Surg. 2019;54(9):1731–1735. doi:10.1016/j.jpedsurg.2018.10.105
60. Coccolini F, Ceresoli M, Kluger Y, et al. Open abdomen and entero-atmospheric fistulae: an interim analysis from the International Register of Open Abdomen (IROA). Injury. 2019;50(1):160–166. doi:10.1016/j.injury.2018.09.040
61. De Waele J, Hoste E, Malbrain M. Decompressive laparotomy for abdominal compartment syndrome–a critical analysis. Crit Care. 2006;10(2):R51. doi:10.1186/cc4870
62. Mentula P. Surgical decompression for abdominal compartment syndrome in severe acute pancreatitis. Arch Surg. 2010;145(8):764. doi:10.1001/archsurg.2010.132
63. De Waele JJ, Kimball E, Malbrain M, et al. Decompressive laparotomy for abdominal compartment syndrome: decompressive laparotomy for abdominal compartment syndrome. Br J Surg. 2016;103(6):709–715. doi:10.1002/bjs.10097
64. Demetriades D. Total management of the open abdomen. Int Wound J. 2012;9:17–24. doi:10.1111/j.1742-481X.2012.01018.x
65. Leppäniemi A, Mentula P, Hienonen P, Kemppainen E. Transverse laparostomy is feasible and effective in the treatment of abdominal compartment syndrome in severe acute pancreatitis. World J Emerg Surg WJES. 2008;3:6. doi:10.1186/1749-7922-3-6
66. Leppäniemi AK, Hienonen PA, Siren JE, Kuitunen AH, Lindström OK, Kemppainen EAJ. Treatment of abdominal compartment syndrome with subcutaneous anterior abdominal fasciotomy in severe acute pancreatitis. World J Surg. 2006;30(10):1922–1924. doi:10.1007/s00268-006-0024-6
67. Chiara O, Cimbanassi S, Biffl W, et al. International consensus conference on open abdomen in trauma. J Trauma Acute Care Surg. 2016;80(1):173–183. doi:10.1097/TA.0000000000000882
68. Coccolini F, Roberts D, Ansaloni L, et al. The open abdomen in trauma and non-trauma patients: WSES guidelines. World J Emerg Surg. 2018;13(1):7. doi:10.1186/s13017-018-0167-4
69. Kubiak BD, Albert SP, Gatto LA, et al. Peritoneal negative pressure therapy prevents multiple organ injury in a chronic porcine sepsis and ischemia/reperfusion model. Shock. 2010;34(5):525–534. doi:10.1097/SHK.0b013e3181e14cd2
70. Fernández LG. Management of the open abdomen: clinical recommendations for the trauma/acute care surgeon and general surgeon: management recommendations for open abdomen. Int Wound J. 2016;13(S3):25–34. doi:10.1111/iwj.12655
71. Cothren CC, Moore EE, Johnson JL, Moore JB, Burch JM. One hundred percent fascial approximation with sequential abdominal closure of the open abdomen. Am J Surg. 2006;192(2):238–242. doi:10.1016/j.amjsurg.2006.04.010
72. Leppäniemi A, Tolonen M, Tarasconi A, et al. 2019 WSES guidelines for the management of severe acute pancreatitis. World J Emerg Surg. 2019;14(1):27. doi:10.1186/s13017-019-0247-0
73. Rogers WK, Garcia L. Intraabdominal hypertension, abdominal compartment syndrome, and the open abdomen. Chest. 2018;153(1):238–250. doi:10.1016/j.chest.2017.07.023
74. Tolonen M, Mentula P, Sallinen V, Rasilainen S, Bäcklund M, Leppäniemi A. Open abdomen with vacuum-assisted wound closure and mesh-mediated fascial traction in patients with complicated diffuse secondary peritonitis: a single-center 8-year experience. J Trauma Acute Care Surg. 2017;82(6):1100–1105. doi:10.1097/TA.0000000000001452
75. Seternes A, Rekstad LC, Mo S, et al. Open abdomen treated with negative pressure wound therapy: indications, management and survival. World J Surg. 2017;41(1):152–161. doi:10.1007/s00268-016-3694-8
76. Kiliç E. The effects of temporary closure methods on mortality and morbidity in patients with open abdomen. Turk J Trauma Emerg Surg. 2018. doi:10.5505/tjtes.2017.95038
77. Miller PR, Meredith JW, Johnson JC, Chang MC. Prospective evaluation of vacuum-assisted fascial closure after open abdomen: planned ventral hernia rate is substantially reduced. Ann Surg. 2004;239(5):608–616. doi:10.1097/01.sla.0000124291.09032.bf
78. Pauli EM, Rosen MJ. Open ventral hernia repair with component separation. Surg Clin North Am. 2013;93(5):1111–1133. doi:10.1016/j.suc.2013.06.010
79. Burger JWA, Luijendijk RW, Hop WCJ, Halm JA, Verdaasdonk EGG, Jeekel J. Long-term follow-up of a randomized controlled trial of suture versus mesh repair of incisional hernia. Trans Meet Am Surg Assoc. 2004;CXXII(NA;):176–183. doi:10.1097/01.sla.0000141193.08524.e7
80. Satterwhite TS, Miri S, Chung C, Spain D, Lorenz HP, Lee GK. Outcomes of complex abdominal herniorrhaphy: experience with 106 cases. Ann Plast Surg. 2012;68(4):382–388. doi:10.1097/SAP.0b013e31823b68b1
81. de Moya MA, Dunham M, Inaba K, et al. Long-term outcome of acellular dermal matrix when used for large traumatic open abdomen. J Trauma Inj Infect Crit Care. 2008;65(2):349–353. doi:10.1097/TA.0b013e31817fb782
82. Bondre IL, Holihan JL, Askenasy EP, et al. Suture, synthetic, or biologic in contaminated ventral hernia repair. J Surg Res. 2016;200(2):488–494. doi:10.1016/j.jss.2015.09.007
83. Holihan JL, Bondre I, Askenasy EP, et al. Sublay versus underlay in open ventral hernia repair. J Surg Res. 2016;202(1):26–32. doi:10.1016/j.jss.2015.12.014
84. Mentula P, Leppäniemi A. Prophylactic open abdomen in patients with postoperative intra-abdominal hypertension. Crit Care. 2010;14(1):111. doi:10.1186/cc8207
85. Beuran M, Iordache F-M. Damage control surgery – physiopathological benchmarks. J Med Life. 2008;1(2):96–100.
86. Leppäniemi A, Kimball EJ, De Laet I, Malbrain MLNG, Balogh ZJ, De Waele JJ. Management of abdominal sepsis – a paradigm shift? Anaesthesiol Intensive Ther. 2015;47(4):400–408. doi:10.5603/AIT.a2015.0026
87. Sartelli M, Abu-Zidan FM, Ansaloni L, et al. The role of the open abdomen procedure in managing severe abdominal sepsis: WSES position paper. World J Emerg Surg. 2015;10(1):35. doi:10.1186/s13017-015-0032-7
88. Robledo FA, Luque-de-León E, Suárez R, et al. Open versus closed management of the abdomen in the surgical treatment of severe secondary peritonitis: a randomized clinical trial. Surg Infect. 2007;8(1):63–72. doi:10.1089/sur.2006.8.016
89. Leber GE. Long-term complications associated with prosthetic repair of incisional hernias. Arch Surg. 1998;133(4):378. doi:10.1001/archsurg.133.4.378
90. Miller K, Junger W. Ileocutaneous fistula formation following laparoscopic polypropylene mesh hernia repair. Surg Endosc. 1997;11(7):772–773. doi:10.1007/s004649900448
91. Kirkpatrick AW, Coccolini F; for The Closed Or Open after Laparotomy (COOL) after Source Control for Severe Complicated Intra-Abdominal Sepsis Investigators, et al. Closed or open after source control laparotomy for severe complicated intra-abdominal sepsis (the COOL trial): study protocol for a randomized controlled trial. World J Emerg Surg. 2018;13(1):26. doi:10.1186/s13017-018-0183-4
92. De Waele JJ, Kaplan M, Sugrue M, Sibaja P, Björck M. How to deal with an open abdomen? Anaesthesiol Intensive Ther. 2015;47(4):372–378. doi:10.5603/AIT.a2015.0023
93. Seternes A, Fasting S, Klepstad P, et al. Bedside dressing changes for open abdomen in the intensive care unit is safe and time and staff efficient. Crit Care. 2016;20(1):164. doi:10.1186/s13054-016-1337-y
94. Chabot E, Nirula R. Open abdomen critical care management principles: resuscitation, fluid balance, nutrition, and ventilator management. Trauma Surg Acute Care Open. 2017;2(1):e000063. doi:10.1136/tsaco-2016-000063
95. Cheatham ML, Safcsak K, Brzezinski SJ, Lube MW. Nitrogen balance, protein loss, and the open abdomen. Crit Care Med. 2007;35(1):127–131. doi:10.1097/01.CCM.0000250390.49380.94
96. Moore SM, Burlew CC. Nutrition support in the open abdomen. Nutr Clin Pract. 2016;31(1):9–13. doi:10.1177/0884533615620420
97. Singer P, Blaser AR, Berger MM, et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019;38(1):48–79. doi:10.1016/j.clnu.2018.08.037
98. Burlew CC, Moore EE, Cuschieri J, et al. Who should we feed? A Western Trauma Association multi-institutional study of enteral nutrition in the open abdomen after injury. J Trauma Acute Care Surg. 2012;73(6):1380–1388. doi:10.1097/TA.0b013e31259924c
99. Picot D, Layec S, Dussaulx L, Trivin F, Thibault R. Chyme reinfusion in patients with intestinal failure due to temporary double enterostomy: a 15-year prospective cohort in a referral centre. Clin Nutr. 2017;36(2):593–600. doi:10.1016/j.clnu.2016.04.020
100. Reintam Blaser A, Deane AM, Starkopf J. Translating the European Society for Clinical Nutrition and Metabolism 2019 guidelines into practice. Curr Opin Crit Care. 2019;25(4):314–321. doi:10.1097/MCC.0000000000000619
101. Heyland DK, Heyland RD, Cahill NE, et al. Creating a culture of clinical excellence in critical care nutrition: the 2008 “best of the best” award. J Parenter Enter Nutr. 2010;34(6):707–715. doi:10.1177/0148607110361901
102. Hodgson CL, Stiller K, Needham DM, et al. Expert consensus and recommendations on safety criteria for active mobilization of mechanically ventilated critically ill adults. Crit Care. 2014;18(6):658. doi:10.1186/s13054-014-0658-y
103. Tipping CJ, Harrold M, Holland A, Romero L, Nisbet T, Hodgson CL. The effects of active mobilisation and rehabilitation in ICU on mortality and function: a systematic review. Intensive Care Med. 2017;43(2):171–183. doi:10.1007/s00134-016-4612-0
104. Arai M, Kim S, Ishii H, Hagiwara J, Kushimoto S, Yokota H. The long-term outcomes of early abdominal wall reconstruction by bilateral anterior rectus abdominis sheath turnover flap method in critically ill patients requiring open abdomen. World J Emerg Surg. 2018;13(1):39. doi:10.1186/s13017-018-0200-7
105. Willms A, Schaaf S, Schwab R, et al. Intensive care and health outcomes of open abdominal treatment: long-term results of vacuum-assisted wound closure and mesh-mediated fascial traction (VAWCM). Langenbecks Arch Surg. 2017;402(3):481–492. doi:10.1007/s00423-017-1575-8
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.