Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 7
A single center, pilot, double-blinded, randomized, comparative, prospective clinical study to evaluate improvements in the structure and function of facial skin with tazarotene 0.1% cream alone and in combination with GliSODin® Skin Nutrients Advanced Anti-Aging Formula
Authors Goldberg L, Crysler C
Received 15 November 2013
Accepted for publication 31 January 2014
Published 14 May 2014 Volume 2014:7 Pages 139—144
DOI https://doi.org/10.2147/CCID.S57600
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Lawrence D Goldberg, Corina Crysler
Shaft Medical San Diego, San Diego, CA, USA
Background: Superoxide dismutase (SOD) reduces the reactive oxygen species formation associated with oxidative stress. An imbalance between free radicals and antioxidants can lead to accelerated aging. GliSODin® Skin Nutrients Advanced Anti-Aging Formula (GAAF) is an SOD-containing dietary nutricosmetic formulated with other nutraceuticals that promote improvements in the structure and function of the skin, including hydration, elasticity, structural integrity, and photoaging caused by oxidative stress. Tazarotene cream 0.1% (TAZ) is a United States Food and Drug Administration-approved drug indicated for use in the mitigation of facial fine wrinkling, facial mottled hyper- and hypopigmentation, and benign facial lentigines when taken in conjunction with a comprehensive skin care and sun avoidance program.
Objective: To determine if the antioxidant, anti-aging, hydrating and skin-rejuvenating properties of GAAF complement the retinoic actions of TAZ to improve the structure and function of facial skin.
Method: A 90-day comparative study of ten subjects with facial photodamage; daily topical application of TAZ was used in combination with three capsules of GAAF (780 mg each) or placebo orally, with food, per the randomization allocation.
Results: After 90 days of treatment, TAZ alone and in combination with GAAF improved fine wrinkles (↓1.2 versus 2.0), mottled hyperpigmentation (↓2.2 versus 2.8) and overall photodamage (↓1.0 versus 1.8), as well as patient-reported response to treatment (↓2.0 versus 1.6). At week 12, TAZ/GAAF combination treatment (Group A) versus TAZ treatment alone (Group C) was of significant clinical benefit, with respect to fine wrinkling (14.7%/41.7%), overall photodamage (15.6%/53.0%), skin moisture (19.1%/103.2%), skin elasticity (12.8%/87.7%), and response to treatment (8.8%/21.4%).
Conclusion: The study suggests GAAF in combination with TAZ is safe and provides significant clinical benefit with relative improvement in facial fine wrinkling, overall photodamage, skin moisture and elasticity.
Keywords: antioxidant, superoxide dismutase, anti-aging, retinoic, combined therapy, facial photodamage, nutraceuticals
Introduction
Facial skin can be especially susceptible to the effects of photodamage and aging. A familiar but proven way1,2 to ameliorate those effects is to use the retinoid actions of creams such as tazarotene (TAZ) cream 0.1% (Avage®; Allergan Inc., Irvine, CA, USA), a compound that is related to vitamin A and has been approved by the United States Food and Drug Administration (FDA) to treat some of the effects of aging in facial skin.
Another effective treatment relies on the use of dietary supplements that contain superoxide dismutase (SOD), an enzyme which reduces the reactive oxygen species formation associated with oxidative stress. Aging diminishes the overall activity of SOD and increases sensitivity to oxidative stress. GliSODin® Skin Nutrients Advanced Anti-Aging Formula (GAAF; Isocell North America Inc., Toronto, ON, Canada) is a bioactive SOD-containing dietary nutricosmetic combined with other nutraceuticals that improve skin hydration, elasticity, firmness, and structural integrity; it also helps alleviate the symptoms of photoaging caused by oxidative stress. Taken orally, nutricosmetics contain precisely tailored formulas that combine the nutritional components of nutraceuticals, such as antioxidants and vitamins, with the pharmaceutical and skin-enhancing properties of cosmetics.
This study tested whether the combined use of GAAF with TAZ cream would offer a more effective way to reduce wrinkles, dark skin patches, mottled hyperpigmentation, and overall photodamage. We also looked at whether it would improve patient-reported response to treatment and patients’ self-image.
This study found that daily intake of the GAAF formula boosts the anti-aging effects of using TAZ cream daily. The combined treatment proved significantly more effective than the use of TAZ treatment alone, particularly in improving the extent of fine wrinkling and overall photodamage, skin moisture and elasticity, and response to treatment. Complementary studies suggest that because supplements continuously deliver bioactive compounds to all skin compartments through the blood, the ingestion of antioxidants such as GAAF could play a vital role in enhancing the effect of anti-aging creams.
Material and methods
As this was a pilot study, the statistical analyses were descriptive and were intended to provide only estimates of key trial parameters and to inform power calculations for a potential definitive trial. A formal sample size calculation and formal statistical comparisons between randomization groups were not undertaken. Potential risks associated with this study included redness, burning, dryness, stinging, and irritated and peeling skin. Because TAZ can harm a fetus if administered during pregnancy, pregnant and nursing women were precluded from participating in the study. Adverse reactions were mild and the study did not record any instances of medication discontinuation or study withdrawals.
Use of TAZ and GAAF
Related to vitamin A, TAZ is a compound cream that has been approved by the FDA to mitigate fine facial wrinkles and treat mottled light and dark skin patches on the face and noncancerous freckles; it is intended for use in conjunction with a comprehensive skin care and sun avoidance program.
SOD reduces reactive oxygen species formation associated with oxidative stress. Aging decreases SOD activity and increases sensitivity to oxidative stress.
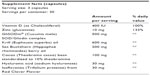
GAAF contains a bioactive form of SOD called GliSODin® that can improve hydration, elasticity, structural integrity, and reduce photoaging caused by oxidative stress. The unique combination of primary ingredients in GAAF – which include krill oil, sea buckthorn berry oil, cacao bean extract, and hyaluronic acid – work together to keep skin moist, reduce redness and skin irritation, promote skin clarity and immunity, and improve skin firmness. The active ingredients of the GAAF formula include 1) GliSODin®, which provides the SOD that boosts the body’s antioxidant defense system and helps neutralize free radicals in the skin and reduces inflammation; 2) krill oil, another antioxidant whose omega-3s hydrate the skin and also reduces inflammation; 3) zinc, which reduces inflammation within the skin (and thereby ameliorates such conditions as adult acne); 4) vitamin D, which significantly enhances skin metabolism and growth; 5) sea buckthorn berry oil, which hydrates skin and helps make skin tone more uniform; 6) cacao (cocoa) bean extract, which improves dermal circulation and thereby helps deliver key nutrients to the skin; 7) hyaluronic acid, which hydrates skin and enriches its elasticity; and 8) red clover isoflavones, which both hydrate skin and help modulate the hormonal fluctuations that can mar skin complexion. In addition to helping protect the skin from environmental damage and free radicals (which harm cell membranes, proteins, and DNA, thereby causing skin aging and wrinkling), these ingredients significantly help rehydrate and rejuvenate skin.
The proven antioxidant properties of these ingredients also reduce fine lines and wrinkles, skin irritation, hyperpigmentation, and the inflammation that causes dry flaky skin, rashes, and redness. Conversely, the GliSODin® formula increases the skin-enhancing activities of SOD, improves skin microcirculation, elasticity and healing, maintains the structural integrity of collagen and skin, and serves as an immunomodulator that can boost the functioning of the immune system. The purpose of this study is to determine if treatment with TAZ cream will be enhanced if used with the SOD-containing GAAF nutricosmetic capsule.
All subjects used a pea-sized amount of TAZ nightly on their face and were required to use sun protection factor (SPF)-15 sunscreen, daily. All subjects took either placebo or GAAF capsules (780 mg each) orally three times a day with food.
The study relied on both visual evaluations of the subjects’ faces and measurements of their facial skin attributes. Researchers took digital photographs of the subjects’ faces at each of the four visits to measure damage to skin caused by the sun and responses to treatment. They also measured the amount of moisture and other properties of subjects’ skin by placing a probe on different portions of their faces.
Independent consultants conducted blind reviews of the subjects’ photos to evaluate, at each stage, facial fine wrinkling, mottled hyperpigmentation, overall photodamage, and global response to treatment. They also tested facial skin for moisture, sebum, and elasticity. The measurement of skin moisture was based on the capacitive (Corneometer®; Courage + Khazaka Electronic Gmbh, Cologne, Germany) method. An adequate moisture content of the stratum corneum is essential for a well-functioning skin barrier. Only an intact natural moisturizing factor provides an effective scavenging function against radicals and nitrogen oxides penetrating from the outside; it consequently protects the skin against premature skin aging. Hence, the moisture content is the most important parameter of skin diagnosis and measured with the Corneometer®. The analysis of the moisture retention capacity of the skin is easy to perform, based on the dielectric constant of the water and measured in the superficial layers of the stratum corneum as deep as 10–20 μm to ensure that the measurement is not influenced by capillary blood vessels.3 Moisture measurements of the forehead, both cheeks, and the chin were taken for women; moisture measurement of men’s chins was not recommended because of the possibility of excessive facial hair.
Researchers measured sebum on the forehead and left cheek using the photometric (Sebumeter® Courage + Khazaka Electronic Gmbh) method, the grease spot photometer. They measured skin elasticity around the left eye area (the “crow’s feet” region) based on the suction method; in this method, skin is drawn slightly into the aperture of the probe via negative pressure. After a few seconds, the negative pressure ceases, the skin relaxes, and the penetration depth of the skin is determined optically during suction and relaxation. Melanin was assessed at baseline, based on the absorption principle, using the Multi Skin Test Center® MC 750 (Courage + Khazaka Electronic GmbH, Cologne, Germany). The Sebumeter® provides the most widely-used method to reproducibly and accurately determine the sebum level of the skin surface, as well as on scalp and hair. This is documented by the numerous mentions in dermatology and cosmetology literature in which the terms “sebumetry” and sebum measurements are inseparable.4 A researcher uses a probe to emit a defined quantity of light to the face in three defined wavelengths, and a receiver measures the light the skin reflects and absorbs.
Subjects answered questions about their appearance, body image, and response to treatment on three separate questionnaires. In addition, subjects assessed their own distress and dysfunction regarding problems of appearance using the Derriford Appearance Scale 24 (DAS24),5 to issues regarding body image using the Body Image Concern Inventory (BICI),6,7 and to facial overall photodamage using a five-point scale.
Inclusion versus exclusion
To participate in the study, subjects had to be healthy males or healthy non-pregnant, non-lactating females over the age of 18 years with a Fitzpatrick skin type of I to IV and no upper age limit. Facial measurement of melanin was used to help determine Fitzpatrick skin type. Subjects all had at least mildly severe fine wrinkling and mottled hyperpigmentation. The subjects could not be using photosensitizing medications, anticoagulation or cortisone therapies, or facial photoaging or hyperpigmentation treatments. They had to be free of skin diseases, any known metabolic disorders such as diabetes, and any known malignancies. They could not have recent or current histories of chemotherapy, inflammation within the treatment area, or allergies to gluten or known hypersensitivity to any component(s) or excipient(s) of the study products. All participants had to agree to use a sunscreen with SPF ≥15 and avoid prolonged exposure to ultraviolet (UV) light during the study. Potential risks associated with this study included redness, burning, dryness, stinging, and irritated and peeling skin. Because TAZ can harm a fetus if administered during pregnancy, pregnant and nursing women were precluded from participating in the study. Adverse reactions were mild and the study did not record any instances of medication discontinuation or study withdrawals.
Subjects who scored >72 at screening on the BICI6,7 were excluded from the study as these scores indicated body dysmorphic disorder. Subjects who missed either, more than 2 consecutive days of dosing, or more than four total doses of their study agent per monthly interval were considered non-compliant and not included as part of the efficacy analyses.
Procedures
Prior to initiation of any study-related procedures, subjects signed and dated a WIRB Institutional Review Board approved written informed consent to participate in the study. The study contained ten subjects – eight females and two males. The eight females included four Caucasian, two Hispanic, and two Asian women. The two males were Caucasian, one presenting in the study with facial hair that may have prevented some evaluations completed by evaluators.
Subjects were randomized in a 1:1 ratio of TAZ + GAAF and TAZ + placebo. Five subjects were given TAZ + GAAF; four females and one male. Five subjects were given TAZ + placebo; four females and one male.
Researchers evaluated changes, relative to baseline, in fine facial wrinkling and mottled hyperpigmentation, and in facial skin moisture, sebum, and elasticity over 3 months.
They also evaluated, relative to baseline, changes in overall photodamage and global response to treatment over that period. Finally, they scored changes, relative to baseline, in individual and total BICI and DAS24 self-scoring and in facial skin treatment response scales over the same period.
Blinded reviewer assessments addressed the facial fine wrinkling, mottled hyperpigmentation, overall photodamage, and global response to treatment scores using absolute scores and percent change from baseline as follows: ([mean post-treatment score − mean baseline score]/baseline score) ×100. Negative values reflected improvement and positive values indicated worsening compared to baseline.
The number and percentage of subjects with improvement of at least one-grade (clinical improvement) were summarized by treatment group and visit. A one-grade improvement was considered to be significant and relevant.
Results
TAZ by itself, and in combination with GAAF, improved fine wrinkles (↓1.2 versus 2.0), mottled hyperpigmentation (↓2.2 versus 2.8), and overall photodamage (↓1.0 versus 1.8), as well as patient-reported response to treatment (↓2.0 versus 1.6). At 3 months, the efficacy of the TAZ/GAAF combination treatment was significantly greater than the TAZ treatment alone, as measured by absolute and relative comparative improvement with regard to the parameters of fine wrinkling (14.7%/41.7%), overall photodamage (15.6%/53.0%), skin moisture (19.1%/103.2%), skin elasticity (12.8%/87.7%), and response to treatment (8.8%/21.4%). The combination of GAAF and TAZ was shown to reduce skin inflammation, help repair damaged skin, and reduce the effects of various serious skin conditions, such as psoriasis, rosacea, and eczema (Tables 1 and 2).
  | Table 1 Data summary of change in endpoints from baseline to 90 days |
Absolute scores and percent change from baseline in blinded reviewer assessments (facial fine wrinkling, mottled hyperpigmentation, overall photodamage, and global response to treatment scores) were summarized by treatment group and visit using descriptive statistics. Percent changes in score have been calculated as follows: ([mean post-treatment score − mean baseline score]/baseline score) ×100. Negative values indicated improvement and positive values indicated worsening compared to baseline.
The blinded reviewer assessments for the number and percentage of subjects with improvement of at least one-grade (clinical improvement) were summarized by treatment group and visit. A one-grade improvement was considered to be both noticeable and relevant to the subject as well as to the investigator.
Discussion
The purpose of this study was to determine if the antioxidant properties of GAAF would complement the retinoic actions of TAZ to improve the structure and function of facial skin.
Subjects were encouraged to use moisturizers or emollients before or after applying TAZ to increase its efficiency, but such usage could also have skewed results.
Clinicians evaluated facial conditions using objective criteria to avoid subjective interpolations; they evaluated fine wrinkling and mottled hyperpigmentation using a five-point scale, overall photodamage using a six-point scale, and global response to treatment using a seven-point scale. The evaluator controlled for variability by undertaking all facial skin measurements at normal room conditions (20°C ±2°C and 40%–60% air humidity). Efficacy was evaluated by an independent consultant through blinded review of photos to assess facial fine wrinkling, mottled hyperpigmentation, overall photodamage, and global response to treatment.
Nutricosmetics, which are already widely used throughout Europe and Asia, have the potential to dramatically improve skin care throughout North America. Numerous studies have shown that the ingredients in GAAF offer a full spectrum of anti-aging and skin enhancing features. Krill oil, for example, has been documented to reduce chronic skin inflammation (as well as numerous symptoms of arthritis) within short treatment periods.8 It is an excellent source of the antioxidant carotenoid astaxanthin, which contains ten times more antioxidant activity than beta-carotene and up to 1,000 times more than vitamin E; krill oil also contains more eicosapentaenoic acid per gram than most fish oil capsules.9 In an in vitro study, the astaxanthin in krill oil was found to be a highly effective antioxidant and to protect against the effects of the sun’s UV radiation.10
Hyaluronic acid can benefit any tissue that needs hydration (especially aging skin) or lubrication, reduces inflammation, and provides notable antioxidant and free radical scavenging effects.11 It also has been demonstrated to improve and accelerate the healing process of chronic wounds and the healing of acute wounds.12 Other studies have shown that hyaluronic acid also improves the functioning of inflammatory mediators to reduce cell and skin degradation, and serves as an antioxidant that reduces the amount of reactive oxygen species.13
Enhancing antioxidant and immune functions, zinc also maintains the integrity of biological membranes, plays a critical role in skin metabolism and repair, and has been shown to reduce inflammatory acne.14,20 Zinc has also been found to be an important antioxidant that protects against free radical-induced oxidative damage and UV radiation; it too, improves the healing of skin.15
Isoflavones have been shown to help inhibit the aging of skin induced by UV light in mice, and photodamage caused to skin, and attendant discomfort, in humans.16 They also significantly reduce fine wrinkles and improve skin elasticity.17 The consumption of flavanol-rich cocoa has been demonstrated to acutely increase dermal blood flow and oxygen saturation; it has also been shown to increase endogenous photoprotection, enhance dermal blood circulation, reduce the roughness and scaling of skin surfaces, and improve hydration variables.18,21
The GAAF containing the unique combination of the above ingredients (and others) is available commercially from GliSODin Skin Nutrients, Professional Nutricosmetics (Isocell North America Inc.).
The concept of dietary antioxidants has been studied previously as a means to provide nutritional support for aging skin. In their study, Buonocore et al began by observing that the traditional way to administer antioxidants was through topical application; they proposed that dietary supplements might be more efficacious, however, because administration into the blood delivers nutraceutical bioactive compounds continuously to all skin compartments.19 If, as Buonocore et al propose, dietary antioxidants can reduce the adverse effects of reactive oxygen species on skin aging, antioxidant substances and bioactive food compounds administered at pharmacological doses in combination with TAZ and GAAF could be especially efficacious.
Conclusion
The results of this study suggest that the addition of the antioxidant GAAF to the treatment of facial photodamage with TAZ is safe and provides significant clinical benefit with respect to key observed physician-reported scales and patient-reported response to treatment, with relative improvement in facial fine wrinkling, overall photodamage, skin moisture and elasticity. The antioxidant properties of GAAF can provide a safe and effective treatment for facial skin when combined with the retinoic actions of TAZ cream based on this clinical study. When supplemented by other preventive measures, combined use of TAZ and GAAF could help people with sun-damaged facial skin.
Acknowledgments
The study was funded by a grant from Isocell North America Inc., the manufacturer of GliSODin® products.
Disclosure
CC is an employee of Isocell. The authors report no other conflicts of interest in this work.
References
Lowe N, Gifford M, Tangehtti E et al. Tazarotene 0.1% cream versus tretinoin 0.05% emollient cream in the treatment of photodamaged facial skin: a multicenter, double-blind, randomized, parallel-group study. J Cosmet Laser Ther. 2004;6(2):79–85. | |
Phillips TJ, Gottlieb AB, Leyen JJ et al. Tazarotene Cream Photodamage Clinical Study Group. Efficacy of 0.1% tazarotene cream for the treatment of photodamage: a 12-month multicenter, randomized trial. Arch Dermatol. 2002;138(11):1486–1493. | |
Corneometer® CM 825 [webpage on the internet]. Leichlingen, Germany: Kosmetik Konzept KOKO GmbH and Co; 2012. Available from: http://www.dermaviduals.com/english/skin-testing/lab-probes/corneometer-cm-825.html. Accessed June 23, 2013. | |
Sebumeter® SM 815 [webpage on the internet]. Leichlingen, Germany: Kosmetik Konzept KOKO GmbH and Co; 2012. Available from: http://www.dermaviduals.com/english/skin-testing/lab-probes/sebumeter-sm-815.html. Accessed June 23, 2013. | |
Carr T, Moss T, Harris D. The DAS24: A short form of the Derriford Appearance Scale DAS59 to measure individual responses to living with problems of appearance. Br J Health Psychol. 2005;10:285–298. | |
Littleton HL, Axsom D, Pury CLS. Development of the body image concern inventory. Behav Res Ther. 2005;43:229–2241. | |
Littleton H, Breitkopf CR. The Body Image Concern Inventory: Validation in a multiethnic sample and initial development of a Spanish language version. Body Image. 2008;5:381–388. | |
Deutsch L. Evaluation of the effect of Neptune Krill Oil on chronic inflammation and arthritic symptoms. J Am Coll Nutr. 2007;26(1): 39–48. | |
Alternative sources of omega-3 fatty acids [webpage on the internet]. Boulder, CO: NewHope360; 2013. Available from: http://newhope360.com/news-amp-analysis/alternative-sources-omega-3-fatty-acids. Accessed June 23, 2013. | |
Natural Standard Professional Monograph. Available from: http://www.naturalstandard.com/index-abstract.asp?create-abstract=patient-astaxanthin.asp&title=Astaxanthin. Accessed June 23, 2013. | |
Nick GL. The Disease-Fighting Effects of Hyaluronan. Townsend Letter for Doctors and Patients. 2004;249:131–132. | |
Voinchet V, Vasseur P, Kern J. Efficacy and safety of hyaluronic acid in the management of acute wounds. Am J Clin Dermatol. 2006;7(6): 353–357. | |
Moreland L. Intra-articular hyaluronan (hyaluronic acid) and hylans for the treatment of osteoarthritis: mechanisms of action. Arthritis Res Ther. 2003;5(2):54–67. | |
Chien XX, Zafra-Stone S, Bagchi M, Bagchi D. Bioavailability, antioxidant and immune-enhancing properties of zinc methionine. Biofactors. 2006;27(1–4):231–244. | |
Rostan EF, DeBuys HV, Madey DL, Pinnell SR. Evidence supporting zinc as an important antioxidant for skin. Int J Dermatol. 2002;41(9): 606–611. | |
Skin health [webpage on the internet]. March 2007. Available from: http://www.pharmpress.com/shop/samples/nutraceuticals_2ed_sample.pdf. Accessed June 23, 2013. | |
Aging Skin [webpage on the internet]. Moreno Valley, CA: iHerb Inc.; 2013. Available from: http://healthlibrary.epnet.com/GetContent.aspx?token=e0498803-7f62-4563-8d47-5fe33da65dd4&chunkiid=38389. Accessed June 23, 2013. | |
Neukam K, Stahl W, Tronnier H, Sies H, Heinrich U. Consumption of flavanol-rich cocoa acutely increases microcirculation in human skin. Eur J Nutr. 2007;46(1):53–56. | |
Buonocore D, Lazzeretti A, Tocabens P, et al. Resveratrol-procyanidin blend: nutraceutical and antiaging efficacy evaluated in a placebo-controlled, double-blind study. Clin, Cosmet Investig Dermatol. 2012;5: 159–165. | |
Dreno B, Amblard P, Agache P, Sirot S, Litoux P. Low doses of zinc gluconate for inflammatory acne. Acta Derm Venereol. 1989;69(6): 541–543. | |
Heinrich U, Neukam K, Tronnier H, Sies H, Stahl W. Long-term ingestion of high flavanol cocoa provides photoprotection against UV-induced erythema and improves skin condition in women. J Nutr. 2006;136(6): 1565–1569. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.