Back to Journals » Journal of Pain Research » Volume 12
A single session of hyperbaric oxygen therapy demonstrates acute and long-lasting neuroplasticity effects in humans: a replicated, randomized controlled clinical trial
Authors Wahl AM , Bidstrup D , Smidt-Nielsen IG , Werner MU , Hyldegaard O , Rotbøll-Nielsen P
Received 15 December 2018
Accepted for publication 24 April 2019
Published 31 July 2019 Volume 2019:12 Pages 2337—2348
DOI https://doi.org/10.2147/JPR.S198359
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Katherine Hanlon
Supplementary video of ID 198359
Views: 1395
Anna M Wahl,1 Daniel Bidstrup,1 Isabel G Smidt-Nielsen,1 Mads U Werner,2 Ole Hyldegaard,1,3 Per Rotbøll-Nielsen2
1Hyperbaric Unit, Department of Anesthesia, Head and Orthopedic Center, Rigshospitalet, Copenhagen University Hospitals, Copenhagen, Denmark; 2Multidisciplinary Pain Center, Neuroscience Center, Rigshospitalet, Copenhagen University Hospitals, Copenhagen, Denmark; 3Institute of Clinical Medicine, University of Copenhagen, Copenhagen, Denmark
Purpose: Animal studies have demonstrated anti-inflammatory, and anti-nociceptive properties of hyperbaric oxygen therapy (HBOT). However, physiological data are scarce in humans. In a recent experimental study, the authors used the burn injury (BI) model observing a decrease in secondary hyperalgesia areas (SHA) in the HBOT-group compared to a control-group. Surprisingly, a long-lasting neuroplasticity effect mitigating the BI-induced SHA-response was seen in the HBOT-preconditioned group. The objective of the present study, therefore, was to confirm our previous findings using an examiner-blinded, block-randomized, controlled, crossover study design.
Patients and methods: Nineteen healthy subjects attended two BI-sessions with an inter-session interval of ≥28 days. The BIs were induced on the lower legs by a contact thermode (12.5 cm,2 47C°, 420 s). The subjects were block-randomized to receive HBOT (2.4 ATA, 100% O2, 90 min) or ambient conditions ([AC]; 1 ATA, 21% O2), dividing cohorts equally into two sequence allocations: HBOT-AC or AC-HBOT. All sensory assessments performed during baseline, BI, and post-intervention phases were at homologous time points irrespective of sequence allocation. The primary outcome was SHA, comparing interventions and sequence allocations.
Results: Data are mean (95% CI). During HBOT-sessions a mitigating effect on SHA was demonstrated compared to AC-sessions, ie, 18.8 (10.5–27.0) cm2 vs 32.0 (20.1–43.9) cm2 (P=0.021), respectively. In subjects allocated to the sequence AC-HBOT a significantly larger mean difference in SHA in the AC-session vs the HBOT-session was seen 25.0 (5.4–44.7) cm2 (P=0.019). In subjects allocated to the reverse sequence, HBOT-AC, no difference in SHA between sessions was observed (P=0.55), confirming a preconditioning, long-lasting (≥28 days) effect of HBOT.
Conclusion: Our data demonstrate that a single HBOT-session compared to control is associated with both acute and long-lasting mitigating effects on BI-induced SHA, confirming central anti-inflammatory, neuroplasticity effects of hyperbaric oxygen therapy.
Keywords: burns, hyperbaric oxygenation, inflammation, pathophysiology, secondary hyperalgesia
Plain language summary
Following hyperbaric oxygen therapy in animals, studies demonstrate anti-inflammatory and analgesic effects. However, human studies are surprisingly scarce in this field. The authors in a recent novel study showed that hyperbaric oxygen therapy in humans was associated with a long-lasting reduction of pain sensitivity in the skin surrounding an injured area. The objective of the present study was to further examine and validate these findings using an improved methodological design. An experimental first degree burn injury was induced on the lower leg in 19 healthy male subjects followed by an intervention of either hyperbaric oxygen therapy (2.4 ATA, 100% oxygen, 90 min) or normal ambient conditions (1 ATA, 21% oxygen [corresponds to conditions at sea level]) serving as a control. Quantitative sensory skin assessments were made at standardized time intervals. The test subjects received each treatment at study sessions separated by ≥28 days. The hyperbaric session was compared to the ambient control session allowing the subjects to be their own control. Data from the present study confirms our previous findings showing a long-lasting (≥1 month) reduction of pain sensitivity in the skin around the burn injury. These findings indicate that hyperbaric oxygen therapy could be an interesting venue for clinical pain research in persistent postsurgical pain and phantom limb pain.
Introduction
Hyperbaric oxygen therapy (HBOT) is a recognized treatment form1,2 and has been considered an adjunctive treatment for chronic pain conditions.3,4 Several experimental studies, using different methods and treatment dosages, have shown anti-inflammatory effects of HBOT,5–7 but there is a paucity of studies investigating these effects in humans. A previous study,8 carried out by the present authors, was the first in humans to demonstrate an ameliorating effect of HBOT on the pathophysiological consequences following a previously validated inflammatory burn injury (BI) model.9–11 We demonstrated an attenuation of secondary hyperalgesia areas (SHA) in normal skin surrounding the injury, likely reflecting a central anti-nociceptive effect. Interestingly, a preconditioning, protective effect of HBOT on the development of secondary hyperalgesia was also seen, more than one month after the primary injury. Both phenomena indicated an effect on central sensitization.12,13
The original study was a randomized, controlled, crossover study with an open therapeutic design.8 The objective of the present study was to replicate our findings, adding an improved single-blinded design, ie, blinding for examiner bias. The primary outcome was an assessment of SHA, a measure of central sensitization. The secondary outcomes were measures of peripheral inflammation and sensitization, ie edema, erythema, mechanical pain thresholds, and, thermal detection and pain thresholds. In addition, exploratory analyses of the combined data of the previous and present studies were made.
Methods
Approvals
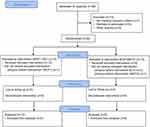
The study was approved by the Committee of Health Research Ethics of the Capital Region of Denmark (no. H-6–2014-089), the Danish Data Protection Agency (no. 30–1332) and the Danish Health Authority (no. 2014–003858-14). The study was registered before patient enrollment at EudraCT (no. 2014–003858-14, https://www.clinicaltrialsregister.eu/ctr-search/search?query=2014-003858-14), principal investigator: Ole Hyldegaard, registration date: September 23rd 2014. The study was also registered in clinicaltrials.gov (clinicaltrials.gov identifier: NCT02397343, https://clinicaltrials.gov/ct2/show/NCT02397343?term=NCT02397343&rank=1). The study complied with regulations of Good Clinical Practice (GCP) and was monitored by the GCP-unit of Copenhagen University Hospitals. The manuscript adheres to the applicable CONSORT guidelines (Figure 1). No amendment to the original protocol was submitted.
Study design
A randomized (1:1 block allocation), controlled, crossover, single-blinded design was used. Details are in Supplemental Document 1, Supplemental Figure 1, Supplemental Table 1 and Supplemental Video 1. The subjects were randomized to hyperbaric oxygen treatment (HBOT: 2.4 ATA, 90 min, no air-breaks) or treatment in an open environment during ambient pressure conditions (ambient pressure conditions [AC]: 1 ATA).
Subjects
Subjects were recruited using www.forsoegsperson.dk. For inclusion and exclusion criteria see Supplemental Table 2. Before inclusion, the subjects received written and oral study information and provided informed written consent. A physical health examination including the completion of a Professional Association of Diving Instructors (PADI) health declaration was performed by a senior medical specialist in diving- and hyperbaric medicine (OH).
All treatment sessions were made at the Hyperbaric Unit, Department of Anesthesia, Head and Orthopedic Center, Rigshospitalet, Copenhagen University Hospitals. The subjects received a compensation of USD 316 (EUR 268) after completion of both sessions.
Study algorithm
The study included two sessions, identical except for the type of intervention treatment (Figure 2A). Each session started with baseline measurements, in chronological order, skin-erythema (SE) and dermal-thickness (DT), and, assessments of quantitative sensory testing (QST) variables: mechanical thresholds and thermal thresholds. Baseline assessments were followed by a standardized first-degree BI.14,15 Following the intervention treatment (HBOT/AC) assessments were repeated at timepoints 130, 190 and 250 min (Figure 2A). The two sessions were separated by an interval of ≥28 days to minimize the risk of carry-over effects from the previous BI.
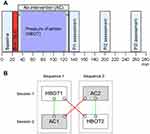 |
Figure 2 (A and B) Study setup. Notes: (A) Study algorithm. Each session followed the same study algorithm except for type of intervention: baseline assessments (0–20 min); burn injury (20–30 min); HBOT-/AC-intervention (30–130 min); and PI1-3 including measurements of skin-erythema (SE) and dermal-thickness (DT), and QST assessments, mechanical and thermal thresholds (130–150 min, 190–210 min, 250–270 min). HBOT-intervention included a compression phase (C; 30–35 min); a therapy phase of 100% O2; 2.4 ATA (35–125 min); and a decompression phase (125–130 min). AC-intervention included control-therapy with 21% O2, 1.0 ATA (30–130 min). Each subject received both treatments during two individual sessions but was randomized to either HBOT1-AC1 or AC2-HBOT2 sequence (Figure 2B). (B) Sequences, Sessions, and within-/between-sequence comparisons. Subject-flows were divided into "sequences" (Sequence 1: HBOT1+ AC1; Sequence 2: AC2+ HBOT2) and "sessions" (Session 1: HBOT1+ AC2; Session 2: AC1+ HBOT2). Within-sequence comparisons are comparisons of data within the same sequence (green lines) (HBOT1 vs AC1; AC2 vs HBOT2) whereas the between-sequence comparisons are comparisons of data between sequences (red lines) (HBOT1 vs HBOT2; AC1 vs AC2).Abbreviations: AC, ambient pressure conditions; HBOT, hyperbaric oxygen therapy; PI, post-injury; QST, quantitative sensory testing. |
Randomization procedure
Two randomizations were made using www.randomizer.org. The first randomization decided the order of treatment. Subjects were separated into the two sequences; one sequence starting with the HBOT-session and one starting with the AC-session (Sequence 1: HBOT1-AC1 sequence; Sequence 2: AC2-HBOT2 sequence; Figure 2B), dividing the study into sessions and sequences. The second randomization decided upon the left- or right-sided location of the first of the two BIs.
Randomization allocations and master randomization lists were kept concealed in opaque envelopes marked with consecutive numbers. Randomization procedures were conducted by a medical staff member, otherwise not associated with this study. The sealed envelopes were opened, when appropriate by the unblinded co-examinators (DB, ISN) and allocation instructions were followed.
Hyperbaric oxygen therapy procedure
The HBOT procedure was performed as previously reported in detail.8 The procedure consisted of an initial compression period lasting 5 min (Figure 2A), reaching a plateau of 2.4 ATA during which HBOT was administered for 90 min (100% O2) using no air-breaks, followed by a short decompression period (Figure 2A).
Sensitization method
The BIs were induced by the co-examinator using a Peltier-based contact thermode (active area 2.5×5.0 cm2, 47°C, 420 s; Thermotest, MSA, Somedic AB, Hörby, Sweden). The thermode was positioned at a prespecified site on the calf, using the homologous anatomical site on the contralateral side during the second session. An elastic compression bandage kept the thermode in position maintaining a constant application pressure during all assessments. The subject rated the intensity of pain, using a horizontally held, visual analog scale (VAS; 0= no pain, 10= worst imaginable pain) at baseline, and, at timepoints 0, and 15, 30, 45, 60, 120, 180, 240, 300, 419 and 425 s after the thermode had reached 47°C.
Measurements and assessments
Skin-erythema and dermal-thickness
Measurements of SE and DT were made inside and outside the testing area (10 cm distal from the center) using a combined non-invasive, skin-reflectance spectrophotometer and a high-resolution ultrasound scanner (Derma-Lab Combo, Cortex Technology ApS, Hadsund, Denmark).16,17 The spectrophotometer was calibrated prior to all assessments. The SE was measured as the erythema index (arbitrary units), and the DT was measured in the testing area as previously described.15 Measurements were each made in triplicates using the mean value in further analysis.
Mechanical thresholds
Assessments of pin-prick pain threshold (PPT) were made in the testing area using “weighted-pins” (8, 16, 32, 64, 128, 256 and 512 mN; MRC Systems, Heidelberg, Germany) according to a modified Dixon’s “up-and-down method”,18–20 as previously reported.8 The PPT was determined four times, and the median value was used in further analysis.
Secondary hyperalgesia areas
Assessment of the SHA was by a 512 mN “weighted-pin” stimulator.19 The area surrounding the BI was stimulated clockwise along eight symmetrical radials converging towards the center of the BI, as previously reported.8 The stimulation began in normal skin with an application rate of 0.5 Hz and a distance of 1–2 cm between each pin-prick, projecting in towards the center. The subject indicated when a definite change in perception, from a non-noxious to an uncomfortable or stinging sensation occurred. The octagonal corners of secondary hyperalgesia were marked on the skin. The markings were then transferred by manual copying onto a transparent overhead sheet and digitally transferred to a vector-based computer program (Canvas 8.0, ACD Systems International, Victoria, Canada) for area calculations. The SHA were calculated by subtracting the testing area from the total area.
Thermal Thresholds
Warmth detection threshold (WDT), cool detection threshold (CDT) and heat pain threshold (HPT) were assessed using the contact thermode system and determined according to the-method-of-limits,21 as previously reported.8 Threshold assessments were made in triplicate using the mean value in further analysis.
Statistics
Statistical analyses
Data were manually checked for errors by one of the investigators (AW) and by the GCP-unit. Statistical analyses were made using the MedCalc Statistical Software (Version 15.11.4, MedCalc Software bvba, Ostend, Belgium). During the statistical analyses, data were first partially unblinded dividing the subjects into two groups, A and B. Data were unblinded only after completion of the analysis. Normality of the data was tested using the Kolmogorov-Smirnov test and visual inspection of residual plots. When relevant, data were corrected with baseline values obtained at the beginning of each session to avoid potential carryover effects between the two sessions. To avoid mass significance in multiple comparisons, a summated AUC-measure (area-under-the-curve min−1) was used where appropriate. For normally distributed data, significance was determined using the paired or unpaired t-tests, and for non-normally distributed data the Wilcoxon or Mann-Whitney tests, respectively, were used as appropriately. Comparisons across sessions and sequences were made to explore potential preconditioning effects (Figure 2B). In analysis of the consistency of baseline assessments, intraclass correlation coefficients (ICC [2,1; two-way random single measures, consistency]) were used, categorized as previously reported.22 Depending on normality, data are presented as mean or median (95% CI). The level of statistical significance was a priori set at <0.05. The Bonferroni correction was used for multiple comparisons for the primary outcome, the statistical significance therefore was set at 0.025.
Sample size estimates
Based on the primary outcome, SHA, and the results from our original study,8 an a priori sample size estimation was made using a minimal relevant difference of 19 cm2 and a standard deviation of 28 cm2. The significance level was set at 0.05 (α) and the power at 0.8 (β=0.2). A randomized, single-blinded, crossover design was used. The calculations yielded an estimated sample size of 19 per-protocol subjects, but to compensate for dropouts the number of intention-to-treat subjects was 26.
Exploratory analyses of combined data
Exploratory analyses were made by combined data from the present study with data from our original study.8 Statistical analyses were made using the same methods as described in the previous paragraph.
Results
Subjects
Subject flow
The first study session was on February 16, 2015, and the last study session was on May 13, 2015. The study was completed when the a priori estimated sample size had been reached. Twenty-six subjects were included in the study following the initial visit, however, five subjects did not complete the study (Figure 1; CONSORT 2010 Flow Diagram). Two subjects (#3, #6) never showed up after the initial visit. One subject (#26) decided, due to time restraints, not to take part in the study. One subject (#13) had to withdraw before the intervention, due to medical reasons not associated with the study. One subject (#1) never showed up at the second study session (HBOT2-session). Data from these five subjects were not included in the statistical analyses. Thus, 21 subjects completed the trial. No adverse effects or complications were seen during the study.
Missing data
Data sets from these 21 subjects were incomplete for subjects #10, #16 and #25. Subjects #10 and #16 were unable to consistently perceive secondary hyperalgesia, and their SHA-assessments were excluded from the analysis, leaving 19 data sets available for the primary outcome analysis. Subject #25 presented an incomplete data set regarding SE-measurements, leaving 20 data sets for analysis of SE-data (n=20). The missing SE-data were attributed to human error, constituting only 4/3,864 (0.1%) of the total number of data entries.
Anthropometrics
Anthropometrics are shown in Table 1.38
 |
Table 1 Anthropometric data |
Burn injury
The median time interval between the two BIs (sessions) was 35 days (34–39 days). The mean pain intensity during the BIs, calculated as AUC, was 3.3 (2.4–4.1) VAS-units during the AC-sessions, and 3.0 (2.3–3.6) VAS-units during the HBOT-sessions (P=0.59).
Primary outcome (secondary hyperalgesia areas)
Baseline data
Data consistency during Session 1 vs Session 2 (Figure 2B) was examined by ICC’s (0.85 [0.64–0.94]) and by comparisons of baseline assessments using simple paired sample tests demonstrating a highly significant difference in baseline assessments between the sessions (Wilcoxon: P=0.0017). The median values for SHA were significantly larger in Session 1 (26.6 [12.5–37.6] cm2) compared to Session 2 (15.9 [12.5–23.4] cm2).
Post-injury vs baseline assessments
Data consistency of BI-induced changes in sensory assessments were examined by comparisons between baseline and post-injury 1–3 (PI1-3) AUC-values. Significantly increased BI-induced SHA-values were seen in both sessions (HBOT-session, P=0.0002; AC-session, P=0.0001).
HBOT vs AC-sessions: SHA-values of the HBOT- and AC-sessions, respectively, were calculated as AUC corrected for the respective baseline values and given as the mean. HBOT-sessions showed a SHA of 18.8 (10.5–27.0) cm2, whereas the AC-sessions had an area of 32.0 (20.1–43.9) cm2 (P=0.021; Figure 3A), indicating a significant mitigating effect of HBOT-sessions compared to AC-sessions.
Within-sequence and between-sequence effects
Within-sequence effects were examined, in Sequence 1 comparing session HBOT1 with session AC1, and, in Sequence 2 comparing session AC2 with session HBOT2 (Figure 2B). The comparison in Sequence 1 (HBOT1 vs AC1; Figure 4A) did not yield any statistical difference (P=0.55) while in Sequence 2 (AC2 vs HBOT2), a significantly larger area in the AC2-session was demonstrated, corresponding to a mean difference of 25.0 (5.4–44.7) cm2, compared to the HBOT2-session (P=0.019).
Between-sequence effects were examined by comparing session HBOT1 with session HBOT2, and, by comparing session AC1 with session AC2 (Figure 2B). While the comparison of HBOT-sessions did not yield any statistical significance (P=0.73), comparison of the AC-sessions demonstrated a statistically significant larger area in the AC2-session, corresponding to a mean difference of 25.5 (4.9–46.1) cm2, compared to the AC1-session (P=0.018).
Summary
The analyses demonstrated a mitigating effect on SHA of HBOT-sessions vis-á-vis AC-sessions. Furthermore, they demonstrated a significant preconditioning effect in Sequence 1 when the HBOT-session was administered prior to the AC-session, compared to Sequence 2, when the AC-session preceded the HBOT-session (Figure 2B).
Secondary outcomes (SE, DT, mechanical and thermal thresholds)
Baseline data
Data consistency for secondary outcomes are available in Supplemental Document 2.
Post-injury vs baseline assessments
Comparisons between baseline values and post-injury values are available in Supplemental Document 2.
HBOT vs AC-sessions
No statistically significant differences between sessions, calculated as AUC corrected for the respective baseline values, were found regarding the mean values in SE, DT, PPT or the median values in CDT. A significantly lower mean WDT was found in the HBOT-session compared to the AC-session (P=0.011). A significantly lower mean value was also found regarding HPT in the HBOT-session, compared to the AC-session (P=0.045). For detailed secondary outcome analyses see Supplemental Document 2.
Combined primary outcome data from original and present study
Subjects
Nineteen subjects were included from the present study and 17 subjects from the original study,8 making a total of 36 subjects available for the combined data analysis.
Baseline data
The original study showed no baseline SHA and, therefore, the combined baseline data are identical to results of the present study.
Post-injury vs baseline assessments
Significantly increased BI-induced SHA-values were seen in both sessions (HBOT-session and AC-session, P=0.0001).
HBOT vs AC-sessions
Significantly smaller mean SHA were found in the HBOT-group (26.4 [19.7–33.0]: Figure 3B) compared to the AC-group (40.9 [31.8–50.0]: P=0.0018), corroborating a mitigating effect of HBOT.
Within-sequence and between-sequence effects
Within-sequence effects showed no statistical difference in Sequence 1 (HBOT1 vs AC1; Figure 4B) (P=0.96) while significantly larger SHA were found in the AC2-session compared to the HBOT2-session, corresponding to a mean difference of 30.5 cm2 (17.9–43.2 cm2; P=0.0001).
Between-sequence effects comparing the HBOT-sessions (HBOT1 vs HBOT2; Figure 4B) did not yield any statistical difference (P=0.47). However, a significant difference was found when comparing the AC-sessions (AC1 vs AC2) showing a statistically significantly larger area in the AC2-session, corresponding to a mean difference of 29.7 cm2 (14.9–44.4 cm2; P=0.0006), compared to the AC1-session, corroborating the preconditioning effect of the HBOT1-session.
Summary
The analyses of the combined data demonstrated first, a highly significant decrease in SHA related to HBOT compared to AC (P=0.0018). Second, a highly significant preconditioning effect on the development of SHA related to HBOT (P=0.0001). Third, the combined study results corroborated the data consistency between the studies.
Protocol violations
During data collection, the examiner noticed a number of baseline pre-burn SHA. However, according to our experience, this is rarely observed in healthy skin, and therefore a short questionnaire was made to evaluate if the subjects were, in fact, able to detect SHA. Most subjects were able to discriminate secondary hyperalgesia from normal sensation. However, two subjects (#10, #16) were not able to discriminate or could not recall a perceptual difference between pre- and post-burn SHA and were therefore excluded in the data analysis of SHA (cf. Missing data). The implementation of the questionnaire had not been described in the protocol and is, therefore, a minor violation of protocol.
Discussion
In the human experimental BI-model, we demonstrated that HBOT mitigates inflammation-induced secondary hyperalgesia. Remarkably, a single session of HBOT also has a preconditioning, long-lasting, pre-emptive anti-hyperalgesic effect on a contralateral repeat injury. Since no signs of mitigation of the primary hyperalgesia in the injured areas were found, our data clearly demonstrate that HBOT has acute and long-lasting neuroplasticity effects on central sensitization.23,24
Mitigation of secondary hyperalgesia
Secondary hyperalgesia is a centrally induced phenomenon12,13,23 where the conditioning input from nociceptors by heterosynaptic potentiation amplifies the response from segmentally connected afferent non-nociceptive and nociceptive nerve fibers. Although the clinical role of central sensitization has been debated,23,25 it may contribute to the pain trajectories of osteoarthritis, temporomandibular joint disorders, neuropathic pain, visceral pain hypersensitivity disorders and persistent post-surgical pain.12 Neuroplasticity represents a physiological adaption in the nervous system responding to perturbations in the external environment.26,27 Neuroplasticity leads to functional and structural alterations in the nervous system and is intimately linked to memory processing, storage and consolidation. In the nociceptive system, modulation of ascending and descending control pathways are by neuroplasticity mechanisms.28 In humans central sensitization of pain, eg, secondary hyperalgesia, temporal summation, and long term potentiation,12,29 is often coined a maladaptive neuroplasticity response, mainly affecting the descending inhibition system.27,28 The available evidence from this study, therefore, substantiate the presence of a potential neuroplasticity mechanism in the phenotypic changes: the mitigation of secondary hyperalgesia. However, it would be interesting to obtain further proof of neuronal plasticity with MRI using the BOLD-technique.30
Interestingly, the time frame of neuroplasticity changes in pain phenotype is neatly demonstrated in the present study. The acute mitigating effect on injury induced SHA is seen hours after the injury while the preemptive, protective effect on development of SHA is demonstrated four weeks following preconditioning with a single session HBOT. Recent clinical studies indicate that HBOT attenuates chronic pain in fibromyalgia by inducing neuroplasticity following a series of 40 treatment sessions, thereby rectifying abnormal brain activity in pain related areas.3 One animal study found a two-phase antinociceptive effect, with the second phase lasting up to three weeks after four HBOT-sessions by a mechanism mediated through the NO dependent release of endogenous opiods.31 As pain disorders are not congenital but evolve over time due to multifactorial causes, the concept of neuroplasticity is inherent in the development of pain disorders. Since HBOT has been shown to induce neuroplasticity in the chronically injured brain even months to years after the acute insult,3,32 understanding the specific mitigating mechanisms of HBOT on the pathophysiological perturbations following tissue injury may shed some light on future management strategies as well as indicating rational and targeted use of HBOT33 in various pain disorders.
Effects of HBOT on the primary burn injury area
A reduction in WDT and HPT in the HBOT-sessions compared to the AC-sessions was demonstrated, but no other significant differences in QST-indices in the primary BI-area were found. Multiple comparisons were made in the secondary analysis without using Bonferroni correction, leaving a considerable risk of type 1 error. Therefore, our results hardly support any effect of HBOT on the primary BI-area.
Combined data from original and present study
Methodological differences
We decided to combine the data of the present study with data from our original study8 in order to cautiously examine the statistical consistency of the studies. The two studies are methodologically almost identical, but few important differences do exist. First, the roles of examiner and co-examinator, and, the blinding procedure, are novel in the present study. Second, the delineating-method of SHA using pin-prick instruments vs polyamide monofilaments in the original study differed. However, this unlikely influences the results, since we used paired data analysis with each subject being his control and furthermore, it has been demonstrated that the results obtained by the two delineating-methods are reproducible and inter-correlated.19 We, therefore, consider combining the results to be appropriate and methodologically interesting.
Mitigation of secondary hyperalgesia and preconditioning
When combining the data, the results had the same general tendency as in the individual studies, but with a markedly increased statistical significance. Although the interpretation of post-hoc analyses should be made with diligence the effect sizes of the present study and the combined studies, were very large, 2.5 and 3.5, respectively. The statistical significance increased with P-values from 0.021 (n=19) to 0.0018 (n=36), making a type I error very unlikely.
Data consistency
In the present study and the combined studies, we found a statistically significant difference in SHA baseline values between Session 1 and Session 2, probably indicating a habituation phenomenon. However, this does not affect our results, since corrections with baseline values have been made. The ICC’s showed excellent data consistency.
Limitations of the study
Blinding conditions
During this single-blinded, block-randomized, controlled, crossover study, the examiner was blinded to the subject’s allocation, but the design did not prevent the development of subject bias. While it is highly unlikely that the subject could consciously replicate the preconditioning effects with the sparse information received in combination with the randomization- and group allocation procedures performed (Figure 2B), we cannot exclude subject bias. Double-blind designs have been reported to be feasible in a hyperbaric environment.1,34,35 However, to do this, a single-seat hyperbaric chamber would be needed or, alternatively, a study design with seven participants receiving treatment simultaneously requiring a huge number of examiners and co-examiners to do the assessments. Unfortunately, neither of these setup designs were possible during the present study.
Multiple comparisons
Multiple comparisons were made in the statistical sub-analyses of the primary outcome increasing the likelihood of introducing a type 1 error. In spite of the conservative Bonferroni correction, the results continued to be statistically significant in the present study and the combined analyses, indicating that a type 1 error would be highly unlikely.
Advantages of the study
Growing concerns about the reproducibility in scientific research has been expressed during the last decade.36,37 In the previous study the small number of subjects and the single-session procedure seemed potential confounding factors. Our principal objective of the present study was to replicate and validate our previous study results on the effect of HBOT on human burn pathophysiology.
Conclusion
The present study demonstrates that hyperbaric oxygen therapy has an immediate mitigating effect, as well as a long-lasting preconditioning effect, on secondary hyperalgesia induced by a repeat burn injury. These are the first studies in humans to demonstrate ameliorating effects of hyperbaric oxygen therapy on central sensitization, and the results corroborate experimental findings from animal studies. Hyperbaric oxygen therapy seems an interesting venue for future preventive measures in severe pain conditions characterized by central sensitization, eg, persistent postsurgical pain and phantom limb pain.
Abbreviation list
AC, ambient conditions; AUC, area-under-the-curve per minute; BI, burn injury; CDT, cool detection threshold; DT, dermal-thickness; HBOT, hyperbaric oxygen therapy; HPT, heat pain threshold; ICC, intraclass correlation coefficients; PADI, Professional Association of Diving Instructors; PPT, pin-prick pain threshold; SE, skin-erythema; SHA, secondary hyperalgesia areas; WDT, warmth detection threshold.
Data availability
The complete dataset is available in Supplemental File 1.
Acknowledgments
The authors thank Sohail Asghar, MD PhD, Department of Neuroanesthesia, Neuroscience Center, Copenhagen University Hospitals, Copenhagen, Denmark, for valuable discussions concerning the manuscript. AMW is supported by unrestricted grants from P. Carl Petersens Fond, and C.C. Klestrup og hustru Henriette Klestrups Mindelegat grant no. 10761. Mads U. Werner is supported by NIHDA37621.
Author contributions
AW, MW and PRN conceived the study. AW, DB, MW, OH and PRN designed the study. AW, DB and ISN conducted the study. MW, OH and PRN supervised the study. AW and MW analyzed and interpreted data, performed statistical analyses and wrote the manuscript. All authors critically revised the manuscript, gave final approval for publication and agree to be accountable for all aspects of the work.
Disclosure
Mrs Anna M Wahl reports grants from P. Carl Petersens Fond and grants from C.C. Klestrup og hustru Henriette Klestrups Mindelegat, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Weaver LK, Churchill SK, Bell J, Deru K, Snow GL. A blinded trial to investigate whether ‘pressure-familiar’ individuals can determine chamber pressure. Undersea Hyperb Med. 2012;39(4):801–805.
2. Mathieu D, Marroni A, Kot J. Tenth European Consensus Conference on Hyperbaric Medicine: recommendations for accepted and non-accepted clinical indications and practice of hyperbaric oxygen treatment. Diving Hyperb Med. 2017;47(1):24–32. doi:10.28920/dhm47.1.24-32
3. Efrati S, Golan H, Bechor Y, et al. Hyperbaric oxygen therapy can diminish fibromyalgia syndrome–prospective clinical trial. PLoS One. 2015;10(5):e0127012. doi:10.1371/journal.pone.0127012
4. Sutherland AM, Clarke HA, Katz J, Katznelson R. Hyperbaric oxygen therapy: a new treatment for chronic pain? Pain Practice. 2016;16(5):620–628. doi:10.1111/papr.12312
5. Hui J, Zhang ZJ, Zhang X, Shen Y, Gao YJ. Repetitive hyperbaric oxygen treatment attenuates complete Freund’s adjuvant-induced pain and reduces glia-mediated neuroinflammation in the spinal cord. J Pain. 2013;14(7):747–758. doi:10.1016/j.jpain.2013.02.003
6. Zhao BS, Meng LX, Ding YY, Cao YY. Hyperbaric oxygen treatment produces an antinociceptive response phase and inhibits astrocyte activation and inflammatory response in a rat model of neuropathic pain. J Mol Neurosci. 2014;53(2):251–261. doi:10.1007/s12031-013-0213-3
7. Gu N, Niu JY, Liu WT, et al. Hyperbaric oxygen therapy attenuates neuropathic hyperalgesia in rats and idiopathic trigeminal neuralgia in patients. Eur J Pain. 2012;16(8):1094–1105. doi:10.1002/j.1532-2149.2012.00113.x
8. Rasmussen VM, Borgen AE, Jansen EC, Rotboll Nielsen PH, Werner MU. Hyperbaric oxygen therapy attenuates central sensitization induced by a thermal injury in humans. Acta Anaesthesiol Scand. 2015;59(6):749–762. doi:10.1111/aas.12492
9. Pedersen JL, Kehlet H. Hyperalgesia in a human model of acute inflammatory pain: a methodological study. Pain. 1998;74(2–3):139–151.
10. Naert AL, Kehlet H, Kupers R. Characterization of a novel model of tonic heat pain stimulation in healthy volunteers. Pain. 2008;138(1):163–171. doi:10.1016/j.pain.2007.11.018
11. Knutti IA, Suter MR, Opsommer E. Test-retest reliability of thermal quantitative sensory testing on two sites within the L5 dermatome of the lumbar spine and lower extremity. Neurosci Lett. 2014;579:157–162. doi:10.1016/j.neulet.2014.07.023
12. Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152(3 Suppl):S2–S15. doi:10.1016/j.pain.2010.09.030
13. Ziegler EA, Magerl W, Meyer RA, Treede RD. Secondary hyperalgesia to punctate mechanical stimuli. Central sensitization to A-fibre nociceptor input. Brain. 1999;122(Pt 12):2245–2257. doi:10.1093/brain/122.12.2245
14. Werner MU, Petersen KL, Rowbotham MC, Dahl JB. Healthy volunteers can be phenotyped using cutaneous sensitization pain models. PLoS One. 2013;8(5):e62733. doi:10.1371/journal.pone.0062733
15. Andersen LP, Gogenur I, Fenger AQ, Petersen MC, Rosenberg J, Werner MU. Analgesic and antihyperalgesic effects of melatonin in a human inflammatory pain model: a randomized, double-blind, placebo-controlled, three-arm crossover study. Pain. 2015;156(11):2286–2294. doi:10.1097/j.pain.0000000000000284
16. Clarys P, Alewaeters K, Lambrecht R, Barel AO. Skin color measurements: comparison between three instruments: the Chromameter(R), the DermaSpectrometer(R) and the Mexameter(R). Skin Res Technol. 2000;6(4):230–238.
17. Olsen LO, Takiwaki H, Serup J. High-frequency ultrasound characterization of normal skin. Skin thickness and echographic density of 22 anatomical sites. Skin Res Technol. 1995;1(2):74–80. doi:10.1111/j.1600-0846.1995.tb00021.x
18. Dixon WJ. Staircase bioassay: the up-and-down method. Neurosci Biobehav Rev. 1991;15(1):47–50.
19. Ringsted TK, Enghuus C, Petersen MA, Werner MU. Demarcation of secondary hyperalgesia zones: punctate stimulation pressure matters. J Neurosci Methods. 2015;256:74–81. doi:10.1016/j.jneumeth.2015.08.018
20. Dixon WJ, The up-and-down method for small samples. Am Stat Assoc J. 1965;60:967–978. doi:10.1080/01621459.1965.10480843
21. Yarnitsky D, Sprecher E. Different algorithms for thermal threshold measurements. In: Boivie J, Hansson P, Lindblom U, editors. Touch, Temperature, and Pain in Health and Disease: Mechanisms and Assessments. Seattle: IASP Press; 1994:105–112.
22. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174.
23. Woolf CJ. What to call the amplification of nociceptive signals in the central nervous system that contribute to widespread pain? Pain. 2014;155(10):1911–1912. doi:10.1016/j.pain.2014.07.021
24. Slimani H, Plaghki L, Valenti P, Werner MU, Kehlet H, Kupers R. Adelta and not C fibers mediate thermal hyperalgesia to short laser stimuli after burn injury in man. Pain. 2018. doi:10.1097/j.pain.0000000000001339
25. Hansson P. Translational aspects of central sensitization induced by primary afferent activity: what it is and what it is not. Pain. 2014;155(10):1932–1934. doi:10.1016/j.pain.2014.07.016
26. Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10(9):895–926. doi:10.1016/j.jpain.2009.06.012
27. Pace MC, Passavanti MB, De Nardis L, et al. Nociceptor plasticity: a closer look. J Cell Physiol. 2018;233(4):2824–2838. doi:10.1002/jcp.25993
28. Bannister K, Dickenson AH. The plasticity of descending controls in pain: translational probing. J Physiol. 2017;595(13):4159–4166. doi:10.1113/JP274165
29. Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1–32. doi:10.1146/annurev.neuro.051508.135531
30. Asghar MS, Pereira MP, Werner MU, Martensson J, Larsson HB, Dahl JB. Secondary hyperalgesia phenotypes exhibit differences in brain activation during noxious stimulation. PLoS One. 2015;10(1):e0114840. doi:10.1371/journal.pone.0114840
31. Chung E, Zelinski LM, Ohgami Y, Shirachi DY, Quock RM. Hyperbaric oxygen treatment induces a 2-phase antinociceptive response of unusually long duration in mice. J Pain. 2010;11(9):847–853. doi:10.1016/j.jpain.2009.12.004
32. Efrati S, Fishlev G, Bechor Y, et al. Hyperbaric oxygen induces late neuroplasticity in post stroke patients–randomized, prospective trial. PLoS One. 2013;8(1):e53716. doi:10.1371/journal.pone.0053716
33. Ablin JN, Efrati S, Buskila D. Building up the pressure on chronic pain. Clin Exp Rheumatol. 2016;34(2 Suppl 96):S3–5.
34. Jansen T, Mortensen CR, Tvede MF. It is possible to perform a double-blind hyperbaric session: a double-blinded randomized trial performed on healthy volunteers. Undersea Hyperb Med. 2009;36(5):347–351.
35. Clarke D. Effective patient blinding during hyperbaric trials. Undersea Hyperb Med. 2009;36(1):13–17.
36. PSYCHOLOGY. Estimating the reproducibility of psychological science. Science. 2015;349(6251):aac4716. doi:10.1126/science.aac4716
37. Richarson AD, Scott DA, Zagnitko O, Aza-Blanc P, Chang CC, Russler-Germain DA. Registered report: IDH mutation impairs histone demethylation and results in a block to cell differentiation. Elife. 2016;5:e10860. doi:10.7554/eLife.10860
38. Verbraecken J, Van de Heyning P, De Backer W, Van Gaal L. Body surface area in normal-weight, overweight, and obese adults. A comparison study. Metabolism. 2006;55(4):515–524. doi:10.1016/j.metabol.2005.11.004
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.