Back to Journals » Drug Design, Development and Therapy » Volume 16
A Systematic Review of the Global Intervention for SARS-CoV-2 Combating: From Drugs Repurposing to Molnupiravir Approval
Authors Ashour NA, Abo Elmaaty A, Sarhan AA, Elkaeed EB , Moussa AM, Erfan IA, Al-Karmalawy AA
Received 19 December 2021
Accepted for publication 26 February 2022
Published 15 March 2022 Volume 2022:16 Pages 685—715
DOI https://doi.org/10.2147/DDDT.S354841
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Georgios Panos
Nada A Ashour,1,* Ayman Abo Elmaaty,2,* Amany A Sarhan,3 Eslam B Elkaeed,4 Ahmed M Moussa,3 Ibrahim Ali Erfan,5 Ahmed A Al-Karmalawy3
1Department of Clinical Pharmacology, Faculty of Pharmacy, Horus University-Egypt, New Damietta, 34518, Egypt; 2Department of Medicinal Chemistry, Faculty of Pharmacy, Port Said University, Port Said, 42526, Egypt; 3Department of Pharmaceutical Medicinal Chemistry, Faculty of Pharmacy, Horus University-Egypt, New Damietta, 34518, Egypt; 4Department of Pharmaceutical Sciences, College of Pharmacy, AlMaarefa University, Ad Diriyah, 13713, Riyadh, Saudi Arabia; 5Department of Pharmacology and Biochemistry, Faculty of Pharmacy, Horus University-Egypt, New Damietta, 34518, Egypt
*These authors contributed equally to this work
Correspondence: Ahmed A Al-Karmalawy, Email [email protected]
Abstract: The rising outbreak of SARS-CoV-2 continues to unfold all over the world. The development of novel effective antiviral drugs to fight against SARS-CoV-2 is a time cost. As a result, some specific FDA-approved drugs have already been repurposed and authorized for COVID-19 treatment. The repurposed drugs used were either antiviral or non-antiviral drugs. Accordingly, the present review thoroughly focuses on the repurposing efficacy of these drugs including clinical trials experienced, the combination therapies used, the novel methods followed for treatment, and their future perspective. Therefore, drug repurposing was regarded as an effective avenue for COVID-19 treatment. Recently, molnupiravir is a prodrug antiviral medication that was approved in the United Kingdom in November 2021 for the treatment of COVID-19. On the other hand, PF-07321332 is an oral antiviral drug developed by Pfizer. For the treatment of COVID-19, the PF-07321332/ritonavir combination medication is used in Phase III studies and was marketed as Paxlovid. Herein, we represented the almost history of combating COVID-19 from repurposing to the recently available oral anti-SARS-CoV-2 candidates, as a new hope to end the current pandemic.
Keywords: SARS-CoV-2, drug repurposing, clinical trials, molnupiravir, paxlovid
Graphical Abstract:
Introduction
The human respiratory system is targeted by coronaviruses (CoV). Coronaviruses are responsible for these dangerous outbreaks, such as the Middle East Respiratory Syndrome (MERS-CoVs), Severe Acute Respiratory Syndrome (SARS-CoV),1 and finally, the novel Severe Acute Respiratory Syndrome (SARS-CoV-2) causing COVID-19 disease.2–6 COVID-19 was nominated for the identification of the SARS-CoV-2 in Wuhan city (China) by December 2019.1,2,7–10 Thus, the World Health Organization (WHO) guided to do some tests about the recent emergence of this outbreak, infection control, and its abolishment.10,11 The first appearance of COVID-19 was through an unknown source of an animal at a seafood market and that was responsible for the emerged outbreak.10 However, it was enrolled that the main source of contagion was from animals (zoonotic)12 including intermediate hosts like a bat.8 Hence, SARS-CoV-2 is a positive single-RNA stranded virus that may provoke diseases in humans or animals. There are 4 -CoV subfamilies (gamma, delta, alpha, and beta -CoV), but SARS-CoV-2 belongs to the beta-CoV subfamily. Although mammals are affected by beta and alpha -CoV viruses, however birds are affected by delta and gamma -CoV viruses.6,13,14
The studies on SARS-CoV-2 propose that most cases may be started by comparatively low loads of virus with syndromes starting from asymptomatic to mild/moderate diseases having different durations.15 Common typical symptoms like cough and fever are the beginning of the appearance of a more serious virus that has a higher load.1,12 As the disease proceeds, some symptoms may appear, such as hypoxemia, fever, pneumonia, and inflammatory responses. Hence, the patient should go to the hospital.3,15 The majority of COVID-19 cases are either asymptomatic or experience moderate disease only. However, most cases that have a respiratory illness are usually required to go to hospitals.16,17
COVID-19 has been an important overt issue all over the world since December (2019). The number of COVID-19 cases in territories and about 224 countries (Worldometer, 2021) has reached about 251,337,194 cases on the 9th of November 2021.18 The global breakthrough of this novel virus urges scientists all over the world to develop new vaccines and test them, ensuring their efficacy.19–22
It is very important to point out the mutations experienced by SARS-CoV-2. Because extremely detrimental changes are swiftly purged, most mutations found in circulating SARS-CoV-2 virions’ genomes are likely to be neutral or modestly deleterious.23 This is because, while high-effect mutations that aid virus adaptation and fitness do occur, they are rare as compared to tolerable low-effect or no-effect “neutral” amino acid alterations. In at least certain settings, a tiny proportion of mutations are likely to affect virus phenotypic in a way that offers a fitness advantage. Pathogenicity, infectivity, transmissibility, and/or antigenicity of viruses may all be affected by such alterations.23 In South Africa, a novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variant B.1.1.529 was recently reported, causing a surge in COVID-19 cases.24 The World Health Organization identified B.1.1.529, also known as Omicron, as a variant under monitoring (VUM) on November 24, 2021. The Omicron variation was identified as a variant of concern (VOC) two days later. This variation has a lot of mutations, including 15 alterations in the spike receptor-binding domain (RBD).24
Drug repurposing – so-called therapeutic switching – is the new application of already existing therapeutics to another disease indication.25,26 The drug repurposing approach represents a rapid and low-cost drug discovery and development.27 For example, recently repurposed drugs that were approved for treating coronary artery disease and erectile dysfunction were aspirin and sildenafil, respectively.28
There are many claimed strategies introduced to combat SARS-CoV-2. An attractive one is to target ACE2 either directly through supplementation or indirectly through drugs that stimulate its downstream players.29 In addition, the main protease (Mpro) is considered to be one of the main targets for drug development against SARS-COV-2.5 Moreover, the anti-inflammatory strategies like nuclear factor erythroid 2 p45-related factor 2 (NRF2) activators can be deployed against the virus.30
Based on the above, we can conclude the great importance of collecting and understanding the previously published articles regarding the efforts and trials for combating COVID-19. Herein, we reviewed most of the previously repurposed FDA-approved drugs, their clinical trials, and the newly introduced candidates to fight SARS-CoV-2. This may help scientists all over the world to make a broad scientific insight concerning this pandemic disease and the possible routes for its defeating.
SARS-CoV-2 Life Cycle and Its Potential Targets
Drugs target SARS-CoV-2 can be classified as (Figure 1):
- Fusion inhibitors: inhibit the fusion process of viral entry
- protease inhibitors: target some proteases,
- Transcription inhibitors: target the reverse transcription step by blocking RNA-dependent RNA polymerase and preventing viral replication,
- Some of the transcriptase inhibitors are nucleoside reverse transcriptase,
- Some antivirals target M2 channel protein.
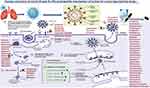 |
Figure 1 The life cycle of SARS-CoV-2, the potential targets of antiviral drugs, and their classification according to the mechanism of action. Note: Created with BioRender.com. |
The nucleocapsid (N) protein comprising the viral genome resides in the innermost layer of SARS-CoV-2. As described, SARS-CoV-2 is an RNA-positive-sense virus that helps it to translate its genome immediately upon entering the cell using host ribosomes. Thus, 29 distinct proteins are encoded in the genome.
The nucleocapsid contains the envelope (E) protein,31 and the entire envelope of the virus contains the membrane (M) protein. Besides, the spike (S) protein in the enveloped membrane on its surface is used by the virus when bound to host cells.31 Moreover, an angiotensin-converting enzyme-2 (ACE2) receptor is a protein that expresses itself in different types of cells, such as esophageal cells, absorbent enterocytes, myocardial cells, alveolar cells, and proximal cells of the kidneys.32 Thus, transmembrane serine protease 2 (TMPRSS2) is primed with the (S) protein, and SARS-CoV-2 infection in the cells of the lungs has been seen to be avoided by the TMPRSS2 inhibitor.
The cathepsin B/L – cysteine proteases – can promote the cleavage of (S) protein in TMPRSS2-negative cells33 given the non-structural seven proteins are as follows: (ORF)7a, PLpro, Nsp12, Nsp3c, Mpro, Nsp1, and Nsp13. However, the four structural proteins are M, S, N, and E that is encoded by SARS-CoV-2.34
The rationale behind the major components and biochemical events in the coronavirus replication cycle is considered as a crucial goal to develop new antiviral drugs. These include proteolytic enzymes and RNA-dependent RNA polymerases.35 SARS-CoV-2 is transmitted in humans mainly by respiratory droplets, yet it might also go ahead in an airborne transition mode.36 The virus comes into the steward cells during two pathways, either via plasma membrane fusibility or endosomes. Through both mechanisms, it engages ACE2 as an entry sensor and the viral S protein, which mediates binding to the host cell membrane.33,37
A recent study showed that the attachment between S protein and ACE2 is activated by a host protease called TMPRSS2.33 The virus uses S protein to neutralize antibodies, making it easier to bind to the host receptors.38 Although the detailed fusion machinery of SARS-CoV-2 is not fully understood, the beta coronaviruses mostly use hemagglutinin-esterase (HE) to link to sialic acid on the glycoprotein surface. These fusion steps could be inhibited using fusion inhibitors. New insights into the antiviral effects of chloroquine against coronavirus what to expect for COVID-19?39
After the fusion completion, the envelope is peeled off and the genome of SARS-CoV-2 along with its nucleocapsid penetrates the cytoplasm of the host cell.33,40 Its genome contains open reading frames 1a and 1b- (ORF1a and ORF1b) genes that produce two polyproteins (pp), pp1a and pp1b, that help in hijacking host ribosomes for the viral translation process.41
Then, papain-like protease (Ppro) and Mpro cleave these polyproteins to produce many non-structural proteins. Besides Ppro and Mpro, 3C-like cysteine protease (3CLPro) is suggested to exist in SARS-CoV-2 based on about 96% similarity with SARS-CoV by using a three-dimensional analysis model.42 These proteases play an extremely important role in viral transcription and replication. In addition, the design of protease inhibitors that inhibit these proteases are potential antivirals against SARS-CoV-2.43
Since SARS-CoV-2 complete mechanisms have not been well understood yet, its replication explanation was based on MERS-CoV and SARS-CoV models because SARS-CoV-2 non-structural and structural proteins are similar to the proteins of these two kinds of viruses. A non-structural protein known as nsp12 forms a transcription and replication complex known as RNA-dependent RNA polymerases (RdRp).44 Nsp12 is paired with its cofactor (nsp7 and nsp8) in SARS-CoV. This protein complex generates a negative-sense complementary RNA using the initial positive RNA as a reference.45 The virus then uses the negative-strand RNA to synthesize new positive RNA molecules to process another stage of translation and replication and synthesize the genomes of the newest viral particles. In SARS-CoV, this process is regulated by topoisomerase III-beta. Using reverse transcription inhibitors, these stages can be disrupted by budding and assembly of the enveloped virus.46 As post-translational alteration is necessary, the structural protein complexes (M, N, E, and S) form the sub-genomic RNA and then join the endoplasmic reticulum.47 In the cytoplasm, N forms a nucleoprotein complex and the positive-strand RNA. In the endoplasmic reticulum-Golgi apparatus compartment (ERGIC), both complexes combine to achieve the virus replication process.48 They are excreted via the vesicles and Golgi apparatus as mature viruses into the extracellular zone and liberated from cells to infect new ones.49
Drug Repurposing Against SARS-CoV-2
On the other hand, drug repurposing26,28,50–56 is emerging as a good choice for COVID-19 treatment.57–65 Drug repurposing strategy can be utilized via the use of already-approved effective drugs that show a promising avenue for their reuse for other indications, such as combating COVID-19.22,66–75 Countries around the world are intensively trying to invent treatment or preventive measures against SARS-CoV-2.1 To date, there is no existing evidence that suggests any specific, completely effective medication for the treatment of COVID-19 patients.19,76 More than 300 clinical research studies have already evaluated possible therapeutic strategies for COVID-19 prevention and/or treatment.31 The Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO) have proposed many guidelines for treatment including immediate intensive care, experimental antimicrobial agents, immune system boost, oxygen therapy, and others.2
Those facts motivated us to utilize the lessons learned during the coronavirus pandemic, review the latest developments, and forecast future research and technological development needs. So, many questions were discussed such as:
- What are the mechanisms of the action and examples of antiviral materials found in technological and natural environments?
- What are the farthest effective morphological and structural targets of SARS-CoV-2 that feasible antiviral agents’ modes of action can be used for?
- What are the potential determinations required to evaluate the capability of existing antivirals to affect SARS-CoV-2 specific targets?
- What are the specific mechanisms and structural features that must be taken into consideration when developing new antiviral drugs in the future?32
Despite this not being the first review article discussing the aforementioned issue,77 in this article, we tried to be more professional in introducing facts, collecting nearly all the available data, and trying to be as up-to-date as possible. Therefore, we classified the FDA-approved repurposed drugs against SARS-CoV-2 into three main categories, antiviral drugs and non-antiviral drugs, besides the recently introduced convalescent plasma as well (Figure 2).
 |
Figure 2 FDA-approved repurposed drugs against SARS-CoV-2. |
Antiviral Drugs for the Fight Against COVID-19 (Table 1)
Targeting RNA-Dependent RNA Polymerase (Reverse Transcriptase Inhibitors)
Remdesivir
Remdesivir is expanded to be used for Ebola treatment.78 Ebola is a single-stranded RNA virus. Remdesivir appears as an adenosine analog that interferes with the viral RNA-dependent RNA-polymerase (RdRp)79 and induces premature or delayed termination of the RNA chain.15,80,81 In vitro, its action is confirmed as an antiviral against respiratory viral pathogens including SARS-CoV-2. Similar protective effects are seen in vivo in SARS-CoV- and MERS-CoV-infected mice with low airway inflammation and improved lung function. Essentially, the effectiveness of post-exposure therapy depends on the time of drug administration.15
 |  |  |  |  |  |
Table 1 Antiviral Drugs and Non-Antiviral Drugs That Could Be Repurposed Against COVID-19 Include Their Mode of Action, Original Use, and Chemical Structure |
Furthermore, remdesivir’s mechanism of action depends on the fact that nucleoside analogs have been licensed to treat DNA and RNA viruses. However, CoVs have been reported to be resistant to some nucleoside analog inhibitors.78,82 As a nucleoside analog, remdesivir acts as an RdRp inhibitor, targeting the viral genome responsible for the replication process. So, it inhibits the protein complex of CoVs in the RdRp process. After metabolizing remdesivir by the host to its active metabolite nortriptyline (NTP), this metabolite is conjugated to ATP, which incorporates it into the nascent RNA strand. This incorporation of the new strand results in RNA synthesis termination, halting the RNA strand growth after adding more nucleotides.
All CoVs have a proofread process capable of detecting and removing another nucleoside analogd activity, thus preserving its antiviral activity. Unsurprisingly, Agostini et al noted that the mutant Murine hepatitis virus (MHV) was freed from proofread ability and was largely susceptible to remdesivir. It is also possible that mutations that improve the proofread or increase the accuracy of the base-pairing process may lead to remdesivir resistance. Indeed, Agostini et al also induced mutations in MHV (bypassing into remdesivir) that awarded sturdy resistance against the medication. It is unclear to what extent this experiment could represent a cause in which a resistance mutation would naturally develop. Furthermore, some evidence indicated that remdesivir might have another mechanism of action that might allow partial antiviral vitality to continue despite viral mutations.82
Remdesivir was approved or authorized for emergency use by WHO. In November 2020, WHO updated a conditional recommendation against remdesivir in hospitalized patients. In this case, there is a conditional recommendation against the use of remdesivir.83
Clinical Trials
To fight this outbreak, the phase III clinical studies on remdesivir, especially with its previous test findings and implementation, are of practical significance to obtain the more powerful antiviral drug for the potential tactical and political dominance of SARS-CoV-2.81
- Clinical progress was documented in a series of 61 hospitalized patients receiving remdesivir off-license in 36 out of 53 patients with adequate data to examine. However, these results are difficult to understand without a placebo group. An initial randomized controlled study was inaccurate, preferring remdesivir, with a non-significant direction towards the decreased time to clinical changes. This study was inadequate; however, it revealed improved healing in patients treated with remdesivir (average recovery time of 11 days vs placebo of 15 days). Moreover, on day 14, there was progress for better survival. For those with high-flow oxygen, invasive ventilation, or non-invasive ventilation, the subgroup study showed a bit little advantage, indicating that antivirals such as remdesivir would have minimal effectiveness in late diseases where the phenotype is expected to be inflammatory. It should be noted that the analysis was published early and further follow-up research is required to yield entire results.15
- Several clinical research types about the use of remdesivir for the prophylaxis from COVID-19 are pending. The National Institutes of Health (NIH) sponsored a clinical study in the USA and Korea. It is a double-blinded and placebo-controlled experiment under which participants are randomized to administer either a placebo or a preliminary dose of 200 mg of intravenous remdesivir on the first day, preceded by a controlled dose of 100 mg a day, up to a limit of 10 cumulative days of care by discharge. As described in the United States National Library of Medicine clinical trials registry, the primary trial outcome is expressed as the proportion of patients in each category (seven-category clinical severity scale is used) till the fifteenth-day post-treatment initiation. Also, Gilead Sciences funds a remdesivir analysis with a combined primary outcome test of fever and oxygen normalization in patients with extreme COVID-19.80
- Two double-blinded placebo-controlled trials are also recruiting in Hubei Province, China: One study was for mild-to-moderate COVID-19 admitted patients, while the other was based on serious cases.80 The primary success indicator for the mild-to-moderate research study is timely clinical rehabilitation, as described by the normalization of the body temperature, breathing rate, oxygen consumption, and coughing recovery for at least 72 hours. In the extreme case study, the primary result is time for health progress and is described using a six-category ratio scale from discharge to mortality.80
- Previous research has also revealed that remdesivir was effective against MERS-CoV, decreasing the viral loads in the infected portion of mice, and helping in normal lung-based function restoration.84 Also, It is considered to be a treatment-assisted agent versus SARS-CoV-2.78 Preliminary research showed that remdesivir administration for about 12 days could decrease the viral load in oropharyngeal and nasopharyngeal swabs.85
- In an in vitro analysis, chloroquine – an anti-malarial drug – and remdesivir combination can effectively block the development of SARS-CoV-2 in cells of Vero E6.86 Clinical studies in Norway, France, and the USA are underway to determine the potency of remdesivir in COVID-19. COVID-19 Cases in Singapore and the USA were treated with remdesivir, and the first patient got recovered in the USA by administering it intravenously.85
- Another study among 596 patients, 584 received remdesivir or continued standard care, and 533 (91%) completed the trial. The average length of treatment was 5 days for patients in the 5-day remdesivir group and 6 days for patients in the 10-day remdesivir group. On day 11, patients in the 5-day remdesivir group had statistically significantly higher odds of a better clinical status distribution than those receiving standard care.87
- However, a trial outcome concerning safety, viral load, and secondary outcomes. Twenty-two (14%) of 158 patients on remdesivir died versus ten (13%) of 78 on placebo, and there was no signal that viral load decreased differentially over time between remdesivir and placebo groups.88
However, the prophylactic use of remdesivir is generally restricted by its poor oral bioavailability. Further pharmacological efforts are needed to make the drug accessible to the outpatient population. Recently, the manufacturer announced in an open letter that a Phase 1 trial with remdesivir inhalation is being planned and already accepted by the FDA.89
The dosage type of remdesivir used during clinical studies is a freeze-dried powder injection. Thus, the dosage system used is: on the first day, 200 mg as an initial dose of remdesivir is administered by intravenous dripping. Then, 100 mg as a maintenance dose is administered by intravenous dripping per day for 9 days.90
Favipiravir
Favipiravir is a purine analog. It was approved in Japan to treat influenza. Also, it is selective against some RNA viruses in vitro, including SARS-CoV-2. In mice, favipiravir increased the survival of the influenza A virus.15 It is known to be active in vitro against oseltamivir-resistant A, B, and C viruses. After its transformation to the active phosphoribosylated form, favipiravir is considered as a substrate of viral RNA polymerase in many RNA viruses.91
In the tissue, the enzyme phosphoribosylated it to its active form favipiravir-RTP. The following different pathways explain the favipiravir mechanism of action:
- It is mistaken as a purine nucleotide by the RNA-dependent RNA-polymerase (RdRp) enzyme, which acts as a substrate molecule, thus its action is stopped putting an end to viral protein replication.92
- This mode of action along with maintaining the RdRp enzyme by the catalytic domain illustrates the wide variety of activities this compound can do by preventing further extension and integrating into the RNA viral chain.93
- Favipiravir is a virucidal drug. It is committed to causing in vitro fatal mutagenesis during influenza infection. However, it is not known whether Favipiravir has a similar activity against SARS-CoV-2 or not yet.94
Favipiravir is a drug given orally and has a similar mechanism of action to remdesivir. Favipiravir has less supporting evidence to justify its application. However, it is still emerging as an agent that is worth trying out for mild-to-moderate situations.94 Favipiravir’s main advantage is primarily oral administration. So, it can be given as a part of the hospital treatment for cases having symptoms but are not extremely ill. Given that so many COVID-19 cases have mild to moderate accompanying diseases and care should be received at home, hence this medication may likely be used in multiple cases. Viremia reduction is observed when favipiravir is early administered after COVID-19 symptoms appear. In an ongoing experiment, the action of favipiravir on prophylaxis is also investigated.94 On the other hand, its main disadvantage is heavy pill pressure, which results in 18 tablets filled on the first day and 8 tablets per day for the remainder of the treatment course. These worries about the heavy pill pressure are being partly alleviated with the recent introduction of a 400 mg dose. A disadvantage can also be a prescribed medication period of up to 2 weeks. Favipiravir’s teratogenicity is the most important adverse effect.
Besides, other adverse effects like increased uric acid, neutropenia, diarrhea, and elevated ALT and AST during phase III clinical study in the global multi-center patients in Japan. In a randomized controlled trial of favipiravir in cases with COVID-19, psychiatric, hepatic enzyme disorders, serum elevation of uric acid, and gastrointestinal symptoms were the most common adverse effects. The overall favipiravir side effects were moderate even though it should not be taken by pregnant women.90,94
Clinical Trials and Combination Therapy
Favipiravir is approved for the care of novel or persistent influenza in Japan. Besides, it was one of the first approved drugs for controlling COVID-19.10,90,94 In China, the prescribed dosage of favipiravir in influenza is 1600 mg delivered orally every 12 h on the 1st day, followed by 600 mg orally every 12 h, and 600 mg every 24 h on the 6th day.90
Moreover, randomized trials containing (interferon-α and favipiravir) or (baloxavir, marboxil, and favipiravir) were recruited for patients with COVID-19 infections.91 The combination with other antivirals like umifenovir is often tested to assess whether the treatments are synergistic or complementary.92
In an open-label non-randomized study in China, SARS-CoV-2 patients who were treated with a double combination interferon-α/favipiravir in contrast to triple combination interferon-α/ritonavir/lopinavir showed a shorter time for viral clearance (four days vs eleven days, respectively) and a much better improvement of the chest X-ray.15
Ribavirin
Ribavirin is an analog of guanosine90,91 with a wide range of RNA antiviral activity. It is used in the treatment of many viral diseases, including respiratory syncytial virus (RSV), hepatitis C virus (HCV), and some hemorrhagic viruses. It was determined to have 50 mg/mL of in vitro antiviral activity with SARS-CoV. However, lowering hemoglobin, which is harmful to people suffering from respiratory failure, is an undesirable side effect.91 The most extreme adverse effects of ribavirin are hemolytic anemia and reproductive toxicity.95 Lethal mutagenesis, non-specific or specific chain end-up and nucleotide biosynthesis inhibition are involved in the antiviral processes of ribavirin.96
Ribavirin is used in China for COVID-19 cases listed in the Chinese government. The dose for adults is 500 mg, from 2 to 3 times daily by intravenous infusion, and for a duration not exceeding 10 days. It is advised to use ribavirin in combination with ritonavir/lopinavir or interferon.90
Clinical Trials and Combination Therapy
In another retrospective review of 126 patients undergoing therapy with ribavirin (the dose is 2000 mg), the side effects of the drug were tracked. Some patients showed elevated transaminase (40%), hemolytic bleeding, and bradyarrhythmia (14%).97 Therefore, the dose of ribavirin must be monitored cautiously to treat COVID-19 patients.80 Ribavirin was also tested with contradictory findings in the past outbreaks of MERS-CoV and SARS-CoV. Concerning SARS-CoV-2, a randomized Phase II clinical trial of a total of 127 patients recruited with mild/moderate COVID-19 was performed to suppress virus replication in the micromolar stages.98
Applying ribavirin/interferon-beta to ritonavir/lopinavir is more recommended than ritonavir/lopinavir alone.99
Furthermore, Ivermectin and nitazoxanide, anti-parasitic drugs, are shown to potentiate interferon-alpha and beta effects and subsequently the immune responses. They were studied with ribavirin for COVID-19 cases without comorbidities. However, the clinical trials described above are not adequate to explain the successful ribavirin contribution to the progression of the disease. In the case of hospitalizing adult cases with COVID-19, a Phase I review, on the other hand, assessed inhaled ribavirin preparation as a sole agent.100
Tenofovir
It is a nuclear adenosine analog prescribed for the treatment of chronic HBV or HIV infections. Tenofovir can efficiently integrate into RNA-dependent RNA polymerase terminating the polymerase reaction. In combination with emtricitabine, tenofovir is considered a highly effective portion of HIV-contaminated antiretroviral treatment and a first-line alternative to HIV prophylaxis before and after exposure.100
Clinical Trials
Inconsistent with the above therapeutic method, two randomized phase III clinical studies using emtricitabine/tenofovir were carried out for prophylaxis purposes before the exposure to COVID-19 in health care staff.100
Galidesivir
Galidesivir is an analog of adenosine90,91 that replaces carbon with nitrogen on a base at position 7 and replaces nitrogen with oxygen on a ribose ring at position 1.92 The structural alteration changes the electrostatic interaction with the viral RNA polymerase, leading to the untimely end-station of the extended RNA strand.101 Galidesivir has shown a broad spectrum of antiviral activities,100–102 including Togavirus, Filovirus, Arenavirus, Bunyavirus, Paramyxovirus, Coronavirus, Flavivirus, one Picornavirus, and one Orthomyxovirus, and it is currently ongoing as an antiviral treatment for Ebola.101,103 This drug prevents SARS-CoV, SARS-CoV-2, and MERS-CoV infection in primary human cell cultures, thereby improving pulmonary function and decreasing the viral load. The antiviral results showed its ability to incorporate several lethal mutations through viral RNA, however being not through host RNA, hence that would cause a high barrier to drug resistance development.100
Clinical Trials
In a pilot sample, galidesivir was also demonstrated to provide partial protection for Rift Valley Fever Virus (RVFV) infection in the mouse model.102 Effectiveness has been documented more recently in models for tick-borne Flavivirus and Zika infection. Besides, phase I human research on galidesivir administered intramuscularly against placebo in healthy people concluded strong tolerability and promising pharmacokinetics characteristics.101,103
Other Nucleoside/Nucleotide Analogs (Other Transcription Inhibitors)
Other nucleoside and nucleotide analog drugs can be considered. They are either dedicated to various viral infections (eg telbivudine, sofosbuvir, tenofovir, and ribavirin) or scientifically investigated (eg EIDD–2801, and galidesivir).100 Due to their related structural properties with either ribavirin or remdesivir, they are likely to have antiviral action against SARS-CoV-2. Some have been approved by FDA as Nucleoside Analog Reverse Transcriptase Inhibitors (NtRtIs), such as tenofovir, alafenamide, adefovir, tenofovir, disoproxil, didanosine, ganciclovir, and abacavir. Furthermore, the antiviral activity against SARS-CoV-2 can also be revealed with other inhibitors including Non-Nucleoside Reverse Transcriptase Inhibitors (NNRTIs) such as nevirapine, efavirenz, rilpivirine, and delavirdine, and Nucleoside Reverse Transcriptase Inhibitors (NRTIs), such as stavudine, lamivudine, emtricitabine, azvudine, and zalcitabine. In addition, although some in silico trials have already been assessed by molecular docking, further preclinical and clinical trials should be carried out to assess their clinical effectiveness.49
In conclusion, ribavirin and sofosbuvir can be closely bound to the newly developed RdRp coronavirus and hence interfere with the protein function resulting in viral eradication. It is worth mentioning that Sofosbuvir acts as a potent inhibitor of the recently emerging COVID-19 type of HCoV.10
Targeting Spike Protein (Fusion Inhibitors)
Umifenovir
Umifenovir is a short indole derivative that provides a range of action against RNA and DNA viruses. These viruses are host targeting and directing, thus preventing the viral entry via host cell (internalization/attachment).90
It is approved (only in China and Russia) for the prophylaxis and treatment of influenza A and B infections.104 However, it has demonstrated an in vitro efficacy against infections caused by Hepatitis B and C viruses (HBV and HCV), Ebola, and other viruses.
Clinical Trials
In Russia, Umifenovir was initially approved to treat influenza in 1993. The medication is now sold in Russia and China to treat upper respiratory influenza A and B infections.94 In the “Treatment Scheme and New Coronavirus Pneumonia Diagnosis”, umifenovir is recommended for patients with COVID-19. It is administered for adults as 0.2 g, 3 times daily, for 10 days.90
In the clinical studies, oral therapy with umifenovir was shown to suppress mortality and viral load in comparison to other unspecified antiviral drugs or the control group obtaining interferon.105 It has moderate overall side effects, such as abdominal discomfort, nausea, headache, leukopenia, elevated bilirubin, and elevated alkaline phosphatase (AKP). Retrospective trials to determine the safety and efficacy of umifenovir therapy in patients with COVID-19 showed no significant adverse effects in the course of treatment.90
Camostat Mesylate
Camostat mesylate is an inhibitor of serine protease106 and is another medication that targets the fusion of viruses. Inside the targeted host cells, SARS-CoV-2 gains access to TMPRSS2 and/or ACE-2 receptors.96 Camostat mesylate works as a TMPRSS2 inhibitor.107 The SARS-CoV-2 spike (S) protein is downregulated to inhibit the virus from entering the cell and thus inhibit surface fusion.108
Clinical Trials
One previous research showed that camostat mesylate blocked the entry of SARS-CoV into human epithelial bronchial cells.109 In a further in vitro analysis, camostat mesylate and E-64d (a cysteine protease inhibitor) have shown effective SARS-CoV-2 TMPRSS2 inhibition.33
The efficacy of hydroxychloroquine and camostat mesylate combination therapy in Denmark and Germany has been tested.49 A 15-fold greater efficiency for SARS-CoV-2 virus entry into the host cells was observed in another serine protease inhibitor, nafamostat mesylate. Nafamostat mesylate can be considered as a safer alternative to camostat mesylate because nafamostat has more efficient antiviral activity and a favorable safety profile.110 Besides, disseminated intravascular coagulation (DIC) with increased fibrinolysis shown in COVID-19 patients is also treated with nafamostat mesylate.49
Neuraminidase Inhibitors
Oseltamivir
Oseltamivir is a neuraminidase inhibitor.111 It is successful in avoiding influenza and is effective in the treatment of influenza in children.112 Neuraminidase inhibitor medicines like zanamivir, peramivir, and oseltamivir are not anticipated to be effective for SARS-CoV-2 and are not recommended for COVID-19 patients treatment113,114 because SARS-COV-2 neuraminidase has not been found yet.115
Clinical Trials and Combination Therapy
Studies showed that ganciclovir with oseltamivir or ritonavir/lopinavir with oseltamivir is used for the treatment of COVID-19 in Wuhan patients.115 The synergistic effects of ritonavir/lopinavir combination with oseltamivir in SARS-CoV-2 were also confirmed by computational studies.116 In Afghanistan, oseltamivir was used for treating COVID-19 cases with terbutaline and ceftriaxone. Moreover, a case study also showed that the CT scan of the patients’ lungs was greatly improved after three days of oseltamivir administration. Oseltamivir is used in Singapore and Indonesia as the recommended alternative to treat COVID-19.49
Oseltamivir is used by the oral route of administration for the treatment of COVID-19 and suspected patients in Chinese hospitals, but until now, there is no conclusive evidence that it has a true effect on COVID‐19 patients’ recovery.114
Zanamivir and Peramivir
Zanamivir solution is also one of the neuraminidase inhibitors that can be applied for ventilated COVID-19 patients resistant to oseltamivir treatment. Also, Peramivir is used by intravenous route of administration as an antiviral drug. It has some benefits for patients who give no response to zanamivir or oseltamivir.113,114
Oseltamivir was orally used in Chinese hospitals to treat 2019-nCoV confirmed cases. However, there is no definitive proof that oseltamivir may be useful for COVID‐19 patients’ treatment. In addition, neuraminidase inhibitors including zanamivir, peramivir, and oseltamivir have recently been proposed to be ineffective against nCoV‐2019 and hence are not recommended to be used for treatment protocols.113
Protease Inhibitors
Lopinavir
Lopinavir is used with ritonavir to treat and prevent HIV infection. Furthermore, Lopinavir is reported to inhibit SARS-CoV-2 at a half-maximal efficient concentration (EC50) of 2.660/1.671.100 Its administration as an emergency drug in China elevated the eosinophil count among COVID-19 cases.117 An in silico study showed promising effects of ritonavir and lopinavir combination (both are HIV protease inhibitors) as SARS-CoV-2 main protease (Mpro) inhibitors.9 An earlier study showed that a specific combination of lopinavir/ritonavir, known as kaletra® showed antiviral effects against SARS-CoV in both in vitro and clinical trials.118 Therefore, this combination is also being used as a contingency treatment for COVID-19 patients in several countries.119
Clinical Trials and Combination Therapy
A previous study showed that treatment with lopinavir/ritonavir was correlated with better results but did not enhance the clinical recovery of the seriously infected COVID-19 patients.119 Although the effectiveness of lopinavir for COVID-19 treatment has not been tested yet, the combination of ritonavir/lopinavir is used for treating COVID-19 patients in some countries like Japan, Singapore, and the USA. Clinical studies are underway to determine the effectiveness of lopinavir/ritonavir for COVID-19 in Thailand, Spain, Canada, China, Hong Kong, France, and the United States.49
In addition, interferon (INF)-β (an inflammation-regulating molecule) alone or in combination with lopinavir/ritonavir was a “solidarity” clinical trial for coronavirus that has been listed by the WHO.100 Besides, the lopinavir/ritonavir combination can improve the clinical symptoms and reduce the viral load of COVID-19 patients.105
Lopinavir/Ritonavir + Ribavirin
The protease inhibitors recommended in a combination form (Kaletra®) are ritonavir (RTV) and lopinavir (LPV).90 Kaletra® is a specific preparation to treat HIV.15
Lopinavir is an inhibitor of the HIV protease,111 which may weaken the virus infection rate and affect the formation of mature virus particles. Short half-life and low bioavailability are characteristic of lopinavir.90 Lopinavir is an HIV protease inhibitor, which can influence the formation of mature virus particles and weaken the infectivity of the virus.
On the other hand, Ritonavir is an inhibitor of the cytochrome CYP3A4 enzyme, which decreases the metabolism of lopinavir by suppressing the cytochrome P450. The combined treatment of ritonavir and lopinavir can increase the bioavailability of lopinavir in vivo.14,90 Ribavirin is an analog of guanosine that can stop RdRp-mediated RNA viral chain elongation.15
Clinical Trials
Ritonavir and lopinavir treatment combination dramatically increase lopinavir’s bioavailability and increase the antiviral activity in vivo.90
Prophylactic ritonavir/lopinavir-Interferon-β was moderate in viral load decrease for Murine’s infection of MERS-CoV relative to remdesivir. However, no major post-infection effects on viral load, acute lung damage, or lung bleeding were observed.15,120
Ritonavir and lopinavir tablets can be taken every 12 hours for not less than 10 days and a total of 14 days (400 mg/100 mg of lopinavir/ritonavir). Children’s doses are regulated based on body weight: 10 mg/kg for 15−40 kg and 12 mg/kg for 7−15 kg at the full dose of 400/100 mg a time. If the history of symptoms is <7 days, interferon-β1a is given in combination with ritonavir/lopinavir on a 44 μg dosage for 3 doses every six days.121
Lopinavir/Ritonavir + Umifenovir
Patients who received the triple combination treatment of lopinavir/ritonavir plus umifenovir showed an advantage to curb disease development compared to lopinavir/ritonavir alone. Also, the combination of ritonavir/lopinavir and umifenovir significantly stopped the advancement of lung damage.49
Danoprevir
Danoprevir is a potent inhibitor of hepatitis C proteases.122,123 It has been approved as an oral antiviral medication in China by 2018 for the treatment of hepatitis C. Hence, it was repurposed as a drug for the treatment of COVID-19.124
Clinical Trials
Phase III clinical studies on 140 cases showed that a triple combination of pegylated-interferon α-2a, ritonavir-boosted danoprevir, plus ribavirin achieved a sustained virologic response within 12-week (SVR12) after treatment in Chinese infected cases with non-cirrhotic hepatitis C virus.124 In the other two clinical trials, phase II and Phase III, the rate of SVR12 in infected cases was about 99% in the complete oral treatment of non-cirrhotic HCV using the triple combination of ribavirin with ritonavir-boosted danoprevir and ravidasvir (an inhibitor of HCV NS5A).125
Darunavir
Darunavir, an anti-HIV drug, has been recommended for the treatment of COVID-19 in Italy.126 It can be used in a concerted regimen with cytochrome P-450 inhibitors such as cobicistat or ritonavir. Moreover, in vitro studies have shown its promising anti-proliferative effect against SARS-CoVs.126
Clinical Trials
In Thailand, a clinical trial is underway to evaluate the efficacy of darunavir’s combination with other drugs, such as hydroxychloroquine and antivirals for coronavirus patients. Besides, the combination of cobicistat and darunavir was examined in an outstanding clinical trial in China. Thus, a fixed-dose integration of cobicistat and darunavir, known as prezcobix®, may also be used for COVID-19 treatment.49
Newly, HIV-positive patients who were already undergoing treatment with darunavir were subjected to COVID-19 infection, raising the interest in the effectiveness of this HIV protease inhibitor.127 This indicates that darunavir may not be effective for SARS-CoV-2 infection prevention at the present approved dose of 800 mg.49
Atazanavir
An in-silico study reported that atazanavir is stronger than lopinavir at the binding site in SARS-CoV-2 MPro. Besides, an in-vitro study revealed that SRS-CoV-2 replication was inhibited using atazanavir.128
Clinical Trials
Research on HIV-infected people revealed that increased lipid parameters and glucose absorption are associated with ritonavir/atazanavir use compared to lopinavir/ritonavir.129 Also, it is found that if atazanavir is paired with ritonavir it will give the same action in the combination of lopinavir/ritonavir, hence atazanavir could be an alternative to lopinavir. However, further research trials should be carried out. This antiviral medicine is currently an alternative for the treatment of COVID-19.130
Saquinavir and Other Protease Inhibitors
Due to the high degree of similarity between their chemical structures, saquinavir and other protease inhibitors, such as indinavir, amprenavir, and nelfinavir, can also exhibit similar effects against COVID-19 as protease inhibitors as described earlier.131
Clinical Trials
An in-silico study showed that 3CLPro activity in SARS-CoV-2 could be inhibited by saquinavir and indinavir.59 Another research study found that SARS-CoV-2 was inhibited in-vitro by saquinavir, indinavir, amprenavir, and nelfinavir.132 However, saquinavir provides the strongest inhibition compared to others. Saquinavir has been used in the treatment of Singapore’s COVID-19 patients. In addition, two more candidates, raltegravir and paritaprevir, were studied in another in silico study and demonstrated inhibitory effects on 3CLpro of SARS-CoV-2.133
Potent anti-viral phytochemicals that may have inhibitory action against SARS-CoV-2 3CLpro134 have recently been detected through medicinal plant library screening. Hence, the top reported antiviral candidates could be further tested by performing in-vitro studies.49,133
Niclosamide
Niclosamide is an FDA approved anthelmintic drug used for the treatment of different nanomolar to micromolar viral infections including Middle East respiratory syndrome coronavirus (MERS-CoV), severe acute respiratory syndrome coronavirus (SARS-CoV), Japanese encephalitis virus (JEV), Zika virus (ZIKV), Ebola virus (EBOV), hepatitis C virus (HCV), Chikungunya virus (CHIKV), human rhinoviruses (HRVs), Epstein−Barr virus (EBV), and human adenovirus (HAdV). Because of its low cost and low in vivo toxicity profiles as clinically recognized FDA products, niclosamide’s wide spectrum antiviral action can give it the therapeutic potential for the battle of rapidly propagating COVID-19.58
Clinical Trials
Wu et al observed that, after the screening of a limited commercialized drug library, niclosamide could prevent SARS-CoV replication and eliminate the viral antigen replication.135
The cytopathic effect (CPE) of SARS-CoV was blocked by niclosamide, and the virus replication was inhibited in Vero E6 cells. The SARS-CoV 3CLpro has an important role in the production of polyproteins and serves as a central tool for the detection of anti-SARS medications.136–138
A series of 2-chloro-4-nitroaniline derivatives were revealed as potent inhibitors against SARS-CoV 3CLpro. Interestingly, niclosamide showed no obvious inhibitory activity against SARS-CoV 3CLpro up to 50 μM, and it may exert its anti-SARS activity mechanistically via other modes of action. Furthermore, it has been documented that niclosamide inhibits MERS-CoV replication by a factor of up to 1000 at 48 h.p.i.58
Nelfinavir
Nelfinavir is an inhibitor of HIV-1 protease intended for use as an antiviral that attacks HIV through alternative pathways. It has gone out of favor with the advent of novel hybrid antivirals for HIV.139
Nelfinavir’s mechanism of action includes that it prevents the processing of working proteins essential for HIV and binds to the HIV-1 protease active site. So, what is left are unsustainable, non-infectious HIV spores.139 It was revealed as a possible agent with SARS-CoV inhibitory activities based on in-vitro experiments during the SARS epidemic.140 It was reported to prevent the replication of SARS CoV.141
Nelfinavir has the most positive effect against SARS-CoV-2 out of the 30 pharmaceuticals evaluated. Its effectiveness against SARS-CoV or SARS-CoV-2 in individuals has not been tested yet. It is advised to use 750 mg per mouth 3 times daily or 1250 mg per mouth twice daily for the treatment of HIV. Its effective dose for the treatment of COVID-19 is unknown.142 Pharmacokinetics can be variable in nelfinavir patients with chronic hepatic disease.143,144 It has common side effects like gastrointestinal intolerance, especially flatulence, diarrhea, and nausea. There may also be a non-specific rash.142
Ebselen
Ebselen is a compound of organoselenium that decreases peroxynitrite and hydroperoxides and acts as a peroxiredoxin mimetic enzyme and glutathione peroxidase.145 Some protein thiols interact with ebselen through a selenosulfide link, resulting in pleiotropic, anti-inflammatory, and antibacterial actions besides its main activity as an antiviral drug. A possible drug target is the Mpro of SARS-CoV-2, and ebselen was described as a promising inhibitor of Mpro by a panel of over 10,000 substances.100
Ebselen’s function is recognizing the cysteine proteases in viruses and other micro-organism infections. Besides, it affects the inflammation of the lung. Also, ebselen can act as an effective lead compound to suppress Mpro in SARS-CoV-2, and its inhibitory action was reported in vivo in adherent cells. Regardless of this action, modulation of cytokines and inflammatory oxidants are important pathogenetic goals, which are credited in COVID-19 to make potential benefits.100
Clinical Trials
Early research of comprehensive ebselen reported various biological pathway targets, such as enzymes associated with inflammatory reactions, were identified for different clinical conditions.146–148 Tolerability and safety are strong, and no scientific trials have demonstrated any side effects.149 It is discarded and metabolized as glucuronidated and methylated conjugates without selenium in its portion.100,150
Ebselen also has an anti-inflammatory function in vivo, leading to its possible involvement in host reactions to viral infections.90 It also blocks the development of oxidized compounds in brain tissue from HIV encephalitis and macaques with Simian immune deficiency virus encephalitis.96 It is proposed that the therapeutic ability of ebselen could be used to block the symptoms of neurological HIV-1 infection in the brain.90
M2 Ion-Channel Protein Blockers
Adamantane, Amantadine, and Rimantadine
The M2 channel protein on the viral sheath is essential for maintaining the pH across the viral sheath. It is critical for cell entrance and movement through the trans-Golgi membrane of steward cells pending viral maturation. Ion channel protein M2 is one of the targets for fighting influenza viruses.151
Clinical Trials
An early study showed that amantadine can block the p7 protein of HCV that is important for making ion channels in host cell membranes. A report in 1973 claimed that amantadine has a strong in vitro effect against coronavirus.151 Moreover, a recent report showed that amantadine can block protein-membrane channel activity for SARS-CoVs.152 Despite mounting evidence indicating that amantadine has an antiviral efficacy suitable for treating COVID-19,153 other studies are pledged to evaluate its effectiveness.49
Non-Antiviral Drugs That Have Antiviral Activity Against SARS-CoV-2 Infection
Importin α/β1-Mediated Nuclear Import Inhibitors (Ivermectin)
Ivermectin is an FDA-approved anti-parasitic drug. It has also been shown to be a successful anti-viral for humans (HIV) as well as the Dengue virus.100,154 Thus, a single dose of the medication has lowered viral RNA about 5000 times.155 Besides, it can isolate the pre-formed importin α/β1 heterodimer that transmits the viral protein charge nuclearly.49 Since the suppression of nuclear transportation of viral protein is crucial to the host antiviral activity, so ivermectin is considered a therapeutic potential inhibitor to RNA viruses targeting the nuclear transport mechanism.155,156
Clinical Trials and Combination Therapy
Recently, an in vivo study demonstrated the activity of ivermectin to minimize the viral RNA to 5000 times after 48 h of SARS-CoV-2 infection.100 Another trial to show ivermectin’s effectiveness in treating COVID-19 includes research to determine the optimal dosage using the proven safety profile for anti-parasitic treatment.44,157 However, further assessment is required for its effectiveness in the battle against COVID-19.
Ivermectin also showed a wide-spectrum antiviral activity. It inhibits the replication of the yellow fever virus directly by inhibiting the activity of NS3 helicase.158 It also prevents the replication of HIV-1 and dengue viruses154 by preventing importin α/β1, which promotes the movement of proteins between the nucleus and cytoplasm, essential for the replication of these viruses.45,158
In a recent case-controlled retrospective study, it was proposed that the length of the hospital stays and the risk of mortality decreased by ivermectin treatment at a dose of 150 mcg/Kg. Further, randomized controlled clinical trials are needed in patients infected with SARS-CoV-2 before confirming the effectiveness of ivermectin.49
Combination therapy of hydroxychloroquine and ivermectin was suggested for the treatment or prophylaxis of COVID-19. This combination may have a synergistic impact due to its dual action on both viral replication and viral assembly.159 Although the pharmacokinetic review suggested the need for higher doses for attaining the antiviral action, it is also extremely hard to administer the prescribed inhibitory concentration in humans.160
Interferon α and β
Interferons (IFNs) are cytokines that respond to viral infection by stimulating the innate immune system. IFN itself is a broad-spectrum antiviral drug but has no direct action. However, IFN can induce protein synthesis with immunomodulatory and antiviral effects. IFN could also improve the unique cytotoxic effect of the host cell immune cells.160 Moreover, the first vital line of defense against viruses is the interferon (IFN) reaction. Recognition of the innate immune sensing stimulates type I and type III IFN response to viral infections.161 PEGylated and recombinant IFN-α and IFN-β have already been used in the treatment of numerous diseases, including viral hepatitis and multiple sclerosis (MS). Thus, global concern has been revealed for the propositioning of INF against the current COVID-19.153,162 Hence, to incorporate therapeutic rational methods and determine their clinical effectiveness in COVID-19, the biology of coronavirus infections should be closely studied.163
Clinical Trials and Combination Therapy
Clinical trials have shown that IFN reaction in SARS patients is not caused by substantial IFN-I development despite the robust chemokine or cytokine development.155 Serum analysis of COVID-19 patients revealed that the pro-inflammatory chemokines and cytokines were high without any observable amount of type I inflammatory chemokines and cytokines, however, no significant amounts were found. Other experiments showed that the effect of IFN on the virus can be postponed instead of its full absence by studying SARS-CoV-infected cells’ transcriptome over time. Also, it was revealed that IFNs can delay the pro-inflammatory cytokines.164
Moreover, interferon combination with ribavirin caused adverse effects in a systematic review of 8 kinds of research (116 patients) of MERS-CoV interferon treatment: one patient had apparent hemolysis, and two patients had an elevation in the pancreatic enzymes.165
Chlorpromazine
Chlorpromazine (CPZ), a phenothiazine derivative, was selected for Largactil as a French commercial name because it has a wide variety of properties: antiemetic, antiviral, anxiolytic, antifungal properties, immunomodulatory impact, clathrin-mediated endocytosis inhibition, blood–brain barrier modulating function, etc. It acts by inhibiting the assembly of the modulator on cell surfaces and endosomes by CPZ-HCl and thereby prevents the entry of the virus into host cells. Besides, chlorpromazine is used to treat psychotic disorders such as manic-depression or schizophrenia and also treats vomiting, nausea, anxiety, symptoms of tetanus, acute intermittent porphyria, and chronic hiccups.100
Clinical Trials
The CPZ displayed anti-MERS-CoV and anti-SARS-CoV-1 activity, as noted in recent in vitro studies. It is believed that CPZ may reduce COVID-19 infection in patients who require respiratory assistance without ICU.100
Emetine
Emetine is an inhibitor of protein synthesis used in the treatment of amebiasis as an anti-protozoan; it can also prevent malaria by binding the Plasmodium falciparum ribosomal E site. Its possible cardiotoxicity in recent years has limited its therapeutic application. Its antiviral effects were tested against a wide variety of RNA and DNA viruses including Ebola virus, Zika virus, HIV-1, Cytomegalovirus, Poxvirus buffalo, Echovirus-1, Plague of the tiny ruminants virus, Herpesvirus bovine 1, Newcastle virus, Metapneumovirus, Herpes simplex virus-2, Fever virus of the Rift River, and influenza viruses.166
Clinical Trials and Combination Therapy
Emetine was also reported to inhibit SARS-CoV, MERS-CoV, and MHV-A59 in vitro. It was found to inhibit SARS-CoV-2 replication at 0.5 μM efficiently. Its therapeutic plasma levels for SARS-CoV-2 can exceed 0.075 μg/mL below EC50 in vitro. The poisonous concentration in the plasma is 0.5 μg/mL.100 Remdesivir at 6.25 μM may hit 64.9% of viral production inhibition in combination with emetine at 0.195 μm and should be further evaluated in vivo.100
Aplidin
In March 2020, it was announced that aplidin has antiviral activity. It is widely used in the treatment of multiple myeloma.167
Clinical Trials
In-vitro studies have shown that aplidin affects Elongation Factor 1 Alpha (EF1A), the key to the multiplication and spread of the virus. The antiviral effect of aplidin was first studied in HCoV-229E-GFP like virus and human hepatoma cell line. Concerning SARS-CoV-2, the preliminary findings are pretty good. Furthermore, a randomized multi-center proof of the concept-clinical research is pending.167
Teicoplanin
It is a glycopeptide antibiotic that is commonly used for the treatment of bacterial infections. Besides, it is actively used against SARS‐CoV and is included in the prescription list for COVID‐19 treatment. Teicoplanin can treat the infections of gram-positive bacteria, especially streptococcal and staphylococcal infections, and is also widely employed for treating viruses like HIV, Influenza virus, Hepatitis C virus, Flavivirus, Ebola, and Coronaviruses like SARS-CoV, and MERS-CoV.168
Teicoplanin works at the early viral replication stages, preventing the release of the viral genome and the viral replication cycle by low-pH cleavage of the viral spike protein with cathepsin L at the late endosomes (Cathepsin L calls for the incorporation of the 2019-nCoV virus into the cell, and its main target region is the S protein).169
Clinical Trials
Junsong Zhang found that the cleavage site cathepsin L has been maintained among SARS‐CoV and 2019‐nCoV S protein. In vitro, the required IC50 of teicoplanin concentration is 1.66 μM, which is far below the human blood level of 8.78 μM for a 400 mg daily dosage.113
Baricitinib
Baricitinib has a high binding ability for the Janus kinase (JAK) inhibitor through which it binds and inhibits Adaptor Associated Protein Kinase 1 (AAK1). Therefore, both viral penetration and inflammatory reaction associated with SARS-CoV-2 infection may be impaired by its use. JAK inhibitors for cancer and inflammatory disorders like rheumatoid arthritis are employed.170 JAK inhibitors like fedratinib and ruxolitinib, which are closely related to baricitinib, increase the level of clathrin-mediated endocytosis and thus may not be effective in reducing the viral infectivity at tolerable levels. Its medicinal use is associated with neutropenia, lymphocytopenia, and viral reactivation.49 New evidence of the antiviral Remdesivir and the immunomodulatory Baricitinib’s good therapeutic activity against COVID-19 has recently emerged.171
Clinical Trials
Baricitinib is expected to treat the cytokine storm triggered by COVID-19 by suppressing JAK1/JAK2. There have been a variety of clinical trials all over the world, one of which has revealed promising outcomes in which baricitinib (2–4 mg) was prescribed for 1–2 weeks uniformly. However, Deep Vein Thrombosis and Pulmonary Embolism (DVT/PE), excessive liver enzymes, and irregular blood routine were typical and significant adverse reactions. In patients with the aforementioned risk factors, baricitinib should be used with caution. Human data are not adequate for its use in pregnant women. Thus, discontinuation or dose modification should be cautiously considered when using it in patients with renal insufficiency.172
Rapamycin
The repositioning of rapamycin has been a long history. It was first used as an antifungal and eventually as an immunosuppressive agent for organ transplant patients.173 It triggers the downstream interruption of the signal transduction pathway to mTOR phosphorylation (mTORC1). Inhibition of mTOR counteracts viral duplication and improves the outcomes of cases that have some viral infections, such as H1N1 pneumonia,174 MERS-CoV,175 Andes virus,176 and HCV.177
Lianhuaqingwen Capsule
Lianhuaqingwen (LH) is used specifically for the treatment of influenza. It suppresses symptoms like hyperpyrexia or fever, nasal obstruction, muscle soreness, running nose, aversion to cold, headache, and cough. So, adults are advised to take four capsules 3 times daily.90
Clinical Trials
Chinese doctors used LH for the treatment of cases with common and severe COVID-19. LH has shown good anti-inflammatory and antiviral activities against novel coronaviruses in vitro as a potential agent for the treatment of SARS-CoV-2.178 Thus, a separate randomized controlled trial (RCT) has demonstrated that antiviral treatment with LH can greatly improve the symptoms during COVID-19, such as cough, fever, and tiredness in most cases. It may also alleviate symptoms of pneumonia and shorten pneumonia symptoms considerably without any significant adverse effects detected.179 Furthermore, the antiviral effectiveness of LH and other traditional Chinese medications during a pandemic also played a reliable role in SARs-CoV-2 treatment.90
Convalescent Plasma
COVID-19 convalescent plasma, also known as “survivor’s plasma,” is blood plasma derived from patients who have recovered from COVID-19. Recently, the FDA issued an Emergency Use Authorization to allow its use in hospitalized patients with COVID-19.180 This technique shows that the plasma or clean monoclonal antibodies developed against COVID-19 may be taken from fully recovered patients and then delivered as a treatment to new patients.181
Clinical Trials
In a new study, 5 seriously ill COVID-19 cases were treated with convalescent plasma from 20 January to 25 March 2020 in China, Shenzhen.182 At this trial, patients gained the specific antibody of SARS-CoV-2 from 10 to 22 days posts the treatment by convalescent plasma. Four of the five cases experienced reduced virus loads and better outcomes in sequential organ dysfunction. In the last 12 days post the transfusion, their viral test was still negative. The four patients were all removed from artificial ventilation after 2 weeks of admission. But, in nearly 50 days, three patients were eventually released from the hospital.183 Although the sample size of this trial is incredibly limited, the effects of convalescent plasma therapy are encouraging. Hence, this method of care is suggested in the US.181
Some Recently Synthesized Compounds and Approved for COVID-19 Treatment (Table 2)
Molnupiravir
Recently, molnupiravir is a prodrug antiviral medication that was approved in the United Kingdom in November 2021. It is a synthetic nucleoside derivative and works by inhibiting certain RNA viruses through copying errors during RNA replication. Thereby, molnupiravir is used to treat COVID-19 as depicted in Table 2.184–186
 |
Table 2 Some Recently Synthesized Compounds for COVID-19 Treatment Including Their Mode of Action and Chemical Structure |
Pf-07321332
PF-07321332 is an oral antiviral drug developed by Pfizer. It works as an active main protease inhibitor as depicted in Table 2. For the treatment of COVID-19, the PF-07321332/ritonavir combination medication is in phase III studies and will be marketed as Paxlovid.187–189 Ritonavir slows the metabolism of PF-07321332 by cytochrome enzymes in this combination, maintaining higher circulating concentrations of the main drug.
Clinical Trials
Pfizer published Phase 2/3 data in November 2021, which included an 89% decrease in hospitalizations when given within three days of symptom onset.190,191
Conclusions
This article shows an overview that summarizes the efficacy of diverse drugs, classified according to their mechanism of action, against the SARS-CoV-2. In this review, antiviral drugs including fusion inhibitors, protease inhibitors, neuraminidase inhibitors, M2 ion-channel protein blockers, and other non-antiviral drugs that might have activities against SARS-CoV-2 were thoroughly discussed. Besides, the newly FDA-approved molnupiravir and PF-07321332 were discussed shedding light on their mechanism of action and eligibility as novel oral pills combating SARS-CoV-2 by decreasing hospitalizations for COVID-19 patients.
Acknowledgment
The authors extend their appreciation to the research center at Almaarefa University for funding this work.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Tripathy S, Dassarma B, Roy S, Chabalala H, Matsabisa MG. A review on possible modes of action of chloroquine/hydroxychloroquine: repurposing against SAR-CoV-2 (COVID-19) pandemic. Int J Antimicrob Agents. 2020;56(2):106028. doi:10.1016/j.ijantimicag.2020.106028
2. Tufan A, Güler AA, Matucci-Cerinic M. COVID-19, immune system response, hyperinflammation and repurposing antirheumatic drugs. Turkish J Med Sci. 2020;50(SI–1):620–632. doi:10.3906/sag-2004-168
3. Hong SI, Ryu B-H, Chong YP, et al. Five severe COVID-19 pneumonia patients treated with triple combination therapy with lopinavir/ritonavir, hydroxychloroquine, and interferon β-1b. Int J Antimicrob Agents. 2020;56(2):106052. doi:10.1016/j.ijantimicag.2020.106052
4. Saha BK, Bonnier A, Chong W. Antimalarials as antivirals for COVID-19: believe it or not! Am J Med Sci. 2020;360(6):618–630. doi:10.1016/j.amjms.2020.08.019
5. El-Masry R, Al-Karmalawy AA, Alnajjar RA, et al. Newly synthesized series of oxoindole-oxadiazole conjugates as potential anti-SARS-CoV-2 agents: in silico and in vitro studies. New J Chem. 2022. doi:10.1039/D1NJ04816C
6. Roshdy WH, Khalifa MK, San JE, et al. SARS-CoV-2 genetic diversity and lineage dynamics of in Egypt. medRxiv. 2022. doi:10.1101/2022.01.05.22268646
7. Hosseini FS, Amanlou M. Anti-HCV and anti-malaria agent, potential candidates to repurpose for coronavirus infection: virtual screening, molecular docking, and molecular dynamics simulation study. Life Sci. 2020;258:118205. doi:10.1016/j.lfs.2020.118205
8. Kandeel M, Al-Nazawi M. Virtual screening and repurposing of FDA approved drugs against COVID-19 main protease. Life Sci. 2020;251:117627. doi:10.1016/j.lfs.2020.117627
9. Liu X, Wang X-J. Potential inhibitors against 2019-nCoV coronavirus M protease from clinically approved medicines. J Genet Genomics. 2020;47(2):119. doi:10.1016/j.jgg.2020.02.001
10. Elfiky AA. Anti-HCV, nucleotide inhibitors, repurposing against COVID-19. Life Sci. 2020;248:117477. doi:10.1016/j.lfs.2020.117477
11. Li F, Michelson AP, Foraker R, Zhan M, Payne PR. Repurposing drugs for COVID-19 based on transcriptional response of host cells to sars-cov-2. arXiv e-prints. 2020. doi:10.48550/arXiv.2006.01226
12. Samaee H, Mohsenzadegan M, Ala S, Maroufi SS, Moradimajd P. Tocilizumab for treatment patients with COVID-19: recommended medication for novel disease. Int Immunopharmacol. 2020;89:107018. doi:10.1016/j.intimp.2020.107018
13. El kantar S, Nehmeh B, Saad P, et al. Derivatization and combination therapy of current COVID-19 therapeutic agents: a review of mechanistic pathways, adverse effects, and binding sites. Drug Discov Today. 2020;25(10):1822–1838. doi:10.1016/j.drudis.2020.08.002
14. Peretto G, Sala S, Caforio ALP. Acute myocardial injury, MINOCA, or myocarditis? Improving characterization of coronavirus-associated myocardial involvement. Eur Heart J. 2020;41(22):2124–2125. doi:10.1093/eurheartj/ehaa396
15. Farne H, Kumar K, Ritchie AI, Finney LJ, Johnston SL, Singanayagam A. Repurposing existing drugs for the treatment of COVID-19. Ann Am Thorac Soc. 2020;17(10):1186–1194. doi:10.1513/AnnalsATS.202005-566FR
16. Group RC. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693–704.
17. Mahmoud DB, Bakr MM, Al-karmalawy AA, Moatasim Y, El Taweel A, Mostafa A. Scrutinizing the feasibility of nonionic surfactants to form isotropic bicelles of curcumin: a potential antiviral candidate against COVID-19. AAPS PharmSciTech. 2021;23(1):44. doi:10.1208/s12249-021-02197-2
18. World Health Organization. WHO archived: WHO timeline-COVID-19. Available from: https://www.who.int/news/item/27-04-2020-who-timeline—covid-19.
19. Pokhrel R, Chapagain P, Siltberg-Liberles J. Potential RNA-dependent RNA polymerase inhibitors as prospective therapeutics against SARS-CoV-2. J Med Microbiol. 2020;69(6):864. doi:10.1099/jmm.0.001203
20. Shehata MM, Mahmoud SH, Tarek M, et al. In silico and in vivo evaluation of SARS-CoV-2 predicted epitopes-based candidate vaccine. Molecules. 2021;26(20):6182. doi:10.3390/molecules26206182
21. Vitiello A. Sars-Cov-2 and risk of antiviral drug resistance. Ir J Med Sci. 2021;1–2. doi:10.1007/s11845-021-02820-y
22. Mahmoud DB, Ismail WM, Moatasim Y, et al. Delineating a potent antiviral activity of Cuphea ignea extract loaded nano-formulation against SARS-CoV-2: in silico and in vitro studies. J Drug Deliv Sci Technol. 2021;66:102845. doi:10.1016/j.jddst.2021.102845
23. Harvey WT, Carabelli AM, Jackson B, et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol. 2021;19(7):409–424. doi:10.1038/s41579-021-00573-0
24. He X, Hong W, Pan X, Lu G, Wei X. SARS‐CoV‐2 Omicron variant: characteristics and prevention. MedComm. 2021;2(4):838–845. doi:10.1002/mco2.110
25. Elmaaty AA, Darwish KM, Chrouda A, et al. In silico and in vitro studies for benzimidazole anthelmintics repurposing as VEGFR-2 antagonists: novel mebendazole-loaded mixed micelles with enhanced dissolution and anticancer activity. ACS Omega. 2021. doi:10.1021/acsomega.1c05519
26. Elia SG, Al-Karmalawy AA, Nasr MY, Elshal MF. Loperamide potentiates doxorubicin sensitivity in triple-negative breast cancer cells by targeting MDR1 and JNK and suppressing mTOR and Bcl-2: in vitro and molecular docking study. J Biochem Mol Toxicol. 2021;36(1):e22938. doi:10.1002/jbt.22938
27. Ghanem A, Al-Karmalawy AA, Abd El Maksoud AI, et al. Rumex Vesicarius L. extract improves the efficacy of doxorubicin in triple-negative breast cancer through inhibiting Bcl2, mTOR, JNK1 and augmenting p21 expression. Inform Med Unlocked. 2022;29:100869. doi:10.1016/j.imu.2022.100869
28. Al-Karmalawy AA, Khattab MJN. Molecular modelling of mebendazole polymorphs as a potential colchicine binding site inhibitor. New J Chem. 2020;44(33):13990–13996. doi:10.1039/D0NJ02844D
29. Kaur U, Acharya K, Mondal R, et al. Should ACE2 be given a chance in COVID-19 therapeutics: a semi-systematic review of strategies enhancing ACE2. Eur J Pharmacol. 2020;887:173545. doi:10.1016/j.ejphar.2020.173545
30. Cuadrado A, Pajares M, Benito C, et al. Can activation of NRF2 be a strategy against COVID-19? Trends Pharmacol Sci. 2020;41(9):598–610. doi:10.1016/j.tips.2020.07.003
31. Pastick K, Okafor E, Wang F, et al. hydroxychloroquine and chloroquine for treatment of SARS-CoV-2 (COVID-19). In: Open Forum Infect Dis. US: Oxford University Press; 2020:ofaa130.
32. Sun Z, Ostrikov K. Future antiviral surfaces: lessons from COVID-19 pandemic. Sustain Mater Technol. 2020;25:e00203. doi:10.1016/j.susmat.2020.e00203
33. Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. cell. 2020;181(2):271–280. e8. doi:10.1016/j.cell.2020.02.052
34. Wu C, Liu Y, Yang Y, et al. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharmaceutica Sinica B. 2020;10(5):766–788. doi:10.1016/j.apsb.2020.02.008
35. Ghosh AK, Brindisi M, Shahabi D, Chapman ME, Mesecar AD. Drug development and medicinal chemistry efforts toward SARS‐coronavirus and Covid‐19 therapeutics. ChemMedChem. 2020;15(11):907–932. doi:10.1002/cmdc.202000223
36. Li Z, Wang Y, Zhu J, et al. Emerging well-tailored nanoparticulate delivery system based on in situ regulation of the protein Corona. J Control Release. 2020;320:1–18. doi:10.1016/j.jconrel.2020.01.007
37. Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367(6485):1444–1448. doi:10.1126/science.abb2762
38. Shang J, Ye G, Shi K, et al. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581(7807):221–224. doi:10.1038/s41586-020-2179-y
39. Devaux CA, Rolain J-M, Colson P, Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int J Antimicrob Agents. 2020;55(5):105938. doi:10.1016/j.ijantimicag.2020.105938
40. Ahmed T, Noman M, Almatroudi A, et al. Coronavirus disease 2019 associated pneumonia in China: current status and future prospects. 2020. doi:10.20944/preprints202002.0358.v3
41. Zhang T, Wu Q, Zhang Z. Probable pangolin origin of SARS-CoV-2 associated with the COVID-19 outbreak. Curr Biol. 2020;30(7):1346–1351. e2. doi:10.1016/j.cub.2020.03.022
42. Hilgenfeld R. From SARS to MERS: crystallographic studies on coronaviral proteases enable antiviral drug design. FEBS J. 2014;281(18):4085–4096. doi:10.1111/febs.12936
43. Wang H, Xue S, Yang H, Chen C. Recent progress in the discovery of inhibitors targeting coronavirus proteases. Virol Sin. 2016;31(1):24–30. doi:10.1007/s12250-015-3711-3
44. Shannon A, Le N-T-T, Selisko B, et al. Remdesivir and SARS-CoV-2: structural requirements at both nsp12 RdRp and nsp14 Exonuclease active-sites. Antiviral Res. 2020;178:104793. doi:10.1016/j.antiviral.2020.104793
45. Ferron F, Subissi L, De Morais ATS, et al. Structural and molecular basis of mismatch correction and ribavirin excision from coronavirus RNA. Proc Natl Acad Sci. 2018;115(2):E162–E171. doi:10.1073/pnas.1718806115
46. Nagy PD, Pogany J. The dependence of viral RNA replication on co-opted host factors. Nat Rev Microbiol. 2012;10(2):137–149. doi:10.1038/nrmicro2692
47. Nal B, Chan C, Kien F, et al. Differential maturation and subcellular localization of severe acute respiratory syndrome coronavirus surface proteins S, M and E. J Gen Virol. 2005;86(5):1423–1434. doi:10.1099/vir.0.80671-0
48. Klumperman J, Locker JK, Meijer A, Horzinek MC, Geuze HJ, Rottier P. Coronavirus M proteins accumulate in the Golgi complex beyond the site of virion budding. J Virol. 1994;68(10):6523–6534. doi:10.1128/jvi.68.10.6523-6534.1994
49. Frediansyah A, Tiwari R, Sharun K, Dhama K, Harapan H. Antivirals for COVID-19: a critical review. Clin Epidemiol Glob Health. 2020. doi:10.1016/j.cegh.2020.07.006
50. Khattab M, Al‐Karmalawy AA. Revisiting activity of some nocodazole analogues as a potential anticancer drugs using molecular docking and DFT calculations. Front Chem. 2021;9:92. doi:10.3389/fchem.2021.628398
51. Khattab M, Al-Karmalawy AA. Computational repurposing of benzimidazole anthelmintic drugs as potential colchicine binding site inhibitors. Future Med Chem. 2021;13(19):1623–1638. doi:10.4155/fmc-2020-0273
52. El-Demerdash A, Al-Karmalawy AA, Abdel-Aziz TM, Elhady SS, Darwish KM, Hassan AHE. Investigating the structure–activity relationship of marine natural polyketides as promising SARS-CoV-2 main protease inhibitors. RSC Adv. 2021;11(50):31339–31363. doi:10.1039/D1RA05817G
53. Hazem RM, Antar SA, Nafea YK, Al-Karmalawy AA, Saleh MA, El-Azab MF. Pirfenidone and vitamin D mitigate renal fibrosis induced by doxorubicin in mice with Ehrlich solid tumor. Life Sci. 2021;288:120185. doi:10.1016/j.lfs.2021.120185
54. Elebeedy D, Badawy I, Elmaaty AA, et al. In vitro and computational insights revealing the potential inhibitory effect of Tanshinone IIA against influenza A virus. Comput Biol Med. 2021;141:105149. doi:10.1016/j.compbiomed.2021.105149
55. Siddiqui AJ, Jahan S, Ashraf SA, et al. Current status and strategic possibilities on potential use of combinational drug therapy against COVID-19 caused by SARS-CoV-2. J Biomol Struct Dyn. 2021;39(17):6828–6841. doi:10.1080/07391102.2020.1802345
56. Siddiqui AJ, Danciu C, Ashraf SA, et al. Plants-derived biomolecules as potent antiviral phytomedicines: new insights on ethnobotanical evidences against coronaviruses. Plants. 2020;9(9):1244. doi:10.3390/plants9091244
57. Eliaa SG, Al-Karmalawy AA, Saleh RM, Elshal MF. Empagliflozin and doxorubicin synergistically inhibit the survival of triple-negative breast cancer cells via interfering with the mTOR pathway and inhibition of calmodulin: in vitro and molecular docking studies. ACS Pharmacol Transl Sci. 2020;3(6):1330–1338. doi:10.1021/acsptsci.0c00144
58. Ghanem A, Emara HA, Muawia S, Abd El Maksoud AI, Al-Karmalawy AA, Elshal M. Tanshinone IIA synergistically enhances the antitumor activity of doxorubicin by interfering with the PI3K/AKT/mTOR pathway and inhibition of topoisomerase II: in vitro and molecular docking studies. New J Chem. 2020;44(40):17374–17381. doi:10.1039/D0NJ04088F
59. Al-Karmalawy AA, Alnajjar R, Dahab M, Metwaly A, Eissa I. Molecular docking and dynamics simulations reveal the potential of anti-HCV drugs to inhibit COVID-19 main protease. Pharm Sci. 2021. doi:10.34172/PS.2021.3
60. Abo elmaaty A, Hamed MIA, Ismail MI, et al. Computational insights on the potential of some NSaids for treating COVID-19: priority set and lead optimization. Molecules. 2021;26(12):3772. doi:10.3390/molecules26123772
61. Elmaaty AA, Darwish KM, Khattab M, et al. In a search for potential drug candidates for combating COVID-19: computational study revealed salvianolic acid B as a potential therapeutic targeting 3CLpro and spike proteins. J Biomol Struct Dyn. 2021;1–28. doi:10.1080/07391102.2021.1918256
62. Kandeil A, Mostafa A, Kutkat O, et al. Bioactive polyphenolic compounds showing strong antiviral activities against severe acute respiratory syndrome coronavirus 2. Pathogens. 2021;10(6):758. doi:10.3390/pathogens10060758
63. Sarhan AA, Ashour NA, Al‐Karmalawy AA. The journey of antimalarial drugs against SARS-CoV-2: review article. Inform Med Unlocked. 2021;24:100604. doi:10.1016/j.imu.2021.100604
64. Al-Karmalawy AA, Dahab MA, Metwaly AM, et al. Molecular docking and dynamics simulation revealed the potential inhibitory activity of ACEIs against SARS-CoV-2 targeting the hACE2 receptor. Front Chem. 2021;9:227. doi:10.3389/fchem.2021.661230
65. Alnajjar R, Mostafa A, Kandeil A, Al-Karmalawy AAJH. Molecular docking, molecular dynamics, and in vitro studies reveal the potential of angiotensin II receptor blockers to inhibit the COVID-19 main protease. Heliyon. 2020;6(12):e05641. doi:10.1016/j.heliyon.2020.e05641
66. Zeng X, Song X, Ma T, et al. Repurpose open data to discover therapeutics for COVID-19 using deep learning. J Proteome Res. 2020;19(11):4624–4636. doi:10.1021/acs.jproteome.0c00316
67. Soltane R, Chrouda A, Mostafa A, et al. Strong inhibitory activity and action modes of synthetic maslinic acid derivative on highly pathogenic coronaviruses: COVID-19 drug candidate. Pathogens. 2021;10(5):623. doi:10.3390/pathogens10050623
68. Zaki AA, Ashour A, Elhady SS, Darwish KM, Al-Karmalawy AA. Calendulaglycoside A showing potential activity against SARS-CoV-2 main protease: molecular docking, molecular dynamics, and SAR studies. J Tradit Complement Med. 2021. doi:10.1016/j.jtcme.2021.05.001
69. Elmaaty AA, Alnajjar R, Hamed MI, Khattab M, Khalifa MM, Al-Karmalawy AA. Revisiting activity of some glucocorticoids as a potential inhibitor of SARS-CoV-2 main protease: theoretical study. RSC Adv. 2021;11(17):10027–10042. doi:10.1039/D0RA10674G
70. Zaki AA, Al-Karmalawy AA, El-Amier YA, Ashour AJN. Molecular docking reveals the potential of Cleome amblyocarpa isolated compounds to inhibit COVID-19 virus main protease. New J Chem. 2020;44(39):16752–16758. doi:10.1039/D0NJ03611K
71. Mahmoud A, Mostafa A, Al-Karmalawy AA, et al. Telaprevir is a potential drug for repurposing against SARS-CoV-2: computational and in vitro studies. Heliyon. 2021;7:9.
72. Elebeedy D, Elkhatib WF, Kandeil A, et al. Anti-SARS-CoV-2 activities of tanshinone IIA, carnosic acid, rosmarinic acid, salvianolic acid, baicalein, and glycyrrhetinic acid between computational and in vitro insights. RSC Adv. 2021;11(47):29267–29286. doi:10.1039/D1RA05268C
73. Hamed MIA, Darwish KM, Soltane R, et al. β-Blockers bearing hydroxyethylamine and hydroxyethylene as potential SARS-CoV-2 Mpro inhibitors: rational based design, in silico, in vitro, and SAR studies for lead optimization. RSC Adv. 2021;11(56):35536–35558. doi:10.1039/D1RA04820A
74. El Gizawy HA, Boshra SA, Mostafa A, et al. Pimenta dioica (L.) Merr. bioactive constituents exert anti-SARS-CoV-2 and anti-inflammatory activities: molecular docking and dynamics, in vitro, and in vivo studies. Molecules. 2021;26(19):5844. doi:10.3390/molecules26195844
75. Al-Karmalawy AA, Farid MM, Mostafa A, et al. Naturally available flavonoid aglycones as potential antiviral drug candidates against SARS-CoV-2. Molecules. 2021;26(21):6559. doi:10.3390/molecules26216559
76. Kelly M, O’Connor R, Townsend L, et al. Clinical outcomes and adverse events in patients hospitalised with COVID‐19, treated with off‐label hydroxychloroquine and azithromycin. Br J Clin Pharmacol. 2021;87(3):1150–1154. doi:10.1111/bcp.14482
77. Sanghai N, Shafiq K, Tranmer GK. Drug discovery by drug repurposing: combating COVID-19 in the 21st century. Mini Rev Med Chem. 2021;21(1):3–9. doi:10.2174/1389557520999200824103803
78. Martinez MA. Compounds with therapeutic potential against novel respiratory 2019 coronavirus. Antimicrob Agents Chemother. 2020;64(5). doi:10.1128/AAC.00399-20
79. Elfiky AA. Ribavirin, Remdesivir, Sofosbuvir, Galidesivir, and Tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): a molecular docking study. Life Sci. 2020;253:117592. doi:10.1016/j.lfs.2020.117592
80. Amirian ES, Levy JK. Current knowledge about the antivirals remdesivir (GS-5734) and GS-441524 as therapeutic options for coronaviruses. One Health. 2020;9:100128. doi:10.1016/j.onehlt.2020.100128
81. Cao YC, Deng QX, Dai SX. Remdesivir for severe acute respiratory syndrome coronavirus 2 causing COVID-19: an evaluation of the evidence. Travel Med Infect Dis. 2020;35:101647. doi:10.1016/j.tmaid.2020.101647
82. Agostini ML, Andres EL, Sims AC, et al. Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. MBio. 2018;9(2). doi:10.1128/mBio.00221-18
83. World Health Organization. WHO medical product alert N°4/2021: falsified remdesivir. Available from: https://www.who.int/home/search?indexCatalogue=genericsearchindex1&searchQuery=Remdesivir&wordsMode=AllWords.
84. Sheahan TP, Sims AC, Graham RL, et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med. 2017;9:396. doi:10.1126/scitranslmed.aal3653
85. Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382(10):929–936. doi:10.1056/NEJMoa2001191
86. Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. doi:10.1038/s41586-020-2008-3
87. Spinner CD, Gottlieb RL, Criner GJ, et al. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial. JAMA. 2020;324(11):1048–1057. doi:10.1001/jama.2020.16349
88. Norrie JD. Remdesivir for COVID-19: challenges of underpowered studies. Lancet. 2020;395(10236):1525–1527. doi:10.1016/S0140-6736(20)31023-0
89. Malin JJ, Suárez I, Priesner V, Fätkenheuer G, Rybniker J. Remdesivir against COVID-19 and other viral diseases. Clin Microbiol Rev. 2021;34(1):e00162–20.
90. Dongyuan W, Zigang L, Yihui L. An overview of the safety, clinical application and antiviral research of the COVID-19 therapeutics. J Infect Public Health. 2020. doi:10.1016/j.jiph.2020.07.004
91. Jean SS, Lee P-I, Hsueh P-R. Treatment options for COVID-19: the reality and challenges. J Microbiol Immunol Infect. 2020;53(3):436–443. doi:10.1016/j.jmii.2020.03.034
92. Furuta Y, Gowen BB, Takahashi K, Shiraki K, Smee DF, Barnard DL. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antiviral Res. 2013;100(2):446–454. doi:10.1016/j.antiviral.2013.09.015
93. Jin Z, Smith LK, Rajwanshi VK, Kim B, Deval J. The ambiguous base-pairing and high substrate efficiency of T-705 (favipiravir) ribofuranosyl 5′-triphosphate towards influenza A virus polymerase. PLoS One. 2013;8(7):e68347. doi:10.1371/journal.pone.0068347
94. Agrawal U, Raju R, Udwadia ZF. Favipiravir: a new and emerging antiviral option in COVID-19. Med J Armed Forces India. 2020;76(4):370–376. doi:10.1016/j.mjafi.2020.08.004
95. Booth CM, Matukas LM, Tomlinson GA, et al. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA. 2003;289(21):2801–2809. doi:10.1001/jama.289.21.JOC30885
96. Vanderlinden E, Vrancken B, Van Houdt J, et al. Distinct effects of T-705 (favipiravir) and ribavirin on influenza virus replication and viral RNA synthesis. Antimicrob Agents Chemother. 2016;60(11):6679. doi:10.1128/AAC.01156-16
97. Sung J, Wu A, Joynt G, et al. Severe acute respiratory syndrome: report of treatment and outcome after a major outbreak. Thorax. 2004;59(5):414–420. doi:10.1136/thx.2003.014076
98. Pandey A, Nikam AN, Shreya AB, et al. Potential therapeutic targets for combating SARS-CoV-2: drug repurposing, clinical trials and recent advancements. Life Sci. 2020;256:117883. doi:10.1016/j.lfs.2020.117883
99. Cheng C-Y, Lee Y-L, Chen C-P, et al. Lopinavir/ritonavir did not shorten the duration of SARS CoV-2 shedding in patients with mild pneumonia in Taiwan. J Microbiol Immunol Infect. 2020;53(3):488–492. doi:10.1016/j.jmii.2020.03.032
100. Elsevier novel coronavirus information center. Available from: https://www.elsevier.com/connect/coronavirus-information-center.
101. Hung IF-N, Lung K-C, Tso EY-K, et al. Triple combination of interferon beta-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395(10238):1695–1704. doi:10.1016/S0140-6736(20)31042-4
102. Warren TK, Wells J, Panchal RG, et al. Protection against filovirus diseases by a novel broad-spectrum nucleoside analogue BCX4430. Nature. 2014;508(7496):402–405. doi:10.1038/nature13027
103. Taylor R, Kotian P, Warren T, et al. BCX4430–a broad-spectrum antiviral adenosine nucleoside analog under development for the treatment of Ebola virus disease. J Infect Public Health. 2016;9(3):220–226. doi:10.1016/j.jiph.2016.04.002
104. Kadam RU, Wilson IA. Structural basis of influenza virus fusion inhibition by the antiviral drug Arbidol. Proc Natl Acad Sci. 2017;114(2):206–214. doi:10.1073/pnas.1617020114
105. Zhu Z, Lu Z, Xu T, et al. Arbidol monotherapy is superior to lopinavir/ritonavir in treating COVID-19. J Infect. 2020;81(1):e21–e23. doi:10.1016/j.jinf.2020.03.060
106. Saul S, Einav S. Old drugs for a new virus: repurposed approaches for combating COVID-19. ACS Infect Dis. 2020;6(9):2304–2318. doi:10.1021/acsinfecdis.0c00343
107. Uno Y. Camostat mesilate therapy for COVID-19. Intern Emerg Med. 2020;15(8):1577–1578. doi:10.1007/s11739-020-02345-9
108. Gasmi A, Noor S, Tippairote T, Dadar M, Menzel A, Bjørklund G. Individual risk management strategy and potential therapeutic options for the COVID-19 pandemic. Clin Immunol. 2020;215:108409. doi:10.1016/j.clim.2020.108409
109. Kawase M, Shirato K, van der Hoek L, Taguchi F, Matsuyama S. Simultaneous treatment of human bronchial epithelial cells with serine and cysteine protease inhibitors prevents severe acute respiratory syndrome coronavirus entry. J Virol. 2012;86(12):6537. doi:10.1128/JVI.00094-12
110. Hoffmann M, Schroeder S, Kleine-Weber H, Müller MA, Drosten C, Pöhlmann S. Nafamostat mesylate blocks activation of SARS-CoV-2: new treatment option for COVID-19. Antimicrob Agents Chemother. 2020;64(6). doi:10.1128/AAC.00754-20
111. Phadke M, Saunik S. COVID‐19 treatment by repurposing drugs until the vaccine is in sight. Drug Dev Res. 2020;81(5):541–543. doi:10.1002/ddr.21666
112. Welliver R, Monto AS, Carewicz O, et al. Effectiveness of oseltamivir in preventing influenza in household contacts: a randomized controlled trial. JAMA. 2001;285(6):748–754. doi:10.1001/jama.285.6.748
113. Yousefi B, Valizadeh S, Ghaffari H, Vahedi A, Karbalaei M, Eslami M. A global treatments for coronaviruses including COVID‐19. J Cell Physiol. 2020;235(12):9133–9142. doi:10.1002/jcp.29785
114. Li G, De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV). Nat Rev Drug Discov. 2020;19(3):149–150. doi:10.1038/d41573-020-00016-0
115. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi:10.1016/S0140-6736(20)30183-5
116. Muralidharan N, Sakthivel R, Velmurugan D, Gromiha MM. Computational studies of drug repurposing and synergism of lopinavir, oseltamivir and ritonavir binding with SARS-CoV-2 protease against COVID-19. J Biomol Struct Dyn. 2020;1–6. doi:10.1080/07391102.2020.1752802.
117. Liu F, Xu A, Zhang Y, et al. Patients of COVID-19 may benefit from sustained lopinavir-combined regimen and the increase of eosinophil may predict the outcome of COVID-19 progression. Int J Infect Dis. 2020;95:183–191. doi:10.1016/j.ijid.2020.03.013
118. Chu C, Cheng V, Hung I, et al. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59(3):252–256. doi:10.1136/thorax.2003.012658
119. Cao B, Wang Y, Wen D, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):1787–1799. doi:10.1056/NEJMoa2001282
120. Sheahan TP, Sims AC, Leist SR, et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. 2020;11(1):1–14. doi:10.1038/s41467-019-13940-6
121. Eastman RT, Roth JS, Brimacombe KR, et al. Remdesivir: a review of its discovery and development leading to emergency use authorization for treatment of COVID-19. ACS Cent Sci. 2020;6(5):672–683. doi:10.1021/acscentsci.0c00489
122. Seiwert SD, Andrews SW, Jiang Y, et al. Preclinical characteristics of the hepatitis C virus NS3/4A protease inhibitor ITMN-191 (R7227). Antimicrob Agents Chemother. 2008;52(12):4432. doi:10.1128/AAC.00699-08
123. Altay O, Mohammadi E, Lam S, et al. Current status of COVID-19 therapies and drug repositioning applications. Iscience. 2020;23(7):101303. doi:10.1016/j.isci.2020.101303
124. Wei L, Shang J, Ma Y, et al. Efficacy and safety of 12-week interferon-based danoprevir regimen in patients with genotype 1 chronic hepatitis C. J Clin Transl Hepatol. 2019;7(3):221. doi:10.14218/JCTH.2019.00018
125. Xu X, Feng B, Guan Y, et al. Efficacy and safety of all-oral, 12-week ravidasvir plus ritonavir-boosted danoprevir and ribavirin in treatment-naive noncirrhotic HCV genotype 1 patients: results from a phase 2/3 clinical trial in China. J Clin Transl Hepatol. 2019;7(3):213. doi:10.14218/JCTH.2019.00033
126. Nicastri E, Petrosillo N, Ascoli Bartoli T, et al. National institute for the infectious diseases “L. Spallanzani” IRCCS. Recommendations for COVID-19 clinical management. Infect Dis Rep. 2020;12(1):3–9. doi:10.4081/idr.2020.8543
127. Riva A, Conti F, Bernacchia D, et al. Darunavir does not prevent SARS-CoV-2 infection in HIV patients. Pharmacol Res. 2020;157:104826. doi:10.1016/j.phrs.2020.104826
128. Fintelman-Rodrigues N, Sacramento CQ, Lima CR, et al. Atazanavir inhibits SARS-CoV-2 replication and pro-inflammatory cytokine production. BioRxiv. 2020. doi:10.1128/AAC.00825-20
129. Stanley TL, Joy T, Hadigan CM, et al. Effects of switching from lopinavir/ritonavir to atazanavir/ritonavir on muscle glucose uptake and visceral fat in HIV infected patients. AIDS. 2009;23(11):1349. doi:10.1097/QAD.0b013e32832ba904
130. Vatansever EC, Yang KS, Drelich AK, et al. Bepridil is potent against SARS-CoV-2 in vitro. Proc Natl Acad Sci. 2021;118(10):e2012201118. doi:10.1073/pnas.2012201118
131. Hall DC, Ji H-F. A search for medications to treat COVID-19 via in silico molecular docking models of the SARS-CoV-2 spike glycoprotein and 3CL protease. Travel Med Infect Dis. 2020;35:101646. doi:10.1016/j.tmaid.2020.101646
132. Yamamoto N, Matsuyama S, Hoshino T, Yamamoto N. Nelfinavir inhibits replication of severe acute respiratory syndrome coronavirus 2 in vitro. BioRxiv. 2020. doi:10.1101/2020.04.06.026476
133. Ul Qamar MT, Alqahtani SM, Alamri MA, Chen -L-L. Structural basis of SARS-CoV-2 3CLpro and anti-COVID-19 drug discovery from medicinal plants. J Pharm Anal. 2020;10(4):313–319. doi:10.1016/j.jpha.2020.03.009
134. Khan RJ, Jha RK, Amera GM, et al. Targeting SARS-CoV-2: a systematic drug repurposing approach to identify promising inhibitors against 3C-like proteinase and 2′-O-ribose methyltransferase. J Biomol Struct Dyn. 2020:1–14. doi:10.1080/07391102.2020.1753577
135. Wu C-J, Jan J-T, Chen C-M, et al. Inhibition of severe acute respiratory syndrome coronavirus replication by niclosamide. Antimicrob Agents Chemother. 2004;48(7):2693. doi:10.1128/AAC.48.7.2693-2696.2004
136. Anand K, Ziebuhr J, Wadhwani P, Mesters JR, Hilgenfeld R. Coronavirus main proteinase (3CLpro) structure: basis for design of anti-SARS drugs. Science. 2003;300(5626):1763–1767. doi:10.1126/science.1085658
137. Yang H, Yang M, Ding Y, et al. The crystal structures of severe acute respiratory syndrome virus main protease and its complex with an inhibitor. Proc Natl Acad Sci. 2003;100(23):13190–13195. doi:10.1073/pnas.1835675100
138. Chou K-C, Wei D-Q, Zhong W-Z. Binding mechanism of coronavirus main proteinase with ligands and its implication to drug design against SARS. Biochem Biophys Res Commun. 2003;308(1):148–151. doi:10.1016/S0006-291X(03)01342-1
139. Barlow A, Landolf KM, Barlow B, et al. Review of emerging pharmacotherapy for the treatment of coronavirus disease 2019. Pharmacotherapy. 2020;40(5):416–437. doi:10.1002/phar.2398
140. Stockman LJ, Bellamy R, Garner P. SARS: systematic review of treatment effects. PLoS med. 2006;3(9):e343. doi:10.1371/journal.pmed.0030343
141. Xu Z, Peng C, Shi Y, et al. Nelfinavir was predicted to be a potential inhibitor of 2019-nCov main protease by an integrative approach combining homology modelling, molecular docking and binding free energy calculation. BioRxiv. 2020. doi:10.1101/2020.05.09.086165
142. Burns-Naas L, Zorbas M, Jessen B, et al. Increase in thyroid follicular cell tumors in nelfinavir-treated rats observed in a 2-year carcinogenicity study is consistent with a rat-specific mechanism of thyroid neoplasia. Hum Exp Toxicol. 2005;24(12):643–654. doi:10.1191/0960327105ht568oa
143. Khaliq Y, Gallicano K, Seguin I, et al. Single and multiple dose pharmacokinetics of nelfinavir and CYP2C19 activity in human immunodeficiency virus‐infected patients with chronic liver disease. Br J Clin Pharmacol. 2000;50(2):108–115. doi:10.1046/j.1365-2125.2000.00238.x
144. Trapé M, Barnosky S. Nelfinavir in expanded postexposure prophylaxis causing acute hepatitis with cholestatic features two case reports. Infect Control Hosp Epidemiol. 2001;22(6):333–334. doi:10.1017/S0195941700075767
145. Müller A, Cadenas E, Graf P, Sies H. A novel biologically active seleno-organic compound—1: glutathione peroxidase-like activity in vitro and antioxidant capacity of PZ 51 (Ebselen). Biochem Pharmacol. 1984;33(20):3235–3239. doi:10.1016/0006-2952(84)90083-2
146. Parnham MJ, Sies H. The early research and development of ebselen. Biochem Pharmacol. 2013;86(9):1248–1253. doi:10.1016/j.bcp.2013.08.028
147. Sies H. Ebselen, a selenoorganic compound as glutathione peroxidase mimic. Free Radic Biol Med. 1993;14(3):313–323. doi:10.1016/0891-5849(93)90028-S
148. Azad GK, Tomar RS. Ebselen, a promising antioxidant drug: mechanisms of action and targets of biological pathways. Mol Biol Rep. 2014;41(8):4865–4879. doi:10.1007/s11033-014-3417-x
149. Kil J, Lobarinas E, Spankovich C, et al. Safety and efficacy of ebselen for the prevention of noise-induced hearing loss: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2017;390(10098):969–979. doi:10.1016/S0140-6736(17)31791-9
150. Wendel A, Fausel M, Safayhi H, Tiegs G, Otter R. A novel biologically active seleno-organic compound—II: activity of PZ 51 in relation to Glutathione Peroxidase. Biochem Pharmacol. 1984;33(20):3241–3245. doi:10.1016/0006-2952(84)90084-4
151. Griffin SD, Beales LP, Clarke DS, et al. The p7 protein of hepatitis C virus forms an ion channel that is blocked by the antiviral drug, Amantadine. FEBS Lett. 2003;535(1–3):34–38. doi:10.1016/S0014-5793(02)03851-6
152. Torres J, Maheswari U, Parthasarathy K, Ng L, Liu DX, Gong X. Conductance and amantadine binding of a pore formed by a lysine‐flanked transmembrane domain of SARS coronavirus envelope protein. Protein Sci. 2007;16(9):2065–2071. doi:10.1110/ps.062730007
153. Smieszek SP, Przychodzen BP, Polymeropoulos MH. Amantadine disrupts lysosomal gene expression: a hypothesis for COVID19 treatment. Int J Antimicrob Agents. 2020;55(6):106004. doi:10.1016/j.ijantimicag.2020.106004
154. Wagstaff KM, Sivakumaran H, Heaton SM, Harrich D, Jans DA. Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus. Biochem J. 2012;443(3):851–856. doi:10.1042/BJ20120150
155. Caly L, Wagstaff KM, Jans DA. Nuclear trafficking of proteins from RNA viruses: potential target for antivirals? Antiviral Res. 2012;95(3):202–206. doi:10.1016/j.antiviral.2012.06.008
156. Yang SN, Atkinson SC, Wang C, et al. The broad spectrum antiviral ivermectin targets the host nuclear transport importin α/β1 heterodimer. Antiviral Res. 2020;177:104760. doi:10.1016/j.antiviral.2020.104760
157. Tu Y-F, Chien C-S, Yarmishyn AA, et al. A review of SARS-CoV-2 and the ongoing clinical trials. Int J Mol Sci. 2020;21(7):2657. doi:10.3390/ijms21072657
158. Ali MJ, Hanif M, Haider MA, et al. Treatment options for COVID-19: a review. Front Med. 2020;7:480. doi:10.3389/fmed.2020.00480
159. Patrì A, Fabbrocini G. Hydroxychloroquine and ivermectin: a synergistic combination for COVID-19 chemoprophylaxis and treatment? J Am Acad Dermatol. 2020;82(6):e221. doi:10.1016/j.jaad.2020.04.017
160. Momekov G, Momekova D. Ivermectin as a potential COVID-19 treatment from the pharmacokinetic point of view: antiviral levels are not likely attainable with known dosing regimens. Biotechnol Biotechnol Equip. 2020;34(1):469–474. doi:10.1080/13102818.2020.1775118
161. Park A, Iwasaki A. Type I and type III interferons–induction, signaling, evasion, and application to combat COVID-19. Cell Host Microbe. 2020;27(6):870–878. doi:10.1016/j.chom.2020.05.008
162. Scheuplein VA, Seifried J, Malczyk AH, et al. High secretion of interferons by human plasmacytoid dendritic cells upon recognition of Middle East respiratory syndrome coronavirus. J Virol. 2015;89(7):3859. doi:10.1128/JVI.03607-14
163. Lazear HM, Schoggins JW, Diamond MS. Shared and distinct functions of type I and type III interferons. Immunity. 2019;50(4):907–923. doi:10.1016/j.immuni.2019.03.025
164. Yoshikawa T, Hill TE, Yoshikawa N, et al. Dynamic innate immune responses of human bronchial epithelial cells to severe acute respiratory syndrome-associated coronavirus infection. PLoS One. 2010;5(1):e8729. doi:10.1371/journal.pone.0008729
165. Uzé G, Tavernier J. High efficiency targeting of IFN-α activity: possible applications in fighting tumours and infections. Cytokine Growth Factor Rev. 2015;26(2):179–182. doi:10.1016/j.cytogfr.2014.10.006
166. Andersen PI, Krpina K, Ianevski A, et al. Novel antiviral activities of obatoclax, emetine, niclosamide, brequinar, and homoharringtonine. Viruses. 2019;11(10):964. doi:10.3390/v11100964
167. Drożdżal S, Rosik J, Lechowicz K, et al. FDA approved drugs with pharmacotherapeutic potential for SARS-CoV-2 (COVID-19) therapy. Drug Resist Updat. 2020;53:100719. doi:10.1016/j.drup.2020.100719
168. Raad I, Darouiche R, Vazquez J, et al. Efficacy and safety of weekly dalbavancin therapy for catheter-related bloodstream infection caused by gram-positive pathogens. Clin Infect Dis. 2005;40(3):374–380. doi:10.1086/427283
169. Pouya MA, Afshani SM, Maghsoudi AS, Hassani S, Mirnia K. Classification of the present pharmaceutical agents based on the possible effective mechanism on the COVID-19 infection. DARU J Pharm Sci. 2020;28:1–20. doi:10.1007/s40199-018-0218-0
170. Russell B, Moss C, George G, et al. Associations between immune-suppressive and stimulating drugs and novel COVID-19—a systematic review of current evidence. ecancermedicalscience. 2020;14. doi:10.3332/ecancer.2020.1022
171. Vitiello A, Ferrara F. Perspectives of association Baricitinib/Remdesivir for adults with Covid-19 infection. Mol Biol Rep. 2022;49(1):827–831. doi:10.1007/s11033-021-06888-8
172. Zhang X, Zhang Y, Qiao W, Zhang J, Qi Z. Baricitinib, a drug with potential effect to prevent SARS-COV-2 from entering target cells and control cytokine storm induced by COVID-19. Int Immunopharmacol. 2020;86:106749. doi:10.1016/j.intimp.2020.106749
173. Dancey J. mTOR signaling and drug development in cancer. Nat Rev Clin Oncol. 2010;7(4):209. doi:10.1038/nrclinonc.2010.21
174. Ciliberto G, Mancini R, Paggi MG. Drug repurposing against COVID-19: focus on anticancer agents. J Exp Clin Cancer Res. 2020;39:1–9. doi:10.1186/s13046-020-01590-2
175. Kindrachuk J, Ork B, Hart BJ, et al. Antiviral potential of ERK/MAPK and PI3K/AKT/mTOR signaling modulation for Middle East respiratory syndrome coronavirus infection as identified by temporal kinome analysis. Antimicrob Agents Chemother. 2015;59(2):1088. doi:10.1128/AAC.03659-14
176. McNulty S, Flint M, Nichol ST, Spiropoulou CF. Host mTORC1 signaling regulates Andes virus replication. J Virol. 2013;87(2):912. doi:10.1128/JVI.02415-12
177. Stöhr S, Costa R, Sandmann L, et al. Host cell mTORC1 is required for HCV RNA replication. Gut. 2016;65(12):2017–2028. doi:10.1136/gutjnl-2014-308971
178. Runfeng L, Yunlong H, Jicheng H, et al. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2). Pharmacol Res. 2020;156:104761. doi:10.1016/j.phrs.2020.104761
179. Hu K, Guan WJ, Bi Y, et al. Efficacy and safety of Lianhuaqingwen capsules, a repurposed Chinese herb, in patients with coronavirus disease 2019: a multicenter, prospective, randomized controlled trial. Phytomedicine. 2021;85:153242. doi:10.1016/j.phymed.2020.153242
180. Al-Karmalawy AA, Soltane R, Abo Elmaaty A, et al. Coronavirus disease (COVID-19) control between drug repurposing and vaccination: a comprehensive overview. Vaccines. 2021;9(11):1317. doi:10.3390/vaccines9111317
181. Kumar S, Zhi K, Mukherji A, Gerth K. Repurposing antiviral protease inhibitors using extracellular vesicles for potential therapy of COVID-19. Viruses. 2020;12(5):486. doi:10.3390/v12050486
182. Shen C, Wang Z, Zhao F, et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323(16):1582–1589. doi:10.1001/jama.2020.4783
183. Chen L, Xiong J, Bao L, Shi Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect Dis. 2020;20(4):398–400. doi:10.1016/S1473-3099(20)30141-9
184. GOV.UK. Summary of product characteristics for Lagevrio. Available from: https://www.gov.uk/government/publications/regulatory-approval-of-lagevrio-molnupiravir/summary-of-product-characteristics-for-lagevrio.
185. GOV.UK. First oral antiviral for COVID-19, Lagevrio (molnupiravir), approved by MHRA. Available from: https://www.gov.uk/government/news/first-oral-antiviral-for-covid-19-lagevrio-molnupiravir-approved-by-mhra.
186. Kabinger F, Stiller C, Schmitzová J, et al. Mechanism of molnupiravir-induced SARS-CoV-2 mutagenesis. Nat Struct Mol Biol. 2021;28(9):740–746. doi:10.1038/s41594-021-00651-0
187. Vandyck K, Deval J. Considerations for the discovery and development of 3-chymotrypsin-like cysteine protease inhibitors targeting SARS-CoV-2 infection. Curr Opin Virol. 2021;49:36–40. doi:10.1016/j.coviro.2021.04.006
188. Schooley RT, Carlin AF, Beadle JR, et al. Rethinking remdesivir: synthesis, antiviral activity and pharmacokinetics of oral lipid prodrugs. bioRxiv. 2021. doi:10.1128/AAC.01155-21
189. Ahmad B, Batool M, Kim MS, Choi S. Exploring the binding mechanism of PF-07321332 SARS-CoV-2 protease inhibitor through molecular dynamics and binding free energy simulations. Int J Mol Sci. 2021;22(17):9124. doi:10.3390/ijms22179124
190. Pfizer. Pfizer’s novel COVID-19 oral antiviral treatment candidate reduced risk of hospitalization or death by 89% in interim analysis of phase 2/3 epic-HR study. Available from: https://www.pfizer.com/news/press-release/press-release-detail/pfizers-novel-covid-19-oral-antiviral-treatment-candidate.
191. Mahase E. COVID-19: pfizer’s paxlovid is 89% effective in patients at risk of serious illness, company reports. British Medical Journal Publishing Group; 2021.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
